Abstract
Skin barrier keeps the “inside-in” and the “outside-out” forming a protective blanket against external insults. Epidermal lipids such as ceramides, fatty acids, triglycerides and cholesterol are integral components in driving the formation and maintenance of epidermal permeability barrier (EPB). A breach in this lipid barrier sets the platform for the subsequent onset and progression of atopic dermatitis (AD). Such lipids are also important in the normal functioning of different organisms, both plants and animals and in diseases including cancer. A double increase in AD rate in the recent years and the chronic nature of this disorder emphasizes the need of this review to shed light on the multifaceted role of the diverse types of lipids in mediating AD pathogenesis.
Keywords: Atopic Dermatitis (AD), Epidermal Permeability Barrier (EPB), Lipid Gene Regulation, Cutaneous Lipid Metabolism
Beyond the Brick Wall: The Stratum Corneum and Epidermal Barrier Functions
The skin is the frontier between organisms and environment controlling two key functions: 1) maintaining a robust external barrier resilient against environmental stressors and 2) maintaining a water-tight internal barrier that prevents trans-epidermal water loss (TEWL) (see glossary), as well as prevent the colonization of pathogens into the skin and underlying tissues. Together, these two account for the role of the skin as a versatile double barrier with dual external-internal protection [1–3]. Besides being an indispensable barrier, the skin is also involved in the implementation of chief regulatory functions in association with the fundamental organs of the body. Such functions include the ability of the skin to serve as a common platform for signal exchange between internal organs and the environment via the cutaneous neuroendocrine system upon conditions such as immunostimulation or exposure to solar radiation through production of neuropeptides, biogenic amines, melatonin, opioids, cannabinoids, secosteroids as well as growth factors and cytokines to mediate their divergent effector functions [4, 5].
Although the essential barrier functionality is mainly localized to the outermost layer of the skin that is the epidermis or the stratum corneum (SC), sebaceous glands embedded in the deeper layers of the skin are also endowed in part with the responsibility of maintained lubrication and delayed dehydration of the skin along with photoprotection and antimicrobial action thereby serving as another crucial mediator of effective barrier functionality [6]. In this review, we restrict our discussion to the role played by the epidermal lipids in constituting effective epidermal barrier functionality.
The SC, resembling a brick-wall consists of protein enriched corneocytes (analogous to bricks) embedded in intercellular matrix of non-polar lipids (mortar component) present in between the granular and the cornified layers [1–3].Corneocytes as “bricks” represent the cellular complement of SC structure and contain a variety of enzymes, water, and keratin filaments, which gives rise to the mechanical strength of the SC [3, 7, 8]. They are encased in a cross-linked layer of proteins such as filaggrin, loricrin, and involucrin [3, 9–11] which anchor the cells with the lipid rich extracellular matrix, the “mortar” [12]. This highly ordered, hydrophobic lipid scaffolding/matrix is composed of approximately 50% ceramides (CER), 15% free fatty acids (FFAs) and the rest 25% comprising predominantly cholesterol and a small percentage of triacylglycerol (TAG) species, with FFAs and long chain bases liberated from sebaceous TAG and epidermal CER, respectively, in SC serving as potent antimicrobial agents [13–15]. The epidermal permeability barrier (EPB) formation involves proper expression of proteins involved in epidermal terminal differentiation and proper cornified envelope crosslinking [12, 16–19].
Improper EPB formation contributes to TEWL and triggers onset of inflammatory skin disease e.g. Atopic Dermatitis (AD) [20–23].This validates the crucial role of SC in maintaining dual barrier functionality. Also, SC has potent antioxidant properties to prevent oxidative damage to the skin by the secretion of enzymes such as catalase as well as non-enzymatic molecules such as vitamin E, glutathione and uric acid [24].
Indeed, many of the skin diseases such as lamellar ichthyosis (LI), psoriasis, Netherton’s syndrome (NTS), and AD (see Box 1) are all characterized by defective or weakened epidermal barrier functionality [25]. There are various factors and hypotheses governing the final outcome of AD (for details, refer to Box 2). In the following section we have elucidated the role of the key genes involved in the formation of a proper EPB, how their deficiency leads to improper barrier formation and finally how a disrupted barrier results in enhanced Th2 inflammatory response which can thereby act as a positive feedback loop to further worsen the integrity of the barrier leading to the chronic nature of AD.
Box 1: Diseases of Defective Epidermal Barrier Function.
Diseases such as AD, Lamellar ichthyosis (LI), psoriasis and Netherton syndrome (NTS) are all characterized by defective or weakened epidermal barrier functionality.
Atopic Dermatitis
Atopic Dermatitis, also known as eczema. It is a chronic, genetically inherited, multifactorial inflammatory skin disease characterized by dry, itchy and reddened skin [74].
Lamellar Ichthyosis
Lamellar Ichthyosis is a genetically inherited autosomal recessive condition that mainly affects the skin. It becomes apparent at birth and persists throughout life. People suffering from this condition show impaired permeability barrier function. Infants suffering are generally born with glossy, waxy layer of skin which undergoes shedding after the first two weeks of birth. Adults on the other hand show large, dark, plate-like scales covering majority of their skin along with hair loss, reddened skin, decreased sweating among other symptoms [75].
Psoriasis
Psoriasis is an autoimmune condition that is chronic in nature. It is caused by rapid buildup of skin cells that results in scaling on the skin surface. Symptoms include flaking, inflammation, and thick, white, silvery, or red patches of skin. Some common therapeutic strategies include topical application of steroid creams or administration of oral medication [76].
Netherton Syndrome
Netherton syndrome is an inherited autosomal recessive trait characterized by scaly skin, hair anomalies, increased susceptibility to atopic eczema (resulting in dry reddened and scaly skin) along with elevated serum IgE levels. It occurs due to mutation in the gene SPINK5 coding for a protein that acts as a brake on the activity of certain proteases in the skin. Excessive action of proteases such as kallikrein (KLK5/KLK7) results in few outer layers of skin and hence impaired permeability barrier function [77].
Box 2: Major Factors and Hypotheses Governing Atopic Dermatitis.
AD usually occurs as a part of “atopic triad” (including eczema, rhinitis and asthma) and is a multifactorial ailment associated with barrier disruption and severe immune irregularities leading to systemic inflammation [23, 74, 78–81]. Controversy remains regarding the primary cause of AD- (1) immune irregularities leading to chronic inflammation or (2) epidermal barrier breach. The “inside-out” hypothesis states that cutaneous inflammation precedes barrier disruption resulting in an impaired EPB. The “outside-in” hypothesis on the other hand states that skin barrier disruption results in increased cutaneous and systemic Th2 responses with TSLP being the potential activator of type 2 responses [82]. While some cytokines such as TNF-α, IFN-γ and IL-17 are indicators of chronic inflammation, others like IL-1α/β, GMCSF or IL-6 are homeostatic signals that can eventually reach the deeper layers of the skin versus the Th2 cytokines[83]. Inflammatory Th2 cells also predispose patients to the development of atopic triad. Recently a new “inside-outside-inside” hypothesis is suggested, which states that primary immune irregularities elicit secondary barrier disruption effects which can further result in activation of the type 2 immune responses [70, 84]. However, the pathways that are instrumental in driving AD remains to be identified.
Various factors operate through either of the hypotheses to promote the onset of AD as well as their progression. The two major factors include skin pH and stress. Elevated level of pH in the AD skin had been found to be correlated with increased activity of peptidases such as KLK5/KLK7 and concomitant PAR2 activation[85]. Following PAR2 activation, expression of inflammatory mediators predominantly TSLP promotes the recruitment of the Th2 cells to the site of inflammation [85, 86]. The production of cytokines such as IL-4, IL-5, IL-10 and IL-13 by the Th2 cells then serve as further determinants of AD progression [87].PAR2 activity also gets regulated by NFK-β pathway [88]. Environmental stress as well as emotional stress has also been shown to mediate its effect on the clinical manifestation of AD via neuroendocrine signalling pathways involved in aggravating or attenuating inflammatory responses though local production of CRF (Corticotropin Releasing Factor) and POMC peptides [89]. CRF or CRH (Corticotropin Releasing Hormone) acts as a major regulator of the HPA (Hypothalamic-Pituitary-Adrenal) axis and a coordinator of stress response. Neuroendocrine signalling is also known to show its effect on the inflammatory pathway through glucocorticoid, serotonin and melatonin production. Thus, the abundant expression of a cutaneous serotoninergic/melatoninergic system(s) can help counteract external and internal stresses to preserve the vital skin barrier function [90, 91].
Key Genes Involved in EPB Formation and the Th2 Inflammatory Response in AD due to Breaches in the Brick Wall
Firstly, any alteration in the expression of the genes involved in lipid biosynthesis and metabolism pathways of the crucial lipids constituting the EPB results in destroyed barrier functionality and hence AD. Transcriptional regulatory proteins COUP-TF-interacting protein 1 and 2 (CTIP1 and CTIP2) are involved in regulation of epidermal lipid metabolism [26, 27]. A study by Li et al., 2017 in murine models showed altered expression level of several of those genes in Ctip1−/− mice. Expression of genes such as 3-Ketodihydrosphingosine Reductase (Kdsr), UDP-Glucose Ceramide Glucosyltransferase (Ugcg), N-acylsphingosine Amidohydrolase 1 (Asah1) and Ceramide Synthase 6 (Cers6) were found to be downregulated by approximately 0.6-fold, while expression of Sphingomyelin Synthase 2 (Sgms2), ELOVL Fatty Acid Elongase 4 (Elovl4), Sphingomyelin Synthase 1 (Sgms1) and Sphingosine-1-phosphate phosphatase 1 (Sgpp1) were found to be downregulated by 0.5-fold as compared to control. On the other hand, genes such as Sphingosine Kinase 1 (Sphk1) and Sphingosine Kinase 2 (Sphk2) were found to exhibit an upregulated expression by approximately 1-fold, Sphingomyelin Phosphodiesterase (Smpd1) by 0.5-fold, Serine Palmitoyl transferase Long Chain Base Subunit 3 (Sptlc3) and Ceramide Synthase 2 (Cers2) by 0.3-fold in Ctip1−/− [26]. Another study by Wang et al., 2013 showed CTIP2 as a regulator of genes such as Ugcg, Asah1, Cers6 and Sgms2 with Ugcg expression found to be downregulated by 0.2-fold, Asah1 by 0.1-fold and CerS6 by 0.25-fold in Ctip2−/− mice at E16.5. Sgms2 however was found to be expressed at a higher level by 0.5-fold in Ctip2−/− mice at E16.5 [27].Thus, the above studies established the central role played by CTIP1 and CTIP2 in mediating the formation of EPB in adult mouse skin. (All fold changes are taken to the nearest approximation).
Secondly, Filaggrin is another multifunctional protein that plays a key role in EPB formation. There are predominantly two pathways exploited by filaggrin deficiency to act as mediators of AD, (1) by disrupting the normal epidermal terminal differentiation events and, (2) by inducing enhanced expression of Thymic Stromal Lymphopoietin (TSLP) on the epidermis and subsequent aberrant Th2 response [28]. Filaggrin mutations also correlate with asthma and food allergies, independent of AD. Filaggrin generates trans-urocanic acid, the principal UV-B filter in epidermis, and hence, reduced filaggrin predisposes to development of non-melanoma skin cancers. This sums up the most crucial genes and related proteins involved in intact EPB formation.
Studies in the past have successfully established the link between type 2 immune responses and AD pathogenesis. Hatano et al., 2005 first described IL-4 mediated downregulation of ceramide synthesis and barrier function [29]. Berdyshev et al., 2018 showed that altered lipid profiles, notably those with shorter chain FFA species, are linked to IL-13-driven AD skin lesion development. RNA-seq analysis revealed that FFA elongases (ELOVL3 and 6) were inhibited by IL-4/IL-13 cytokines in a STAT6-dependent manner with IL-13 mediated downregulated expression of Elovl3 and Elovl6 by 0.77-fold and 0.57-fold respectively in lesional skin of murine models as compared to control [13]. A parallel study showed that the presence of IL-4 depressed the levels of mRNA for sphingomyelinase (SMASE) by 50% and glucocerebrosidase (GBA) by 70% and upregulated the expression levels of acid ceramidase by 20% resulting in overall decreased levels of ceramides in human (Homo sapiens) epidermal living skin equivalents (LSEs) [30], which was found to be strongly associated with increased TEWL and AD phenotype by tape stripping analysis [3, 31]. In a separate study involving LSEs, a cocktail of Th2 cytokines (IL-4, IL-31, and IL-13) significantly reduced the gene expression levels of the epidermal lipid metabolic enzymes such as those coding for SMASE by 0.7 fold, for GBA by 0.6 fold, and for ELOVL1 by 0.5 fold with respect to control [32]. The lipidomic profiling done in each to analyse the corresponding changes in lipid profile showed significantly downregulated levels of CER [AS] and [NS] (see figure 2 for nomenclature of ceramide subclasses) synthesized respectively by a SMASE and GBA and long chain FFAs and CER species synthesized in part by elongases such as ELOVL1, which correlated significantly with the AD phenotype [32].
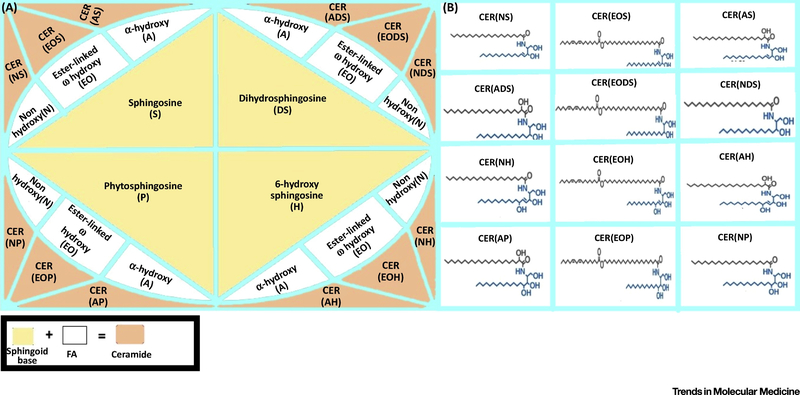
Fig 2. Classification of ceramides and their subclasses.
Three types of fatty acid namely Non-hydroxy (N), Ester-linked ω-hydroxy (EO) and α-hydroxy (A) combine with four different types of sphingoid base namely Sphingosine (S), Dihydro-sphingosine (DS), Phyto-sphingosine (P) and 6-hyrdoxy sphingosine (H) to give rise to twelve classes of ceramides. The ceramide classes being abbreviated as CER(NDS), CER(NS), CER(NP), CER(NH), CER(EODS), CER(EOS), CER(EOP), CER(EOH), CER(ADS), CER(AS), CER(AP) and CER(AH). The structures representing different ceramide classes are depicted in the right panel.
Overall, these studies indicated the role played by various regulatory proteins in the formation of EPB, their regulation as well as how immune irregularities (in regard to ‘inside-out’ hypothesis) such as type 2 hyperactivation serves as an important factor in progression of AD. Extensive research in this area has the potential to offer beneficial therapeutic targets.
Alteration of the Ceramide Composition in AD
Ceramide synthesis occurs through four major pathways in the epidermis: (1) de novo pathway, (2) sphingomyelinase pathway, (3) salvage pathway, and (4) exogenous ceramide recycling pathway [26] (see Fig. 3) (for details refer to Box 3). Till date, twelve classes of ceramide have been identified [33] (see Fig. 2). Ceramides were one of the first lipids to be connected with skin barrier function deficiencies and AD. DiNardo first cited this observation [34]. Later studies showed that deficient barrier function is associated with a general decrease of the so-called ultra-long chain ceramides, those more than 26 carbons in length and an increase in short-chain ceramides [13]. This study was further validated by the decreased expression in the mRNA level of the elongases in patients bearing lesions of AD. Consistently, genetic ablation of either Elovl1 or Elovl4 in mice resulted in decreased levels of ceramides with very-long acyl chains with CER (C30-C36) being reduced by almost 45% in null mice and 5% in heterozygous mice as compared to wild type mice, thereby resulting in increased TEWL and death soon after birth [35, 36].
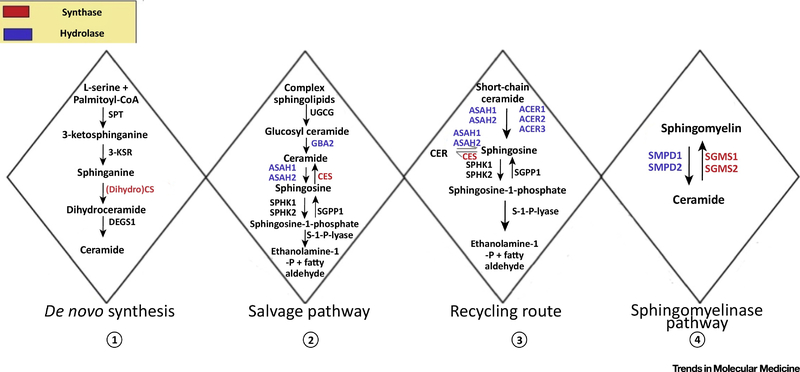
Fig 3. Pathways involved in sphingolipid metabolism.
Synthesis of ceramide occurs via four different pathways (1) De-novo Synthesis (2) Salvage Pathway (3) Exogeneous CER recycling route and (4) Sphingomyelinase pathway. The enzymes responsible for synthesis in the different pathways are highlighted in red and the enzyme responsible for hydrolysis are highlighted in blue. Serine Palmitoyl Transferase (SPT); 3-Ketosphinganine Reductase (3-KSR); Dihydro-ceramide Synthase (DCS); Sphingolipid Delta (4)-Desaturase 1(DEGS1); UDP-Glucose Ceramide Glucosyltransferase (UGCG); Glucocerebrosidase (GAB2); Acid Ceramidase (ASAH1, ASAH2); Ceramide Synthase(CES); Sphingosine Kinase (SPHK1, SPHK2); Sphingosine-1-Phosphate Phosphatase 1 (SGPP1); Sphingosine-1-Phosphate Lyase (S-1-P-Lyase); Alkaline Ceramidase (ACER1, ACER2, ACER3); Acid Sphingomyelinase (SMPD1, SMPD2); Sphingomyelin Synthase (SGMS1, SGMS2).
Box 3: Major Pathways Involved in Sphingolipid Biosynthesis.
Ceramide comprise almost 50% of the total lipid component constituting SC [14]. The first and rate-limiting step in the de novo synthesis of ceramides is catalysed by the serine palmitoyl transferase (SPT) enzyme complex, which catalyses the condensation of palmitoyl-CoA and L-serine in ER to form 3-Ketosphinganine finally giving rise to ceramide. De novo synthesis of ceramide primarily occurs in the ER followed by its transfer to the Golgi apparatus by either vesicular trafficking or the ceramide transfer protein (CERT). They then get further metabolized to other sphingolipids, such as sphingomyelin and complex glycosphingolipids [92]. Hydrolysis of sphingomyelins forms ceramides in the sphingomyelinase pathway [93]. The salvage pathway involves constitutive breakdown of sphingolipids and glycosphingolipids in the late endosomes and the lysosomes, with the final aim of producing sphingosine. Ceramide can also undergo hydrolysis by acid ceramidases present in the lysosome to form sphingosine and FFA. The long-chain sphingoid bases upon release from the lysosome then re-enter pathways for synthesis of ceramide and/or sphingosine-1-phosphate catalysed by ceramide synthase family members accounting for almost 50% to 90% of sphingolipid biosynthesis [94]. Finally, the exogenous recycling pathway allows short chain ceramides to create sphingosine thereby leading to the synthesis of endogenous long chain ceramide species. De novo pathway is the predominant pathway while others serving as secondary ones, since ceramides need to be first incorporated in the derivatised compounds by prior functioning of this pathway [95]. The ceramide species thereafter undergo acylation with linoleoyl-CoA to form acyl-ceramides. Acyl-ceramides are glycosylated in the Golgi and with other lipids are packed into lamellar bodies [12]. The lamellar bodies, containing glycosylated ceramides, acyl ceramides, cholesterol, and VLCFAs, are secreted to the extracellular space of SC, where the ceramides become covalently linked to structural skin proteins such as involucrin, filaggrin, Small Proline Rich Proteins (SPR) to form the EPB [12, 14, 52] (see Fig. 1).
Though ceramides dominate by weight, they are no more important than other key lipids. Application of one or two of these lipids such as a mixture of only ceramide species or ceramide along with FFA to perturbed skin delays barrier recovery; only equimolar mixtures of all the crucial lipids allows normal recovery as measured by TEWL. This shows the crucial role of all four lipids in EPB formation irrespective of their relative abundance within the SC [96]. Drugs such as Epiceram serve as the most representative example of the above observation [97].
The correlation between the chain length of ceramides and development of AD was first elucidated by Ishikawa et al., 2010 [37]. A reduced average chain length of ceramides in AD can be attributed to increases in C34 CER as well as a reduction in the ester linked omega hydroxy (CER[EO]) ultra-long chain subclasses. A human study showed within the C34 CER subgroup, subclasses [NH], [NS], [AS], and [AH] were found to be largely increased with [NH] and [NS] comprising 0.6% and 0.78% respectively of the total CER content in comparison to control in which both [NH] and [NS] comprises 0.18% of the total CER content. [AS] and [AH] in AD subjects were also found to comprise 2% and 0.5% respectively of the total CER content in contrast to healthy subjects where [AS] comprised 0.4% and [AH] comprised 0.2% of the total CER content [38]. Similar trends were also observed in mice skin with germline deletion of Ctip2 (highly expressed in AD skin) and its homolog Ctip1 [26, 27].
Ceramides, especially CER[NS] and CER[EOS], have been seen to be related to the packing of corneocytes in the SC. A study showed that after topical application of a formulation containing either CER[EOS] or CER[NS] to a skin barrier repair model, a higher fraction of lipids was found to be involved in assuming an orthorhombic organization compared to formulations without CER justifying the crucial role of CER[EOS] and CER[NS] in intact EPB formation [39].
ABHD5/CGI-58 is a protein involved in the regulation of an epidermal-specific lipase that liberates linoleic acid from a pool of triglycerides that is enriched in linoleic acid, making it available for transesterification to generate acyl-ceramide crucial to barrier formation (catalysed by PNLPA1). Mutation in the gene coding for ABHD5/CGI-58 results in Chanarin-Dorfman syndrome, characterized by dry scaly skin at birth similar to AD [40]. This further underscores the importance of epidermal CER and TAG metabolism in the generation of proper EPB and how their deficiency leads to AD.
Variation in the FFA Content in AD
FFAs are an integral part of the SC lipid matrix. The epidermis is dynamically engaged in FFA biosynthesis and processing. The disruption of the permeability barrier results in a rapid and marked increase in fatty acid synthesis within the epidermal keratinocytes to restore the integrity of EPB [41, 42]. Barrier disruption increases the activities and mRNA levels of both of the key enzymes required for de novo fatty acid synthesis, acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) [43]. Fatty acids being an important constituent serving towards EPB formation, reduced expression of fatty acid synthesizing enzymes such as elongases, increased expression of desaturases had been found to correlate with the phenotype of shortened fatty acyl chain length and increased level of unsaturated FAs in lesions associated with AD. Studies by Malecheidt et al., 2002 in human models reported an apparent reduction in FFA chain length in AD patients with short chain FAs comprising 7.7% and 7.6% of the total FFA fraction in lesional and non - lesional AD skin, respectively, as compared to healthy subjects where the short chain FAs comprised only 5.4% of the total FFA fraction [44]. FFAs are in general toxic for living cells. Therefore, they are not stored as FFAs in lamellar bodies. they are rather stored as phospholipids and these phospholipids get metabolized at the SG-SC interface with FA as one of their products. Studies have shown phospholipids normally are degraded completely, however they do persist in AD [45] validating the importance of phospholipid metabolism at SG-SC interface for potential release of FFAs in mediating proper barrier formation and how the lack of proper metabolism of phospholipids and their subsequent accumulation serves as a hallmark of AD.
In addition to a well-documented reduction in FFA chain length, there also appears to be distinct alterations in unsaturated FFA levels in AD. Recent reports have observed increase in unsaturated FFA levels including monounsaturated fatty acid species (MUFAs) in human AD skin from 20% in control to almost 30% [46]. Li et al., 2017 however reported a large decrease in the MUFAs C16:1 and C18:1, which corelated to increased susceptibility to Staphylococcus aureus infection and barrier dysfunctions [47]. Thus, while a study in human models by Van smeden et al., 2014 showed an increase in the level of MUFAs in lesional atopic eczema patients as compared to non-lesional atopic eczema and healthy subjects, a 2017 human study by Li et al., showed that the decrease in the level of MUFAs serve as a driving factor leading to increased colonization by S. aureus and resulting impaired EPB. Given the contradictory nature of the two observations, a more extensive study needs to be performed to determine the role of MUFAs in AD. A parallel study by Mojumdar et al., 2014 using in-vitro constituted model SC membrane system mimicking human SC lipid composition and organization showed that increased prevalence of MUFAs disrupt the EPB by promoting hexagonal packing conformation as opposed to the stable orthorhombic packing conformation [48].
Experimental studies in the past have shown the crucial role of epidermal elongases in mediating the lipid composition changes characteristic of AD and have been listed below. Park et al., 2012 demonstrated that the protein levels ELOVL1 and ELOVL4 were significantly reduced in an Oxazolone induced murine model of AD by western blot analysis. Reduced expression level of elongases such as ELOVL1 and ELOVL4 impairs elongation of FA beyond C22 and hence the C22 and C16 CER levels showed an increase of 0.2 and 3-fold respectively with respect to control, while the C24 and C26 CER levels showed a decrease of 0.25 and 0.75-fold respectively with respect to control as observed from liquid chromatography-mass spectrometric (LC-MS) analysis [49]. Further study by Sassa et al., 2013 showed the part played by ELOVL1 and ELOVL4 in the production of less than C26-CoA and more than C28-CoA respectively thus establishing the role of epidermal ELOVL1 in connecting the synthesis of long or very long chain fatty acids (LCFA/VLCFAs) to ultra-long chain fatty acids (ULCFAs) crucial to barrier formation [50]. Enzymatic changes underlying FFA saturation levels have also been characterized in AD. Stearoyl CoA desaturase 1 (SCD-1) is responsible for the formation of unsaturated FFAs 16:1 and 18:1 with other SCDs introducing increasing levels of desaturation. A study showed that the mRNA expression level of Fatty acid desaturase 2 (FADS2) was double the expression observed in control [51]. Detailed quantification of protein level of FADS2 and SCD1 could help ascertain their role in AD.
RNA-seq analysis showed that type 2 inflammatory cytokines IL-4 and IL-13 inhibit the expression of ELOVL3 and ELOVL6 in human keratinocyte culture [13]. Besides, genetic factors influencing epidermal barrier homeostasis may also contribute to altered FFA metabolism. Ctip1 and Ctip2 are two such genes know to play important roles in EPB formation [26, 27]. Li et al., 2017 showed that germline deletion of the Ctip1 in a murine model reduced the gene expression level of Elovl4 by 1-fold and ultimately reduced long chain FFA species in the SC [26]. Overall, FFA metabolism appears to be regulated by Th2-dependent inflammatory cytokines and genes related to barrier formation and maintenance. Current AD management strategies aim at employing anti-inflammatory or immunosuppressive agents to influence downstream SC FFA composition. Future strategies involving direct supplementation of deficient FFA species may serve useful in disease management (see Clinician’s Corner).
Clinician’s Corner.
Defects in epidermal lipid metabolism are strongly associated with AD and manifest in readily detectable changes in epidermal lipid composition. Strategies designed to restore barrier functions at the molecular level may be more effective than those designed to reduce inflammation such as corticosteroids. That approach can involve supplementing the skin with lipid-rich formulations designed to restore proper SC lipid composition and ultimately strengthen EPB integrity.
Preventative reinforcement of the EPB may prove useful in AD management. Treatment of neonates with topical moisturizers has demonstrated a marked reduction in AD prevalence [98]. Such studies underscore the shifting paradigm of EPB integrity, rather than aberrant Th2-mediated inflammation, being the most important determinant in AD development. Accordingly, future treatment strategies should be designed to reflect this paradigm shift.
Topical application of lipids can improve EPB homeostasis and has been useful against AD. Such strategies involve treatment with non-physiologic or physiologic lipid species. Although non-physiologic lipid treatment quickly restores barrier function, they can also inhibit normal barrier repair mechanism and thus not correct the underlying abnormality. Appropriate molar mixes of physiologic lipids, i.e. those containing CER, FFA, CHOL and TAG, can improve EPB homeostasis. Incomplete or misbalanced mixtures of lipids can negatively affect EPB. This underscores the need to develop more personalized and precise lipid formulations [96].
Therapies designed to modulate epidermal lipid enzyme activity could be employed to restore proper healthy lipid composition. This is especially relevant to changes in AD profile involving increased amounts of lipid classes (e.g. increased CER-34, CER-NS, and CER-AS) where levels cannot simply be supplemented by topical lipid application. Topical application of acetyl CoA carboxylase activator and/or fatty acid synthase activator can also increase the rate of de-novo fatty acid synthesis resulting in rapid recovery of barrier functions. Similarly, application of activators involved in stimulating various enzymes involved in CER, FFA, CHOL and TAG synthesis pathway can result in rapid restoration of the EPB integrity.
Lipidomic and biophysical techniques have been used to rigorously evaluate the effects of a topical formulation on epidermal lipid composition. Such knowledge of AD biomarkers may allow the development of personalized topical treatments optimized to remediate a patient’s lipidomic profile [99]. Given the heterogeneity in AD phenotype, such personalized approaches may represent the most effective means of treating AD.
Finally, it should be also noted that treating immune abnormality improves, but does not normalize barrier function, favoring ‘outside-in’ model - disease inevitably rebounds.
Changes in Cholesterol Content in AD
The epidermis is an active site of cholesterol synthesis [52]. There are two primary types of cholesterol found in the SC, cholesterol and cholesterol-3-sulfate. Past studies have shown that cholesterol synthesis is vital to maintaining the homeostasis of the human skin barrier [12]. Disruption to this balance can lead to TEWL and thereby AD.
There are also certain derivatives of cholesterol that are known to play an important role concerning EPB homeostasis. Oxysterols derived either via cholesterol oxidization through enzymatic processes, or as by-products of the cholesterol biosynthetic pathway play an important role in inducing epidermal differentiation and inhibiting proliferation for formation of intact epidermal barrier [53].
CYP11A1 is yet another gene expressed in the skin involved in production of various derivatives of cholesterol. It is important for local glucocorticoid synthesis to attenuate the inflammatory response [54], and also plays a role in activation of vitamin D through noncanonical pathway [55–57] or activation of 7-dehydrocholesterol or lumisterols [57–59]. These issues serve as an important aspect owing to their ability to attenuate inflammatory responses and thereby can also contribute to the maintenance of an intact barrier. Lastly, there are various potential targets for sterols, lipids and secosteroids regulation including a wide range of nuclear receptors [60] or Vitamin D receptors [61] or retinoic orphan acid receptors [62, 63] or aryl hydrocarbon receptor [64].
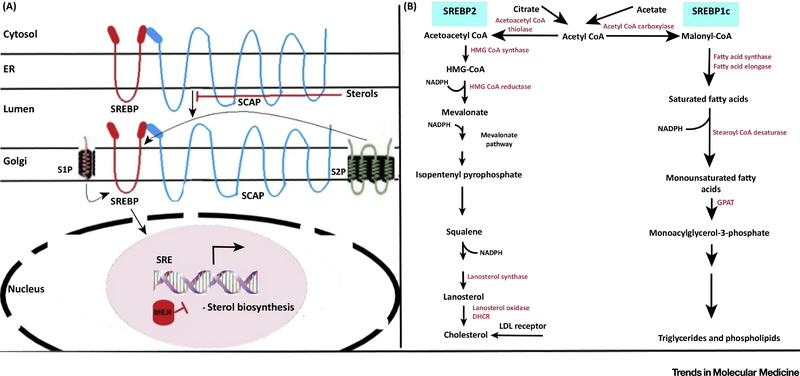
The gene expression level of several enzymes involved in cholesterol biosynthetic pathway were found to increase in upper and lower epidermis following acute barrier disruption in a study concerning murine models. HMG-CoA Reductase (Hmgcr), HMG-CoA Synthase (Hmgcs), Farnesyl Pyrophosphate Synthase (Fdps), Squalene Synthase (Fdft1) were found to increase by approximately 1-fold, 1.5-fold, 0.8-fold and 0.9-fold respectively with respect to control upon acute barrier disruption thereby resulting in enhanced epidermal cholesterol synthesis, mediated via Sterol Regulatory Element Binding Protein (SREBP-1) and (SREBP-2) [43] (For details, see Fig. 4).These observations demonstrated the role of cholesterol in maintaining the integrity of EPB.
Fig 4. SREBP mediated regulation of genes involved in synthesis of cholesterol, fatty acids and triglycerides.
The left panel depicts sterol activated SCAP (SREBP Cleavage Activating Protein) mediated transport of SREBP (Sterol Regulatory Element Binding Protein) from ER (endoplasmic reticulum) to the Golgi Apparatus where two site specific proteases S1P (site-1-protease) and S2P (site-2-protease) stimulate the release of N-terminal bHLH domain from the membrane. The bHLH domain upon being directed to the nucleus bind to the sterol response element (SRE) upstream of the target genes thereby stimulating transcription of genes involved in sterol biosynthesis. Abundance of sterol moieties inhibits this process. The right panel shows the role of isoforms SREBP-2 and SREBP-1c in regulating the fatty acid biosynthetic pathway and cholesterologenesis respectively when expressed at normal cellular levels. Expression at levels higher than normal allows both the isoforms to govern activation of all the enzymes involved in both fatty acid biosynthesis as well as in synthesis of cholesterol. Glycerol-3-Phosphate Acyltransferase (GPAT); 7-Dehydrocholesterol Reductase (DHCR).
Li et al., 2017 found elevated levels of cholesterol-3-sulfate in AD patients and potentially identified it as a contributor to barrier disruption [47]. Recent studies in normal human epidermal keratinocytes (NHEK) have shown cholesterol-3-sulfate as an inducer of the skin barrier protein pro-filaggrin expression in the epidermal keratinocytes [65].10μM and 40μM of cholesterol-3-sulfate downregulated the mRNA expression level of pro-filaggrin by 0.66 fold and 0.86 fold, respectively, with respect to control in RORα knocked down cells justifying the role of cholesterol-3-sulfate in inducing pro-filaggrin expression through increased expression of RORα. However, these observations could not justify the role of cholesterol-3-sulfate as an effective barrier disruptor because increased expression of pro-filaggrin and its conversion to mature filaggrin protein are normally associated with maintained integrity of EPB [66, 67]. Possibility is such that even though increase in cholesterol-3-sulfate in AD patients have been found to be correlated with increased pro-filaggrin expression, maybe the pro-filaggrin protein fail to undergo maturation to the filaggrin protein, and hence barrier breach results. Increased expression of cholesterol-3-sulfate in human AD skin positively corelated to colonization by S. aureus thereby further compounding the severity of the AD disease [47].
Impact of Modified Triglyceride Composition on AD Development
The epidermis is an active site of triglyceride synthesis, but their role in EPB homeostasis is poorly defined. Diacylglycerol acyltransferase (Dgat2) expressed in the epidermis plays a crucial role in the acylation of the linoleoyl CoA containing diacylglycerol (DAG) to TAG [68]. Dgat2 knockout mice showed markedly reduced content of linoleic acid-containing TAGs by a fold change of 0.97 with respect to control and hence reduced synthesis of acyl-ceramides in the skin leading to disrupted EPB and demise soon after birth [68].
Other studies in murine models have also shown an additional role of triglycerides in regulating epidermal proliferation and barrier integrity through controlled calcium influx into epidermal keratinocytes [69].
Radner and Fischer noted that mutations in the gene coding for ABHD5/CGI-58 protein were linked with the degradation of triglycerides in the human skin [2] as is commonly observed in case of neutral lipid storage disorder. Another gene potentially connected with alteration of triglyceride levels in AD skin is filaggrin with a general increase in the number of triglycerides present in AD patients with enhanced filaggrin expression [66, 67, 70]. More specifically, Li et al.,2017 discovered that certain groups of triglycerides, namely 46:1, 48:1, 48:2, 50:1, 50:2, and 50:3 as well as 46:2 and 56:2, were altered in populations of AD patients compromised by S. aureus colonization [47]. Besides, increased TEWL was associated with decreased levels of triglyceride 46:2. A parallel study showed an increase in the triglyceride levels in group 52:2 by a fold change of 2.33 and a decrease in group 52:5 by a fold change of 0.5 in the skin of mice lacking transcription factor CTIP1/BCL11A that control lipid metabolism and barrier functions [26]. Thus, the accumulation of long-chain triglycerides in the SC could impact barrier functions and influence AD pathogenesis.
Concluding remarks
Strategies to counteract AD is an area of extensive research. Initial treatment options included application of prescribed emollients and administration of immunosuppressants and anti-inflammatory drugs such as corticosteroids. However, they were non-specific and exhibited transient efficacy. Topical application of lipids as a treatment strategy could be a promising alternative in mild and moderate AD. These involve the use of either non-physiological lipids such as petrolatum, beeswax, etc. or alternatively lipids similar to the intrinsic pool of lipids present in the lamellar bodies. However, their mechanism of action are still unknown [71]. Yet other therapeutic strategies include using anti—IL-4/13 receptor antibody, anti-IL-13 antibodies in order to reduce the systemic Th2 inflammation reported in severe AD [72]. Additional studies using Omics approach to identify new potential candidate genes associated with the development of various endotypes of AD are necessary [73] (see Outstanding Questions). Addressing how filaggrin mutations affects epidermal lipid metabolism or the role of cholesterol-3-sulfate as a barrier disruptor, can help us gain further insight into the factors governing the pathogenesis of AD and thereby enable us develop better therapies against AD.
Outstanding Questions.
How FLG mutations affects epidermal lipid metabolism? Multiple lines of evidence suggest FLG mutations do not correlate with SC lipid composition. However, there is some correlation with FLG metabolites, namely natural moisturizing factors, and SC lipid composition which suggests the presence of a common factor influencing both.
Will therapies aimed at solely restoring barrier function improve antimicrobial defenses in AD? It is currently unknown whether simply reestablishing a healthy SC lipid profile is sufficient to prevent microbial infiltration.
Could FLG replacement represent a legitimate AD management strategy? One such study suggested that topical application of FLG metabolites might be used to reduce AD symptoms. Although such therapies are likely to have an effect on SC lipid content/organization, it is thought that such a treatment would nonetheless improve overall barrier functions.
It is unclear whether epidermal barrier abnormalities are drivers of AD or consequences of Th2 mediated inflammatory immune response. Likewise, it is not clear whether skin inflammation is a source of, or response to a damaged barrier.
AD patients are characterized by elevated levels of cholesterol-3-sulfate, which was shown to be an inducer of pro-filaggrin gene. Increased level of pro-filaggrin should correlate with increased level of mature filaggrin and hence restore barrier integrity. However, that does not justify the role of cholesterol-3 sulfate as a barrier disruptor. How it directs the disruption of EPB as observed in AD? Is it via a filaggrin mediated pathway where even though there is increased level of pro-filaggrin, they fail to undergo maturation to filaggrin? Or it acts via a filaggrin-independent pathway to act as barrier disruptor? What specific types of inhibitor can help us target its role as a barrier disruptor and hence as a therapy for AD?
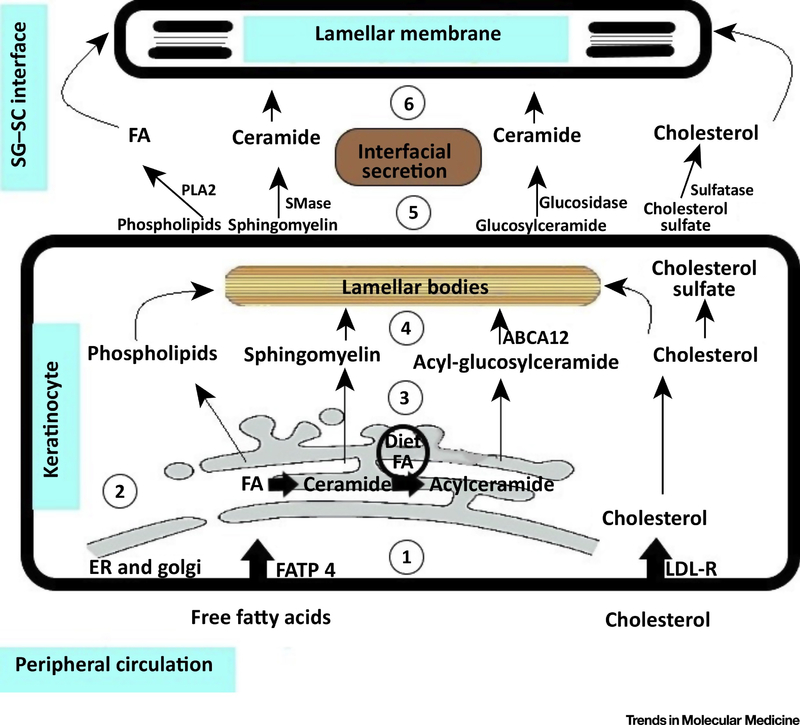
Fig 1. Major lipids constituting epidermal permeability barrier (EPB).
(1) The epidermis can uptake free fatty acids from the peripheral circulation via FATP4 as well as can uptake cholesterol from the peripheral circulation in the form of LDL via the LDL-Rs. (2) In the epidermis, free fatty acids play an important role in the synthesis of ceramides and acyl-ceramides in the endoplasmic reticulum (ER) followed by their conversion to phospholipids, sphingomyelin and acyl-glucosylceramide in the cytosol. The epidermis also serves as an active site for synthesis of cholesterol (3) The phospholipids such as sphingomyelin, acyl-glucosylceramide formed from ceramide and cholesterol inside epidermal keratinocytes of the granular layer undergo processing through the trans-golgi network. (4) They get packaged in the lamellar bodies (5) and get released into the extracellular space from the upper differentiated layers (Stratum granulosum) of the skin epidermis at the interface of the granular and the cornified layer. (6) The secreted lipids then contribute to the formation of the lamellar membrane and thus the EPB. Phospholipase A2 (PLA2); Sphingomyelinase (SMase); Low Density Lipoprotein (LDL); Low Density Lipoprotein Receptor (LDL-R); Fatty Acid Transport Protein 4(FATP 4); ATP Binding Cassette Sub-Family A Member 12 (ABCA12).
Highlights.
An elaborate interaction of extrinsic and intrinsic factors plays an important role in the outcome of AD. Such factors include immune irregularities, genetic level changes, epigenetic alterations such as methylation and histone modifications, the microbiome, the environmental stimuli, the age as well as the ancestry of the subject.
Changes in epidermal lipid metabolism are associated with AD and lipidomic tools such as LC-MS/MS have revealed dramatic changes in lipid composition with respect to AD skin in rodents and humans.
Global analysis of the various biomarkers associated with the varied endotypes of AD involving genomics, epigenomics, proteomics, metabolomics, transcriptomics, lipidomics, microbiomics and exposomics can provide us with new biological insights into the etiology of AD as well as help us detect new AD associated genes and pathways.
Acknowledgements
This work was supported in part by the Oregon State University (OSU) College of Pharmacy (AKI), a R01 grant (AR056008) to AKI and a R15 grant (AR068584-01) to GI both from NIAMS at NIH.
Glossary
- Acyl-ceramide
Acyl-ceramide contain a long chain base and an N-acylated ultralong acyl chain with an additional hydrophobic linoleic acid and it serves an important role in the organization of lipid lamellae and skin barrier formation
- Epidermal Living Skin Equivalents (LSEs)
Co-culture of primary human keratinocyte and fibroblast cells within a collagen gel at the air liquid interface for almost ten days result in a model closely resembling the fully stratified human epidermis
- Genetic ablation
It involves modification of the original DNA sequence in order to disrupt production of a specific type of gene. It is used for knockout studies and is instrumental in determining the function of a particular type of gene
- HPA axis
The hypothalamic-pituitary- adrenal (HPA) axis is also referred to as the pivotal stress response system. This response is characterized by hypothalamic release of corticotropin-releasing factor (CRF). CRF in turn stimulates the release of adrenocorticotropic hormone (ACTH). ACTH upon binding to receptors on the adrenal cortex stimulates release of cortisol. In response to stressors, cortisol continues to be released for several hours in the blood stream up to a certain blood concentration when protection is achieved and the cortisol then exerts negative feedback to the hypothalamic release of CRF and the pituitary release of ACTH (negative feedback) to bring back systemic homeostasis
- Lipidomics
It involves complete characterization of the entire lipid content of a particular species as well as large scale and comprehensive study of the various pathways involved in lipid metabolism and function as well as factors governing regulation of genes involved in those pathways
- Mono-Unsaturated Fatty Acid (MUFA)
Long Chain Fatty Acids bearing double bond at a single position within the chain. For example, C16:1 represents fatty acid with a chain length of 16 carbons and bearing a single double bond
- RNA-Seq
It is used to determine the presence and quantity of total cellular RNA (mRNA, rRNA and tRNA) present in the biological sample. It helps decipher the varying expression pattern of the genes in tissue, time and context dependent manner and hence allows proper determination of the diseased state versus the control conditions
- Sphingolipids
This class of lipids comprise fatty acid derivatives of sphingosine (an amino alcohol) or other sphingoid bases, serving as essential component of biological membranes. The simplest form of sphingolipid is ceramide
- Tape-Stripping
Tape Stripping constitutes an essential methodology in the field of dermatological research to determine the physiology of the stratum corneum. Adhesive films are pressed to the surface of the skin and thereafter can be used for further investigations
- TEWL
Trans-epidermal water loss (TEWL) refers to the water naturally lost through the epidermal layer of the animal skin into the surrounding atmosphere via diffusive and evaporative processes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feingold KR (2009) The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res 50 Suppl, S417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radner FP and Fischer J (2014) The important role of epidermal triacylglycerol metabolism for maintenance of the skin permeability barrier function. Biochim Biophys Acta 1841 (3), 409–15. [DOI] [PubMed] [Google Scholar]
- 3.van Smeden J and Bouwstra JA (2016) Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr Probl Dermatol 49, 8–26. [DOI] [PubMed] [Google Scholar]
- 4.Slominski AT et al. (2012) Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol 212, v, vii, 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slominski AT et al. (2018) How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 159 (5), 1992–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picardo M et al. (2009) Sebaceous gland lipids. Dermatoendocrinol 1 (2), 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feingold KR (2007) Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res 48 (12), 2531–46. [DOI] [PubMed] [Google Scholar]
- 8.Michaels S, A. et al. (1975) Drug permeation through human skin: Theory and invitro experimental measurement. [Google Scholar]
- 9.Armengot-Carbo M et al. (2015) The role of filaggrin in the skin barrier and disease development. Actas Dermosifiliogr 106 (2), 86–95. [DOI] [PubMed] [Google Scholar]
- 10.Palmer CN et al. (2006) Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 38 (4), 441–6. [DOI] [PubMed] [Google Scholar]
- 11.Stubbs CD and Slater SJ (1996) The effects of non-lamellar forming lipids on membrane protein-lipid interactions. Chem Phys Lipids 81 (2), 185–95. [DOI] [PubMed] [Google Scholar]
- 12.Elias PM et al. (2014) Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis. Biochim Biophys Acta 1841 (3), 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berdyshev E et al. (2018) Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 3 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruse V et al. (2017) The Significance of Epidermal Lipid Metabolism in Whole-Body Physiology. Trends Endocrinol Metab 28 (9), 669–683. [DOI] [PubMed] [Google Scholar]
- 15.Wertz PW (2018) Lipids and the Permeability and Antimicrobial Barriers of the Skin. Journal of Lipids 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs E (2015) Cell biology: More than skin deep. J Cell Biol 209 (5), 629–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs E (2016) Epithelial Skin Biology: Three Decades of Developmental Biology, a Hundred Questions Answered and a Thousand New Ones to Address. Curr Top Dev Biol 116, 357–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koster MI and Roop DR (2007) Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol 23, 93–113. [DOI] [PubMed] [Google Scholar]
- 19.Watt FM (2014) Mammalian skin cell biology: at the interface between laboratory and clinic. Science 346 (6212), 937–40. [DOI] [PubMed] [Google Scholar]
- 20.Elias PM (2017) The how, why and clinical importance of stratum corneum acidification. Exp Dermatol 26 (11), 999–1003. [DOI] [PubMed] [Google Scholar]
- 21.Elias PM (2018) Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp Dermatol 27 (8), 847–851. [DOI] [PubMed] [Google Scholar]
- 22.Hsu YC et al. (2014) Emerging interactions between skin stem cells and their niches. Nat Med 20 (8), 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BE and Leung DYM (2018) Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol Res 10 (3), 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiele J et al. (2001) The Antioxidant Network of the Stratum corneum. [DOI] [PubMed] [Google Scholar]
- 25.Weidinger S and Novak N (2016) Atopic dermatitis. Lancet 387 (10023), 1109–1122. [DOI] [PubMed] [Google Scholar]
- 26.Li S et al. (2017) Transcription Factor CTIP1/ BCL11A Regulates Epidermal Differentiation and Lipid Metabolism During Skin Development. Sci Rep 7 (1), 13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z et al. (2013) Transcription factor Ctip2 controls epidermal lipid metabolism and regulates expression of genes involved in sphingolipid biosynthesis during skin development. J Invest Dermatol 133 (3), 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole C et al. (2014) Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol 134 (1), 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatano Y et al. (2005) Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factoralpha and interferon-gamma in human epidermis. J Invest Dermatol 124 (4), 786–92. [DOI] [PubMed] [Google Scholar]
- 30.Hatano Y et al. (2007) Interleukin-4 depresses levels of transcripts for acid-sphingomyelinase and glucocerebrosidase and the amount of ceramide in acetone-wounded epidermis, as demonstrated in a living skin equivalent. J Dermatol Sci 47 (1), 45–7. [DOI] [PubMed] [Google Scholar]
- 31.Angelova-Fischer I et al. (2011) Distinct barrier integrity phenotypes in filaggrin-related atopic eczema following sequential tape stripping and lipid profiling. Exp Dermatol 20 (4), 351–6. [DOI] [PubMed] [Google Scholar]
- 32.Danso M et al. (2017) Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition. J Dermatol Sci 88 (1), 57–66. [DOI] [PubMed] [Google Scholar]
- 33.Masukawa Y et al. (2008) Characterization of overall ceramide species in human stratum corneum. [DOI] [PubMed] [Google Scholar]
- 34.Di Nardo A et al. (1998) Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol 78 (1), 27–30. [DOI] [PubMed] [Google Scholar]
- 35.Li W et al. (2007) Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int J Biol Sci 3 (2), 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogiatzi SI et al. (2007) Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol 178 (6), 3373–7. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa J et al. (2010) Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol 130 (10), 2511–4. [DOI] [PubMed] [Google Scholar]
- 38.Janssens M et al. (2012) Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res 53 (12), 2755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkers T et al. (2018) Topically Applied Ceramides Interact with the Stratum Corneum Lipid Matrix in Compromised Ex Vivo Skin. Pharm Res 35 (3), 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haemmerle G et al. (2011) ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med 17 (9), 1076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grubauer G et al. (1987) Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res 28 (6), 746–52. [PubMed] [Google Scholar]
- 42.Feingold KR (1991) The regulation and role of epidermal lipid synthesis. Adv Lipid Res 24, 57–82. [DOI] [PubMed] [Google Scholar]
- 43.Harris IR et al. (1997) Permeability barrier disruption coordinately regulates mRNA levels for key enzymes of cholesterol, fatty acid, and ceramide synthesis in the epidermis. J Invest Dermatol 109 (6), 783–7. [DOI] [PubMed] [Google Scholar]
- 44.Macheleidt O et al. (2002) Deficiency of Epidermal Protein-Bound ω-Hydroxyceramides in Atopic Dermatitis. Journal of Investigative Dermatology 119 (1), 166–173. [DOI] [PubMed] [Google Scholar]
- 45.Schafer L and Kragballe K (1991) Abnormalities in epidermal lipid metabolism in patients with atopic dermatitis. J Invest Dermatol 96 (1), 10–5. [DOI] [PubMed] [Google Scholar]
- 46.van Smeden J et al. (2014) The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol 23 (1), 45–52. [DOI] [PubMed] [Google Scholar]
- 47.Li S et al. (2017) Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br J Dermatol 177 (4), e125–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mojumdar EH et al. (2014) Monounsaturated fatty acids reduce the barrier of stratum corneum lipid membranes by enhancing the formation of a hexagonal lateral packing. Langmuir 30 (22), 6534–43. [DOI] [PubMed] [Google Scholar]
- 49.Park YH et al. (2012) Decrease of ceramides with very long-chain fatty acids and downregulation of elongases in a murine atopic dermatitis model. J Invest Dermatol 132 (2), 476–9. [DOI] [PubMed] [Google Scholar]
- 50.Sassa T et al. (2013) Impaired epidermal permeability barrier in mice lacking elovl1, the gene responsible for very-long-chain fatty acid production. Mol Cell Biol 33 (14), 2787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mihaly J et al. (2014) Increased FADS2-derived n-6 PUFAs and reduced n-3 PUFAs in plasma of atopic dermatitis patients. Skin Pharmacol Physiol 27 (5), 242–8. [DOI] [PubMed] [Google Scholar]
- 52.Feingold KR (2012) Lamellar bodies: the key to cutaneous barrier function. J Invest Dermatol 132 (8), 1951–3. [DOI] [PubMed] [Google Scholar]
- 53.Sakaki T et al. (2014) CYP24A1 as a potential target for cancer therapy. Anticancer Agents Med Chem 14 (1), 97–108. [DOI] [PubMed] [Google Scholar]
- 54.Slominski A et al. (2004) A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem 271 (21), 4178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slominski A et al. (2005) The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J 272 (16), 4080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slominski AT et al. (2012) In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J 26 (9), 3901–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slominski AT et al. (2015) Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol 151, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slominski AT et al. (2012) Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol 44 (11), 2003–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slominski AT et al. (2017) Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci Rep 7 (1), 11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wollam J and Antebi A (2011) Sterol Regulation of Metabolism, Homeostasis, and Development. 80 (1), 885–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teske KA et al. (2016) Synthesis and evaluation of vitamin D receptor-mediated activities of cholesterol and vitamin D metabolites. Eur J Med Chem 109, 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slominski AT et al. (2014) RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J 28 (7), 2775–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slominski AT et al. (2017) Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORalpha and RORgamma. J Steroid Biochem Mol Biol 173, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slominski AT et al. (2018) Differential and Overlapping Effects of 20,23(OH)(2)D3 and 1,25(OH)(2)D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)(2)D3. Int J Mol Sci 19 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanyu O et al. (2012) Cholesterol sulfate induces expression of the skin barrier protein filaggrin in normal human epidermal keratinocytes through induction of RORalpha. Biochem Biophys Res Commun 428 (1), 99–104. [DOI] [PubMed] [Google Scholar]
- 66.McAleer MA and Irvine AD (2013) The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol 131 (2), 280–91. [DOI] [PubMed] [Google Scholar]
- 67.Irwin McLean WH and Irvine AD (2012) Heritable Filaggrin Disorders: The Paradigm of Atopic Dermatitis. J Invest Dermatol 132 Suppl 3, E20–1. [DOI] [PubMed] [Google Scholar]
- 68.Stone SJ et al. (2004) Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem 279 (12), 11767–76. [DOI] [PubMed] [Google Scholar]
- 69.Katsuta Y et al. (2005) Unsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermis. J Invest Dermatol 124 (5), 1008–13. [DOI] [PubMed] [Google Scholar]
- 70.Irvine AD et al. (2011) Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 365 (14), 1315–27. [DOI] [PubMed] [Google Scholar]
- 71.Ghadially R et al. (1993) Membrane Structural Abnormalities in the Stratum Corneum of the Autosomal Recessive Ichthyoses. [DOI] [PubMed] [Google Scholar]
- 72.Guttman-Yassky E et al. (2013) New era of biologic therapeutics in atopic dermatitis. Expert Opin Biol Ther 13 (4), 549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh D et al. (2018) Leveraging Multilayered “Omics” Data for Atopic Dermatitis: A Road Map to Precision Medicine. 9 (2727). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weidinger S et al. (2018) Atopic dermatitis. Nat Rev Dis Primers 4 (1), 1. [DOI] [PubMed] [Google Scholar]
- 75.Richard G (1993) Autosomal Recessive Congenital Ichthyosis. In GeneReviews((R)) (Adam MP et al. eds). [Google Scholar]
- 76.Boehncke WH and Schon MP (2015) Psoriasis. Lancet 386 (9997), 983–94. [DOI] [PubMed] [Google Scholar]
- 77.Bitoun E et al. (2002) Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol 118 (2), 352–61. [DOI] [PubMed] [Google Scholar]
- 78.Brunner PM et al. (2018) Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol 120 (1), 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eichenfield LF et al. (2012) Atopic dermatitis: epidemiology and pathogenesis update. Semin Cutan Med Surg 31 (3 Suppl), S3–5. [DOI] [PubMed] [Google Scholar]
- 80.Leung DY (2013) New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int 62 (2), 151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leung DY (2016) Clinical implications of new mechanistic insights into atopic dermatitis. Curr Opin Pediatr 28 (4), 456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silverberg NB and Silverberg JI (2015) Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis 96 (6), 359–61. [PubMed] [Google Scholar]
- 83.Feghali CA and Wright TM (1997) Cytokines in acute and chronic inflammation. Front Biosci 2, d12–26. [DOI] [PubMed] [Google Scholar]
- 84.Lowe AJ et al. (2018) The skin as a target for prevention of the atopic march. Ann Allergy Asthma Immunol 120 (2), 145–151. [DOI] [PubMed] [Google Scholar]
- 85.Jang H et al. (2016) Skin pH Is the Master Switch of Kallikrein 5-Mediated Skin Barrier Destruction in a Murine Atopic Dermatitis Model. J Invest Dermatol 136 (1), 127–35. [DOI] [PubMed] [Google Scholar]
- 86.Hachem JP et al. (2006) Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol 126 (9), 2074–86. [DOI] [PubMed] [Google Scholar]
- 87.Werfel T et al. (2016) Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 138 (2), 336–49. [DOI] [PubMed] [Google Scholar]
- 88.Johnson JJ et al. (2016) Protease-activated Receptor-2 (PAR-2)-mediated Nf-kappaB Activation Suppresses Inflammation-associated Tumor Suppressor MicroRNAs in Oral Squamous Cell Carcinoma. J Biol Chem 291 (13), 6936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zbytek B et al. (2013) Key Role of CRF in the Skin Stress Response System. Endocrine Reviews 34 (6), 827–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Slominski A et al. (2005) The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J 19 (2), 176–94. [DOI] [PubMed] [Google Scholar]
- 91.Slominski AT et al. (2018) Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J Invest Dermatol 138 (3), 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hannun YA and Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9 (2), 139–50. [DOI] [PubMed] [Google Scholar]
- 93.Haimovitz-Friedman A et al. (1994) Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med 180 (2), 525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kitatani K et al. (2008) The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 20 (6), 1010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zabielski P et al. (2017) Effect of plasma free fatty acid supply on the rate of ceramide synthesis in different muscle types in the rat. PLoS One 12 (11), e0187136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Man MM et al. (1996) Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol 106 (5), 1096–101. [DOI] [PubMed] [Google Scholar]
- 97.Madaan A (2008) Epiceram for the treatment of atopic dermatitis. Drugs Today (Barc) 44 (10), 751–5. [DOI] [PubMed] [Google Scholar]
- 98.Simpson EL et al. (2014) Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 134 (4), 818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li S et al. (2016) Lipidomic analysis of epidermal lipids: a tool to predict progression of inflammatory skin disease in humans. Expert Rev Proteomics 13 (5), 451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]