ABSTRACT
Mycobacterium tuberculosis is an ancient master of the art of causing human disease. One important weapon within its fully loaded arsenal is the type VII secretion system. M. tuberculosis has five of them: ESAT-6 secretion systems (ESX) 1 to 5. ESX-1 has long been recognized as a major cause of attenuation of the FDA-licensed vaccine Mycobacterium bovis BCG, but its importance in disease progression and transmission has recently been elucidated in more detail. This review summarizes the recent advances in (i) the understanding of the ESX-1 structure and components, (ii) our knowledge of ESX-1’s role in hijacking macrophage function to set a path for infection and dissemination, and (iii) the development of interventions that utilize ESX-1 for diagnosis, drug interventions, host-directed therapies, and vaccines.
INTRODUCTION
Tuberculosis (TB) is a global health problem caused by the airborne pathogen Mycobacterium tuberculosis. Currently, one-third of the world’s population is infected with M. tuberculosis, and this slow, tenacious bacterium kills 1.6 million people around the world each year, equating to over 4,300 deaths every day (1). Failure to eradicate this age-old disease is the result of an ineffective vaccine and extended, often insufficient, chemotherapy. To date, the only licensed vaccine available is Mycobacterium bovis BCG, a live attenuated strain of M. bovis discovered in 1919 by Albert Calmette and Camille Guérin following 230 subcultures of the original virulent isolate (2, 3). Distribution of this vaccine to various countries, and more subculturing, led to genetic variations between different BCG strains. However, all strains possess a common deletion that occurred prior to 1919. The deleted region is called region of difference 1 (RD1), and it encodes a key part of the type VII secretion system known as ESAT-6 secretion system 1 (ESX-1) (Fig. 1A); deletion(s) in this particular region are considered the major cause of BCG attenuation (4–6).
FIGURE 1.
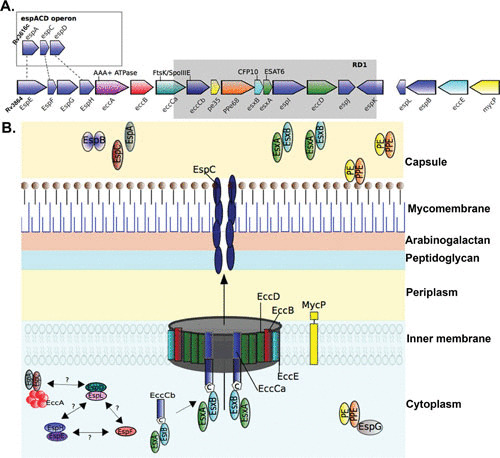
Schematic of the ESX-1 secretion system. (A) Gene map of the esx-1 locus and the espACD operon in M. tuberculosis H37Rv. The esx-1 locus includes esx genes encoding the secreted effector proteins EsxA and EsxB alongside ecc genes encoding ESX-conserved proteins and esp genes encoding ESX secretion-associated proteins (108). The espACD operon is at a locus distinct from the esx-1 locus but shares sequence homology with espE, espF, and espH of esx-1 (dashed lines). The spontaneous deletion (Rv3871 to Rv3878) from esx-1 found in the vaccine strains of M. bovis BCG is known as region of difference 1 (RD1) and is indicated by the gray box. (B) Model of the ESX-1 secretion system in the mycobacterial cell envelope. In common with all ESX systems, the core structure of the ESX-1 secretion apparatus starts with the inner membrane-spanning conserved components EccB, EccC, EccD, and EccE (109). EccC is an ATP-driven translocase consisting of two subunits (a and b) that are assembled following EccB binding of target substrate, in this case, the heterodimer EsxAB, where EccCb interacts with the carboxyl-terminal signal sequence of EsxB (labeled “C”) (21, 110). EsxAB secretion is codependent on the secretion of EspC/EspA, which is also dependent on interaction with the cytosolic ATPase EccA (20, 111). EspC polymerizes during secretion, indicating a role for EccA and EspA as cytosolic chaperones (18), and forms a filamentous structure thought to provide a channel for secretion of ESX-1 substrates (18). Other important ESX-1 substrates include the PE and PPE families of proteins, which form heterodimers and are recruited by the putative cytosolic chaperone EspG to initiate interaction with the core complex of proteins within the inner membrane (112–114). EspB is also secreted by ESX-1 and forms a PE-PPE-like fold, containing a C-terminal domain that is processed by the MycP1 protease during secretion (26, 115). EspD to -F and EspH proteins are cytosolic and were recently shown to be stabilized by the cytosolic chaperone EspL (24–26).
Brief History of ESX-1
ESX-1 is considered omnipresent in terms of scientific publications and functionalities, with studies showing its involvement in intercellular conjugation (7), membrane escape (8), and passage through the lung interstitium (9). ESX-1 is a complex, multifunctional type VII secretion system, producing and releasing a plethora of proteins, many of which are required for its own secretion.
Mahairas et al. first observed RD1 (Rv3871-9) at the genetic level in 1996 while comparing genetic differences between BCG, its parent M. bovis, and its cousin M. tuberculosis (4). Following this seminal study, comparative genomics of numerous BCG strains determined that RD1 was the first region of difference that occurred prior to attenuation in 1921 (10). ESX-1 and its four closely related homologues in M. tuberculosis were identified as potential secretion systems in an organism that was originally believed to have none, with additional homologues identified in other Gram-positive bacteria (11). Similar to Calmette and Guérin a century earlier, several groups saw the potential for a novel vaccine strategy, postulating that the removal of this particular attenuating region from the backbone of the strains that cause human TB, i.e., M. tuberculosis, would result in a vaccine that would work better than BCG. In 2003, the Jacobs, Sherman, and Cole groups were the first to create vaccine candidates based on this hypothesis, with the first two labs knocking out RD1 in M. tuberculosis (9, 12) and the latter taking an alternative approach by adding RD1 to BCG (13). Interestingly, RD1 knockdown strain studies in mice revealed intriguing results, wherein the bacterial count was the same but the pathology was dramatically different (9). Protection was observed, but it never achieved the levels found with BCG vaccination in murine models of infection. However, Koch’s molecular postulates were fulfilled, telling us that the removal of RD1 resulted in attenuation (14). Interestingly, a recently isolated clinical strain defective in this region has shown similar results, where there is no significant difference in bacterial count but pathology and cytokine response are remarkably different (15).
Although these studies did not lead to novel vaccine candidates, they did provide tools to study M. tuberculosis virulence, which may help us design better vaccines and treatment strategies. Recent advances in our understanding of TB disease and ESX-1 are discussed. However, care should be taken to differentiate between cellular functions as they pertain to different organisms; for example, there have been significant advances in Mycobacterium marinum studies in its zebrafish host, which have shown that ESX-1 is not virulence related in this particular species of mycobacteria (16).
Vaccine Studies That Taught Us More about Pathogenesis than Protection
No ESX-1 story is complete without the original ESX-1 knockout mutant, M. bovis BCG. The tale has been told many times of the 230 subcultures that Albert Calmette and Camille Guérin carried out, culminating in the attenuation of virulent M. bovis. The story of BCG begins at the Pasteur Institute in Lille, France, where Guérin began subculturing a virulent strain of M. bovis isolated from the udder of a tuberculous cow. The culture was passaged every ∼3 weeks on a medium containing potato, glycerine, and ox bile. Mycobacterial cultures are notoriously clumpy, and Calmette and Guérin found that a mycobacterial stew, composed of M. bovis growing on potatoes that were cooked in ox bile and then dipped at one end in glycerinated ox bile, led to an emulsified culture, making it easier to standardize inoculating doses. This serendipitously resulted in attenuation of the strain, which led Calmette and Guérin to produce a vaccine utilizing this bacillus (2). By 1919, 230 subcultures had resulted in a strain that failed to produce tuberculous disease when injected into guinea pigs, rabbits, cattle, and horses (17). Following this success, preparations began for the first human clinical trials.
In the early 20th century, ethical standards were somewhat less stringent than today, and the first human “trial” of BCG was undertaken in an unlikely subject, infants. In 1921, an infant under the care of its grandmother, who had TB, was given three doses of oral BCG to prevent likely infection and possible death (3). This initial foray into attenuated TB vaccines was a success, and the child remained healthy. Following this success, a larger trial began 6 months later in which BCG was orally administered to 317 infants at birth. Ultimately, BCG proved to be safe and effective in protecting against childhood TB (3). However, vaccine safety was severely questioned in 1930 following the Lübeck disaster, in which 250 infants were vaccinated in a northern German city with a contaminated BCG strain, resulting in 73 deaths and 135 additional cases of TB. A lengthy investigation attributed the disaster to negligent vaccine preparation, leading to contamination with virulent tubercle bacilli.
Despite this early setback, BCG has subsequently proven to be one of the safest vaccines ever created and has saved the lives of countless individuals, many of them children. However, TB remains an enormous global problem, bringing attention back to BCG attenuation fundamentals, the loss of RD1, and, in turn, the absence of an ESX-1 secretion system.
THE ESX-1 SECRETION SYSTEM AND ITS PROTEINACEOUS ARMY
Many of the proteins secreted by ESX-1 are immunodominant and are at the forefront of infection and disease. The ESX type VII secretion systems are complex; the current working model of the ESX-1 secretion system is shown schematically in Fig. 1B, with the recent addition of EspC, which forms a filamentous structure spanning the bacterial cell envelope (18).
The main ESX-1 system spans the inner membrane with a channel-like structure composed of conserved ESX components (EccB to -D) that form the membrane core complex. EccB and EccC are ATPases involved in recognizing substrates and providing energy for the secretion of substrates across the mycomembrane (19–21). Interestingly, in ESX-1 and ESX-5, the eccC gene, encoding a FtsK/SpoIIIE-type ATPase, has split into two genes that generate the proteins EccCa and EccCb, which interact and form a functional unit for secretion through the ESX-1 system. Although the mycosin protease MycP1 is not an integral part of the central membrane complex, and its protease activity is dispensable for ESX-1-mediated protein secretion, MycP1 is a conserved membrane component associated with the membrane complex, and this association is essential for the stability of the complex (22).
In addition to membrane components, ESX-1 also has cytosolic components, including cytosolic ATPase (EccA), chaperones (EspD to -H), and secreted substrate proteins (EsxAB, PE35-PPE68, EspA to -C, and EspE) called effectors. EccA is a cytosolic AAA (ATPase associated with diverse cellular activities)-type ATPase. Most cytosolic chaperones associated with the ESX-1 system belong to ESX-1 secretion-associated proteins (Esp). EspI (Rv3876) to -L (Rv3880c) are encoded within an operon that generates ESX conserved components (Rv3868 to Rv3883c), and the EspE to -H (Rv3864 to Rv3867) genes are upstream, whereas the EspACD (3616c to 3614c) operon is at a more distant location in the genome (Fig. 1A). EspD itself is secreted by M. tuberculosis, although not exclusively in an ESX-1-dependent manner, but it is also required for stabilizing (EspC and EspA) and secretion (EsxA) of ESX-1 substrates (23). More recently, the EspD to -F and EspH proteins were shown to be stabilized by the chaperone EspL (24). Although some Esp proteins serve as cytosolic chaperones, some act as effectors (EspA to -C and EspE) of ESX-1 and are secreted (25, 26). EspC has been shown to form filaments in the membrane (18), whereas EspE localizes to the cell wall. Fusion of EspE with a fluorescent marker protein in M. marinum demonstrated that ESX-1 localizes at new poles with active peptidoglycan synthesis following cell division (27). How the Esp proteins encoded within the esx-1 operon or distally in the genome interact with each other and with other components of the ESX-1 system and contribute to its integrity and functionality are still active areas of study.
The other major effectors secreted by the ESX-1 system are key immunogenic, highly secreted ESX proteins, including the early secreted antigenic target of 6 kDa (ESAT-6, also called EsxA) and the culture filtrate protein of 10 kDa (CFP-10 or EsxB). EsxA and EsxB are secreted as a heterodimer (EsxAB) in a codependent complex (28). Binding of the EsxAB heterodimer to the ESX-1 inner membrane core complex component EccC for secretion involves recognition of the bipartite secretion signal motif, consisting of WXG, located on EsxA, and tyrosine-X-X-X-aspartic acid/glutamic acid (YXXXD/E), located on EsxB (21, 25, 29). In contrast, secretion of the PE35-PPE68 heterodimer is dependent on direct binding to EspG (30). The ESX-1 system is highly complex, and this complexity is not restricted to the machinery and effector molecules; the regulation of ESX-1 itself also appears to be multifactorial and is indirectly regulated by PhoPR (31), WhiB6 (32), EspR (33), Lsr2 (34), and MprAB (35).
ESX-1 Lyses Membranes
The macrophage phagosome is a highly inhospitable environment; however, M. tuberculosis survives and replicates within this environment. It has long been debated whether M. tuberculosis can escape the phagosome and replicate within the cytosol, and McDonough et al. were some of the first researchers to demonstrate the controversial escape of M. tuberculosis from phagosomes to the cytosol using electron microscopy (36). This has since been shown to be ESX-1 mediated via ESAT-6 (37). The ESX-1 system was also found to lyse whole cells, causing macrophage and epithelial cell necrosis in in vitro infection experiments (4). Many researchers carried out the obvious experiments, adding ESAT-6 (EsxA) directly to cell cultures to look for membrane lysis, often in terms of measuring the release of cytoplasmic contents. However, no cell lysis was observed, until the missing link of low pH was identified (38, 39). Conrad et al. stated that contact dependence was required for ESAT-6 membrane lysis but then, in the same study, showed that low pH caused this as well (40). The Jacobs laboratory tried this experiment in 2003 before we knew of the low-pH trigger, and although our study was unsuccessful, we did demonstrate total membrane disruption in a simplified artificial membrane model with ESAT-6 or ESAT-6 and CFP-10 together, but not with CFP-10 alone (9). In addition, cryo-electron micrographs of ESAT-6 and CFP-10 proteins showed differently sized pore-like structures (Fig. 2, inset a) (8).
FIGURE 2.
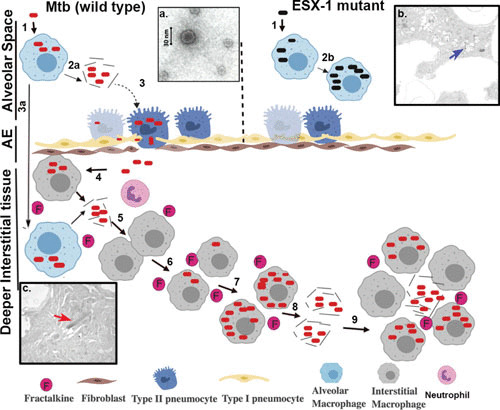
ESX-1-related disease progression within the lung. Steps involved in progression of disease are represented with the numbers 1 to 9. (1) Infection of alveolar macrophages with M. tuberculosis (wild type) or ESX-1 mutant. (2) Lysis of the phagosomal and cellular macrophage membranes is carried out by EsxA from wild-type M. tuberculosis (2a), while the ESX-1 mutant remains trapped in the alveolar macrophages in the alveolar space (2b). (3) Infection of type II pneumocytes in the alveolar epithelium (AE) by M. tuberculosis, with resulting ESAT-6-mediated lysis, allowing passage into the interstitial tissue or (3a) translocation of infected alveolar macrophage to lung interstitial tissue. (4) Translocated bacteria are ingested by and replicate within macrophages, which produce cytokines such as fractalkine. (5) Release of bacilli by necrosis of infection-dependent macrophages. (6) Recruitment of neutrophils and naive macrophages by fractalkine and infection of new macrophages and other cells by phagocytosis. (7) Intracellular replication of bacilli in recruited infected macrophages. (8) Continuation of the cycle, leading to egress of M. tuberculosis from the host cells into deeper interstitial tissue and dissemination within the lungs. (9) Establishment of granulomas and necrosis. (Insets) (a) Electron micrograph of EsxAB. (b) Electron micrograph of the ESX-1 mutant (blue arrow) trapped within the phagosome of an alveolar macrophage in alveolar space in a murine model. (c) Electron micrograph showing wild-type M. tuberculosis (red arrow) following egress to the cytoplasm and interstitial spaces in the murine lung.
Phagosome Permeabilization and Its Prospective Roles
The implications of phagosomal escape have been discussed in many reviews covering mycobacterial species and other intracellular pathogens, such as Salmonella enterica serovar Typhimurium or Listeria monocytogenes (41). The reasoning includes cytoplasmic nutritional availability, antigen presentation to CD8 T cells, and dissemination. In M. tuberculosis, it is clear that this escape happens (37) and that it is ESX-1 mediated; however, some studies suggest that such escape is a temporal by-product of ESX-1 lysing the cell membrane to escape from the cell (42).
Nutrient-rich cytoplasm seems an attractive environment for proliferation, providing an advantage to intracellular pathogens that develop mechanisms to permeabilize, and escape from, the phagosome. Interestingly, to counter host-imposed nutrient restrictions, M. tuberculosis can synthesize most of the essential nutrients for its growth, but it is also capable of acquiring nutrients from the host. M. tuberculosis can obtain carbon, nitrogen, and some amino acids from the host (41, 43, 44), but intriguingly, it is not able to acquire arginine, methionine, or leucine from the host (45–47). In the Jacobs laboratory, we have demonstrated that arginine or methionine auxotrophy is bactericidal to M. tuberculosis in vitro and in vivo, both in macrophages and in mice (45, 46), whereas leucine auxotrophy is bacteriostatic (45–47). This finding was noteworthy, as mouse plasma has an arginine concentration of ∼200 μM under normal conditions, and M. tuberculosis possesses arginine transporters (48, 49). Salmonella Typhimurium establishes an active arginine recovery system using its arginine transporter ArgT and by promoting accumulation of the host arginine transporter (mCAT1) on phagosomes (50), which does not appear to be the case with M. tuberculosis. This amino acid autarky is, as yet, an unexplored metabolic vulnerability. However, it does suggest that if ESX-1 is functional and provides access to the cytosol, M. tuberculosis amino acid auxotrophs should acquire their missing nutrient from the cytosol and proliferate and disseminate as observed with other pathogens (41, 51). However, by utilizing nutrients from the host in this manner, these pathogens also alert the host immune system. It is unclear whether M. tuberculosis’s ability to biosynthesize most of the amino acids and metabolic shutdown during famine of the nutrients provide an evolutionary advantage by enhancing the ability of M. tuberculosis to persist in the host, ultimately evading vaccine and drug treatments. Further studies are needed to better answer these questions and fully elucidate the essentiality and sufficiency of ESX-1 system to lyse phagosomal membranes and gain access to the nutrient-rich cytosol for proliferation and dissemination.
Another implication of phagosome permeabilization by M. tuberculosis is egress of the organism to the cytosol for dissemination and pathogenesis of disease. ESAT-6-mediated phagosome disruption activates the cytosolic inflammasome receptor NLRP3, also triggering increased necrosis (52, 53). Augenstreich et al. suggested that ESX-1, along with phthiocerol dimycocerosates, causes phagosomal rupture but leads to an alternative mode of death, specifically, apoptosis (54). Further, ESX-1-mediated phagosome permeabilization has been shown to enhance type I interferon (IFN) secretion as a pathogenic mechanism for promoting bacterial replication and manipulation of host immunity (8, 55). With so many conflicting studies, it is clear that phagosome disruption remains a much-debated topic in the TB field. Engendering further conflict in the field is the question of whether ESX-1 mediates exit from the cell by the formation of ejectosomes, membrane protrusions containing the bacilli that propel themselves by means of an actin tail. These structures have been observed in M. marinum (56), but whether they occur in M. tuberculosis-infected human macrophages has yet to be determined, particularly as M. tuberculosis does not have the required active tail (57).
ESX-1 at the Site of Disease: Disease Progression via Necrosis
Orme suggested that necrosis is itself a means to progression of TB disease (58), and following intracellular replication within alveolar macrophages at the site of disease, cell death often occurs, with M. tuberculosis ESX-1 promoting necrosis, not apoptosis (4). Indeed, M. tuberculosis actively suppresses macrophage apoptosis, reducing bacterial replication (59) with ESX-1, while ESX-1-mediated necrosis enhances bacterial replication (60). M. tuberculosis also mediates cellular necrosis in other host cells, such as alveolar epithelial cells in the epithelial bilayer, which is needed for infection and disease progression (61–63). King believes the interaction of M. tuberculosis with alveolar epithelial cells is too often overlooked in TB disease, with most researchers conducting studies using macrophages and often missing these short-lived interactions, i.e., the egress across the alveolar interstitium within epithelial cells (63). Notably, studies have shown that type II pneumocytes are highly susceptible to M. tuberculosis infection, with the bacilli translocating into interstitial tissue through the basolateral surfaces of these cells via exocytosis or necrosis (64). In collaboration with our laboratory, King’s group performed screens to find mutants of M. tuberculosis incapable of lysing alveolar epithelial cells, discovering that ESX-1 was required for epithelial cell lysis (9). We also observed ESX-1-mediated macrophage lysis both in vitro and in vivo (Fig. 2), with M. tuberculosis ESX-1 mutants remaining trapped within alveolar macrophages but wild-type bacilli escaping and egressing to the interstitial tissue (9).
Krishnan et al. predicted in 2010 that M. tuberculosis-infected alveolar macrophages may translocate from alveolar spaces to lung interstitium to disseminate M. tuberculosis (65), and recent studies support their hypothesis. Cohen et al. recently provided evidence of M. tuberculosis-infected alveolar macrophages being relocated to the lung interstitium due to ESX-1 and interleukin 1R (IL-1R) dependence (66). ESX-1 effector protein involved in dissemination of infected alveolar macrophages into the interstitium in mice is still undetermined (9, 66).Once M. tuberculosis escapes from the activated macrophage via necrosis, it needs a niche. In this instance, a growth-permissive naive macrophage serves as the perfect host where M. tuberculosis can survive and replicate. Therefore, to attract naive macrophages, M. tuberculosis has evolved a mechanism involving induction of fractalkine production by M. tuberculosis-infected cells.
ESX-1 and the Chemokine Fractalkine
First discovered by Bazan et al. in 1998 (67), fractalkine is a rather unusual chemokine and in a class of its own, having a strange stalk-like structure that can also tether host cells. It has been reported to attract naive macrophages to the lung, and fractalkine production from M. tuberculosis-infected cells has been determined to be ESX-1 regulated (68). This study also linked fractalkine levels to the influx of naive macrophages during TB infection using bronchoalveolar lavage samples taken directly from the lungs of TB patients. Other cytokines are likely involved in this influx, such as ESX-1-regulated IL-1R (66, 68, 69).
If M. tuberculosis ESX-1 mediates fractalkine production from the infected macrophage, what is the effector protein responsible for triggering its production? This appears to be ESAT-6, which activates the tyrosine kinase Syk (53); Syk functions in an upstream activation pathway to produce fractalkine, and its inhibition can stop M. tuberculosis ESX-1-mediated fractalkine production and the resulting monocytic infiltration (68). The fractalkine axis has been proposed as a treatment target via the inhibition of monocyte infiltration and thus inflammation in diseases such as Crohn’s (70), and a vaccination approach has successfully been used to protect against respiratory syncytial virus infection in an animal model (71). Early establishment of infection and subsequent bacillary dissemination relies upon the availability of permissive “niche” cells. Therefore, a chemotactic signal would be a requisite for increasing the number of the cells from the approximate one macrophage per every 9 ml of lung volume (72). The question of whether other ESX-1-dependent effectors also are involved in the induction of fractalkine production by M. tuberculosis-infected macrophages is an open area of investigation.
DO OTHER BACTERIA HAVE ESX-1 SECRETION SYSTEMS?
Homologues of ESX-1 proteins have been detected in various members of the Actinobacteria (other mycobacterial species and Streptomyces coelicolor), Firmicutes (Staphylococcus aureus, L. monocytogenes, Bacillus anthracis, and Bacillus subtilis), and Gammaproteobacteria, such as Helicobacter pylori (73). These secretion systems contain at least one FtsK-SpoIII ATPase, plus one member of the WXG100 protein family (73). A type VII-like secretion system in S. aureus is composed of five membrane proteins (EsaA and EssA to -D), three cytosolic proteins (EsaB, -E, and -G), and five secreted virulence factors (EsxA to -D and EsaD) (74). In S. aureus, this specialized type VII secretion system is rapidly induced in response to interaction of the bacteria with host fluids, including blood serum, nasal secretions, and pulmonary surfactant (74). Furthermore, by generating mutants with deletions of genes homologous to the M. tuberculosis ESX-1 membrane core complex genes essA, essB, and essC in two S. aureus strains, Kneuper et al. demonstrated that these genes play a major role in nasal colonization and in development of pneumonia in cystic fibrosis murine infection models (75). Deletion of this secretion system in S. aureus leads to its attenuation via decreased bacterial growth and a subsequent decrease in the number of abscesses in host kidneys, spleen, and liver in mice (76, 77). Conversely, an ESX-1 secretion system found in L. monocytogenes is not required for epithelial cell invasion and intracellular multiplication in macrophages in vitro; indeed, in an in vivo murine model, the expression of the ESAT-6 homologue EsxA was detrimental to L. monocytogenes, resulting in decreased infection (78). Whether deletion of ESX secretion systems from these pathogens can be exploited for live-vaccine design to protect against infection remains to be investigated.
PRACTICAL IMPLICATIONS OF ESX-1
Drug Interventions and Diagnostics
Studies on ESX-1 proteins have been of continuous interest for the development of drug interventions, diagnostic markers, and vaccines. EsxA and EsxB, two of the most highly secreted proteins of M. tuberculosis, have formed the basis for a major breakthrough in TB diagnostics, the IFN-γ release assays (IGRAs). The addition of another ESX-1 protein, EspC, which is present in BCG but not secreted, may potentially enhance the sensitivity and specificity of these assays (79). Interestingly, lysis of M. tuberculosis phagosomal membranes via EsxA and EsxB is associated with NOD2-RIP2- or cGAS-STING-dependent or -independent activation of type I interferon IFN-α and IFN-β induction (80). Furthermore, Barczak et al. showed that PDIM production and export are required for coordinated secretion of ESX-1 substrates for phagosome permeabilization and induction of type I IFN response (81).
Several studies have shown that induction of type I IFNs can worsen the outcome of TB (80, 82–84). Berry et al. performed whole-blood transcript signature studies on patients with active and latent TB to identify signatures linked to the progression of active disease (85). Recently, Singhania et al. identified type I IFN as a part of the transcriptome signature that can differentiate between patients with active and latent TB (86). These studies suggest that inhibitors of M. tuberculosis pertaining to IFN-α and IFN-β induction are good targets for novel immunotherapeutic strategies to combat M. tuberculosis. In addition to the effector proteins EsxA and EsxB, potential targets include immunogenic PE (Pro-Glu) and PPE (Pro-Pro-Glu) proteins and Esp proteins. Effectors such as EspE that are localized on the M. tuberculosis cell surface are potential therapeutic targets for antibodies, generating antibody-dependent cell cytotoxic responses, with the aim of eliminating extracellular M. tuberculosis. In a recent study, IL-2 induced by stimulation of whole blood with ESX-1-secreted PE35 and PPE68, using a technique similar to that used for IGRAs, was capable of discriminating between patients latently infected with M. tuberculosis and those with active TB (87).
The use of ESX-1 proteins distinguishes between BCG-vaccinated and nonvaccinated humans, as BCG does not possess or secrete these proteins. Determining the difference between latent TB infection, BCG vaccination, and active TB infection could revolutionize TB treatment strategies, allowing the development of differentiating biomarkers. Better characterizations of the T-cell-stimulatory proteins secreted by ESX-1 will lead to development of improved IGRAs with enhanced prognostic value.
Why Do We Need a New Vaccine?
BCG is good at protecting a range of animals, from badgers to horses. Unfortunately, that same coverage does not translate to adult humans, resulting in a failure to eradicate TB. However, there is a silver lining with BCG, as it is able to elicit some protection against the disease in children (88). However, if we are to rid the world of TB by 2020, which is the aim of the World Health Organization, we need a better vaccine, and luckily, there are several in the clinical pipeline that are related to ESX-1 or other type VII secretion systems.
In 2012, the Tuberculosis Vaccine Initiative led the TB vaccine community to generate a blueprint for TB vaccine development (https://www.who.int/immunization/sage/3_TB_Vaccines_Strategic_Blueprint_draft_Oct2011_nov11.pdf). TB vaccine development is difficult, as there is still no known immune correlate of protection. In 2012, there were 13 candidate TB vaccines undergoing clinical trials. In the 5 years that followed, progress of these candidates through the TB vaccine pipeline slowed or failed altogether, with very few preclinical candidates emerging (89). This impeded progress resulted in the current pipeline of 12 TB vaccine candidates that are currently in phase 1 to 3 clinical trials (Fig. 3). This pipeline consists of a variety of delivery methods (protein/adjuvant, attenuated/killed cells or cell extract, and viral vectors), but the range of antigens and vaccine technologies is actually quite narrow; most rely on eliciting a strong T-helper 1 (Th1) and cell-mediated immune response, known to be important in anti-TB immunity, based on the attenuated M. tuberculosis vaccine strain (BCG).
FIGURE 3.
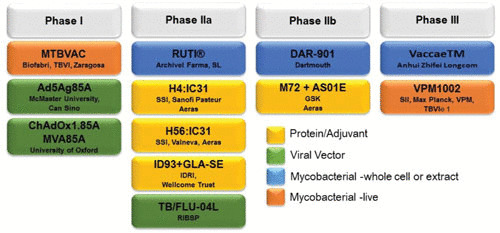
TB vaccines in the pipeline, undergoing phase 1 to 3 clinical trials. Current vaccine candidates in the pipeline include protein/adjuvant-based, attenuated/killed or cell extract-based, and viral vector-based vaccines.
Approaches that focus on ESX-1 and other type VII secretion systems include live, attenuated MTBVAC (90) and VPM1002 (91) and the protein subunit vaccines ID93+GLA-SE (92), H4:IC31 (93), H56:IC31 (94), and M72+AS01E (95). ID93+GLA-SE, H4:IC31, and H56:IC31 have ESAT-6 as one of the antigens secreted by ESX-1, while M72+AS01E has PPE18 secreted by ESX-5 (92–95). MTBVAC was developed from an attenuated M. tuberculosis clinical isolate that retained most of the discovered M. tuberculosis T-cell epitopes, including the immunodominant antigens EsxA and EsxB of the RD1 region deleted from BCG (Fig. 1A). MTBVAC has entered clinical trials as a preventive vaccine in newborns, adolescents, and adults. It is believed that by targeting the virulence-specific epitopes missing from the BCG vaccine, MTBVAC might afford better protection against TB in human hosts (96). VPM1002 is a recombinant BCG strain with the urease gene (ureC) replaced by the listeriolysin O gene hly from L. monocytogenes (91). In phase II clinical trials, VPM1002 afforded safety and immunogenicity to newborn infants as well as adults. Furthermore, the incidence of abscess formation was lower with VPM1002 than BCG (97).
However, it may be possible that M. tuberculosis whole-cell vaccines alone cannot confer the desired level of protection and need to be combined with novel approaches involved in enhancing host immune response, such as that taken in the development of the ID93+GLA-SE and M72+AS01E vaccine candidates. ID93+GLA-SE vaccine was rationally designed as a fusion protein of four immunogenic protein targets that are associated with either virulence (Rv3619, Rv3620, and Rv2608) or latency (Rv1813) (92, 98). Rv3619 and Rv3620 are the ESX-5 ESX protein pair (ESXM/N) paralogs called EsxV and EsxW and are uniquely expressed by M. tuberculosis, not M. bovis or BCG, while Rv2608 (PPE42) and Rv1813 are common to M. tuberculosis, M. bovis, and BCG (99–101). The immunogenicity of this vaccine candidate has been boosted by using a Toll-like receptor 4 agonist as an adjuvant, resulting in the induction of a humoral response with a preferential increase in IgG1 and IgG3 subclasses and a Th1-type cellular response (102). Similarly, M72+AS01E includes the antigens Rv1196 (PPE18) and Rv0125, along with the adjuvant AS01E, and showed an efficacy of 54% efficacy in phase 2b trials against M. tuberculosis (103). Although this candidate includes an alternative agonist and is designed to promote T cell and antibody responses, we do not know if these are the only correlates that will protect.
There are also questions regarding the role of chemokines in the vaccine response. This subclass of proteins is responsible for recruiting host immune cells and has been largely neglected by TB vaccinologists. Perhaps a better chemokine-centered vaccine could be developed that could halt or contain the spread of M. tuberculosis upon initial infection. M. tuberculosis uses ESX-1 to spread into the lung interstitium from its initial encounter with the alveolar macrophage (66), in granuloma formation in a human lung tissue model (104), and to modulate the infected macrophage to produce the chemokine fractalkine, which calls in permissive macrophages that can lead to M. tuberculosis progression (68). Altering this chemotactic call may switch the immune response, favoring the host. It is therefore possible that ESX systems will lead the way for novel vaccine development. If so, we need to further understand how they work before we can harness their secretory host-controlling powers.
Moreover, multiple strains have been observed in the same patient (105), with mixed infections being massively underrepresented in the majority of diagnostic methods used today (106). Such incomplete diagnosis is, in itself, a huge issue with regard to curbing the spread of TB, potentially resulting in incorrect treatment regimens and enhanced TB rates, but this topic is beyond the scope of this article. In addition, many of the circulating strains causing TB may be other members of the M. tuberculosis complex (107), indicating that we may need to look for vaccines that will also protect against other members of the M. tuberculosis complex, including Mycobacterium africanum, M. bovis, etc.
CONCLUDING REMARKS
Without ESX-1, M. tuberculosis is highly attenuated. M. tuberculosis uses virulence-associated ESX-1 to lyse membranes, egress through cells and lung tissue, and cause tuberculous disease. We need to understand the exact role of each of the plethora of proteins that ESX-1 employs to manipulate and modulate. We urgently need novel strategies to protect against and prevent M. tuberculosis infections. Furthering our understanding of this proteinaceous army could enable us to target specific ESX-1 proteins involved in the hijacking of the host pathways and ultimately halt the spread of disease. Perhaps this will open new avenues leading to the development of novel immunotherapeutic strategies for TB and a variety of other bacterial diseases.
ACKNOWLEDGMENTS
We thank Keely R. Redhage for her help with proofreading and useful comments. We thank Tsungda Hsu for useful discussions related to RD1. This work was supported by grant R01AI026170.
REFERENCES
- 1.World Health Organization. 2018. Global tuberculosis report 2018. World Health Organization, Geneva, Switzerland. https://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Calmette A. 1922. L’infection Bacillaire et la Tuberculose chez l’Homme et chez les Animaux, 2nd ed. Masson et Cie, Paris, France. [Google Scholar]
- 3.Calmette A. 1931. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med 24:1481–1490. [PMC free article] [PubMed] [Google Scholar]
- 4.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol 178:1274–1282 10.1128/jb.178.5.1274-1282.1996. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gröschel MI, Sayes F, Simeone R, Majlessi L, Brosch R. 2016. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol 14:677–691 10.1038/nrmicro.2016.131. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.van Pinxteren LA, Ravn P, Agger EM, Pollock J, Andersen P. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin Diagn Lab Immunol 7:155–160. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray TA, Clark RR, Boucher N, Lapierre P, Smith C, Derbyshire KM. 2016. Intercellular communication and conjugation are mediated by ESX secretion systems in mycobacteria. Science 354:347–350 10.1126/science.aag0828. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. 2012. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11:469–480 10.1016/j.chom.2012.03.007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA 100:12420–12425 10.1073/pnas.1635213100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520–1523 10.1126/science.284.5419.1520. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Pallen MJ. 2002. The ESAT-6/WXG100 superfamily—and a new Gram-positive secretion system? Trends Microbiol 10:209–212 10.1016/S0966-842X(02)02345-4. [DOI] [PubMed] [Google Scholar]
- 12.Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J Infect Dis 187:117–123 10.1086/345862. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9:533–539 10.1038/nm859. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Falkow S. 2004. Molecular Koch’s postulates applied to bacterial pathogenicity--a personal recollection 15 years later. Nat Rev Microbiol 2:67–72 10.1038/nrmicro799. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Clemmensen HS, Knudsen NPH, Rasmussen EM, Winkler J, Rosenkrands I, Ahmad A, Lillebaek T, Sherman DR, Andersen PL, Aagaard C. 2017. An attenuated Mycobacterium tuberculosis clinical strain with a defect in ESX-1 secretion induces minimal host immune responses and pathology. Sci Rep 7:46666 10.1038/srep46666. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosserman RE, Thompson CR, Nicholson KR, Champion PA. 2018. Esx paralogs are functionally equivalent to ESX-1 proteins but are dispensable for virulence in Mycobacterium marinum. J Bacteriol 200:e00726-17 10.1128/JB.00726-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakula A. 1983. BCG: who were Calmette and Guérin? Thorax 38:806–812 10.1136/thx.38.11.806. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou Y, Rybniker J, Sala C, Cole ST. 2017. EspC forms a filamentous structure in the cell envelope of Mycobacterium tuberculosis and impacts ESX-1 secretion. Mol Microbiol 103:26–38 10.1111/mmi.13575. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Zhang XL, Li DF, Fleming J, Wang LW, Zhou Y, Wang DC, Zhang XE, Bi LJ. 2015. Core component EccB1 of the Mycobacterium tuberculosis type VII secretion system is a periplasmic ATPase. FASEB J 29:4804–4814 10.1096/fj.15-270843. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Champion PA, Champion MM, Manzanillo P, Cox JS. 2009. ESX-1 secreted virulence factors are recognized by multiple cytosolic AAA ATPases in pathogenic mycobacteria. Mol Microbiol 73:950–962 10.1111/j.1365-2958.2009.06821.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Champion PA, Stanley SA, Champion MM, Brown EJ, Cox JS. 2006. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313:1632–1636 10.1126/science.1131167. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.van Winden VJ, Ummels R, Piersma SR, Jiménez CR, Korotkov KV, Bitter W, Houben EN. 2016. Mycosins are required for the stabilization of the ESX-1 and ESX-5 type VII secretion membrane complexes. mBio 7:e01471-16 10.1128/mBio.01471-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JM, Boy-Röttger S, Dhar N, Sweeney N, Buxton RS, Pojer F, Rosenkrands I, Cole ST. 2012. EspD is critical for the virulence-mediating ESX-1 secretion system in Mycobacterium tuberculosis. J Bacteriol 194:884–893 10.1128/JB.06417-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sala C, Odermatt NT, Soler-Arnedo P, Gülen MF, von Schultz S, Benjak A, Cole ST. 2018. EspL is essential for virulence and stabilizes EspE, EspF and EspH levels in Mycobacterium tuberculosis. PLoS Pathog 14:e1007491 10.1371/journal.ppat.1007491. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fortune SM, Jaeger A, Sarracino DA, Chase MR, Sassetti CM, Sherman DR, Bloom BR, Rubin EJ. 2005. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci USA 102:10676–10681 10.1073/pnas.0504922102. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomonson M, Setiaputra D, Makepeace KAT, Lameignere E, Petrotchenko EV, Conrady DG, Bergeron JR, Vuckovic M, DiMaio F, Borchers CH, Yip CK, Strynadka NCJ. 2015. Structure of EspB from the ESX-1 type VII secretion system and insights into its export mechanism. Structure 23:571–583 10.1016/j.str.2015.01.002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Carlsson F, Joshi SA, Rangell L, Brown EJ. 2009. Polar localization of virulence-related Esx-1 secretion in mycobacteria. PLoS Pathog 5:e1000285 10.1371/journal.ppat.1000285. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renshaw PS, Lightbody KL, Veverka V, Muskett FW, Kelly G, Frenkiel TA, Gordon SV, Hewinson RG, Burke B, Norman J, Williamson RA, Carr MD. 2005. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J 24:2491–2498 10.1038/sj.emboj.7600732. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daleke MH, Ummels R, Bawono P, Heringa J, Vandenbroucke-Grauls CM, Luirink J, Bitter W. 2012. General secretion signal for the mycobacterial type VII secretion pathway. Proc Natl Acad Sci USA 109:11342–11347 10.1073/pnas.1119453109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ates LS, Houben EN, Bitter W. 2016. Type VII secretion: a highly versatile secretion system. Microbiol Spectr 4:VMBF-0011-2015. [DOI] [PubMed] [Google Scholar]
- 31.Frigui W, Bottai D, Majlessi L, Monot M, Josselin E, Brodin P, Garnier T, Gicquel B, Martin C, Leclerc C, Cole ST, Brosch R. 2008. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog 4:e33 10.1371/journal.ppat.0040033. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solans L, Aguiló N, Samper S, Pawlik A, Frigui W, Martín C, Brosch R, Gonzalo-Asensio J. 2014. A specific polymorphism in Mycobacterium tuberculosis H37Rv causes differential ESAT-6 expression and identifies WhiB6 as a novel ESX-1 component. Infect Immun 82:3446–3456 10.1128/IAI.01824-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghavan S, Manzanillo P, Chan K, Dovey C, Cox JS. 2008. Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature 454:717–721 10.1038/nature07219. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon BR, Li Y, Wang L, Sintsova A, van Bakel H, Tian S, Navarre WW, Xia B, Liu J. 2010. Lsr2 is a nucleoid-associated protein that targets AT-rich sequences and virulence genes in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 107:5154–5159. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang X, Samten B, Cao G, Wang X, Tvinnereim AR, Chen XL, Howard ST. 2013. MprAB regulates the espA operon in Mycobacterium tuberculosis and modulates ESX-1 function and host cytokine response. J Bacteriol 195:66–75 10.1128/JB.01067-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonough KA, Kress Y, Bloom BR. 1993. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun 61:2763–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houben D, Demangel C, van Ingen J, Perez J, Baldeón L, Abdallah AM, Caleechurn L, Bottai D, van Zon M, de Punder K, van der Laan T, Kant A, Bossers-de Vries R, Willemsen P, Bitter W, van Soolingen D, Brosch R, van der Wel N, Peters PJ. 2012. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol 14:1287–1298 10.1111/j.1462-5822.2012.01799.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.De Leon J, Jiang G, Ma Y, Rubin E, Fortune S, Sun J. 2012. Mycobacterium tuberculosis ESAT-6 exhibits a unique membrane-interacting activity that is not found in its ortholog from non-pathogenic Mycobacterium smegmatis. J Biol Chem 287:44184–44191 10.1074/jbc.M112.420869. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jonge MI, Pehau-Arnaudet G, Fretz MM, Romain F, Bottai D, Brodin P, Honoré N, Marchal G, Jiskoot W, England P, Cole ST, Brosch R. 2007. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol 189:6028–6034 10.1128/JB.00469-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conrad WH, Osman MM, Shanahan JK, Chu F, Takaki KK, Cameron J, Hopkinson-Woolley D, Brosch R, Ramakrishnan L. 2017. Mycobacterial ESX-1 secretion system mediates host cell lysis through bacterium contact-dependent gross membrane disruptions. Proc Natl Acad Sci USA 114:1371–1376 10.1073/pnas.1620133114. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gouzy A, Poquet Y, Neyrolles O. 2014. Nitrogen metabolism in Mycobacterium tuberculosis physiology and virulence. Nat Rev Microbiol 12:729–737 10.1038/nrmicro3349. [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. 2012. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 8:e1002507 10.1371/journal.ppat.1002507. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beste DJ, Nöh K, Niedenführ S, Mendum TA, Hawkins ND, Ward JL, Beale MH, Wiechert W, McFadden J. 2013. 13C-flux spectral analysis of host-pathogen metabolism reveals a mixed diet for intracellular Mycobacterium tuberculosis. Chem Biol 20:1012–1021 10.1016/j.chembiol.2013.06.012. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baughn AD, Rhee KY. 2014. Metabolomics of central carbon metabolism in Mycobacterium tuberculosis. Microbiol Spectr 2:MGM2-0026-2013. 10.1128/microbiolspec.MGM2-0026-2013. [PubMed] [DOI] [PubMed] [Google Scholar]
- 45.Berney M, Berney-Meyer L, Wong KW, Chen B, Chen M, Kim J, Wang J, Harris D, Parkhill J, Chan J, Wang F, Jacobs WR Jr. 2015. Essential roles of methionine and S-adenosylmethionine in the autarkic lifestyle of Mycobacterium tuberculosis. Proc Natl Acad Sci USA 112:10008–10013 10.1073/pnas.1513033112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiwari S, van Tonder AJ, Vilchèze C, Mendes V, Thomas SE, Malek A, Chen B, Chen M, Kim J, Blundell TL, Parkhill J, Weinrick B, Berney M, Jacobs WR Jr. 2018. Arginine-deprivation-induced oxidative damage sterilizes Mycobacterium tuberculosis. Proc Natl Acad Sci USA 115:9779–9784 10.1073/pnas.1808874115. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McAdam RA, Weisbrod TR, Martin J, Scuderi JD, Brown AM, Cirillo JD, Bloom BR, Jacobs WR Jr. 1995. In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect Immun 63:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobert AP, Daulouede S, Lepoivre M, Boucher JL, Bouteille B, Buguet A, Cespuglio R, Veyret B, Vincendeau P. 2000. l-Arginine availability modulates local nitric oxide production and parasite killing in experimental trypanosomiasis. Infect Immun 68:4653–4657 10.1128/IAI.68.8.4653-4657.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peteroy-Kelly M, Venketaraman V, Connell ND. 2001. Effects of Mycobacterium bovis BCG infection on regulation of l-arginine uptake and synthesis of reactive nitrogen intermediates in J774.1 murine macrophages. Infect Immun 69:5823–5831 10.1128/IAI.69.9.5823-5831.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das P, Lahiri A, Lahiri A, Sen M, Iyer N, Kapoor N, Balaji KN, Chakravortty D. 2010. Cationic amino acid transporters and Salmonella Typhimurium ArgT collectively regulate arginine availability towards intracellular Salmonella growth. PLoS One 5:e15466 10.1371/journal.pone.0015466. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang YJ, Rubin EJ. 2013. Feast or famine: the host-pathogen battle over amino acids. Cell Microbiol 15:1079–1087 10.1111/cmi.12140. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong KW. 2017. The role of ESX-1 in Mycobacterium tuberculosis pathogenesis. Microbiol Spectr 5:TBTB2-0001-2015 10.1128/microbiolspec.TBTB2-0001-2015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.Wong KW, Jacobs WR Jr. 2011. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol 13:1371–1384 10.1111/j.1462-5822.2011.01625.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Augenstreich J, Arbues A, Simeone R, Haanappel E, Wegener A, Sayes F, Le Chevalier F, Chalut C, Malaga W, Guilhot C, Brosch R, Astarie-Dequeker C. 2017. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol 19:e12726 10.1111/cmi.12726. [PubMed] [DOI] [PubMed] [Google Scholar]
- 55.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. 2007. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol 178:3143–3152 10.4049/jimmunol.178.5.3143. [PubMed] [DOI] [PubMed] [Google Scholar]
- 56.Hagedorn M, Rohde KH, Russell DG, Soldati T. 2009. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 323:1729–1733 10.1126/science.1169381. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stamm LM, Brown EJ. 2004. Mycobacterium marinum: the generalization and specialization of a pathogenic mycobacterium. Microbes Infect 6:1418–1428 10.1016/j.micinf.2004.10.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Orme IM. 2014. A new unifying theory of the pathogenesis of tuberculosis. Tuberculosis (Edinb) 94:8–14 10.1016/j.tube.2013.07.004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR. 2003. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol 170:430–437 10.4049/jimmunol.170.1.430. [PubMed] [DOI] [PubMed] [Google Scholar]
- 60.Dallenga T, Repnik U, Corleis B, Eich J, Reimer R, Griffiths GW, Schaible UE. 2017. M. tuberculosis-induced necrosis of infected neutrophils promotes bacterial growth following phagocytosis by nacrophages. Cell Host Microbe 22:519–530.e3. [PubMed] [DOI] [PubMed] [Google Scholar]
- 61.Bermudez LE, Sangari FJ, Kolonoski P, Petrofsky M, Goodman J. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect Immun 70:140–146 10.1128/IAI.70.1.140-146.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sasindran SJ, Torrelles JB. 2011. Mycobacterium tuberculosis infection and inflammation: what is beneficial for the host and for the bacterium? Front Microbiol 2:2 10.3389/fmicb.2011.00002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dobos KM, Spotts EA, Quinn FD, King CH. 2000. Necrosis of lung epithelial cells during infection with Mycobacterium tuberculosis is preceded by cell permeation. Infect Immun 68:6300–6310 10.1128/IAI.68.11.6300-6310.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scordo JM, Knoell DL, Torrelles JB. 2016. Alveolar epithelial cells in Mycobacterium tuberculosis infection: active players or innocent bystanders? J Innate Immun 8:3–14 10.1159/000439275. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krishnan N, Robertson BD, Thwaites G. 2010. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis (Edinb) 90:361–366 10.1016/j.tube.2010.08.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.Cohen SB, Gern BH, Delahave JL, Adams KN, Plumlee CR, Winkler JK, Sherman DR, Gerner MY, Urdahl KB. 2018. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 24:439–446.e4. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature 385:640–644 10.1038/385640a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Hingley-Wilson SM, Connell D, Pollock K, Hsu T, Tchilian E, Sykes A, Grass L, Potiphar L, Bremang S, Kon OM, Jacobs WR Jr, Lalvani A. 2014. ESX1-dependent fractalkine mediates chemotaxis and Mycobacterium tuberculosis infection in humans. Tuberculosis (Edinb) 94:262–270 10.1016/j.tube.2014.01.004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. 2010. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327:466–469 10.1126/science.1179663. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brand S, Hofbauer K, Dambacher J, Schnitzler F, Staudinger T, Pfennig S, Seiderer J, Tillack C, Konrad A, Göke B, Ochsenkühn T, Lohse P. 2006. Increased expression of the chemokine fractalkine in Crohn’s disease and association of the fractalkine receptor T280M polymorphism with a fibrostenosing disease phenotype. Am J Gastroenterol 101:99–106 10.1111/j.1572-0241.2005.00361.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, Tripp RA. 2010. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol 84:1148–1157 10.1128/JVI.01755-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. 1982. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 125:740–745. [DOI] [PubMed] [Google Scholar]
- 73.Unnikrishnan M, Constantinidou C, Palmer T, Pallen MJ. 2017. The enigmatic Esx proteins: looking beyond mycobacteria. Trends Microbiol 25:192–204 10.1016/j.tim.2016.11.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 74.Lopez MS, Tan IS, Yan D, Kang J, McCreary M, Modrusan Z, Austin CD, Xu M, Brown EJ. 2017. Host-derived fatty acids activate type VII secretion in Staphylococcus aureus. Proc Natl Acad Sci USA 114:11223–11228 10.1073/pnas.1700627114. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kneuper H, Cao ZP, Twomey KB, Zoltner M, Jäger F, Cargill JS, Chalmers J, van der Kooi-Pol MM, van Dijl JM, Ryan RP, Hunter WN, Palmer T. 2014. Heterogeneity in ess transcriptional organization and variable contribution of the Ess/Type VII protein secretion system to virulence across closely related Staphylocccus aureus strains. Mol Microbiol 93:928–943 10.1111/mmi.12707. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burts ML, Williams WA, DeBord K, Missiakas DM. 2005. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci USA 102:1169–1174 10.1073/pnas.0405620102. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Hu M, Liu Q, Qin J, Dai Y, He L, Li T, Zheng B, Zhou F, Yu K, Fang J, Liu X, Otto M, Li M. 2016. Role of the ESAT-6 secretion system in virulence of the emerging community-associated Staphylococcus aureus lineage ST398. Sci Rep 6:25163 10.1038/srep25163. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinheiro J, Reis O, Vieira A, Moura IM, Zanolli Moreno L, Carvalho F, Pucciarelli MG, García-Del Portillo F, Sousa S, Cabanes D. 2017. Listeria monocytogenes encodes a functional ESX-1 secretion system whose expression is detrimental to in vivo infection. Virulence 8:993–1004 10.1080/21505594.2016.1244589. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Millington KA, Fortune SM, Low J, Garces A, Hingley-Wilson SM, Wickremasinghe M, Kon OM, Lalvani A. 2011. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA 108:5730–5735 10.1073/pnas.1015153108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donovan ML, Schultz TE, Duke TJ, Blumenthal A. 2017. Type I interferons in the pathogenesis of tuberculosis: molecular drivers and immunological consequences. Front Immunol 8:1633 10.3389/fimmu.2017.01633. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barczak AK, Avraham R, Singh S, Luo SS, Zhang WR, Bray MA, Hinman AE, Thompson M, Nietupski RM, Golas A, Montgomery P, Fitzgerald M, Smith RS, White DW, Tischler AD, Carpenter AE, Hung DT. 2017. Systematic, multiparametric analysis of Mycobacterium tuberculosis intracellular infection offers insight into coordinated virulence. PLoS Pathog 13:e1006363 10.1371/journal.ppat.1006363. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE III, Freedman VH, Kaplan G. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci USA 98:5752–5757 10.1073/pnas.091096998. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Antonelli LR, Gigliotti Rothfuchs A, Gonçalves R, Roffê E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. 2010. Intranasal poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest 120:1674–1682 10.1172/JCI40817. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, Derrick SC, Shi R, Kumar NP, Wei W, Yuan X, Zhang G, Cai Y, Babu S, Catalfamo M, Salazar AM, Via LE, Barry CE III, Sher A. 2014. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511:99–103 10.1038/nature13489. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O’Garra A. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466:973–977 10.1038/nature09247. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singhania A, Verma R, Graham CM, Lee J, Tran T, Richardson M, Lecine P, Leissner P, Berry MPR, Wilkinson RJ, Kaiser K, Rodrigue M, Woltmann G, Haldar P, O’Garra A. 2018. A modular transcriptional signature identifies phenotypic heterogeneity of human tuberculosis infection. Nat Commun 9:2308 10.1038/s41467-018-04579-w. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pourakbari B, Mamishi S, Marjani M, Rasulinejad M, Mariotti S, Mahmoudi S. 2015. Novel T-cell assays for the discrimination of active and latent tuberculosis infection: the diagnostic value of PPE family. Mol Diagn Ther 19:309–316 10.1007/s40291-015-0157-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 88.Abdallah AM, Bestebroer J, Savage ND, de Punder K, van Zon M, Wilson L, Korbee CJ, van der Sar AM, Ottenhoff TH, van der Wel NN, Bitter W, Peters PJ. 2011. Mycobacterial secretion systems ESX-1 and ESX-5 play distinct roles in host cell death and inflammasome activation. J Immunol 187:4744–4753 10.4049/jimmunol.1101457. [PubMed] [DOI] [PubMed] [Google Scholar]
- 89.Voss G, Casimiro D, Neyrolles O, Williams A, Kaufmann SHE, McShane H, Hatherill M, Fletcher HA. 2018. Progress and challenges in TB vaccine development. F1000 Res 7:199 10.12688/f1000research.13588.1. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marinova D, Gonzalo-Asensio J, Aguilo N, Martin C. 2017. MTBVAC from discovery to clinical trials in tuberculosis-endemic countries. Expert Rev Vaccines 16:565–576 10.1080/14760584.2017.1324303. [PubMed] [DOI] [PubMed] [Google Scholar]
- 91.Grode L, Ganoza CA, Brohm C, Weiner J III, Eisele B, Kaufmann SH. 2013. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 31:1340–1348 10.1016/j.vaccine.2012.12.053. [PubMed] [DOI] [PubMed] [Google Scholar]
- 92.Penn-Nicholson A, Tameris M, Smit E, Day TA, Musvosvi M, Jayashankar L, Vergara J, Mabwe S, Bilek N, Geldenhuys H, Luabeya AK, Ellis R, Ginsberg AM, Hanekom WA, Reed SG, Coler RN, Scriba TJ, Hatherill M, TBVPX-114 study team. 2018. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir Med 6:287–298 10.1016/S2213-2600(18)30077-8. [DOI] [PubMed] [Google Scholar]
- 93.Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, Mabwe S, Makhethe L, Erasmus M, Toefy A, Mulenga H, Hanekom WA, Self SG, Bekker LG, Ryall R, Gurunathan S, DiazGranados CA, Andersen P, Kromann I, Evans T, Ellis RD, Landry B, Hokey DA, Hopkins R, Ginsberg AM, Scriba TJ, Hatherill M, C-040-404 Study Team. 2018. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med 379:138–149 10.1056/NEJMoa1714021. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suliman S, Luabeya AKK, Geldenhuys H, Tameris M, Hoff ST, Shi Z, Tait D, Kromann I, Ruhwald M, Rutkowski KT, Shepherd B, Hokey D, Ginsberg AM, Hanekom WA, Andersen P, Scriba TJ, Hatherill M, Oelofse RE, Stone L, Swarts AM, Onrust R, Jacobs G, Coetzee L, Khomba G, Diamond B, Companie A, Veldsman A, Mulenga H, Cloete Y, Steyn M, Africa H, Nkantsu L, Smit E, Botes J, Bilek N, Mabwe S, H56-035 Trial Group. 2019. Dose optimization of H56:IC31 vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial. Am J Respir Crit Care Med 199:220–231 10.1164/rccm.201802-0366OC. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Penn-Nicholson A, Geldenhuys H, Burny W, van der Most R, Day CL, Jongert E, Moris P, Hatherill M, Ofori-Anyinam O, Hanekom W, Bollaerts A, Demoitie MA, Kany Luabeya AK, De Ruymaeker E, Tameris M, Lapierre D, Scriba TJ, Vaccine Study Team. 2015. Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting.Vaccine 33:4025–4034 10.1016/j.vaccine.2015.05.088. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonzalo-Asensio J, Marinova D, Martin C, Aguilo N. 2017. MTBVAC: attenuating the human pathogen of tuberculosis (TB) toward a promising vaccine against the TB epidemic. Front Immunol 8:1803 10.3389/fimmu.2017.01803. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loxton AG, Knaul JK, Grode L, Gutschmidt A, Meller C, Eisele B, Johnstone H, van der Spuy G, Maertzdorf J, Kaufmann SHE, Hesseling AC, Walzl G, Cotton MF. 2017. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin Vaccine Immunol 24:e00439-16 10.1128/CVI.00439-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN, Reed SG. 2008. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol 181:7948–7957 10.4049/jimmunol.181.11.7948. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shah S, Briken V. 2016. Modular organization of the ESX-5 secretion system in Mycobacterium tuberculosis. Front Cell Infect Microbiol 6:49 10.3389/fcimb.2016.00049. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Copin R, Coscollá M, Efstathiadis E, Gagneux S, Ernst JD. 2014. Impact of in vitro evolution on antigenic diversity of Mycobacterium bovis bacillus Calmette-Guerin (BCG). Vaccine 32:5998–6004 10.1016/j.vaccine.2014.07.113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, Simon S, Harris B, Atkin R, Doggett J, Mayes R, Keating L, Wheeler PR, Parkhill J, Barrell BG, Cole ST, Gordon SV, Hewinson RG. 2003. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci USA 100:7877–7882 10.1073/pnas.1130426100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, Rolf T, Lu L, Alter G, Hokey D, Jayashankar L, Walker R, Snowden MA, Evans T, Ginsberg A, Reed SG, TBVPX-113 Study Team. 2018. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. NPJ Vaccines 3:34 10.1038/s41541-018-0057-5. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, Ayles HM, Henostroza G, Thienemann F, Scriba TJ, Diacon A, Blatner GL, Demoitié MA, Tameris M, Malahleha M, Innes JC, Hellström E, Martinson N, Singh T, Akite EJ, Khatoon Azam A, Bollaerts A, Ginsberg AM, Evans TG, Gillard P, Tait DR. 2018. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 379:1621–1634 10.1056/NEJMoa1803484. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parasa VR, Rahman MJ, Ngyuen Hoang AT, Svensson M, Brighenti S, Lerm M. 2014. Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue. Dis Model Mech 7:281–288 10.1242/dmm.013854. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hingley-Wilson SM, Casey R, Connell D, Bremang S, Evans JT, Hawkey PM, Smith GE, Jepson A, Philip S, Kon OM, Lalvani A. 2013. Undetected multidrug-resistant tuberculosis amplified by first-line therapy in mixed infection. Emerg Infect Dis 19:1138–1141 10.3201/eid1907.130313. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McIvor A, Koornhof H, Kana BD. 2017. Relapse, re-infection and mixed infections in tuberculosis disease. Pathog Dis 75:ftx020 10.1093/femspd/ftx020. [PubMed] [DOI] [PubMed] [Google Scholar]
- 107.Yeboah-Manu D, Asare P, Asante-Poku A, Otchere ID, Osei-Wusu S, Danso E, Forson A, Koram KA, Gagneux S. 2016. Spatio-temporal distribution of Mycobacterium tuberculosis complex strains in Ghana. PLoS One 11:e0161892 10.1371/journal.pone.0161892. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bitter W, Houben EN, Bottai D, Brodin P, Brown EJ, Cox JS, Derbyshire K, Fortune SM, Gao LY, Liu J, Gey van Pittius NC, Pym AS, Rubin EJ, Sherman DR, Cole ST, Brosch R. 2009. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog 5:e1000507 10.1371/journal.ppat.1000507. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Houben EN, Bestebroer J, Ummels R, Wilson L, Piersma SR, Jiménez CR, Ottenhoff TH, Luirink J, Bitter W. 2012. Composition of the type VII secretion system membrane complex. Mol Microbiol 86:472–484 10.1111/j.1365-2958.2012.08206.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 110.Rosenberg OS, Dovala D, Li X, Connolly L, Bendebury A, Finer-Moore J, Holton J, Cheng Y, Stroud RM, Cox JS. 2015. Substrates control multimerization and activation of the multi-domain ATPase motor of type VII secretion. Cell 161:501–512 10.1016/j.cell.2015.03.040. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Das C, Ghosh TS, Mande SS. 2011. Computational analysis of the ESX-1 region of Mycobacterium tuberculosis: insights into the mechanism of type VII secretion system. PLoS One 6:e27980 10.1371/journal.pone.0027980. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daleke MH, van der Woude AD, Parret AH, Ummels R, de Groot AM, Watson D, Piersma SR, Jiménez CR, Luirink J, Bitter W, Houben EN. 2012. Specific chaperones for the type VII protein secretion pathway. J Biol Chem 287:31939–31947 10.1074/jbc.M112.397596. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ekiert DC, Cox JS. 2014. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci USA 111:14758–14763 10.1073/pnas.1409345111. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Korotkova N, Freire D, Phan TH, Ummels R, Creekmore CC, Evans TJ, Wilmanns M, Bitter W, Parret AH, Houben EN, Korotkov KV. 2014. Structure of the Mycobacterium tuberculosis type VII secretion system chaperone EspG5 in complex with PE25-PPE41 dimer. Mol Microbiol 94:367–382 10.1111/mmi.12770. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Korotkova N, Piton J, Wagner JM, Boy-Röttger S, Japaridze A, Evans TJ, Cole ST, Pojer F, Korotkov KV. 2015. Structure of EspB, a secreted substrate of the ESX-1 secretion system of Mycobacterium tuberculosis. J Struct Biol 191:236–244 10.1016/j.jsb.2015.06.003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]


