Adenine phosphoribosyltransferase (APRT) enzyme deficiency is an important and potentially reversible cause of progressive chronic kidney disease. It is a rare, autosomal recessive disorder that was first described in 19741 with more than 40 known mutations2, 3 and estimated prevalence of 1 in 50,000 to 100,000 persons.2, 4, 5 The APRT enzyme is important for the conversion of adenine to adenosine monophosphate in the salvage purine pathway. Deficiency of this enzyme results in the metabolism of adenine into 2,8-dihydroxyadenine (DHA) by xanthine oxidase (xanthine dehydrogenase) (Figure 1a). DHA is highly insoluble in urine and will precipitate to cause either urolithiasis or crystal nephropathy. There are no known extrarenal manifestations and reasons for this remains unclear given the extensive tissue distribution of this enzyme.
Figure 1.
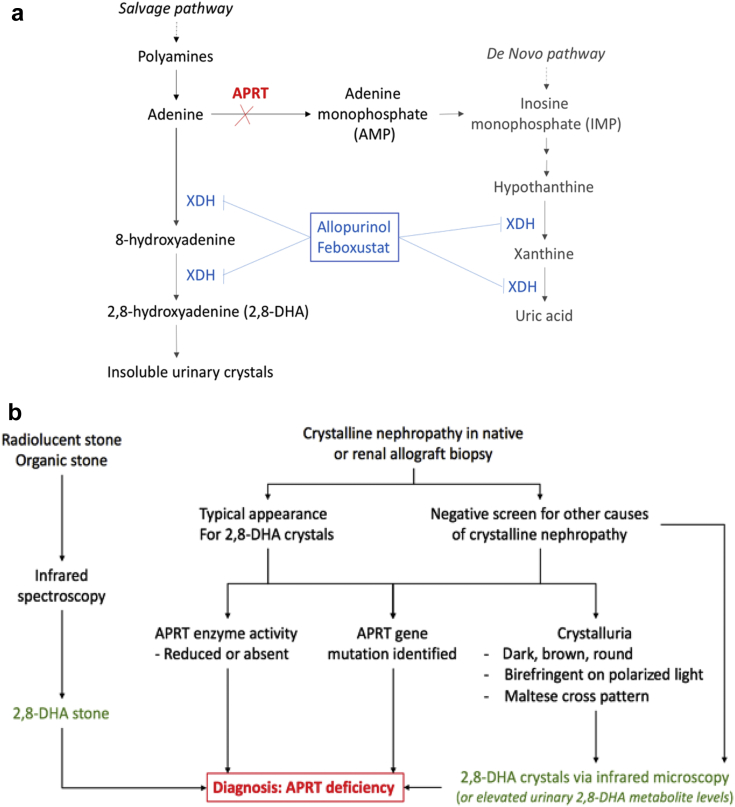
(a) Purine metabolism pathway. In the absence of adenine phosphoribosyltransferase (APRT) enzyme activity, adenine is converted into 8-hydroxyadenine and 2,8-dihydroxyadenine (DHA) by xanthine oxidase (XDH, also known as xanthine dehydrogenase). DHA becomes insoluble and precipitates in the urine to cause crystal nephropathy and/or urolithiasis. (b) Suggested diagnostic pathway for APRT deficiency. Patients presenting with urolithiasis and found to have “organic” stone on analysis ideally should have the stone analyzed by infrared mass spectroscopy to confirm DHA composition. Patients presenting with crystalline nephropathy of uncertain cause, or with the typical appearance for DHA crystals, should have confirmation testing by (i) demonstration of reduced or absent APRT enzyme activity on red cell lysates, (ii) identification of homozygous gene mutation, or (iii) crystalluria sent for infrared mass spectroscopy to confirm composition.
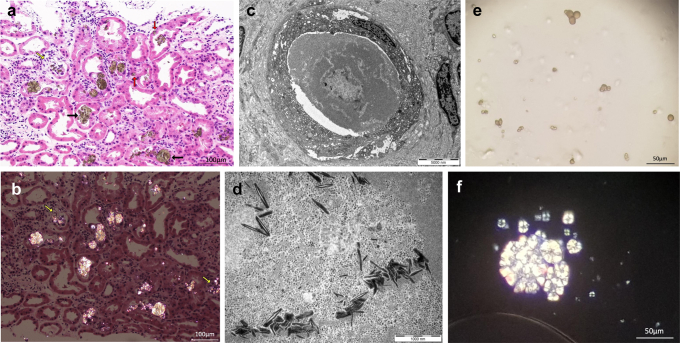
The typical appearances of DHA crystals on renal biopsy are of yellow-brown needle-shaped crystals, which are arranged in spherical, radial, or irregular aggregates and are birefringent under polarized light. Tubular injury, deposition, and obstruction can occur, and foreign body reaction with histiocytes can be seen surrounding some of the crystals. Left untreated, chronic crystal deposition in the kidney can lead to irreversible tubular atrophy and interstitial fibrosis. Crystalluria is characterized by birefringent yellow-brown crystals that are round in appearance and display a Maltese cross pattern on polarized light microscopy.
Although it can present in any age group and stage of disease, it is most commonly diagnosed in adults (median age 36 years2), with up to 15% of patients diagnosed either in end-stage renal failure or following kidney transplantation.3, 4 APRT deficiency also may be misdiagnosed, as other forms of renal stone disease, crystal nephropathy, or chronic kidney disease of unknown etiology.2 The diagnosis of APRT deficiency in patients with urolithiasis or crystal nephropathy is based on the following: (i) genetic mutation testing; (ii) absent or reduced APRT enzyme activity in red cell lysates; or (iii) confirmation of DHA crystal composition by infrared spectroscopy (Figure 1b).
Reducing DHA production by xanthine oxidase inhibition is the cornerstone of pharmacological therapy for APRT deficiency. Allopurinol is the most used drug in this instance, whereas the addition or switch to febuxostat has been used in several cases.2, 6 Supportive therapy includes low purine diet and high fluid intake. Urinary alkalinization is ineffective, as DHA remains insoluble at physiological urine pH ranges.5
We present 3 cases (Tables 1 and 2) that highlight the diagnostic pitfalls and challenges in management, particularly in the transplantation setting. For further details to this article, please refer to the Supplementary Methods and Supplementary References.
Table 1.
Baseline characteristics of the 3 patients, before transplantation in cases 1 and 2
| Patient characteristics | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age, yr, at diagnosis | 47 | 52 | 23 |
| Gender | Male | Female | Male |
| Race | Caucasian | Lebanese | Lebanese |
| Comorbidities | Obesity, hypertension, atrial fibrillation | Graves disease | Obesity, atrial flutter |
| Urolithiasis | No | No | Yes |
| Family history of kidney disease | No | No | No |
| Creatinine at presentation, μmol/l | 356 | 268 | 1020 |
| Proteinuria, g/24 h | 0.92 | 1.09 | 0.23 |
| Microscopic hematuria | No | No | No |
| Native kidney biopsy | LM: 15/18 globally sclerosed glomeruli; tubular, epithelial and interstitial birefringent, brown crystal deposition with diffuse interstitial inflammation and fibrosis. IF: nondiagnostic EM: Needle-shaped crystals in some tubules |
LM: 6 of 10 globally sclerosed glomeruli; golden brown, birefringent tubular crystals with associated diffuse interstitial fibrosis and inflammation. IF: nondiagnostic EM: nondiagnostic |
LM: 5 of 17 globally sclerosed glomeruli; yellow- brown, birefringent tubular crystals with associated diffuse interstitial fibrosis. IF: nondiagnostic EM: nondiagnostic |
| Provisional diagnosis | APRT deficiency | Chronic interstitial nephritis | Oxalate nephropathy |
| Time from presentation to dialysis (modality) | 2 mo (hemodialysis) | 3 yr (peritoneal dialysis) | <1 wk (hemodialysis) |
| Time from native biopsy to APRT diagnosis | 10 wk | 6 yr | 10 mo |
| Genetic testing | Not done | Homozygous c.188G>A (p.Gly63Asp) | Homozygous c.188G>A (p.Gly63Asp) |
| APRT enzyme activity, nmol/min per mg | 0.1 | 0.11 | 0.09 |
| Urine stone analysis | NA | NA | Organic material |
| Urine 2,8-DHA | 28 mmol/mol | Not available | Not available |
| Urine biochemistry | Hypocitraturia | Hypocitraturia | Hyperoxaluria |
| Urine microscopy | Yes, but assessed only posttransplant | Birefringent crystals | |
APRT, adenine phosphoribosyltransferase; EM, electron microscopy; IF, immunofluorescence; LM, light microscopy; mo, month; NA, not applicable; wk, week; y, yr.
Normal APRT enzyme activity > 0.15 nmol/min per mg and spot urine 2,8-DHA <13 mmol/mol.
Table 2.
Post–kidney transplant course for patients 1 and 2
| Case 1 | Case 2 | |
|---|---|---|
| Transplantation | January 2018 | May 2017 |
| APRT diagnosis before transplantation | Yes | No |
| Allopurinol dose pretransplant | 150 mg (for 4.5 yr) | No |
| Delayed graft function | Yes | Yes |
| Implantation biopsy | Acute tubular injury | Acute tubular injury |
| Time from transplant to crystal detection, d | 10 | 24 |
| Maximal allopurinol dose, mg | 600 | 300 |
| Oxypurinol trough, mg/l | 21–32 | 30–36 |
| Drug reaction with eosinophilia and systemic symptoms or ocular involvement | Nil | Nil |
| Febuxostat | 40 mg | Nil |
| Crystalluria posttransplant | Detected 4 mo post | Not detected 12 mo post |
| Treatment for rejection | Yes, pulse methylprednisolone | Yes, methylprednisolone and 6 mg/kg antithymocyte globulin (day 24 for Banff grade 2A rejection: i1, t1, v1, ptc2, c4d-ve) |
| Other complications | Invasive CMV disease (graft CMV nephritis; colitis) | BK virus–associated nephropathy Shingles (reactivation of varicella zoster) CMV viremia Urinary tract infections |
| Three-mo posttransplant biopsy | Involved 15% of biopsy (less) No rejection: ci2, ct2, cv1, ah1 |
No rejection: 15% crystals ci3, ct3, cv1, ah0 |
| Three-mo posttransplant GFR/DTPA, ml/min per 1.73 m2 | 52 | 42 |
| Twelve-mo posttransplant biopsy | Not available | No rejection: 15% crystals ci3, ct3, cv1, ah0 |
| Last available creatinine level, μmol/l | 164 (6 mo post) | 145 (12 mo post) |
| Maintenance immunosuppression | Tacrolimus, mycophenolate, prednisolone | Tacrolimus, mycophenolate, prednisolone |
APRT, adenine phosphoribosyltransferase; CMV, cytomegalovirus; DTPA, technetium-99m diethylene-triamine-pentaacetic acid; GFR, glomerular filtration rate; mo, month; yr, year.
Results
Case 1
A 47-year-old Caucasian gentleman presented in 2013 for evaluation of renal impairment on a background of obesity, hypertension and atrial fibrillation. At the time of presentation, the serum creatinine (SCr) was 356 μmol/l (estimated glomerular filtration rate 17 ml/min per 1.73 m2) and subnephrotic proteinuria of 0.92 g/d without microscopic hematuria. This is compared with baseline SCr 119 μmol/l measured 5 years previously. The hemoglobin A1c, autoimmune, and myeloma serology were all unremarkable. Computed tomography scan showed 8.5 cm kidneys without evidence of calculi or hydronephrosis. A native renal biopsy revealed global sclerosis in 15 of 18 glomeruli and multiple, yellow-brown tinged crystals in the tubular lumen, epithelial cytoplasm, interstitium, with associated giant cells, marked tubular atrophy, and diffuse interstitial fibrosis. The crystals ranged from needle to rod to rhomboid in shape and were birefringent under polarized light. Congo red was negative and immunofluorescence was nondiagnostic. Electron microscopy revealed unremarkable glomerular features, no evidence of electron-dense deposits, but noted needle-shaped crystals in some tubules. A 24-hour urine collection was unremarkable except for hypocitraturia (0.4 mmol/d).
Given the unusual crystal appearance, APRT deficiency was suspected and subsequent testing confirmed this diagnosis with reduced APRT enzyme activity (0.1 nmol/min per mg; normal >0.15 nmol/min per mg) and elevated urinary 2,8-dihydroxyadenine (28 mmol/mol; normal < 13 mmol/mol). Despite treatment with allopurinol (100 mg/d), he progressed to be dialysis dependent 2 months after presentation.
His allopurinol was increased to 150 mg daily when he received a deceased renal transplant in January 2018. He had delayed graft function and the implant biopsy showed acute tubular injury, and a repeat biopsy at day 10 demonstrated recurrence of crystal nephropathy with borderline rejection with interstitial inflammation and tubulitis. He received intravenous methylprednisolone (total of 1500 mg), and allopurinol was increased to 400 mg daily. Subsequent biopsy on day 21 showed similar findings (crystal nephropathy, borderline rejection) and he received further pulse methylprednisolone. His renal function improved, no longer needing dialysis at 3 weeks posttransplant, and reached a trough SCr 180 μmol/l. His allopurinol was up-titrated to a maximum tolerated dose of 600 mg daily (limited by diarrhea) and trough oxypurinol levels varied between 21 and 32 mg/l. Surveillance urine microscopy (Figure 2e and f) showed persistent crystalluria and 20 mg of feboxustat was introduced, in addition to allopurinol 600 mg daily.
Figure 2.
(a) Light microscopy renal biopsy images of adenine phosphoribosyltransferase (APRT) deficiency. Hematoxlyn and eosin stain (H&E; original magnification ×20) of the 3-month protocol transplant biopsy of case 1 showing ongoing crystal deposits, predominantly in the tubular lumen (black arrows) and associated with foreign body reaction and acute tubular injury. Crystals also are seen within the epithelial layer in some of the injured or atrophic tubules (red arrows). One tubule demonstrated the disruption of the tubular basement membrane and the release of crystalline material into the intersititum (yellow arrow). (b) Polarized image of the renal biopsy (of Figure 1a) demonstrating birefringent crystals and highlighting the locations of the crystals, which are more subtle on the H&E sections. The areas of tubular rupture and the release of crystals into the interstitium are highlighted (yellow arrows). Bar = 100 μm. (c) Electron microscopy of APRT deficiency demonstrating luminal obstruction with the crystalline aggregates. Bar = 5000 nm. (d) Higher-power view of the electron microscopy image within the luminal aggregate (in Figure 2c) revealing needle-like substructures. Bar = 1000 nm. (e) Crystalluria in APRT deficiency. Typical appearance of spherical, brown crystals seen on urine microscopy at original magnification of ×20. (f) Crystalluria in APRT deficiency under polarized light demonstrating birefringent crystals with a maltese cross pattern at original magnification of ×40.
Four-months after transplantation, he developed invasive cytomegalovirus (CMV) disease and a repeat biopsy (Figure 2a–d) with SCr 270 μmol/l showed acute tubular injury, crystalline nephropathy, and CMV inclusions. His renal function improved to SCr 164 μmol/l following treatment of CMV disease and up-titration of feboxustat to 60 mg daily. He developed a facial rash, which was diagnosed as rosacea and resolved with a short course of doxycycline.
Baseline characteristics of all 3 cases and the posttransplant course are summarized in Tables 1 and 2.
Case 2
A 52-year-old Lebanese woman was investigated for progressive kidney disease with SCr 97 μmol/l rising to 268 μmol/l (estimated glomerular filtration rate 54 and 17 ml/min per 1.73 m2, respectively) over 4 months. She had subnephrotic proteinuria (1.09 g/24 hours) without microscopic hematuria, and the renal ultrasound showed atrophic left kidney and 12.5 cm right kidney. The renal biopsy showed diffuse interstitial inflammation and fibrosis with the occasional golden brown, birefringent intratubular crystals, and this was attributed to ciprofloxacin-associated chronic interstitial disease, and she progressed onto peritoneal dialysis 3 years later.
She received a deceased renal transplant and required 2 sessions of hemodialysis for delayed graft function. A biopsy day 3 posttransplant showed acute tubular injury and borderline rejection, which was treated with methylprednisolone. Her renal function improved to SCr 220 μmol/l but then deteriorated to SCr 262 μmol/l after a period of subtherapeutic tacrolimus levels. A biopsy at day 24 showed Banff grade 2A T-cell–mediated rejection (Banff scores: i1, t1, v1, ptc2), positive simian virus (SV40) stain, and tubular and interstitial crystal deposition.7 She received antithymocyte globulin (total 6 mg/kg) with improvement from peak SCr 327 μmol/l to SCr 243 μmol/l.
Oxalate nephropathy was suspected, but 24-hour urine metabolic screen was unremarkable except for hypocitraturia (<2 mmol/d). With the experience in case 1, we tested for and confirmed APRT deficiency with reduced APRT enzyme activity (0.11 nmol/min per mg). Genetic testing confirmed homozygous c.188G>A; p.Gly63Asp mutation (an identified pathogenic APRT gene mutation). Urine DHA testing was now unavailable. The patient was commenced on allopurinol at 5 weeks posttransplant, tolerating 300 mg/d (corresponding trough oxypurinol levels 35–36 mg/l), again, limited by diarrheal symptoms.
Despite the positive SV40 stain, both blood and urine polymerase chain reaction testing for polyoma and BK virus were negative and hence not initially treated. A repeat biopsy 2 weeks later showed persistent crystals and positive SV40 in 2 nuclei and despite repeatedly negative blood and urine polyoma virus on polymerase chain reaction, so she was treated with intravenous immunoglobulin (IV.Ig) with improvement of SCr to 130 to 150 μmol/l. The 3-month protocol glomerular filtration rate of 42 ml/min per 1.73 m2 (technetium-99m diethylene-triamine-pentaacetic acid) and protocol biopsy was clear from rejection, SV40 staining but had persistence of tubular crystal deposition (associated with detectable crystalluria).
Over the next 9 months, she developed CMV viremia, reactivation of varicella zoster infection, and recurrent urinary tract infections. Her 1-year protocol biopsy (SCr 136 μmol/l, no detectable crystalluria on polarized light microscopy) showed no evidence of rejection or SV40 staining, but persistently detectable birefringent crystals on the biopsy. These were predominantly distributed in the tubular epithelial cells and interstitium; and associated with significant tubular atrophy and interstitial fibrosis. She also developed a mild facial rash around this time, hence the allopurinol was reduced to 200 mg daily (corresponding oxypurinol level 24 ng/ml) with the introduction of feboxustat 40 mg daily.
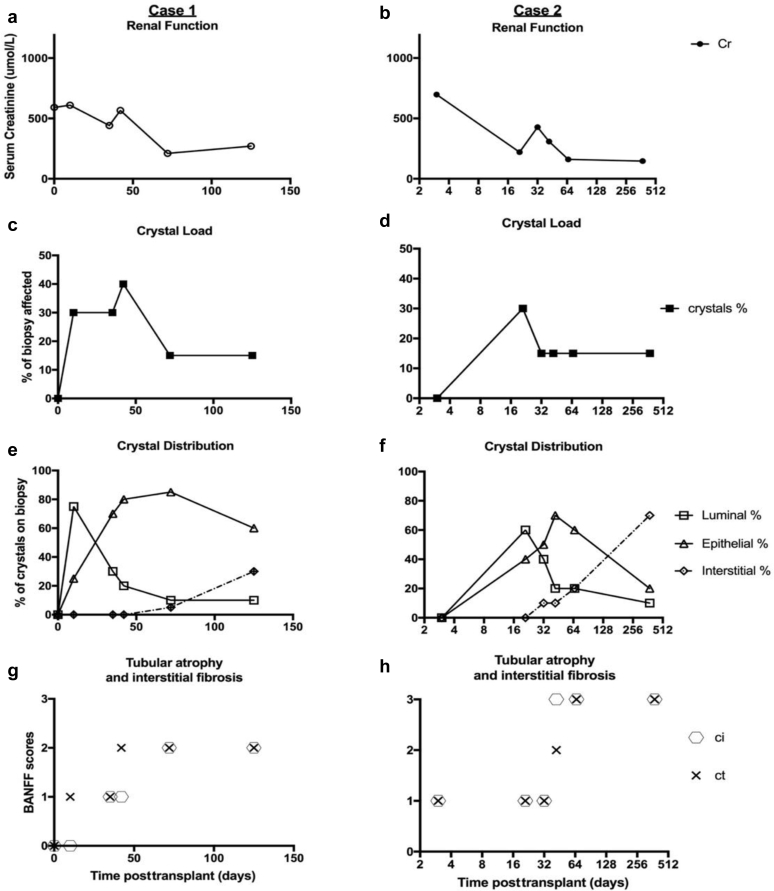
The results of the serum creatinine, crystal load, and tissue distribution at time of transplant (indication and protocol) biopsy of both cases 1 and 2 are presented in Figure 3. The biopsies of both patients showed similar pattern: early in the disease course, during maximal DHA excretion and crystal formation, most crystals are seen in the luminal space. On maximal allopurinol (and/or feboxustat) therapy, there was a progressive decline in the number of crystals seen in the luminal space, with more found in the epithelial layer and interstitial space.
Figure 3.
(a–h) Posttransplant biopsy characteristics (results from all indications and protocol [1-, 3-, and 12-month] biopsies up to July 2018). Case 1 is represented on the left-hand column and case 2 is represented on the right-hand column. Graphs (a) and (b) show the serum creatinine at time of biopsy for case 1 and 2, respectively. Graphs (c) and (d) are the estimated portion of the biopsy section that is affected by crystals and show initial decline, followed by stable levels after allopurinol and/or feboxustat therapy in both patients. Graphs (e) and (f) show the estimated distribution of crystals seen on the renal biopsy: luminal, epithelial, and interstitial compartments. Graphs (g) and (h) demonstrate the tubular atrophy and interstitial fibrosis associated with crystal nephropathy, with severe chronic scores (Banff criteria) seen within the first 12 months posttransplantation.
Case 3
A 23-year-old man of Lebanese descent, who experienced 1 episode of urolithiasis in childhood, presented with oliguric acute kidney injury with SCr 1020 μmol/l (12 months prior with SCr 92 μmol/l). The patient commenced acute hemodialysis given no reversible factors were identified and the renal biopsy showed global sclerosis in 5 of 17 glomeruli, ischemic changes in the viable glomeruli, and birefringent tubular crystals with associated tubular atrophy and severe interstitial fibrosis. The patient’s mother had kept this stone and analysis of this revealed “organic” material. Twenty-four hour urine testing revealed 0.23 g/24 hours proteinuria, no hematuria, and borderline elevated urine oxalate 532 μmol/d (normal < 500 μmol/d). Primary hyperoxaluria was ruled out (negative genetic testing of AGXT, GRHPR, and DHDPSL genes) and no obvious secondary causes were detected on further clinical interrogation. Unexpectedly, this patient coincidentally had the same surname as Case 2 (but not immediately related) and the pathologist suspected APRT deficiency. The diagnosis was confirmed with low enzyme activity (0.09 nmol/min per mg) and a homozygous genetic mutation was confirmed (c.188G>A; p.Gly63Asp). Crystalluria also was detected in this patient on polarized light microscopy.
Discussion
These cases highlight challenging aspects in management of APRT deficiency, particularly given the lack of clinical suspicion, limited access to confirmatory testing, and difficulties in defining target levels of monitoring of disease and response to treatment.
Diagnostic Issues
The condition was initially misdiagnosed in cases 2 and 3, as chronic interstitial nephritis and oxalate nephropathy. In case 2, the diagnosis was made only after detection of recurrence of crystals in the allograft biopsy; and again, this was initially thought to be oxalate nephropathy. This highlights 2 difficulties with histological diagnosis: (i) native renal biopsies in the late stages of disease may be nonspecific due to long-term scarring; and (ii) it may be misdiagnosed as other forms of crystal nephropathy and requires close examination of the features to differentiate between the causes.
Owing to the rare nature of the disease, there is a low index of clinical suspicion. Clues that should alert the clinician to consider APRT deficiency include histological findings of tubular and/or interstitial deposits of crystalline material, crystalluria, and negative screening (and unresponsiveness to treatment) for other more common causes of crystalline nephropathy.8
Access to confirmatory testing also may be a limiting factor. Testing for urinary DHA was not available after the first case (5 years ago). Crystal and stone analysis with infrared mass spectroscopy is also not widely available and would have been useful particularly for case 3, where stone analysis was inconclusive as “organic material.” APRT enzyme activity measured in red cell lysates are usually absent or reduced, but there exist a number of rare pathogenic variants that confer disease but do not lower red cell lysate APRT enzyme activity.9
Targeted Therapy, Dosing, and Monitoring of Symptomatic Individuals
Cornerstone of therapy is to reduce systemic DHA production by reducing purine intake (although most production is via salvage pathway); and inhibition of xanthine oxidase (or xanthine dehydrogenase) with allopurinol or febuxostat (a nonpurine selective inhibitor).
There currently is no effective intervention for removal of DHA metabolite, or increasing its urinary solubility. Dialysis is ineffective in preventing DHA, as it is usually protein bound, and any removal on dialysis would be unable to keep up with the high rate of production given the ubiquitous nature of the purine salvage pathway.
The dosing of allopurinol is not straightforward. High doses of allopurinol2,4,8,S1 (up to 600 mg) and febuxostat (80 mg)6 have been required to achieve effective inhibition of DHA crystalluria. Icelandic nephrologists at Landspitali Hospital (who also run the Rare Stone Group Consortium APRT registry) also have used a combination of high-dose 800 mg allopurinol and 80 mg febuxostat in some cases. These doses are much higher than the recommended dose for patients with significant renal impairment or on dialysis, often leading to concerns of allopurinol hypersensitivity or drug reaction with eosinophilia and systemic symptoms. This limitation also applies in the use of febuxostat, although there have been examples of febuxostat being safely used in patients with chronic kidney diseaseS5 stage 5 (up to 80 mg daily)S2–S4 and transplant patients in the management of gout. With these concerns in mind, close monitoring is required for high-dose therapy of either agent, with particular attention to drug reaction with eosinophilia and systemic symptoms/rash, liver dysfunction, or gastrointestinal side effects.
Oxypurinol is the active metabolite of allopurinol and is renally excreted (minimal excretion when creatinine clearance is <10 ml/min, but dialyzable). Oxypurinol levels have been used to help guide the management of gout (recommended trough levels 5–10 mg), but no target levels exist for APRT deficiency.S6,S7 We believe this can be a useful measure to assess therapeutic dosing in future patients, particularly to account for variability with compliance, absorption, metabolism, and renal function rather than a target or weight-based allopurinol dose alone. We measured oxypurinol levels in both transplant patients and found high levels in both despite the different doses: case 1 on 600 mg daily with trough levels 21 to 32 mg/l; and case 2 on 300 mg daily with trough levels above 30 mg/l; the maximum allopurinol dose in both patients was limited by diarrhea. Both patients described mild, erythematous, nonpruritic, nonpalpable facial rash while on long-term therapy. Case 1 was treated for rosacea without change in therapy otherwise; and in case 2, the mild rash resolved with small reduction of allopurinol therapy.
Histological analysis on renal biopsy is the gold standard to assess therapeutic response, although this is limited by the invasiveness, sample error, and the kinetics of crystal formation (new crystal deposition vs. excretion/removal of existing crystals).
Urine microscopy and DHA metabolites are the only noninvasive methods to monitor therapy. Absence of crystals under conventional and polarized microscopy (and if possible, confirmation with infrared microscopy) has been used as a marker for adequate therapy. Measurement of urine DHA metabolites is an attractive option, as it is more sensitive (before formation of crystals) and can be used with allopurinol or feboxustat (no therapeutic level monitoring), but this is measured only through select laboratories. A recent study of 10 patients with APRT deficiency demonstrated marked reduction of urine DHA (24-hour sample and DHA:creatitine ratio) when treated with allopurinol or febuxostat and will be a promising area once validated and more accessible.S8 The same group is also conducting research into measurement of DHA metabolites in blood, which will be useful in the setting of anuric patients. Uric acid levels are not useful for APRT deficiency (urate levels < 0.25 mmol/l in our patients).
Issues in Renal Transplantation
Recurrence of disease after transplantation is a major issue, and there have been relatively poor outcomes reported to date for patients with APRT deficiency. In the largest case series (by Zaidan et al.4) involving 9 patients with renal transplantation, the median delay to diagnosis was 5 weeks after transplantation; and despite allopurinol or feboxustat therapy, 7 of 9 patients developed chronic allograft nephropathy or graft loss. One case series (by Nasr et al.8) reported a patient treated with combination prednisolone and allopurinol (400 mg/d) before transplantation, with no evidence of recurrence, but unfortunately died with multiorgan failure from unrelated reasons; and another patient treated with allopurinol (200 mg/d) who suffered from recurrence of disease.
As described earlier, the optimal dosing of allopurinol or feboxustat is unclear and clearly poses a challenge in future renal transplant candidates given the risk of early recurrence, chronic graft dysfunction, and loss. Although our patient in case 1 was already on allopurinol before transplantation, he was probably underdosed for his weight; and likely needed additional postdialysis dosing, given the active metabolite (oxypurinol) was dialyzable.
In the early posttransplant setting, there is high risk of acute graft dysfunction from a “dumping” phenomenon, with vast amounts of accumulated systemic DHA excreted into the urine by the new allograft kidney. The risk of tubular crystal formation is higher in the setting of oliguric or low flow states; and both our patients were found to have hypocitraturia.
Early crystal formation and deposition is associated with acute tubular injury, tubular obstruction, foreign body reaction, and tubulitis. It was difficult to distinguish if inflammation on the biopsies was due to crystalline disease or if there was concurrent early or “borderline rejection” (tubulitis and interstitial infiltrates but not meeting Banff Criteria for type 1A rejection). Given they both suffered from delayed graft function, inflammation on the early biopsies was treated with intravenous methylprednisolone, and 1 patient progressed to develop unequivocal Banff grade 2A rejection. This dilemma is not a benign problem, and the clinical assessment will need to be individualized to balance the risk of long-term adverse graft outcomes of borderline/subclinical rejection or tubulitis secondary to crystalline disease versus the well-established complications of overimmunosuppression.S10,S11 Both patients suffered infectious complications: case 1 with invasive CMV; and case 2 with BK nephropathy, varicella zoster reactivation, CMV viremia, and recurrent urinary tract infections.
Disease Course and Kinetics
We reviewed the posttransplant biopsies of our patients and found that early in the disease course, crystals were almost exclusively seen in the proximal tubules. With the initiation of allopurinol therapy, there was at least no further increase in overall crystal burden, with very slow, small decrement over several months for both cases.
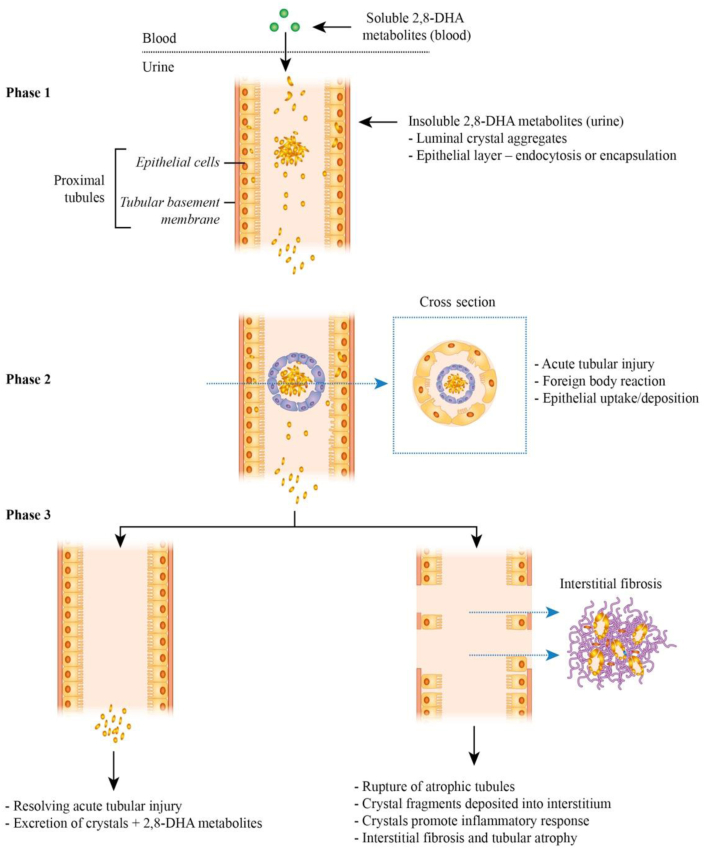
Sequential biopsies in both our transplant patients (on maximal tolerated allopurinol) showed changes to the distribution of crystal load. We hypothesize based on the results presented in Figures 2a and b and 3e and f, following early luminal deposition, the crystals are then also incorporated into the tubular epithelial layer by either endocytosis, or encapsulation (formation of new layer of epithelial cells).S9
This is then followed by atrophy or rupture of the tubular basement membrane and deposition of crystals into the interstitial compartment (Figure 4). Interstitial crystal deposition sustains an ongoing inflammatory response, leading to chronic tubular atrophy and interstitial fibrosis and is an important contributor to the progressive chronic tubular and interstitial Banff scores in our patients (Figure 3g and h).S9
Figure 4.
Proposed natural history and kinetics of 2,8-dihydroxyadenine (DHA) crystal deposition in the kidney in adenine phosphoribosyltransferase (APRT) deficiency. The 2,8-DHA is soluble in the systemic circulation, but crystallizes in the urine. Phase 1: these crystals can either form spherical aggregates in the lumen of the proximal tubules (leading to obstruction) or be incorporated into the epithelial layer. Phase 2: these crystals induce (and sustain) a proinflammatory environment, leading to a foreign body reaction and tubular injury. Phase 3: these crystals are either excreted in the urine or are deposited into the interstitial space due to disruption to the tubular basement membrane from chronic inflammation and/or tubular rupture. Interstitial crystals continue to provoke a proinflammatory environment, leading to interstitial infiltration followed by chronic fibrosis.
Screening and Genetic Counseling
Genetic testing in family members of a patient diagnosed with APRT deficiency relies on pathogenic variants being found in the proband. The large majority of probands will have mutations detectable by sequencing, although rare individuals may have pathogenic deletions that may be missed by Sanger sequencing or exome capture–based techniques. Diagnosis in other family members, particularly at-risk siblings, may then proceed using the known mutations.
Alternatively, APRT enzyme activity or urinary purine metabolite analysis might be more appropriate dependent on cost and availability. Genetic counseling should be offered to family members.
Management of patients with asymptomatic APRT deficiency found on either genetic or enzymatic testing in unclear. There is a risk of silent disease, as some cases manifest in childhood,2,S12 whereas some are detected only when patients are found to have late-stage chronic kidney disease in adulthood. It is tempting to treat all patients regardless of symptoms, particularly because allopurinol is a relatively inexpensive drug, but it may be useful to consider renal function and urinary screening (crystalluria or DHA metabolites) to decide whether long-term exposure to high-dose allopurinol is warranted.
Conclusion
APRT deficiency is an important differential to consider in patients with undetermined crystal nephropathy or nephrolithiasis. It is potentially reversible, but the current treatment strategies are far from satisfactory, and this has implications on long-term graft survival in transplant recipients.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Jennifer Li is recipient of the Australian National Health and Medical Research Council (NHMRC) postgraduate scholarship; Germaine Wong, Gopala Rangan, and Jeremy Chapman are all recipients of NHMRC research grants; and Gopala Rangan also receives research funding from Otsuka, Danone Nutricia, PKD Foundation Australia, and the University of Sydney.
Footnotes
Supplementary Material
References
- 1.Simmonds H.A., Van Acker K.J., Cameron J.S., Snedden W. The identification of 2,8-dihydroxyadenine, a new component of urinary stones. Biochem J. 1976;157:485–487. doi: 10.1042/bj1570485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Runolfsdottir H.L., Palsson R., Agustsdottir I.M. Kidney disease in adenine phosphoribosyltransferase deficiency. Am J Kidney Dis. 2016;67:431–438. doi: 10.1053/j.ajkd.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollée G., Dollinger C., Boutaud L. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol. 2010;21:679–688. doi: 10.1681/ASN.2009080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaidan M., Palsson R., Meriequ E. Recurrent 2,8-dihydroxyadenine nephropathy: a rare but preventable cause of renal allograft failure. Am J Transplant. 2014;14:2623–2632. doi: 10.1111/ajt.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollée G., Harambat J., Bensman A. Adenine phosphoribosyltransferase deficiency. Clin J Am Soc Nephrol. 2012;7:1521–1527. doi: 10.2215/CJN.02320312. [DOI] [PubMed] [Google Scholar]
- 6.Nanmoku K., Kurwosawa A., Shinzato T. Febuxostat for the prevention of recurrent 2,8-dihydroxyadenine nephropathy due to adenine phosphoribosyltransferase deficiency following kidney transplantation. Intern Med. 2017;56:1387–1391. doi: 10.2169/internalmedicine.56.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas M., Loupy A., Lefaucheur C. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell–mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2017;18:293–307. doi: 10.1111/ajt.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasr S.H., Sethi S., Cornell L.D. Crystalline nephropathy due to 2,8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrol Dial Transplant. 2010;25:1909–1915. doi: 10.1093/ndt/gfp711. [DOI] [PubMed] [Google Scholar]
- 9.Deng L., Yang M., Frund S. 2,8-Dihydroxyadenine urolithiasis in a patient with considerable residual adenine phosphoribosyltransferase activity in cell extracts but with mutations in both copies of APRT. Mol Genet Metabol. 2001;72:260–264. doi: 10.1006/mgme.2000.3142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.