Abstract
Hyperphosphatemia is a common complication in patients with chronic kidney disease (CKD), particularly in those requiring renal replacement therapy. The importance of controlling serum phosphate has long been recognized based on observational epidemiological studies that linked increased phosphate levels to adverse outcomes and higher mortality risk. Experimental data further supported the role of phosphate in the development of bone and cardiovascular diseases. Recent advances in our understanding of the mechanisms involved in phosphate homeostasis have made it clear that the serum phosphate concentration depends on a complex interplay among the kidneys, intestinal tract, and bone, and is tightly regulated by a complex endocrine system. Moreover, the source of dietary phosphate and the use of phosphate-based additives in industrialized foods are additional factors that are of particular importance in CKD. Not surprisingly, the management of hyperphosphatemia is difficult, and, despite a multifaceted approach, it remains unsuccessful in many patients. An additional issue is the fact that the supposedly beneficial effect of phosphate lowering on hard clinical outcomes in interventional trials is a matter of ongoing debate. In this review, we discuss currently available treatment approaches for controlling hyperphosphatemia, including dietary phosphate restriction, reduction of intestinal phosphate absorption, phosphate removal by dialysis, and management of renal osteodystrophy, with particular focus on practical challenges and limitations, and on potential benefits and harms.
Keywords: chronic kidney disease, dialysis, diet, hyperphosphatemia, phosphate binders, renal osteodysthrophy
Hyperphosphatemia is a common complication of patients with CKD, particularly in those with end-stage renal disease (ESRD). In general, high serum phosphate levels are observed only in late stages of CKD. An earlier increase in the course of CKD is prevented by the activation of powerful compensatory mechanisms, including an increase in fibroblast growth factor 23 (FGF23) and parathyroid hormone (PTH) secretion. Both hormones enhance the fractional excretion of phosphate per functioning nephron, compensating for the progressive loss of functioning nephron mass.1 In advanced CKD, these mechanisms become gradually unable to overcome the continuous input of phosphate from dietary intake, leading to a positive phosphate balance and hyperphosphatemia. The use of certain medications, mainly vitamin D and its active derivatives, may further aggravate the positive balance by increasing intestinal absorption of phosphate. Altered bone metabolism may also play a part in hyperphosphatemia through 2 distinct pathways: on the 1 hand, the increase in bone resorption characteristic of high bone turnover states augments phosphate efflux from bone to blood in advanced CKD stages; on the other hand, the loss of bone buffering capacity characteristic of low bone turnover states decreases the skeletal buffering capacity of increased extracellular phosphate levels already in earlier CKD stages. However, the concentration of serum phosphate is not only the net result of phosphate ingestion, absorption, and excretion. A recent study has demonstrated that the nicotinamide phosphoribosyl transferase (Nampt)/(NAD+) intracellular pathway plays a fundamental role in activity of renal and intestinal phosphate transporters. Thereby it regulates transcellular phosphate shifts independent of oral phosphate ingestion, and contributes to the diurnal variation in serum phosphate concentration.2, 3 Moreover, in some clinical conditions, serum phosphate can vary due to shifts of phosphate from the extracellular to the intracellular space, or vice versa. Most notably, massive cellular shifts of phosphate out of the cells may occur in association with lactic acidosis and diabetic ketoacidosis, causing severe acute hyperphosphatemia. In addition to promoting cellular phosphate exit, metabolic acidosis can diminish glycolysis and therefore cellular phosphate utilization, resulting in an increase in serum phosphate.4, 5, 6
In clinical practice, the deleterious effects of high serum phosphate levels in CKD were underestimated for many years. Despite the well-known contribution of phosphate retention to the development of secondary hyperparathyroidism, it was only in the late 1990s that hyperphosphatemia began to be widely appreciated as a potentially major cardiovascular villain. Using data from the US Renal Data System, Block et al. found an increased risk of death (relative risk, 1.27) associated with serum phosphate levels >6.5 mg/dl. The increased risk remained statistically significant even after adjustment for confounders.7 Subsequently, numerous epidemiological studies, both in the general population and in CKD patients, have tightened the knot between phosphate excess and adverse outcomes.8, 9, 10, 11 Experimental studies have shed light on the mechanisms by which phosphate may adversely affect the cardiovascular system. Briefly, phosphate may directly contribute to vascular damage by inflammatory actions on the vascular smooth muscle cell, the induction of endothelial dysfunction, and the promotion of vascular calcification.12, 13, 14 Furthermore, a high dietary phosphate content may contribute to atherogenesis.15 Besides its cardiovascular toxicity, hyperphosphatemia has also been linked to a more rapid progression of CKD.11, 16 Phosphate excess may also indirectly exert noxious effects, for example, by inhibiting the renal transformation of 25(OH) vitamin D to 1,25(OH)2vitamin D, and by stimulating both FGF23 and parathyroid hormone (PTH) secretion.17, 18, 19
Based on a large body of clinical and experimental evidence, the control of hyperphosphatemia has emerged as a key element in the management of CKD patients. However, the optimal range for serum phosphate levels in CKD patients is still controversial. The KDOQI guidelines of 2011 suggested that phosphate levels should be kept between 3.5 and 5.5 mg/dl, whereas the subsequent KDIGO guideline of 2009 and its recent update in 2017 opted for a less strict control, suggesting that elevated phosphate levels should be lowered toward the normal range.20
In daily clinical practice, the management of hyperphosphatemia is based on 4 main strategies: (i) restriction of dietary phosphate intake; (ii) reduction of its intestinal absorption; (iii) phosphate removal by dialysis; and (iv) treatment and prevention of renal osteodystrophy.
This review will discuss these treatment approaches, addressing their potential benefits, harms, and limitations in light of the many practical challenges that arise when managing hyperphosphatemia in patients with CKD.
Dietary Phosphate Restriction
Reducing phosphate intake is a widely accepted strategy to aid in the control of hyperphosphatemia. It is a fundamental part of the recommendations issued by both KDIGO and KDOQI guidelines, with a daily phosphate intake of 800 to 1000 mg/d, and a daily protein intake (as the major source of dietary phosphate) of 1.2 g/kg body weight.21, 22 However, one should be aware of several important issues when proposing dietary phosphate restriction.
First, the bioavailability of phosphate needs to be taken into account, and not the phosphate content of food alone. In general, (i) phosphate bioavailability is very low for plant-derived phosphate, probably due to a lower phosphate:protein ratio and to the fact that phosphate from vegetable origin (phytate) is less well absorbed (usually <50%) because humans do not express the degrading enzyme phytase23; (ii) its bioavailability is much greater for processed food; and (iii) its bioavailability appears to be intermediate for animal-derived unprocessed meat. The impact of the source of phosphate was examined in a crossover trial in 9 CKD patients (mean estimated glomerular filtration rate 32 ml/min) that compared vegetarian and meat diets with equivalents nutrients.24 After 1 week on the vegetarian diet, patients had lower serum phosphorus levels, a trend toward decreased urinary 24-hour phosphorus excretion, and significantly lower FGF23 levels in comparison to patients with the meat-based diet.
Second, there are sources of phosphate that are commonly overlooked, mainly from processed foods and medications. Almost all processed foods contain phosphate additives, such as disodium phosphate, monosodium phosphate, and potassium triphosphate, to preserve their color and shelf lives. Inorganic phosphorus, present in phosphate additives, is not protein bound. It dissociates easily in the gut lumen and therefore is more readily absorbed across the intestinal wall. In healthy humans, the fractional absorption rate of phosphate is approximately 70%.25 A study comparing the phosphate content of similar processed food items with and without additives showed that both total and soluble phosphate content were greater in the foods containing phosphate additives, leading to a 70% higher phosphate uptake per gram of protein.26 Another recent report has estimated that as much as 40% of total phosphate intake may be attributable to phosphate-containing additives.27 This problem seems to be even more worrisome in individuals living in low-income communities.28 Importantly, dietary intake of inorganic phosphate has been linked to higher carotid-intima thickness.29 Furthermore, as the phosphate content in processed foods is often hidden, total phosphate intake is generally underestimated. Additional concern stems from the possible contribution of medication excipients to phosphate overload. The phosphate content of commonly prescribed drugs may be very high, for instance 111.5 mg for 40 mg of paroxetine, 32.6 mg for 10 mg of lisinopril, and 40.1 mg for 10 mg of amlodipine.30
Most importantly, although phosphate lowering by tight dietary restriction is often effective both in predialysis and in dialysis patients,31, 32, 33 a beneficial effect on clinical outcomes remains to be demonstrated.33 Moreover, severe protein restriction increases the risk of malnutrition and eventually poorer outcomes.34 An alternative strategy for reducing phosphate content of food is the manner of cooking, as cooking procedures have been demonstrated to affect the bioavailability of phosphate by breaking down food structure and changing mineral solubility. In this regard, boiling sliced meat for 30 minutes in soft water reduced its phosphate content by 50%, with no significant changes in the protein content.35 Finally, recent studies further demonstrated the difficulty of controlling serum phosphate with dietary phosphate restriction. For instance, in a crossover feeding study in 11 CKD patients (estimated glomerular filtration rate 30–45 ml/min per 1.73 m2), a low-phosphate diet (1000 mg/d) lowered serum phosphate levels in comparison to a high-phosphate diet (2500 mg/d), with no change in PTH or FGF23 levels.36 On the other hand, Chang et al. could not demonstrate any effect of consuming a diet with low content of phosphate additives (11 mg/d) for 3 weeks on serum phosphate and FGF23 levels in patients with CKD stage 2 (mean estimated glomerular filtration rate 79.0 ± 25.3 ml/min per 1.73 m2), although urinary phosphate excretion and serum PTH levels decreased significantly.37
With that in mind, dietary phosphate restriction in CKD patients requires a rational approach rather than an indiscriminate prescription of reduced dietary protein intake. The nature of dietary phosphate, including hidden sources, should be carefully examined. Appropriate dietary counseling and educational programs involving patients in their own care are important. Encouraging patients to reduce meat consumption and to shift to a grain-based vegetarian diet may allow sufficient protein intake without adversely affecting serum phosphate. Last but not least, it must be stressed that dietary phosphate restriction is difficult to accept and usually insufficient to achieve adequate control of serum phosphate. Therefore, other strategies such as phosphate binders, and phosphate removal by dialysis in patients with ESRD, need to be discussed and prescribed on an individual basis when deemed necessary.
Pharmacologic Approaches
Phosphate Binders
All effective phosphate binders reduce the absorption of dietary phosphate in the gastrointestinal tract. The mechanism of action is an exchange of the anion phosphate with an active cation (carbonate, acetate, oxyhydroxide, and citrate) to form a nonabsorbable compound that is excreted in the feces.
Insufficient patient adherence is a central problem associated with phosphate binder treatment, given the usually high pill burden, large pill size, and ensuing gastrointestinal adverse events.38 Even under supervised study conditions and short follow-up, more than 3 of 4 patients were found to adhere incompletely to phosphate binder prescription.39
Aluminum-Containing Phosphate Binders
Aluminum-based phosphate binders are among the most effective and best-tolerated chelators as regards acute side effects. In addition, treatment cost is low. However, from the 1970s onward, an increasing number of dialysis patients were found to suffer from severe aluminum intoxication, its major clinical manifestations being encephalopathy, osteomalacia, microcytic anemia, and premature death.40 In the majority of cases, dialysis fluid contamination by aluminum was identified as the main culprit.41 Fortunately, this dramatic epidemic could be solved subsequently by using more appropriate, ultrapure water as dialysis fluid.42 However, even in nondialyzed patients with CKD, sporadic cases of aluminum intoxication due to aluminium-containing phosphate binders were reported as well.43 In this respect, it is noteworthy that citrate has been found to enhance intestinal aluminum absorption considerably, at least in predisposed individuals.44 Uremic infants and children appear to be at particularly high risk for oral aluminum overload, possibly owing to a more permeable intestinal mucosa at young age.45
Therefore, at present, the prolonged use of aluminium-containing phosphate binders in patients with CKD is strongly discouraged, in accordance with recent clinical practice guidelines.20
Calcium Carbonate and Calcium Acetate
Calcium-based binders (calcium carbonate and calcium acetate) became the binders of choice in the 1980s and 1990s. They were found to be effective and to avoid the serious complications sometimes observed with aluminum-containing compounds.46, 47
Calcium acetate is at least as effective as calcium carbonate in lowering serum phosphate in chronic dialysis patients46, 48. However, hypercalcemia episodes have been shown to occur more frequently with calcium acetate.49 In the predialysis setting, calcium acetate was found to be effective in reducing serum phosphorus and intact PTH over a 12-week period in a randomized, double-blind, placebo-controlled trial,50 but calcium carbonate is equally effective.51
A key concern with calcium-containing phosphate binders is the rapid achievement of a positive calcium balance due to daily calcium loading.20 Effectively, in 2 small randomized trials in CKD 3b–4 patients, calcium carbonate supplementation produced a slightly positive calcium balance and did not affect phosphate balance,52, 53 although in 1 of these patients it produced a modest reduction in urinary phosphate excretion compared with placebo.52
The development of ectopic calcification in the media and intima of arterial vessels has been recognized as a major contributing factor for the excess cardiovascular mortality observed in CKD patients.54 Calcium overload may aggravate vascular calcification by inducing positive calcium balance and directly activating the calcification process of the vascular smooth muscle cells.14 The mechanisms leading to vascular calcification include an imbalance between inhibitors and inducers of abnormal mineral deposition in soft tissues, the latter including calcium and phosphate excess, among others.55 Therefore, minimizing exogenous calcium through reducing exposure to calcium-based phosphate binders in the CKD population has been suggested to be beneficial.20
Magnesium-Containing Phosphate Binders
Magnesium carbonate, which exhibits a relatively good gastrointestinal tolerance profile, has been proposed as a valid alternative phosphate binder to calcium acetate, allowing hemodialysis patients to reduce their calcium load.56 In addition, there is experimental evidence that magnesium interferes with hydroxyapatite crystal formation, a key process in vascular calcification.57 In rats, treatment with magnesium carbonate (CaMg) effectively controlled serum phosphate levels and reduced aortic calcification.58 In the clinical setting, an open-label, prospective pilot study that evaluated coronary artery calcification progression in 7 hyperphosphatemic hemodialysis patients treated with magnesium carbonate found no significant progression in the 18-month follow up period.59 Another small, open-label trial evaluated the progression of vascular calcification diagnosed by plain X-ray in 72 hemodialysis patients randomly allocated to receive CaMg plus calcium acetate or calcium acetate alone as a phosphate binders for 12 months. The authors found a small but significant improvement of vascular calcification in the magnesium-treated group.60
Efficacy and tolerability were evaluated in a phase 3 randomized clinical trial comparing a combination of calcium acetate and CaMg with that of sevelamer hydrochloride in 255 hemodialysis patients.61 The authors reported that CaMg was noninferior to sevelamer. Total serum calcium increased slightly in CaMg group, but this was not associated with a higher risk of frank hypercalcemic episodes. An asymptomatic, albeit significant, increase in serum magnesium occurred in the CaMg group as well. Another small clinical trial in peritoneal dialysis patients showed that CaMg was somewhat less effective than calcium carbonate in controlling serum phosphate, and, more importantly, that diarrhea appeared to be a more common dose-limiting adverse effect with CaMg.62
More recently, a randomized, controlled trial, designed to evaluate the effects of magnesium oxide and an oral carbon adsorbent (AST-120) on vascular calcification in predialysis CKD patients, stages 3 to 4, showed that magnesium oxide was capable of slowing down the progression of coronary artery calcification. Notably, Mg oxide had no effect on the serum levels of phosphate or its urinary fractional excretion.63 Moreover, in contrast to a previous smaller study in Chinese predialysis CKD patients that reported a possible effect of oral activate charcoal on phosphate control and vascular calcification,64 Sakaguchi et al. did not notice any effect of AST-120 on these parameters.63 Differences between the chemical composition of the 2 formulations and between characteristics of the study population, such as ethnicity, age, and number of diabetic patients included, might at least partially explain these divergent findings.
Polymeric and Other Calcium-Free, Magnesium-Free Phosphate Binders
Sevelamer
In 2001, sevelamer hydrochloride was launched as the first non−metal-containing, nonabsorbable anion exchange binder. Currently, both sevelamer hydrochloride (HCl) and sevelamer carbonate are used in clinical practice. Sevelamer is a crosslinked polymer that exchanges HCl or carbonate for phosphate in the gastrointestinal tract. The HCl and carbonate moieties are absorbed from the gut, and the resulting phosphate-laden polymer is excreted in the feces.
Early studies demonstrated that sevelamer was effective in controlling hyperphosphatemia in hemodialysis and peritoneal dialysis patients without inducing hypercalcemia as seen with calcium acetate or calcium carbonate.65, 66, 67 In peritoneal dialysis patients, a small crossover study of calcium carbonate versus sevelamer carbonate demonstrated that the compounds were equally effective in reducing phosphate levels. Moreover, sevelamer, but not calcium carbonate, treatment was associated with improvement in endothelial function (endothelin-1, plasminogen activator inhibitor−1), as well as inflammatory markers (C-reactive protein and interleukin-6).68 Clinical and experimental studies also suggested that sevelamer might prevent the accumulation of advanced glycation end-products.69, 70
In addition to chelating phosphate, sevelamer also binds bile salts,71 resulting in a significant reduction in serum total cholesterol and low-density lipoprotein cholesterol in dialysis patients,72 as well as a reduction in plasma glucose in patients with type 2 diabetes.73 One should keep in mind, however, that because of its ability to bind bile salts, sevelamer may interfere with normal fat absorption and the absorption of the fat-soluble vitamins A, D, E, and K.74
The progression of vascular calcification in CKD patients taking calcium-based phosphate binders versus sevelamer has been evaluated in several open-label trials, which led to conflicting results.75, 76, 77, 78 Despite biological plausibility, considering all available data together, the claim that sevelamer is capable of improving coronary calcification compared to calcium-based binders remains a matter of debate according to a recent Cochrane meta-analysis.79 However, the authors concluded that sevelamer might reduce all-cause mortality.
Clinical issues of the treatment with sevelamer are a relatively high pill burden,80 gastrointestinal side effects,81 and cost.82 The cost issue should be solved with severlamer’s patent expiration, depending on country-specific regulations.
Bixalomer
Bixalomer is another amine-functional, nonabsorbable polymer that is currently available only in Japan. It appears to have better gastrointestinal tolerability than sevelamer, as the compound absorbs less water and therefore induces less swelling and consequently higher fluidity.83
Lanthanum Carbonate
In 2004, lanthanum carbonate was launched as the first chewable, calcium-free phosphate binder. It is the first phosphate-binding compound to use the metal lanthanum for phosphate chelation. In the gastrointestinal tract, lanthanum carbonate binds phosphate to form the nonabsorbable compound lanthanum phosphate.
In addition to effective phosphate binding, this compound offers the advantage of a low pill burden. Phase IV studies conducted in different countries demonstrated that phosphate levels were well controlled with a daily pill burden of less than half when compared to those of previous phosphate binders.84, 85, 86 Lanthanum carbonate was also shown to be effective in hyperphosphatemic, nondialysis CKD stage 4 to 5 patients in a multicenter, randomized, double-blind, placebo-controlled trial.87 In hemodialysis patients randomized to lanthanum or calcium carbonate, phosphate levels fell similarly, whereas hypercalcemia was restricted to the calcium carbonate group.88
Key adverse effects of lanthanum reported in a systematic review included vomiting, diarrhea, intradialytic hypotension, cramps, myalgia, and abdominal pain.89 Other problems with lanthanum carbonate relate to its relatively low solubility90.
Lanthanum accumulation in the liver and many other organs has been reported in uremic rats.91 Lanthanum also accumulates in the bone of chronic dialysis patients, with a 50- to 80-fold increase in bone content after 1 to 3 years of lanthanum carbonate therapy.92, 93 However, this was not associated with detectable clinical consequences. Most importantly, there was no increase in the incidence of adverse events associated with any organ function, in particular that of bone, after up to 6 years of treatment of chronic hemodialysis patients.92
Ferric Citrate
In the gastrointestinal tract, ferric citrate binds phosphate in exchange to citrate to form ferric phosphate, which is insoluble and excreted in the feces. Each pill contains 210 mg of elemental iron, which is equivalent to 1 g of ferric citrate.
A phase III randomized, controlled trial compared the effect of ferric citrate with non−iron-containing phosphate binders (sevelamer or calcium-containing) in 441 chronic hemodialysis patients.94 The follow-up was 52 weeks. Ferric citrate was noninferior to the comparator arm in controlling serum phosphate. Furthermore, it increased serum ferritin, reduced the need for intravenous iron and erythropoietin-stimulating agents, and increased hemoglobin levels.94, 95
Sucroferric Oxyhydroxide
Sucroferric oxyhydroxide is a polynuclear, chewable, iron-based phosphate binder. In the gastrointestinal tract, phosphate binds to sucroferric oxyhydroxide to form an insoluble compound. The sucrose and starch components of the tablet are absorbed. These chewable tablets have the advantage of disintegrating rapidly upon contact with water or saliva.96 Sucroferric oxyhydroxide has exhibited a high phosphate-binding capacity across the physiological gastrointestinal pH range and minimal iron release in preclinical studies.97
A large phase III randomized, controlled trial compared sucroferric oxyhydroxide with sevelamer carbonate in 1029 hemodialysis and peritoneal dialysis patients over 12 weeks, with a 24-week extension for safety and 27-week extension for dose finding.98 Sucroferric oxyhydroxide was as effective as sevelamer in lowering serum phosphate in both hemo- and peritoneal dialysis patients at an about 75% lower pill burden and consecutive better adherence. The main gastrointestinal side effect was mild, transient diarrhea, whereas nausea and constipation were more common with sevelamer.99 The phosphate-lowering effect of sucroferric oxyhydroxide was maintained over 1 year of follow-up, and no iron overload was observed. In hemodialysis patients, sucroferric oxyhydroxide may lead to an increase in serum iron parameters, albeit to a much lesser extent than what is observed with ferric citrate.100, 101, 102
Phosphate Binder Choice
Effects on Biochemical and Iintermediate Endpoints
A recent Cochrane review including 104 randomized clinical trials from 29 different countries involving 13,744 adults with CKD concluded that there was no evidence of inferiority or superiority of calcium-based versus non−calcium-based phosphate binders in the control of hyperphosphatemia. In addition, there was no evidence that non−calcium-based phosphate binders were superior in improving the risk of fracture, pruritus, calciphylaxis, or coronary calcification compared to calcium-based binders. As expected, the risk for hypercalcemia was significantly higher with calcium-based phosphate binders.79
Effects on Mortality
In a systematic meta-analysis of randomized trials that included 4622 patients and 936 deaths, there was a 22% reduction in all-cause mortality in patients receiving non−calcium-based phosphate binders (sevelamer or lanthanum) compared with patients assigned to calcium-based regimens.103 Similar reductions in mortality were observed in nonrandomized trials or when nondialysis patients with CKD and dialysis patients were considered separately. Notably, the reduction in mortality associated with non−calcium-based phosphate binders seemed to be independent of the degree of serum phosphate reduction, which did not differ by treatment assignment. The already-mentioned, more recent Cochrane systematic review also led to the conclusion that sevelamer, compared to calcium-based binders, might lower all-cause death in adults with CKD. It was uncertain whether lanthanum carbonate also decreased mortality compared with calcium-based binders, as there was a paucity of data and as comparative data for iron-containing binders were absent.79
A smaller, more recent systematic review of 9 randomized controlled trials involving 2813 adult hemodialysis patients compared the effects of lanthanum carbonate with those of other phosphate binders (calcium carbonate, calcium acetate, and sevelamer). The authors showed that all-cause mortality was significantly lower on lanthanum carbonate than on standard therapy. However, there was no significant difference between the groups in cardiovascular event rate, and serum phosphate control was comparable.104
Table 1 summarizes the characteristics of currently used phosphate binders.
Table 1.
Main advantages and disadvantages of currently used phosphate binders
| Drug | Usual dose (pill burden)a | Advantages | Disadvantages |
|---|---|---|---|
| Calcium carbonate | 500–1250 mg (3–6 tablets) | Lower pill burden | Calcium overload |
| Calcium acetate | 667 mg (6–12 capsules) | As effective as calcium carbonate | Calcium overload High pill burden |
| Magnesium carbonate | 63 mg (2–6 capsules) | Good GI tolerance, lower pill burden | Hypermagnesemia |
| Sevelamer hydrocloride | 800 mg (6–12 capsules) | ↓ LDL-cholesterol levels, better survival in HD | High pill burden, GI side effects, metabolic acidosis |
| Sevelamer carbonate | 800 mg (6–12 capsules) | ↓ LDL-cholesterol levels, better survival in HD | High pill burden, GI side effects |
| Bixalomer | 250 mg (6–14 capsules) | Good GI tolerance | High pill burden |
| Lanthanum carbonate | 250–1000 mg (3–6 chewable tablets) | Lower pill burden, good GI tolerance | Low solubility Tissue accumulation, eg, bone |
| Ferric citrate | 210 mg (4–5 tablets) | Lower pill burden, ↓ iron suplementation ↓ ESA doses |
GI side effects (mild) |
| Sucroferric oxyhydroxide | 500 mg (2–6 chewable tablets) | Lower pill burden | GI side effects (mild) |
ESA, erythropoiesis stimulating agents; GI, gastrointestinal; HD, hemodialysis; LDL, low-density lipoprotein.
Based on package leaftlet information or cited clinical trials.
Drugs Targeting Intestinal Phosphate Transporters
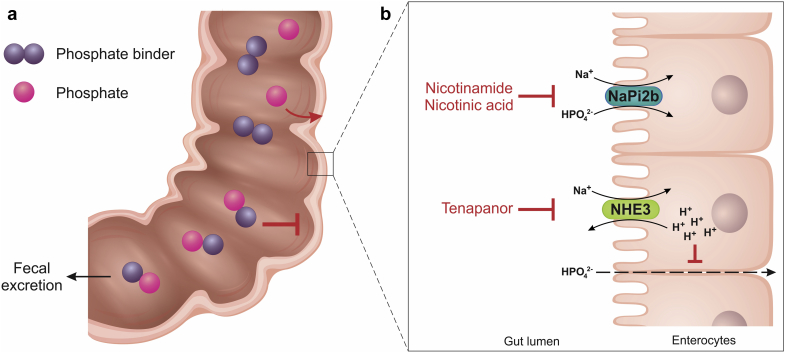
Intestinal phosphate absorption occurs via 2 distinct mechanisms: passive paracellular transport along a concentration gradient, and active sodium-dependent transcellular transport via transporter or exchanger proteins.105 The sodium-dependent inorganic phosphate cotransporter NaPi2b is primarily responsible for phosphate absorption in the gut.106 In addition, the sodium/hydrogen ion-exchanger isoform 3 (NHE3) plays a role in secondary active phosphate absorption.
It has been suggested that, in CKD, phosphate chelation in the gut lumen results in a compensatory upregulation of active transepithelial phosphate transport.107 Therefore, inhibition of phosphate transporter activities, either alone or in conjunction with phosphate binders, has long been viewed as a possible alternative or complementary approach to hyperphosphatemia control in patients with CKD.108
Nicotinic Acid and Nicotinamide
Nicotinic acid (niacin), a water-soluble organic compound that can be metabolized to nicotinamide (also known as niacinamide), lowers sodium-dependent intestinal phosphate absorption via a reduction in NaPi2b expression.109, 110
Several uncontrolled and controlled studies in nondialysis CKD patients and in chronic hemodialysis patients, respectively, showed that niacin or nicotinamide treatment led to reduction in serum phosphate.111, 112, 113, 114 The degree of reduction was generally modest, and side effects were frequent, with high dropout rates. Adverse effects included flushing, nausea, diarrhea, thrombocytopenia, and accumulation of potentially toxic metabolites.115 A recent experimental study in uremic rats showed that nicotinamide unexpectedly increased soft tissue calcification.116 In our opinion, nicotinamide as a single agent is not suited for hyperphosphatemia control in CKD. It has yet to be shown whether there remains any place for low-dose nicotinamide treatment as add-on therapy to established phosphate binders in patients with moderate to severe CKD or in dialysis patients.115
Tenapanor
Tenapanor is an inhibitor of the NHE3 that reduces intestinal sodium and phosphate absorption.117
In a phase II randomized, double-blind, placebo-controlled, dose-finding study that assessed the effects of tenapanor on hyperphosphatemia in 162 patients receiving hemodialysis therapy, this drug induced dose-dependent reductions in mean serum phosphate level from baseline, ranging from −0.47 to −1.98 mg/dl, with the largest reductions occurring in the tenapanor dosing groups, with good drug tolerability. The most common adverse event causing discontinuation was diarrhea.118 These findings have been recently confirmed in a phase III randomized, double-blind, placebo-controlled trial. Tenapanor treatment for 8 weeks significantly reduced phosphate levels by a mean of 1.0–1.2 mg/dl in hyperphosphatemic hemodialysis patients. Adverse events were mainly restricted to stool softening and increased bowel movements.119
Figure 1 illustrates the mechanisms of action of phosphate-lowering pharmacological therapies.
Figure 1.
Mechanisms of action of phosphate-lowering pharmacological agents. (a) Phosphate binders reduce the intestinal absorption of dietary phosphate by forming a nonabsorbable compound in the gastrointestinal tract lumen that is excreted in the feces. (b) Nicotinic acid (niacin) and nicotinamide (niacinamide) inhibit sodium-dependent, active intestinal phosphate absorption via a reduction in NaPi2b expression; tenapanor reduces intestinal sodium and phosphate absorption by inhibiting the sodium/hydrogen ion-exchanger isoform 3 (NHE3), leading to intracellular proton accumulation and inducing a conformational change in tight junction proteins, thereby decreasing permeability to paracellular phosphate transport.
Dialytic Removal of Phosphate by Renal Replacement Therapies
Dialytic removal of phosphate is around 300 mg/d in patients on peritoneal dialysis therapy and 800 mg per session in those receiving hemodialysis therapy on a thrice-weekly regimen, corresponding to approximately 350 mg/d.120, 121 Assuming a constant daily phosphate intake of 1000 mg/d and a net intestinal absorption of 60% to 70%, one easily notices that the amount of phosphate absorbed in a week would range from 4200 mg to 4900 mg, which is far beyond the total amount weekly removed by dialysis: approximately 2400 mg with hemodialysis, and 2100 mg with peritoneal dialysis. As a result, conventional hemodialysis or peritoneal dialysis alone does not allow achieving a neutral phosphate balance.
Alternative renal replacement strategies can be used to improve phosphate control. The Frequent Hemodialysis Network Daily and Nocturnal Trials have demonstrated that more intensive hemodialysis is capable of achieving an increase in phosphate dialytic removal.122 At the end of the study, assignment to daily hemodialysis (for 1.5−2.75 hours, 6 times/wk) or to nocturnal hemodialysis (for 6−8 hours, 6 times/wk) led to a significant decrease in mean serum phosphorus of 0.46 and 1.24 mg/dl, respectively, compared with conventional hemodialysis. Moreover, 42% of patients on nocturnal hemodialysis required the addition of phosphate into the dialysate to prevent hypophosphatemia. Furthermore, both modalities allowed a significant reduction of phosphate binder prescriptions. Therefore, an increase in either the frequency or the duration of hemodialysis may allow a more efficient removal of phosphate.
The possible contribution of greater convective solute removal provided by hemodiafiltration has also been examined. No beneficial effect of hemodiafiltration in comparison to high-flux hemodialysis on the control of mineral metabolism parameters, including phosphate levels, has been demonstrated.123, 124
Preservation of Residual Renal Function
Finally, it should be reinforced that renal function should be preserved along all stages of CKD, including CKD 5D. In ESRD, urinary phosphate excretion relies more on glomerular filtration rate than on tubular function. Moreover, PTH may act on the kidneys even when glomerular filtration rate is as low as 3 ml/min.125 Indeed, hemodialysis and peritoneal dialysis patients with residual renal fucntion have a better control of serum phosphate levels than their counterparts.126, 127
Does Serum Phosphate Alone Matter?
At first glance, the answer seems quite obvious: phosphate matters. Clearly, extremely high as well as extremely low serum phosphate levels are dangerous in the long run. However, this apparently easy answer to the question needs to undergo closer scrutiny because of the complexity of the regulation of phosphate metabolism and its disturbances in CKD. A number of regulatory factors should be taken into account when analysing the level of serum phosphate and before deciding to act on it.20 First of all, serum phosphate exhibits a marked circadian rhythm, with a peak around 3:00 am and a nadir around 11:00 am, which is partially influenced by the time and the type of phosphate ingestion, and partially on the Nampt/NAD+ intracellular pathway.2 Therefore, timing of blood sampling is important. Second, with regard to hemodialysis patients, because phosphate is removed by renal replacement therapy, the distance of blood sampling with respect to the last dialysis session is also important.128, 129 Third, the intraindividual variation of serum phosphate, as for most of the other mineral metabolism parameters, is greater among ESRD patients than in healthy subjects. It has been estimated that at least 8 blood samples are required to estimate the true set point for serum phosphate in such patients.130 Therefore, recommendations for phosphate-lowering treatments and treatment changes should rely on serial measurements and trends rather than on a single estimation.
The serum phosphate level is not a sensitive marker of the state of body phosphate load. Serum phosphate does not rise above normal until the glomerular filtration rate falls below 30 ml/min per 1.73 m2. Urinary total phosphate excretion is not a reliable indicator of phosphate overload either. In contrast, fractional phosphate excretion provides information on the body’s adaptation to the need to excrete increasing amounts of phosphate per remaining functional nephron. Thus, compared with fasting healthy subjects, fasting patients with CKD had significantly higher FGF23 levels and fractional phosphate excretion, although there was no difference in serum phosphate levels.131 Interestingly, in a subsequent 4-hour−postprandial period, urinary phosphate excretion increased despite unchanged serum phosphate and FGF23 levels, but serum PTH was increased. A short-term randomized trial that analyzed the effects of sevelamer and calcium acetate, respectively, on mineral metabolism parameters in 40 patients with CKD stages 3 to 4 showed a progressive decline in both total and fractional phosphate excretion, but no change in serum phosphate, after a 6-week period on either phosphate binder treatment.132 Of note, fractional phosphate excretion can be accurately evaluated in spot urine samples.
Finally, because serum FGF23 increases early in the course of CKD as an adaptive response to prevent phosphate overload, the hypothesis was made that it might be a valuable surrogate marker for long-term serum phosphate fluctuations, like HbA1C for serum glucose, and might provide guidance for clinical interventions. This hypothesis had to be given up with the subsequent advances in our understanding of the numerous mechanisms other than phosphate that play a role in the regulation of FGF23 secretion, such as calcium, PTH, vitamin D, and iron, as well as inflammation.133, 134 In keeping with this knowledge, calcium-based and calcium-free phosphate binders exert different actions on FGF23 secretion despite comparable phosphate-lowering effects.132, 135, 136
Treatment and Prevention of Renal Osteodystrophy
Given that 85% of the human body’s phosphate is located in the bone and teeth, as opposed to an only modest presence in extracellular fluid and soft tissues, bone health is of paramount importance in phosphate homeostasis.137 The disorder of bone morphology and function, termed renal osteodystrophy, is an almost universal finding in patients with CKD.138 It occurs already in early stages of the disease. From a histologic point of view, renal osteodystrophy comprises high-turnover (osteitis fibrosa and mixed renal osteodystrophy) at later CKD stages and low-turnover bone disease (adynamic osteopathy) at early and later CKD stages.125, 126 Both types may contribute to hyperphosphatemia, either by increasing bone resorption and phosphate release, or by reducing bone formation and phosphate uptake.
The main means to reduce high-turnover bone disease consists in treating secondary hyperparathyroidism. Currently available drugs for this purpose differ in their effects on serum phosphate. Active vitamin D derivatives increase intestinal phosphate absorption and, consequently, its serum levels. They also increase serum FGF23 levels. On the other hand, calcimimetics lower both serum PTH and phosphate,139, 140 an effect equally exerted by orally and intravenously administered agents.141 Because calcium is the primary regulator of PTH secretion, it comes as no surprise that calcium-containing phosphate binders also lower serum PTH, and that excessive doses can induce hypoparathyroidism and adynamic osteopathy.
A potential improvement of hyperphosphatemia control by treating low-turnover bone disease has received little attention so far. In chronic hemodialysis or peritoneal dialysis patients, low serum PTH can be raised by lowering dialysate calcium concentration, and this was shown to leave serum phosphate unaltered.142, 143 A plausible explanation is that ameliorating bone remodeling facilitates phosphate uptake by bone via improved bone formation and mineralization; however, concomitantly the increase in serum PTH favors the occurrence of hyperphosphatemia in the absence of functioning kidneys, eventually resulting in a net zero change in serum phosphate. Finally, another issue that might deserve investigation is the role of bone turnover in phosphate mass removal during dialysis therapy, similarly to its reported role in perdialytic calcium mass balance.144
Conclusions
The management of hyperphosphatemia in CKD remains a major challenge for both nephrologists and patients. Because of the complexity of the regulation of phosphate metabolism and its disturbances in CKD, it is of paramount importance to understand the pathogenesis of hyperphosphatemia. Only this will enable an optimal choice among the different, currently available therapeutic modalities (Figure 2) and possibly new options under development. Far from being the only culprit, the amount and type of dietary phosphate requires cautious attention, with special focus on hidden phosphate sources. There is still a widespread lack of awareness and information on the high phosphate content of many industrialized foods and beverages. Gastrointestinal intolerance is induced by most of the phosphate binders. It frequently results in poor compliance. A phosphate binder that is not taken is of no use even if its binding capacity is excellent. This should not be overlooked. In ESRD patients receiving dialysis therapy, all possible efforts should be made to preserve residual renal function, because it contributes to the clearance of uremic solutes, including phosphate, and the adequacy of phosphate removal, along with residual kidney function, needs to be checked when hyperphosphatemia cannot be controlled correctly. Importantly, different factors and disturbances often are simultaneously present in the same patient, but differ from 1 patient to the other. All recognizable factors need to be taken into account when choosing the best possible strategy for the control of hyperphosphatemia, including effectiveness, safety, cost, pill burden, and interference with individual patient’s daily activities. Hyperphosphatemia should be treated only if persistent and/or progressive. Clinical conditions, such as transient organ function disturbances, nutritional status, and residual renal function need to be taken into account to determine the best therapeutic approach. Systematic reviews of currently available data suggest a potential benefit of calcium-free phosphate binders over calcium-based binders for overall survival. There is still a gap in knowledge regarding (i) the optimal timing and strategies to avoid phosphate overload during the course of CKD, (ii) the best approach for monitoring therapy efficacy, and (iii) the effect of bone remodeling on phosphate removal during dialysis. Finally, it remains to be convincingly shown by prospective randomized trials which degree of phosphate control is needed to achieve optimal clinical outcomes in patients with CKD.
Figure 2.
Therapeutic approaches to control serum phosphate in patients with chronic kidney disease.
Disclosure
TBD reports personal fees from Akebia, Amgen, Astellas, Chugai, Fresenius Medical Care, Kyowa Hakko Kirin, Sanofi and Vifor. ZAM reports grants for CKD REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp and Dohme-Chibret, Sanofi-Genzyme, Lilly, Otsuka, and the French government, as well as fees and grants to charities from Astellas, Baxter, Daichii, Medice, and Sanofi-Genzyme. The other authors declared no competing interests.
References
- 1.Isakova T., Wahl P., Vargas G.S. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyagawa A., Tatsumi S., Takahama W. The sodium phosphate cotransporter family and nicotinamide phosphoribosyltransferase contribute to the daily oscillation of plasma inorganic phosphate concentration. Kidney Int. 2018;93:1073–1085. doi: 10.1016/j.kint.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Isakova T., Block G. The phosphate bucket list. Kidney Int. 2018;93:1033–1035. doi: 10.1016/j.kint.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor L.R., Klein K.L., Bethune J.E. Hyperphosphatemia in lactic acidosis. N Engl J Med. 1977;297:707–709. doi: 10.1056/NEJM197709292971307. [DOI] [PubMed] [Google Scholar]
- 5.Sternbach G.L., Varon J. Severe hyperphosphatemia associated with hemorrhagic shock. Am J Emerg Med. 1992;10:331–332. doi: 10.1016/0735-6757(92)90013-n. [DOI] [PubMed] [Google Scholar]
- 6.Kebler R., McDonald F.D., Cadnapaphornchai P. Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am J Med. 1985;79:571–576. doi: 10.1016/0002-9343(85)90053-1. [DOI] [PubMed] [Google Scholar]
- 7.Block G.A., Hulbert-Shearon T.E., Levin N.W., Port F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 8.Kestenbaum B., Sampson J.N., Rudser K.D. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M., Sacks F., Pfeffer M. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 10.Block G.A., Klassen P.S., Lazarus J.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 11.Voormolen N., Noordzij M., Grootendorst D.C. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–2916. doi: 10.1093/ndt/gfm286. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Moreno J.M., Herencia C., de Oca A.M. High phosphate induces a pro-inflammatory response by vascular smooth muscle cells and modulation by vitamin D derivatives. Clin Sci (Lond) 2017;131:1449–1463. doi: 10.1042/CS20160807. [DOI] [PubMed] [Google Scholar]
- 13.Six I., Maizel J., Barreto F.C. Effects of phosphate on vascular function under normal conditions and influence of the uraemic state. Cardiovasc Res. 2012;96:130–139. doi: 10.1093/cvr/cvs240. [DOI] [PubMed] [Google Scholar]
- 14.Shanahan C.M., Crouthamel M.H., Kapustin A., Giachelli C.M. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011:109697–109711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellam T., Wilkie M., Chamberlain J. Dietary phosphate modulates atherogenesis and insulin resistance in apolipoprotein E knockout mice–brief report. Arterioscler Thromb Vasc Biol. 2011;31:1988–1990. doi: 10.1161/ATVBAHA.111.231001. [DOI] [PubMed] [Google Scholar]
- 16.Santamaria R., Diaz-Tocados J.M., Pendon-Ruiz de Mier M.V. Increased phosphaturia accelerates the decline in renal function: a search for mechanisms. Sci Rep. 2018;8:13701. doi: 10.1038/s41598-018-32065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faul C., Amaral A.P., Oskouei B. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez M., Lorenzo V. Parathyroid hormone, a uremic toxin. Semin Dial. 2009;22:363–368. doi: 10.1111/j.1525-139X.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 19.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737–747. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;(113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 23.Lei X.G., Porres J.M. Phytase enzymology, applications, and biotechnology. Biotechnol Lett. 2003;25:1787–1794. doi: 10.1023/a:1026224101580. [DOI] [PubMed] [Google Scholar]
- 24.Moe S.M., Zidehsarai M.P., Chambers M.A. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scanni R., vonRotz M., Jehle S. The human response to acute enteral and parenteral phosphate loads. J Am Soc Nephrol. 2014;25:2730–2739. doi: 10.1681/ASN.2013101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benini O., D'Alessandro C., Gianfaldoni D., Cupisti A. Extra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patients. J Ren Nutr. 2011;21:303–308. doi: 10.1053/j.jrn.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Calvo M.S., Sherman R.A., Uribarri J. Dietary phosphate and the forgotten kidney patient: a critical need for FDA regulatory action. Am J Kidney Dis. 2019;73:542–551. doi: 10.1053/j.ajkd.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez O.M., Anderson C., Isakova T. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol. 2010;21:1953–1960. doi: 10.1681/ASN.2010020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itkonen S.T., Karp H.J., Kemi V.E. Associations among total and food additive phosphorus intake and carotid intima-media thickness–a cross-sectional study in a middle-aged population in southern Finland. Nutr J. 2013;12:94. doi: 10.1186/1475-2891-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman R.A., Ravella S., Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int. 2015;87:1097–1099. doi: 10.1038/ki.2015.67. [DOI] [PubMed] [Google Scholar]
- 31.Combe C., Aparicio M. Phosphorus and protein restriction and parathyroid function in chronic renal failure. Kidney Int. 1994;46:1381–1386. doi: 10.1038/ki.1994.408. [DOI] [PubMed] [Google Scholar]
- 32.Combe C., Morel D., de Precigout V. Long-term control of hyperparathyroidism in advanced renal failure by low-phosphorus low-protein diet supplemented with calcium (without changes in plasma calcitriol) Nephron. 1995;70:287–295. doi: 10.1159/000188606. [DOI] [PubMed] [Google Scholar]
- 33.Klahr S. The modification of diet in renal disease study. N Engl J Med. 1989;320:864–866. doi: 10.1056/NEJM198903303201310. [DOI] [PubMed] [Google Scholar]
- 34.Shinaberger C.S., Greenland S., Kopple J.D. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88:1511–1518. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ando S., Sakuma M., Morimoto Y., Arai H. The effect of various boiling conditions on reduction of phosphorus and protein in meat. J Ren Nutr. 2015;25:504–509. doi: 10.1053/j.jrn.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Ix J.H., Anderson C.A., Smits G. Effect of dietary phosphate intake on the circadian rhythm of serum phosphate concentrations in chronic kidney disease: a crossover study. Am J Clin Nutr. 2014;100:1392–1397. doi: 10.3945/ajcn.114.085498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang A.R., Miller E.R., 3rd, Anderson C.A. Phosphorus additives and albuminuria in early stages of CKD: a randomized controlled trial. Am J Kidney Dis. 2017;69:200–209. doi: 10.1053/j.ajkd.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fissell R.B., Karaboyas A., Bieber B.A. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int. 2016;20:38–49. doi: 10.1111/hdi.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Camp Y.P., Vrijens B., Abraham I. Adherence to phosphate binders in hemodialysis patients: prevalence and determinants. J Nephrol. 2014;27:673–679. doi: 10.1007/s40620-014-0062-3. [DOI] [PubMed] [Google Scholar]
- 40.Alfrey A.C., LeGendre G.R., Kaehny W.D. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976;294:184–188. doi: 10.1056/NEJM197601222940402. [DOI] [PubMed] [Google Scholar]
- 41.Mudge D.W., Johnson D.W., Hawley C.M. Do aluminium-based phosphate binders continue to have a role in contemporary nephrology practice? BMC Nephrol. 2011;12:20. doi: 10.1186/1471-2369-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davison A.M., Walker G.S., Oli H., Lewins A.M. Water supply aluminium concentration, dialysis dementia, and effect of reverse-osmosis water treatment. Lancet. 1982;2:785–787. doi: 10.1016/s0140-6736(82)92678-2. [DOI] [PubMed] [Google Scholar]
- 43.Norris K.C., Crooks P.W., Nebeker H.G. Clinical and laboratory features of aluminum-related bone disease: differences between sporadic and "epidemic" forms of the syndrome. Am J Kidney Dis. 1985;6:342–347. doi: 10.1016/s0272-6386(85)80091-3. [DOI] [PubMed] [Google Scholar]
- 44.Bakir A.A. The fatal interplay of aluminum and citrate in chronic renal failure: a lesson from three decades ago. Artif Organs. 2015;39:87–89. doi: 10.1111/aor.12478. [DOI] [PubMed] [Google Scholar]
- 45.Foley C.M., Polinsky M.S., Gruskin A.B. Encephalopathy in infants and children with chronic renal disease. Arch Neurol. 1981;38:656–658. doi: 10.1001/archneur.1981.00510100084016. [DOI] [PubMed] [Google Scholar]
- 46.Janssen M.J., van der Kuy A., ter Wee P.M., van Boven W.P. Aluminum hydroxide, calcium carbonate and calcium acetate in chronic intermittent hemodialysis patients. Clin Nephrol. 1996;45:111–119. [PubMed] [Google Scholar]
- 47.Jespersen B., Jensen J.D., Nielsen H.K. Comparison of calcium carbonate and aluminium hydroxide as phosphate binders on biochemical bone markers, PTH(1-84), and bone mineral content in dialysis patients. Nephrol Dial Transplant. 1991;6:98–104. doi: 10.1093/ndt/6.2.98. [DOI] [PubMed] [Google Scholar]
- 48.Almirall J., Veciana L., Llibre J. Calcium acetate versus calcium carbonate for the control of serum phosphorus in hemodialysis patients. Am J Nephrol. 1994;14:192–196. doi: 10.1159/000168713. [DOI] [PubMed] [Google Scholar]
- 49.Pflanz S., Henderson I.S., McElduff N., Jones M.C. Calcium acetate versus calcium carbonate as phosphate-binding agents in chronic haemodialysis. Nephrol Dial Transplant. 1994;9:1121–1124. doi: 10.1093/ndt/9.8.1121. [DOI] [PubMed] [Google Scholar]
- 50.Qunibi W., Winkelmayer W.C., Solomon R. A randomized, double-blind, placebo-controlled trial of calcium acetate on serum phosphorus concentrations in patients with advanced non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2011;12:9. doi: 10.1186/1471-2369-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fournier A., Moriniere P., Sebert J.L. Calcium carbonate, an aluminum-free agent for control of hyperphosphatemia, hypocalcemia, and hyperparathyroidism in uremia. Kidney Int Suppl. 1986;18:S114–S119. [PubMed] [Google Scholar]
- 52.Hill K.M., Martin B.R., Wastney M.E. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 2013;83:959–966. doi: 10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiegel D.M., Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int. 2012;81:1116–1122. doi: 10.1038/ki.2011.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.London G.M., Guerin A.P., Marchais S.J. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 55.Paloian N.J., Giachelli C.M. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol. 2014;307:F891–F900. doi: 10.1152/ajprenal.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spiegel D.M., Farmer B., Smits G., Chonchol M. Magnesium carbonate is an effective phosphate binder for chronic hemodialysis patients: a pilot study. J Ren Nutr. 2007;17:416–422. doi: 10.1053/j.jrn.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Massy Z.A., Drueke T.B. Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival. Clin Kidney J. 2012;5(Suppl 1):i52–i61. doi: 10.1093/ndtplus/sfr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Schutter T.M., Behets G.J., Geryl H. Effect of a magnesium-based phosphate binder on medial calcification in a rat model of uremia. Kidney Int. 2013;83:1109–1117. doi: 10.1038/ki.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiegel D.M., Farmer B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int. 2009;13:453–459. doi: 10.1111/j.1542-4758.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 60.Tzanakis I.P., Stamataki E.E., Papadaki A.N. Magnesium retards the progress of the arterial calcifications in hemodialysis patients: a pilot study. Int Urol Nephrol. 2014;46:2199–2205. doi: 10.1007/s11255-014-0751-9. [DOI] [PubMed] [Google Scholar]
- 61.de Francisco A.L., Leidig M., Covic A.C. Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: a controlled randomized study (CALMAG study) assessing efficacy and tolerability. Nephrol Dial Transplant. 2010;25:3707–3717. doi: 10.1093/ndt/gfq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evsanaa B., Liu I., Aliazardeh B. MgCaCO3 versus CaCO3 in peritoneal dialysis patients–a cross-over pilot trial. Perit Dial Int. 2015;35:31–34. doi: 10.3747/pdi.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakaguchi Y., Hamano T., Obi Y. A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol. 2019;30:1073–1085. doi: 10.1681/ASN.2018111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Y., Wang G., Li Y. Effects of oral activated charcoal on hyperphosphatemia and vascular calcification in Chinese patients with stage 3-4 chronic kidney disease. J Nephrol. 2019;32:265–272. doi: 10.1007/s40620-018-00571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bleyer A.J., Burke S.K., Dillon M. A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis. 1999;33:694–701. doi: 10.1016/s0272-6386(99)70221-0. [DOI] [PubMed] [Google Scholar]
- 66.Qunibi W.Y., Hootkins R.E., McDowell L.L. Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study) Kidney Int. 2004;65:1914–1926. doi: 10.1111/j.1523-1755.2004.00590.x. [DOI] [PubMed] [Google Scholar]
- 67.Evenepoel P., Selgas R., Caputo F. Efficacy and safety of sevelamer hydrochloride and calcium acetate in patients on peritoneal dialysis. Nephrol Dial Transplant. 2009;24:278–285. doi: 10.1093/ndt/gfn488. [DOI] [PubMed] [Google Scholar]
- 68.Chennasamudram S.P., Noor T., Vasylyeva T.L. Comparison of sevelamer and calcium carbonate on endothelial function and inflammation in patients on peritoneal dialysis. J Ren Care. 2013;39:82–89. doi: 10.1111/j.1755-6686.2013.12009.x. [DOI] [PubMed] [Google Scholar]
- 69.Kakuta T., Tanaka R., Hyodo T. Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis. 2011;57:422–431. doi: 10.1053/j.ajkd.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 70.Gregorio P.C., Favretto G., Sassaki G.L. Sevelamer reduces endothelial inflammatory response to advanced glycation end products. Clin Kidney J. 2018;11:89–98. doi: 10.1093/ckj/sfx074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braunlin W., Zhorov E., Guo A. Bile acid binding to sevelamer HCl. Kidney Int. 2002;62:611–619. doi: 10.1046/j.1523-1755.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 72.Burke S.K., Dillon M.A., Hemken D.E. Meta-analysis of the effect of sevelamer on phosphorus, calcium, PTH, and serum lipids in dialysis patients. Adv Ren Replace Ther. 2003;10:133–145. doi: 10.1053/jarr.2003.50016. [DOI] [PubMed] [Google Scholar]
- 73.Bronden A., Mikkelsen K., Sonne D.P. Glucose-lowering effects and mechanisms of the bile acid-sequestering resin sevelamer. Diabetes Obes Metab. 2018;20:1623–1631. doi: 10.1111/dom.13272. [DOI] [PubMed] [Google Scholar]
- 74.Susantitaphong P., Jaber B.L. Potential interaction between sevelamer and fat-soluble vitamins: a hypothesis. Am J Kidney Dis. 2012;59:165–167. doi: 10.1053/j.ajkd.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Block G.A., Spiegel D.M., Ehrlich J. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 76.Barreto D.V., de Barreto F.C., de Carvalho A.B. Phosphate binder impact on bone remodeling and coronary calcification–results from the BRiC study. Nephron Clin Pract. 2008;110:c273–c283. doi: 10.1159/000170783. [DOI] [PubMed] [Google Scholar]
- 77.Qunibi W., Moustafa M., Muenz L.R. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 78.Chertow G.M., Burke S.K., Raggi P., Treat to Goal Working Group Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 79.Ruospo M., Palmer S.C., Natale P. Phosphate binders for preventing and treating chronic kidney disease−mineral and bone disorder (CKD-MBD) Cochrane Database Syst Rev. 2018;8:CD006023. doi: 10.1002/14651858.CD006023.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sprague S.M. A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate. Curr Med Res Opin. 2007;23:3167–3175. doi: 10.1185/030079907X242719. [DOI] [PubMed] [Google Scholar]
- 81.Patel L., Bernard L.M., Elder G.J. Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2016;11:232–244. doi: 10.2215/CJN.06800615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park H., Rascati K.L., Keith M.S. Cost-effectiveness of lanthanum carbonate versus sevelamer hydrochloride for the treatment of hyperphosphatemia in patients with end-stage renal disease: a US payer perspective. Value Health. 2011;14:1002–1009. doi: 10.1016/j.jval.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 83.Akizawa T., Origasa H., Kameoka C. Randomized controlled trial of bixalomer versus sevelamer hydrochloride in hemodialysis patients with hyperphosphatemia. Ther Apher Dial. 2014;18:122–131. doi: 10.1111/1744-9987.12068. [DOI] [PubMed] [Google Scholar]
- 84.Vemuri N., Michelis M.F., Matalon A. Conversion to lanthanum carbonate monotherapy effectively controls serum phosphorus with a reduced tablet burden: a multicenter open-label study. BMC Nephrol. 2011;12:49. doi: 10.1186/1471-2369-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dellanna F., Reichel H., Seibt F. Efficacy and safety of lanthanum carbonate in German patients on dialysis. Clin Nephrol. 2012;78:382–390. doi: 10.5414/CN107330. [DOI] [PubMed] [Google Scholar]
- 86.Wilson R.J., Keith M.S., Preston P., Copley J.B. The real-world dose-relativity of sevelamer hydrochloride and lanthanum carbonate monotherapy in patients with end-stage renal disease. Adv Ther. 2013;30:1100–1110. doi: 10.1007/s12325-013-0077-5. [DOI] [PubMed] [Google Scholar]
- 87.Takahara Y., Matsuda Y., Takahashi S. Efficacy and safety of lanthanum carbonate in pre-dialysis CKD patients with hyperphosphatemia: a randomized trial. Clin Nephrol. 2014;82:181–190. doi: 10.5414/cn108269. [DOI] [PubMed] [Google Scholar]
- 88.Toida T., Fukudome K., Fujimoto S. Effect of lanthanum carbonate vs. calcium carbonate on serum calcium in hemodialysis patients: a crossover study. Clin Nephrol. 2012;78:216–223. doi: 10.5414/cn107257. [DOI] [PubMed] [Google Scholar]
- 89.Zhang C., Wen J., Li Z., Fan J. Efficacy and safety of lanthanum carbonate on chronic kidney disease−mineral and bone disorder in dialysis patients: a systematic review. BMC Nephrol. 2013;14:226. doi: 10.1186/1471-2369-14-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Black T., Philips G., Burbridge R. Pharmacobezoar in a patient on an oral phosphate binder. Gastrointest Endosc. 2013;77:511–512. doi: 10.1016/j.gie.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Lacour B., Lucas A., Auchere D. Chronic renal failure is associated with increased tissue deposition of lanthanum after 28-day oral administration. Kidney Int. 2005;67:1062–1069. doi: 10.1111/j.1523-1755.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 92.Hutchison A.J., Barnett M.E., Krause R. Lanthanum carbonate treatment, for up to 6 years, is not associated with adverse effects on the liver in patients with chronic kidney disease stage 5 receiving hemodialysis. Clin Nephrol. 2009;71:286–295. [PubMed] [Google Scholar]
- 93.Spasovski G.B., Sikole A., Gelev S. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up. Nephrol Dial Transplant. 2006;21:2217–2224. doi: 10.1093/ndt/gfl146. [DOI] [PubMed] [Google Scholar]
- 94.Lewis J.B., Sika M., Koury M.J. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26:493–503. doi: 10.1681/ASN.2014020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Umanath K., Jalal D.I., Greco B.A. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol. 2015;26:2578–2587. doi: 10.1681/ASN.2014080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Floege J. Phosphate binders in chronic kidney disease: a systematic review of recent data. J Nephrol. 2016;29:329–340. doi: 10.1007/s40620-016-0266-9. [DOI] [PubMed] [Google Scholar]
- 97.Cozzolino M., Funk F., Rakov V. Preclinical pharmacokinetics, pharmacodynamics and safety of sucroferric oxyhydroxide. Curr Drug Metab. 2014;15:953–965. doi: 10.2174/1389200216666150206124424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Floege J., Covic A.C., Ketteler M. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30:1037–1046. doi: 10.1093/ndt/gfv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Floege J., Covic A.C., Ketteler M. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86:638–647. doi: 10.1038/ki.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Covic A.C., Floege J., Ketteler M. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transplant. 2017;32:1330–1338. doi: 10.1093/ndt/gfw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koiwa F., Yokoyama K., Fukagawa M., Akizawa T. Evaluation of changes in ferritin levels during sucroferric oxyhydroxide treatment. Clin Kidney J. 2019;12:294–299. doi: 10.1093/ckj/sfy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Otsuki T., Utsunomiya K., Moriuchi M. Effect of sucroferric oxyhydroxide on fibroblast growth factor 23 levels in hemodialysis patients. Nephron. 2018;140:161–168. doi: 10.1159/000490903. [DOI] [PubMed] [Google Scholar]
- 103.Jamal S.A., Vandermeer B., Raggi P. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268–1277. doi: 10.1016/S0140-6736(13)60897-1. [DOI] [PubMed] [Google Scholar]
- 104.Wang F., Lu X., Zhang J., Xiong R. Effect of lanthanum carbonate on all-cause mortality in patients receiving maintenance hemodialysis: a meta-analysis of randomized controlled trials. Kidney Blood Press Res. 2018;43:536–544. doi: 10.1159/000488700. [DOI] [PubMed] [Google Scholar]
- 105.Marks J., Debnam E.S., Unwin R.J. Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol. 2010;299:F285–F296. doi: 10.1152/ajprenal.00508.2009. [DOI] [PubMed] [Google Scholar]
- 106.Forster I.C., Hernando N., Biber J., Murer H. Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med. 2013;34:386–395. doi: 10.1016/j.mam.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 107.Block G.A., Wheeler D.C., Persky M.S. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schiavi S.C., Tang W., Bracken C. Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol. 2012;23:1691–1700. doi: 10.1681/ASN.2011121213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Katai K., Tanaka H., Tatsumi S. Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant. 1999;14:1195–1201. doi: 10.1093/ndt/14.5.1195. [DOI] [PubMed] [Google Scholar]
- 110.Eto N., Miyata Y., Ohno H., Yamashita T. Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant. 2005;20:1378–1384. doi: 10.1093/ndt/gfh781. [DOI] [PubMed] [Google Scholar]
- 111.Maccubbin D., Tipping D., Kuznetsova O. Hypophosphatemic effect of niacin in patients without renal failure: a randomized trial. Clin J Am Soc Nephrol. 2010;5:582–589. doi: 10.2215/CJN.07341009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takahashi Y., Tanaka A., Nakamura T. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65:1099–1104. doi: 10.1111/j.1523-1755.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 113.Cheng S.C., Young D.O., Huang Y. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1131–1138. doi: 10.2215/CJN.04211007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lenglet A., Liabeuf S., Esper N.E. Efficacy and safety of nicotinamide in haemodialysis patients: the NICOREN study. Nephrol Dial Transplant. 2017;32:1597. doi: 10.1093/ndt/gfx249. [DOI] [PubMed] [Google Scholar]
- 115.Massy ZA, Drueke TB. Vascular calcification - any place left for nicotinamide [epub ahead of print]? Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfz057. Accessed June 21, 2019. [DOI] [PubMed]
- 116.Kaesler N, Goettsch C, Weis D. et al. Magnesium but not nicotinamide prevents vascular calcification in experimental uraemia [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfy410. Accessed June 21, 2019. [DOI] [PubMed]
- 117.Labonte E.D., Carreras C.W., Leadbetter M.R. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol. 2015;26:1138–1149. doi: 10.1681/ASN.2014030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Block G.A., Rosenbaum D.P., Leonsson-Zachrisson M. Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol. 2017;28:1933–1942. doi: 10.1681/ASN.2016080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Block G.A., Rosenbaum D.P., Yan A., Chertow G.M. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: a randomized phase 3 trial. J Am Soc Nephrol. 2019;30:641–652. doi: 10.1681/ASN.2018080832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramirez J.A., Emmett M., White M.G. The absorption of dietary phosphorus and calcium in hemodialysis patients. Kidney Int. 1986;30:753–759. doi: 10.1038/ki.1986.252. [DOI] [PubMed] [Google Scholar]
- 121.Gutzwiller J.P., Schneditz D., Huber A.R. Estimating phosphate removal in haemodialysis: an additional tool to quantify dialysis dose. Nephrol Dial Transplant. 2002;17:1037–1044. doi: 10.1093/ndt/17.6.1037. [DOI] [PubMed] [Google Scholar]
- 122.Daugirdas J.T., Chertow G.M., Larive B. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol. 2012;23:727–738. doi: 10.1681/ASN.2011070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vilar E., Fry A.C., Wellsted D. Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: a comparative analysis. Clin J Am Soc Nephrol. 2009;4:1944–1953. doi: 10.2215/CJN.05560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Locatelli F., Carfagna F., Del Vecchio L., La Milia V. Haemodialysis or haemodiafiltration: that is the question. Nephrol Dial Transplant. 2018;33:1896–1904. doi: 10.1093/ndt/gfy035. [DOI] [PubMed] [Google Scholar]
- 125.Iwasawa H., Nakao T., Matsumoto H. Phosphate handling by end-stage kidneys and benefits of residual renal function on phosphate removal in patients on haemodialysis. Nephrology (Carlton) 2013;18:285–291. doi: 10.1111/nep.12039. [DOI] [PubMed] [Google Scholar]
- 126.Wang A.Y., Woo J., Sea M.M. Hyperphosphatemia in Chinese peritoneal dialysis patients with and without residual kidney function: what are the implications? Am J Kidney Dis. 2004;43:712–720. [PubMed] [Google Scholar]
- 127.Penne E.L., van der Weerd N.C., Grooteman M.P. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:281–289. doi: 10.2215/CJN.04480510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Becker G.J., Walker R.G., Hewitson T.D., Pedagogos E. Phosphate levels–time for a rethink? Nephrol Dial Transplant. 2009;24:2321–2324. doi: 10.1093/ndt/gfp220. [DOI] [PubMed] [Google Scholar]
- 129.Trivedi H., Szabo A., Zhao S. Circadian variation of mineral and bone parameters in end-stage renal disease. J Nephrol. 2015;28:351–359. doi: 10.1007/s40620-014-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gardham C., Stevens P.E., Delaney M.P. Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin J Am Soc Nephrol. 2010;5:1261–1267. doi: 10.2215/CJN.09471209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Isakova T., Gutierrez O., Shah A. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19:615–623. doi: 10.1681/ASN.2007060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oliveira R.B., Cancela A.L., Graciolli F.G. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Granjon D., Bonny O., Edwards A. Coupling between phosphate and calcium homeostasis: a mathematical model. Am J Physiol Renal Physiol. 2017;313:F1181–F1199. doi: 10.1152/ajprenal.00271.2017. [DOI] [PubMed] [Google Scholar]
- 134.David V., Martin A., Isakova T. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89:135–146. doi: 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cancela A.L., Oliveira R.B., Graciolli F.G. Fibroblast growth factor 23 in hemodialysis patients: effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract. 2011;117:c74–c82. doi: 10.1159/000319650. [DOI] [PubMed] [Google Scholar]
- 136.Ketteler M., Sprague S.M., Covic A.C. Effects of sucroferric oxyhydroxide and sevelamer carbonate on chronic kidney disease−mineral bone disorder parameters in dialysis patients. Nephrol Dial Transplant. 2019;34:1163–1170. doi: 10.1093/ndt/gfy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goretti Penido M., Alon U.S. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27:2039–2048. doi: 10.1007/s00467-012-2175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Drueke T.B., Massy Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89:289–302. doi: 10.1016/j.kint.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 139.Block G.A., Martin K.J., de Francisco A.L. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 140.EVOLVE Trial Investigators. Chertow G.M., Block G.A. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 141.Block G.A., Bushinsky D.A., Cheng S. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317:156–164. doi: 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- 142.Fernandez E., Borras M., Pais B., Montoliu J. Low-calcium dialysate stimulates parathormone secretion and its long-term use worsens secondary hyperparathyroidism. J Am Soc Nephrol. 1995;6:132–135. doi: 10.1681/ASN.V61132. [DOI] [PubMed] [Google Scholar]
- 143.Haris A., Sherrard D.J., Hercz G. Reversal of adynamic bone disease by lowering of dialysate calcium. Kidney Int. 2006;70:931–937. doi: 10.1038/sj.ki.5001666. [DOI] [PubMed] [Google Scholar]
- 144.Karohl C., de Paiva Paschoal J., de Castro M.C. Effects of bone remodelling on calcium mass transfer during haemodialysis. Nephrol Dial Transplant. 2010;25:1244–1251. doi: 10.1093/ndt/gfp597. [DOI] [PubMed] [Google Scholar]