INTRODUCTION
Treatment of unilateral prostate cancer by hemi-gland cryoablation was reported in a pilot study by Onik et al in 20021. The aim was to preserve potency by leaving one neurovascular bundle intact. In subsequent publications, Onik reported an 86% success rate in preserving potency with his method, and he coined the term “male lumpectomy” in a comparison with breast-sparing surgery2. Since then, many patients have been treated with hemi-gland cryoablation, the original form of prostate focal therapy, and intermediate-term follow-up has demonstrated in most cases apparent oncologic control2–7. The procedure is associated with no blood loss and low rates of incontinence and erectile dysfunction; therefore, for certain men, hemi-gland cryoablation may offer an attractive treatment alternative.
For any form of focal therapy or partial gland ablation (PGA), selection criteria and biopsy follow-up are the defining metrics8,9. However, in most studies of focal cryoablation, conventional prostate biopsy has been used to select patients for treatment, and likewise, if obtained at all, for follow-up. Conventional transrectal ultrasound (TRUS) biopsy—systematic, transrectal, ultrasound-guided10—often fails to disclose the underlying pathology11–13. Thus, use of TRUS-guided biopsy to select patients for treatment may under-estimate the disease, and its use in follow-up may over-estimate treatment efficacy. A paucity of follow-up biopsy data may be a reason the ASCO panel, which recently formulated guidelines for treatment of localized prostate cancer, did not endorse cryotherapy as an acceptable option (Grade C evidence)14.
MRI-guided biopsy, which is more accurate than TRUS-guided biopsy15–18, has shown value as a method for selection and follow-up of men undergoing prostate focal therapy with high-intensity focused ultrasound19. Herein is demonstrated the use of the new diagnostic modality in men undergoing hemi-gland cryoablation. In contrast to other cryoablation studies, all patients in the present series had clinically significant disease and all underwent MRI-guided biopsy before and after treatment. The results of the present work suggest that cryotherapy, a relatively old treatment, warrants further study in light of new technology to improve selection and follow-up of patients.
MATERIALS AND METHODS
Patients
Subjects were all 29 men undergoing prostate hemi-gland cryoablation at UCLA in a 14-month period ending in March, 2018. Patients were recruited from a larger pool of men undergoing MRI/US fusion biopsy for suspected prostate cancer, the method of which has been previously published13,18,20. All patients underwent systematic 12-core biopsy and, when an MRI-visible lesion was present, targeted biopsy. All biopsy sites were tracked within the Artemis device, as described previously21. We calculated the change in prostate-specific antigen (PSA), prostate volume and PSA density (PSAD) from baseline to 6-month follow-up for each patient, and conducted a Wilcoxon rank sum test to determine significance of changes. Clinical characteristics of the 29 men are shown in Table 1.
Table 1.
Clinical Characteristics (n = 29)
| Mean Age, years (SD) | 68.7 (6.3) |
|---|---|
| Ethnicity n (%) | |
| Caucasian | 21 (76) |
| Hispanic | 5 (14) |
| Asian | 2 (7) |
| African American | 1 (3) |
| PSA, ng/ml, median (IQR) | 6.6 (5.2–10.3) |
| Prostate Volume, cc, median (IQR) | 37.5 (27.8–50.0) |
| PSA density, ng/ml/cc, median (IQR) | 0.20 (0.14–0.26) |
| PI-RADS™ v2 ROI, n (%) | |
| Grade 5 | 15 (52) |
| Grade 4 | 8 (28) |
| Grade 3 | 3 (10) |
| MRI negative | 3 (10) |
| Gleason Score, n (%) | |
| 3+3 (high volume) | 2 (7) |
| 3+4 | 18 (62) |
| 4+3 | 5 (17) |
| 4+4 | 4 (14) |
Trial Design
The present pilot study was designed as a prospective, open-label, observational trial. Inclusion criteria were as follows: clinically significant prostate cancer, defined as intermediate risk disease (Gleason Score (GS) ≤ 7, N = 23), low-volume high-risk disease (GS8 present in ≤ two cores, N = 4) or high-volume GS6 (> 6 mm cancer core length, N = 2) }22; disease restricted to one half of the prostate (microfoci of contralateral GS6 allowed), PSA ≤ 20, prostate volume ≤ 60 cc, age 40–80. Patients were excluded if they lacked the inclusion criteria or had received prior treatment for prostate cancer or if they were unable to tolerate general anesthesia. MRI-guided biopsy, the focus of the present investigation, was performed in all patients prior to enrollment, within 45 days of treatment, and again in 28/29 patients six months afterward. Quality of life questionnaires (IIEF-5, IPSS, EPIC-26) were completed upon enrollment and at the 6-month follow-up. The trial was approved by the UCLA IRB; a study-specific consent form was signed by all participants; and the trial was registered at clinicaltrials.gov.
Cryoablation Procedure
All procedures were performed on outpatients under general anesthesia at the UCLA Ambulatory Surgical Center by a study co-author. Prior to procedure all men received a cleansing enema and antibiotic prophylaxis. Following induction of anesthesia, patients were placed in the dorsal lithotomy position; genitalia and perineum were prepared in the usual fashion. An 18 French coude-tip Foley catheter was placed in the bladder. Transrectal bi-planar ultrasound was performed using the Flex Focus 800 (BK Medical; Peabody, MA), the probe held in place with a stepper/stabilizer (Civco; Coralville, Iowa). 14-gauge argon gas cryotherapy probes (Galil Medical, Inc; Arden Hills, MN) were introduced through the perineum and spaced to treat the entire hemi-gland. Two or three cryoablation needles were used, depending on prostate volume (average 38cc), ensuring a 10–12 mm zone of ‘lethal ice’ between needles and capsule23. A thermal safety probe was next inserted between the posterior prostate and anterior rectal wall to ensure that the rectum was not cooled below 0°C. Supplementary Figure 1 shows an example ultrasound view of a hemi-gland cryoablation procedure.
Following needle placement, flexible cystoscopy was performed to confirm that no needle had penetrated the urethra or bladder. A guide wire was then inserted into the bladder, over which a urethral warming catheter was placed. 44°C water was then circulated through the warming catheter for prevention of urethral freezing. Cryoablation was initiated anteriorly, with observation of ice ball formation under real-time ultrasound monitoring. Posterior needles were activated after visualization of the developing anterior freeze (approximately 2 minutes). Completion of freezing was determined by observation of rectal wall temperatures and leading edge of the ice ball (another 5–8 minutes). Two cycles of freezing were employed in all patients with an interim thaw sufficient to visualize internal tissue characteristics (approximately 8 minutes). The urethral warming catheter was kept in place for 15 minutes after completion of the second cryoablation cycle. Manual pressure was held for 5 minutes on the perineal puncture sites. After removal of the urethral warming catheter, an 18F indwelling Foley catheter was placed for 48 hours. Operating time (from initial to final catheter insertion) did not exceed 60 minutes.
Follow-up Evaluations
All patients were discharged within a few hours of the procedure with the Foley catheter in place. Discharge medications included a quinolone antibiotic and non-narcotic oral analgesic. Patients returned to clinic at 2 days for voiding trial. PSA, DRE, UF, PVR, and questionnaires were obtained at 3 and 6 months. Repeat multiparametric MRI (mpMRI) and targeted biopsy of the prostate were performed 6 months after treatment using the same MRI/US fusion device, which permitted re-call of stored images and biopsy sites from the initial fusion biopsy. MpMRI was interpreted using PI-RADSv224.
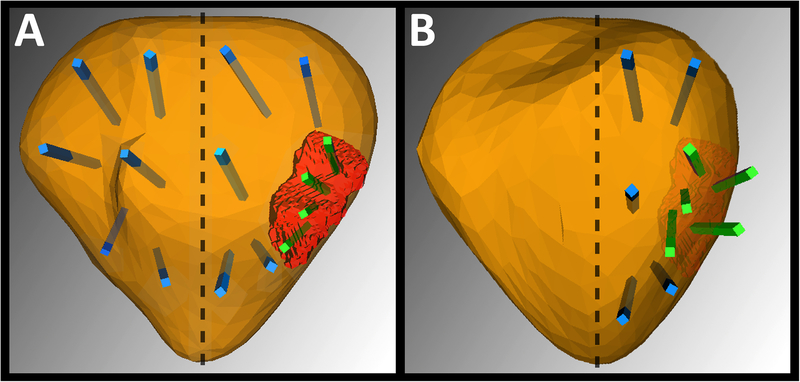
Figure 1 demonstrates how follow-up biopsies were performed. The treated side, including the original MRI-visible region of interest and adjacent ipsilateral sites, was resampled using targeted biopsy. From 4–16 biopsy cores (average 10) were obtained from the treated side of the prostate. the number depending on prostate volume. Contralateral biopsy was deferred at the 6-month point and is planned per protocol for 18-month follow-up. Adverse events were defined and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE).
Figure 1.
Illustration of biopsy method: A, baseline and B, 6-months after successful hemi-gland ablation. Before treatment (A) patient has an MRI-visible lesion on the left (red spot). Targeted cores taken from the lesion (shown in green) were found to contain csCaP. Systematic cores (blue) were found to contain no csCaP outside the lesion, making the patient eligible for focal therapy. After hemi-gland ablation (B), biopsy cores are guided by MRI/US fusion tracking to the region of the prior positive spots; the lesion, which was seen previously, has receded. All cores are now negative for cancer, both targeted and systematic ipsilaterally. In this study of hemi-gland ablation, 6-month biopsy samples were taken from the treated side of the prostate (see text).
RESULTS
Clinical characteristics of the 29 patients are shown in Table 1. The men are a mostly-Caucasian group in their late 60s with moderate PSA elevations. The majority had an MRI-visible lesion containing a clinically-significant prostate cancer (csCaP) on one side of the organ. Before treatment, all patients received mpMRI with combined systematic and targeted biopsy to confirm unilateral disease.
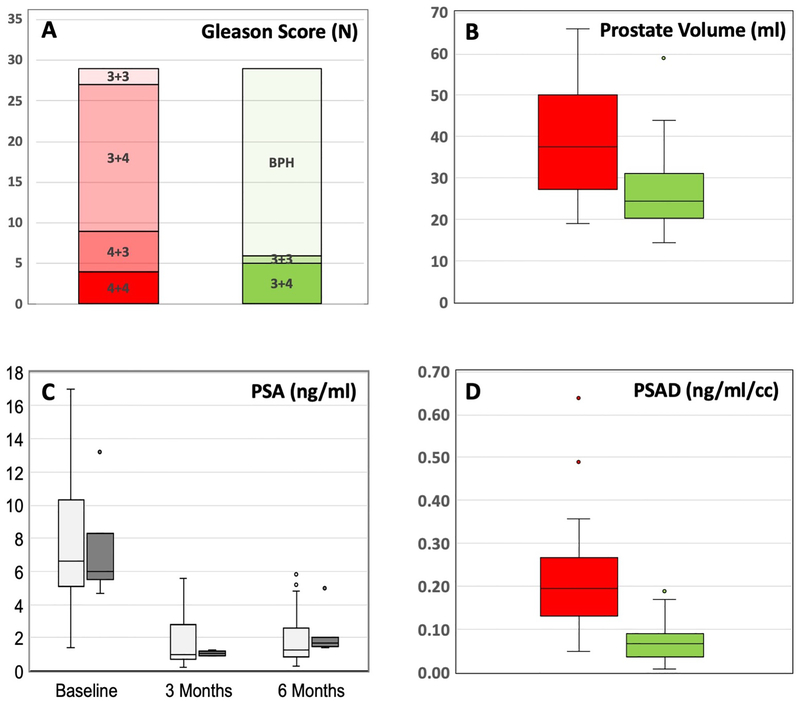
Cryoablation was completed in all 29 patients with no intra-operative complications. At the 6-month mpMRI, the median decrease in prostate volume from baseline was 11.0 cc (IQR = 6–15). The median decrease in PSA at the 6-month follow-up was 5.6 ng/ml (IQR = 2.9–8.2). The median decease in PSA density was 0.14 ng/ml/cc (IQR = 0.07–0.22). All decreases were statistically significant (all P < 0.01). Individual patient changes are shown in Table 2; group changes are shown in Figure 2.
Table 2.
Individual patient metrics before and after treatment
| GLEASON SCORE | PI-RADS™ v2 GRADE | PSA (ng/ml) | PROSTATE VOLUME (cc) | PSAD (ng/ml/cc) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | 6 MO | 6 MO | 6 MO | 6 Months | 6 Months | ||||||
| 1-VC | benign | Negative | 1.1 | 23 | 0.05 | ||||||
| 2-DW | benign | Negative | 1.0 | 26 | 0.04 | ||||||
| 3-AS | benign | Negative | 3.1 | 42 | 0.07 | ||||||
| 4-DC | benign | Negative | 1.0 | 20 | 0.05 | ||||||
| 5-DJ | benign | Negative | 0.3 | 36 | 0.01 | ||||||
| 6-SM | benign | Negative | 1.2 | 17 | 0.07 | ||||||
| 7-EA | benign | Negative | 1.3 | 20 | 0.07 | ||||||
| 8-DL | benign | Negative | 3.3 | 17 | 0.19 | ||||||
| 9-BV | benign | Negative | 0.7 | 25 | 0.03 | ||||||
| 10-MW | benign | Negative | 0.3 | 14 | 0.02 | ||||||
| 11-GB | benign | Negative | 2.4 | 26 | 0.09 | ||||||
| 12-SS | benign | Negative | 0.3 | 16 | 0.02 | ||||||
| 13-JP | benign | Negative | 0.9 | 22 | 0.04 | ||||||
| 14-AN | benign | Negative | 5.2 | 59 | 0.09 | ||||||
| 15-RT | benign | Negative | 4.5 | 28 | 0.16 | ||||||
| 16-AS | benign | Negative | 2.1 | 27 | 0.08 | ||||||
| 17-MW | benign | Negative | 0.9 | 23 | 0.04 | ||||||
| 18-WT | benign | Negative | 1.7 | 31 | 0.06 | ||||||
| 19-JB | benign | Negative | 0.6 | 21 | 0.03 | ||||||
| 20-RG | benign | Negative | 1.3 | 44 | 0.03 | ||||||
| 21-PW | benign | Negative | 1.8 | 23 | 0.08 | ||||||
| 22-PB | benign | 3 | 1.7 | 31 | 0.06 | ||||||
| 23-LM | benign | Negative | 5.8 | 34 | 0.17 | ||||||
| 24-GD | 3+4* | Negative | 4.8 | 44 | 0.11 | ||||||
| 25-JS | 3+4* | Negative | 1.7 | 24 | 0.07 | ||||||
| 26-KL | 3+3* | 5 | 1.5 | 22 | 0.07 | ||||||
| 27-BV | 3+4** | - | 1.4 | - | - | ||||||
| 28-PM | 3+4** | Negative | 2.0 | 15 | 0.13 | ||||||
| 29-DL | 3+4** | Negative | 5.0 | 26 | 0.19 | ||||||
Micro-residual or clinically-insignificant cancer on follow-up biopsy.
Follow-up biopsy positive for csCaP.
Dash indicates missing data.
Figure 2.
Chart showing group changes in Gleason Score (A) and Box & Whisker plots showing group changes in prostate volume (B), PSA (C), and PSA density (D) between baseline (red) and 6 months after hemi-gland ablation (green). In C, open box = treatment success (no cancer at f/u biopsy) and dark box = treatment failure (cancer present at f/u biopsy). All changes are statistically significant (p<0.01).
Histological Changes
All patients underwent repeat fusion biopsy of the treated hemi-gland at 6 months. Targeted cores were taken from the site of the original MRI-visible lesion or tracked sites, adjacent tissue, and systematically from the treated side (Figure 1). At this session, an average of 10 tissue cores (range, 4 to 16) was obtained. Follow-up biopsy was guided by repeat mpMRI at 6 months in all but one patient, who declined re-imaging and underwent fusion biopsy guided by his original MRI. In 23 of 29 patients, follow-up biopsy revealed no cancer. Three patients had persistent csCaP at time of re-biopsy (all with GS 3+4) and underwent further treatment (radiation in two, repeat cryoablation in one). Three patients had residual insignificant or minute CaP as defined by Tay and colleagues25, one GS 3+3 (2mm) and two with GS 3+4 (0.5mm and 1mm), and entered active surveillance. In comparing the 6 men with residual cancer vs the 19 with no residual cancer, no significant difference was found among baseline parameters, including age, Gleason score, MRI grade, PSA, prostate volume, and PSA density.
MRI Changes
Representative examples of MRI findings before and after treatment are shown in Supplementary Figure 2, for both a treatment success (no tumor at follow-up) and a treatment failure (residual csCaP). As shown in Table 2, of the 26 patients with a region of interest (ROI) present on baseline MRI, 23 (88%) showed disappearance of the target on follow-up MRI; in 19 of the 23 (83%), no cancer was found on follow-up biopsy. In the two cases where the target was found to persist, one was biopsy-negative at 6 months and one showed a small amount of Gleason 6; one patient declined follow-up MRI. In four of six men with residual tumor, no lesions were seen on repeat mpMRI. In no case did an ipsilateral target (ROI) develop de novo during the 6-month interval between baseline and follow-up biopsy; two new lesions (both PI-RADSv2 Grade 3) were seen contralaterally at 6 months, and will be evaluated at the planned 18 month follow-up. In the single case in which residual tumor was indicated by MRI (26-KL), mass-like low T2 signal, focal early enhancement with rapid washout, and persistent diffusion restriction were noted. Gross shrinkage of the treated side was often seen.
Quality of Life Measures
24 men reported erections adequate for penetration prior to treatment. Of the 19 men who had attempted intercourse since treatment, 16 (84.2%) reported no change in sexual function. No incidence of incontinence was observed. At 6 months, average IPSS and EPIC-26 scores showed no statistically significant change from pre-treatment scores (p = 0.1 and 0.73 respectively). There was a minor but statistically significant decrease in sexual function and/or satisfaction: average IIEF-5 scores decreased from 18.0 ± 5.6 to 15.5 ± 3.0 (p = 0.04).
Adverse events
One case of transient urinary retention (grade 1), resolving spontaneously at 7 days post-treatment, constituted the sole adverse event. No grade ≥ 2 adverse events were recorded during follow-up.
COMMENT
The present investigation of hemi-gland cryoablation, though limited by short-term study of a relatively small sample, differs from previous work and adds to the knowledge base in several ways. First, the study was prospective, IRB-approved, registered, and featured strict inclusion/exclusion criteria. Many previous studies lack such a design. Second, all patients treated were men with csCaP cancer; in fact, nine men had tumors with primary Gleason score of 4, and only two had Gleason 6 lesions, both of which were high-volume. In many other studies, PGA cryotherapy has been performed for lesions that are now considered clinically insignificant, i.e., low-volume Gleason Score of 6. Thus, the success rate reported herein represents a possible life-altering intervention vis-à-vis treatments of tumors that would likely not affect life expectancy.
Further, and the focus of the present investigation, MRI-guided biopsy, which was both targeted and systematic, was included to bolster patient evaluation by helping to ensure that PGA was appropriate8. In most prior studies of hemi-gland cryoablation, biopsies were performed via ultrasound guidance (i.e., ‘blind’), which often fails to detect clinically significant cancers. In the present study, biopsy follow-up, also MRI-guided, was mandated in the protocol and completed in 28/29 patients. Tracking of biopsy sites, a function of fusion devices, enables re-sampling of previous cancer foci, within or without of MRI-visible lesions21,26, and was used routinely to increase sensitivity of follow-up biopsy. Few prior studies of cryoablation have employed a protocol mandating a follow-up biopsy, and according to a recent literature review25, none by MRI-guided biopsy before and after treatment.
The COLD Registry, established in 2006 and now housed at the Cleveland Clinic, contains data on more than one thousand men who have undergone partial gland ablation (PGA) with cryotherapy4. From treatment observations in this large heterogeneous repository, compiled online by numerous investigators nationwide, the procedure appears relatively safe: incidence of fistula was 0.1% and urinary incontinence 1.6%. The quality of life data, obtained prospectively in the present investigation via validated questionnaires, is consonant with findings reported in the registry. We encountered no fistulae, no urinary incontinence, and little change in sexual function.
In a recent study of 166 men in the registry who underwent PGA for intermediate risk disease, the rate of biochemical-free survival at 2 years was approximately 80%27. PSA data obtained herein confirm a statistically significant decline in levels at 6 months, from 6 ng/ml pre-treatment to 1.5 ng/ml post-treatment (p<0.01); additionally, since prostate volume was available at that interval, PSAD was also determined. Prostate volume declined from 38 to 25 (p<0.01), reflecting the hemi-gland ablation (Figure 2). PSA levels declined more than prostate volume, resulting in a marked decline in PSA density from 2.0 to 0.7 (p<0.01) (Figure 2). Taken altogether, the volume-related PSA changes imply a shift away from a malignant composition of the organ.
However, biopsy data are regarded as the sine qua non for determination of PGA efficacy8. In previous studies of cryotherapy, post-treatment biopsy data, confirming efficacy of PGA, are scarce. In the registry study cited above, biopsy rate was 26% (and many biopsies were ‘for cause’, usually PSA elevation)27. In another recent study, including 393 men undergoing cryotherapy for low and intermediate risk lesions (90% PGA), only 46 (12%) had a follow-up biopsy, all ‘for cause’7. In the present study, 28/29 patients underwent the mandated biopsy at 6 months, and approximately 80% were found to be free of histologic evidence of cancer at that interval; 10% had foci of micro-residual lesions and entered active surveillance; and 10% had persistence of ≥GS7 cancers, requiring further treatment. Thus, the histologic evidence furnished in the present report is compelling, since it was obtained via MRI guidance and electronic tracking of biopsy sites, rather than by US guidance alone. The early histologic results of hemi-gland cryoablation, using contemporary biopsy methods for patient selection and follow-up, are encouraging.
MRI-visible lesions were present before treatment in 26 men, disappearing after treatment in 23/25 pairs available for study. Of the 23 men whose lesion disappeared after treatment, 19 were found to have no tumor on biopsy; 4 men had various amounts of residual tumor. However, among the 6 cases with biopsy-proven persistence of various amounts of cancer, four had no visible lesions at repeat MRI (one patient declined repeat imaging). Thus, disappearance of an MRI-visible lesion was associated with the treatment, but did not specifically indicate eradication of cancer. In a retrospective study with limited biopsy correlations, Kongyuy et al reported a similar observation28. The role of MRI in evaluating response to treatment remains a subject for further investigation.
Earlier applications of cryotherapy often employed whole gland treatment for lesions which may have been inadequately characterized and of high risk. Limiting treatment to lesions of intermediate risk, which are well-defined and localized via MRI-guided biopsy, appears to create opportunity for a safe, effective application of cryotherapy (and other types of focal therapy). As shown here and elsewhere, hemi-gland cryoablation compares favorably with other treatment options regarding quality of life after treatment. Hemi-gland ablation, when used for lesions confined to part of one prostate lobe, offers the additional advantage of wide treatment margins. Further, cryotherapy of prostate cancer, whole gland or partial, is approved under a U.S. FDA action in 2002 and has for years been covered by most third-party payors.
Limitations to the present study include its observational nature, the small sample size (N=29), short follow-up (6 months), and lack of contralateral biopsy at follow-up. However, absence of new MRI lesions at follow-up implies a likelihood that no important cancer developed (or was missed previously). Targeted and systematic tissue sampling, both contralateral and ipsilateral, is planned at the 18-month interval. In the interim, men found to contain no cancer at 6 months (or insignificant amounts) are being followed in an active surveillance program described previously29. Pending results of appropriately-powered, long-term investigations with patient selection and follow-up as indicated herein, hemi-gland cryoablation warrants further study.
CONCLUSION
When patient selection and follow-up employ contemporary biopsy methods, hemi-gland cryoablation of clinically-significant prostate cancer provides a safe treatment option for which a short-term cancer-control rate of 80% may be expected. Complications of treatment are few.
Supplementary Material
Supplementary Figure 1. Intra-operative ultrasound showing example of cryoablation procedure. A) Transverse view, demonstrating placement of cryo-probes for hemi-gland ablation (blue arrows), warming catheter, and thermal probe. B and C) Treatment in progress: leading edge of ice ball has advanced to capsule of prostate, transverse (B), sagittal (C).
Supplementary Figure 2. Examples of multi-parametric MRI (axial views) at baseline and 6 months after hemi-gland cryoablation. Blue arrows point to lesion. A, upper panels, show a patient whose 6-month biopsy was negative, i.e., successful treatment, and B, lower panels, a patient whose 6-month biopsy showed persistent csCaP, i.e., unsuccessful treatment. Note resolution of Grade 5 lesion after successful treatment (A) and persistence of similar lesion after unsuccessful treatment (B).
Acknowledgments
Supported in part by Award R01CA158627 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Other support by UCLA Clinical and Translational Sciences Institute Grant No. UL1TR000124, the Jean Perkins Foundation, the Kent Kresa family foundation, and the Steven C. Gordon Family Foundation.
Footnotes
Disclosures
Dr. Marks is co-founder of Avenda Health, Inc.
This paper was presented in part at 2018 Meeting of American Urological Association, San Francisco, CA.
REFERENCES
- 1.Onik G, narayan P, Vaughan D, Dineen M, brunelle R. Focal “Nerve-Sparing” Cryosurgery for Treatment of Primary Prostate Cancer: A New Approach to Preserving Potency. Urology. 2002;60:109–114. [DOI] [PubMed] [Google Scholar]
- 2.Onik G, Vaughan D, Lotenfoe R, Dineen M, Brady J. “Male Lumpectomy”: Focal Therapy for Prostate Cancer Using Cryoablation. Urology. 2007;70(6):S16–S21. doi: 10.1016/j.urology.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU International. 2011;109(11):1648–1654. doi: 10.1111/j.1464-410X.2011.10578.x. [DOI] [PubMed] [Google Scholar]
- 4.Jones JS. Cryotherapy: Update on the International COLD Registry. Grand Rounds in Urology. April 2017. https://grandroundsinurology.com/cryotherapy-update-international-cold-registry. [Google Scholar]
- 5.Bahn D, de Castro Abreu AL, Gill IS, et al. Focal Cryotherapy for Clinically Unilateral, Low-Intermediate Risk Prostate Cancer in 73 Men with a Median Follow-Up of 3.7 Years. European Urology. 2012;62(1):55–63. doi: 10.1016/j.eururo.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Durand M, Barret E, Galiano M, et al. Focal cryoablation: a treatment option for unilateral low-risk prostate cancer. BJU International. 2013;113(1):56–64. doi: 10.1111/bju.12370. [DOI] [PubMed] [Google Scholar]
- 7.B Barqawi Al, Huebner E, Krughoff K, O’Donnell CI. Prospective Outcome Analysis of the Safety and Efficacy of Partial and Complete Cryoablation in Organ-confined Prostate Cancer. URL. 2018;112:126–131. doi: 10.1016/j.urology.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Jarow JP, Ahmed HU, Choyke PL, Taneja SS, Scardino PT. Partial Gland Ablation for Prostate Cancer: Report of a Food and Drug Administration, American Urological Association, and Society of Urologic Oncology Public Workshop. URL. 2016;88(C):8–13. doi: 10.1016/j.urology.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Tay KJ, Scheltema MJ, Ahmed HU, et al. Patient selection for prostate focal therapy in the era of active surveillance: an International Delphi Consensus Project. 2017;20(3):294–299. doi: 10.1038/pcan.2017.8. [DOI] [PubMed] [Google Scholar]
- 10.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random Systematic Versus Directed Ultrasound Guided Transrectal Core Biopsies of the Prostate. Journal of Urology. 1989;142(1):71–74. doi: 10.1016/S0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 11.Dinh KT, Mahal BA, Ziehr DR, et al. Incidence and Predictors of Upgrading and Up Staging among 10,000 Contemporary Patients with Low Risk Prostate Cancer. Journal of Urology. 2015;194(2):343–349. doi: 10.1016/j.juro.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro RH, Johnstone PAS. Risk of Gleason Grade Inaccuracies in Prostate Cancer Patients Eligible for Active Surveillance. URL. 2012;80(3):661–666. doi: 10.1016/j.urology.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Sonn GA, Chang E, Natarajan S, et al. Value of Targeted Prostate Biopsy Using Magnetic Resonance–Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. European Urology. 2014;65(4):809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bekelman JE, Rumble RB, Chen RC, et al. Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. JCO September 2018:JCO.18.00606–JCO.18.00611. doi: 10.1200/JCO.18.00606. [DOI] [Google Scholar]
- 15.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378(19):1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA. 2015;313(4):390–398. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahar B, Katims A, Barboza MP, et al. Reclassification Rates of Patients Eligible for Active Surveillance After the Addition of Magnetic Resonance Imaging-Ultrasound Fusion Biopsy: An Analysis of 7 Widely Used Eligibility Criteria. URL. 2017;110:134–139. doi: 10.1016/j.urology.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Filson CP, Natarajan S, Margolis DJA, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016;122(6):884–892. doi: 10.1002/cncr.29874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed HU, Dickinson L, Charman S, et al. Focal Ablation Targeted to the Index Lesion in Multifocal Localised Prostate Cancer: a Prospective Development Study. European Urology. 2015;68(6):927–936. doi: 10.1016/j.eururo.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan S, Marks LS, Margolis DJA, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. URO. 2011;29(3):334–342. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang E, Jones TA, Natarajan S, et al. Value of Tracking Biopsy in Men Undergoing Active Surveillance of Prostate Cancer. Journal of Urology. 2018;199(1):98–105. doi: 10.1016/j.juro.2017.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed HU, Hu Y, Carter T, et al. Characterizing Clinically Significant Prostate Cancer Using Template Prostate Mapping Biopsy. Journal of Urology. 2011;186(2):458–464. doi: 10.1016/j.juro.2011.03.147. [DOI] [PubMed] [Google Scholar]
- 23.Shah TT, Arbel U, Foss S, et al. Modeling Cryotherapy Ice Ball Dimensions and Isotherms in a Novel Gel-based Model to Determine Optimal Cryo-needle Configurations and Settings for Potential Use in Clinical Practice. URL. 2016;91(C):234–240. doi: 10.1016/j.urology.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging – Reporting and Data System: 2015, Version 2. European Urology 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tay KJ, Amin MB, Ghai S, et al. Surveillance after prostate focal therapy. World J Urol June 2018:1–11. doi: 10.1007/s00345-018-2363-y. [DOI] [PubMed] [Google Scholar]
- 26.Palapattu GS, Salami SS, Cani AK, et al. Molecular Profiling to Determine Clonality of Serial Magnetic Resonance Imaging/Ultrasound Fusion Biopsies from Men on Active Surveillance for Low-Risk Prostate Cancer. Clin Cancer Res. 2017;23(4):985–991. doi: 10.1158/1078-0432.CCR-16-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tay KJ, Polascik TJ, Elshafei A, Tsivian E, Jones JS. Propensity Score-Matched Comparison of Partial to Whole-Gland Cryotherapy for Intermediate-Risk Prostate Cancer: An Analysis of the Cryo On-Line Data Registry Data. Journal of Endourology. 2017;31(6):564–571. doi: 10.1089/end.2016.0830. [DOI] [PubMed] [Google Scholar]
- 28.Kongnyuy M, Halpern DM, Liu CC, et al. 3-T multiparametric MRI characteristics of prostate cancer patients suspicious for biochemical recurrence after primary focal cryosurgery (hemiablation). International Urology and Nephrology 2017;49(11):1947–1954. doi: 10.1007/s11255-017-1670-3. [DOI] [PubMed] [Google Scholar]
- 29.Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA: A Cancer Journal for Clinicians 2015;65(4):264–282. doi: 10.3322/caac.21278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Intra-operative ultrasound showing example of cryoablation procedure. A) Transverse view, demonstrating placement of cryo-probes for hemi-gland ablation (blue arrows), warming catheter, and thermal probe. B and C) Treatment in progress: leading edge of ice ball has advanced to capsule of prostate, transverse (B), sagittal (C).
Supplementary Figure 2. Examples of multi-parametric MRI (axial views) at baseline and 6 months after hemi-gland cryoablation. Blue arrows point to lesion. A, upper panels, show a patient whose 6-month biopsy was negative, i.e., successful treatment, and B, lower panels, a patient whose 6-month biopsy showed persistent csCaP, i.e., unsuccessful treatment. Note resolution of Grade 5 lesion after successful treatment (A) and persistence of similar lesion after unsuccessful treatment (B).