Abstract
To determine the accuracy and precision of the Icare® TONOVET Plus rebound tonometer for measuring intraocular pressure (IOP) in normal rabbit eyes, as well as compare it to three other commercially available tonometers: the Icare® TONOVET (TV01), Tono-Pen Vet™, and Tono-Pen AVIA Vet™. The anterior chambers of both eyes of three New Zealand White rabbits were cannulated, post-mortem. IOP was measured using each of the above four tonometers at manometric pressures ranging between 5 mmHg and 70 mmHg. Data were analyzed by linear regression, ANOVA, and Bland-Altman plots. A p-value of ≤ 0.05 was considered significant for all statistical tests. IOP values obtained with the TONOVET Plus (in ‘lapine’ mode) were significantly closer to manometric IOP than those obtained with the other tonometers tested. The TV01 (in ‘d’ dog setting) and Tono-Pen AVIA Vet™ were significantly more accurate compared to the Tono-Pen Vet™. All tonometers had high levels of precision, though the TONOVET Plus and TV01 were significantly more precise compared to the Tono-Pen AVIA Vet™. All tonometers tended to underestimate IOP, particularly at high pressures, however the TONOVET Plus was highly correlated with manometric IOP in the clinically relevant range of 5 – 50 mmHg. The TONOVET Plus is an appropriate choice of instrument for measuring IOP in rabbit eyes in both research and clinical settings.
Keywords: Intraocular pressure, rabbit, glaucoma, rebound tonometer
Intraocular pressure (IOP) is considered one of the biggest risk factors for the development of glaucoma and is the most consistent predictor of glaucomatous damage in both animals and humans, thus current treatments for this disease primarily focus on IOP reduction (Wilensky, 1994). Because rabbits are widely used in pharmacological testing and as animal models for the study of glaucoma and ocular diseases (Zhang et al., 2014), and are widely kept as pets, an accurate, reproducible method for measuring IOP in rabbits is vitally important.
Commercially available, hand-held tonometers are frequently used in both veterinary and human medicine, and in research. The two most widely used tonometers in the field of veterinary ophthalmology are the ICare® TONOVET (TV01; Icare Finland Oy, Helsinki, Finland) and the Tono-Pen Vet™ (Reichert, Depew, New York, USA), which operate on the principles of rebound tonometry and applanation tonometry, respectively. Both are lightweight and portable, however the ICare® TV01 is particularly useful, as it does not require topical anesthesia, making it an ideal choice for veterinary clinicians and those using animal models in research. The ICare® TV01 has been validated in rabbits (Kalesnykas and Uusitalo, 2007; Zhang et al., 2014) and in a host of other species, including cats (Rusanen et al., 2010, McLellan et al., 2013), dogs (Gorig et al.,2006; Leiva et al., 2006; Nagata et al., 2011; von Spiessen et al., 2015), and macaques (Elsmo et al., 2011). The TV01 has specific calibrations for dogs/cats (‘d’ mode) and horses (‘h’ mode), but does not have a specific setting for rabbits, therefore previous validation studies in rabbits were done with the TV01 set to the ‘d’ or ‘p’ (un-calibrated) mode (Kalesnykas and Uusitalo, 2007; Zhang et al., 2014). However, the TV01 tends to underestimate IOP in rabbits and a conversion formula is necessary in order to calculate the true IOP (Zhang et al., 2014; Ma et al., 2016). Furthermore, it has been reported that inaccurate readings can result if the TV01 is not used at an appropriate distance (4–8 mm) from the cornea or if the probe is not horizontal and oriented perpendicular to the corneal surface. For example, von Spiessen et al. (2013) found that IOP readings taken with the TV01 were higher when measurements were taken with the device too close to the eye.
Recently, an updated version of the TV01, the Icare® TONOVET Plus (Icare Finland Oy, Helsinki, Finland), has become commercially available. The TONOVET Plus contains upgrades meant to improve accuracy and ease of use. The TONOVET Plus has species-specific settings for both rabbits (‘lapine’) and cats (‘feline’), in addition to dogs and horses. Furthermore, features have been added that ensure readings are only obtained when the tonometer is held an appropriate distance and in the correct plane relative to the ocular surface. Messages indicating “too near” or “too far” appear on the screen when the device is not positioned at the correct distance and a ring surrounding the probe will change from green to red when it is not in the correct plane. In both cases, measurements cannot be taken until the distance or orientation issue has been corrected. The current study was conducted to validate IOP values obtained with the new TONOVET Plus rebound tonometer in normal rabbit eyes and compare its performance with other commonly used hand-held tonometers. To our knowledge, there have been no prior published studies assessing the validity of IOP measurements obtained in normal rabbit eyes using the TONOVET Plus.
Six eyes from three female New Zealand White rabbits, aged 3–5 months, were studied. The eyes were tested in situ within 5 hours post-mortem following humane euthanasia for reasons unrelated to the current study. Carcasses were kept refrigerated between euthanasia and the experimentation.
IOP readings obtained with the TONOVET Plus, TV01, Tono-Pen Vet™ and Tono-Pen AVIA Vet™ (Reichert, Depew, New York, USA) were compared to manometrically-determined IOP values. The anterior chamber of each eye was cannulated with two 25-gauge needles. One needle was placed on the temporal side (~2 o’clock) and the other on the nasal side (~9 o’clock). One needle was connected to a pressure transducer (DTXPlus, Argon Medical Devices, Singapore) and to a continuous physiologic recorder (Dash 4000 Pro, GE Healthcare, Chicago, IL, USA) and the transducer line was filled with Lactated Ringer’s solution. The pressure transducer was calibrated to zero using a mercury manometer connected via polyethylene tubing. The second needle was connected, via infusion set, to a 1 L bag of Lactated Ringer’s solution, which was used to control the pressure in the anterior chamber. A small drop of cyanoacrylate adhesive was applied to needle entry points to prevent fluid leakage. The needles did not affect corneal curvature and no leakage was observed during the procedure. Balanced salt solution or lactated ringers solution was applied to the corneal surface by the observer prior to taking measurements with each tonometer.
IOP measurements were taken at manometric pressures ranging from 5 to 70 mmHg to evaluate tonometer accuracy over a wide IOP range. The pressure was initially set to 5 mmHg and increased in increments of 5 mmHg up to 40 mmHg and then in increments of 10 mmHg up to 70 mmHg. IOP was adjusted by raising or lowering the height of the fluid bag. Three consecutive readings were obtained in each eye using the TONOVET Plus, TV01, Tono-Pen AVIA Vet™ and the Tono-Pen Vet™. Only readings within 5% standard deviation were recorded, as indicated by a low or absent error bar on the TV01 display, a green ring on the TONOVET Plus display, a bar under the <5% standard deviation indicator on the Tono-Pen Vet™ display, or a >95% confidence interval indication on the Tono-Pen AVIA Vet™ display. All measurements were taken by the same observer to avoid any discrepancies caused by different device handling techniques.
The TONOVET Plus was set to ‘lapine’ (rabbit) mode and the TV01 was set to ‘d’ (dogs and cats) mode. The ‘d’ mode was chosen based on results of a previous study showing less deviation from manometric IOP when the TV01 was in the ‘d’ vs. ‘p’ mode when measuring IOP in rabbits (Zhang et al., 2014). Furthermore, according to the TONOVET Users and Maintenance manual, the ‘p’ mode is uncalibrated/undefined and is not intended for use to obtain IOPs (https://tonovet.com/products/icare-vet/vet-brochure). The Tono-Pen Vet ™ and Tono-Pen AVIA Vet™ do not have species-specific settings.
Microsoft Excel™ and Graphpad Prism v7 (GraphPad Software, San Diego, CA, USA) were used for data analysis. Linear regression analyses and ordinary one-way ANOVAs were performed comparing slope and R2 values between devices. ANOVAs were followed by Tukey-Kramer multiple comparisons tests. Bland-Altman plots were used to compare the relationship between tonometer-derived IOP readings and manometric IOP. P ≤ 0.05 was considered statistically significant.
All tonometers showed linear trends when plotted against manometric IOP. The TONOVET Plus was significantly more accurate compared to the other tonometers tested. The linear regression equations for the Icare® TONOVET Plus, TONOVET, Tono-Pen Vet™, and Tono-Pen AVIA Vet™ were y=0.8981x+1.8445, 0.7618x−1.5714, 0.671x+0.6816, and 0.799x−1.5339, respectively (Fig. 1). The TV01 and Tono-Pen AVIA Vet™ were both significantly more accurate compared to the Tono-Pen Vet™, however there was no significant difference in accuracy between the TV01 and Tono-Pen AVIA Vet™.
Figure 1.
IOP measured with the Icare® TONOVET Plus, TONOVET®, Tono-Pen Vet™, and Tono-Pen AVIA™ all showed strong linear correlations with manometric IOP. (A) IOP values taken with the Icare® Tonovet Plus were significantly more accurate than those taken with the (B) TONOVET (p<0.001), (C) Tonopen-Vet (p<0.001) and (D) Tonopen AVIA-Vet (p<0.02). Linear regression lines are shown as dashed and y=x lines shown as solid for reference. Some data points are super-imposed.
The TONOVET Plus, TV01, Tono-Pen Vet™, and Tono-Pen AVIA™ all had high levels of precision, or repeatability (R2=0.985, 0.981, 0.921, and 0.916, respectively). No significant differences in precision were found when comparing the TONOVET Plus, TONOVET, and Tono-Pen Vet™ to each other; however, the TONOVET Plus and TV01 were both significantly more precise than the Tono-Pen AVIA Vet™ (p<0.001). Precision did not differ significantly between the Tono-Pen Vet™ and Tono-Pen AVIA Vet™.
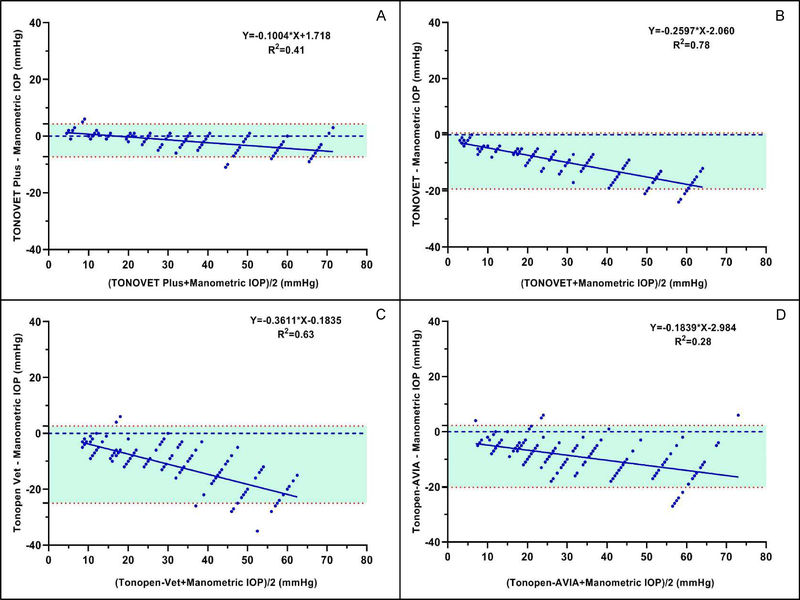
All tonometers tended to underestimate IOP, particularly as manometric pressure increased. Bland-Altman plot regression equations for the TONOVET Plus, TV01, Tono-Pen Vet™, and Tono-Pen AVIA Vet™ were y=−0.1004x+1.718, y=−0.2621x−2.0163, y=−0.3767x+0.441, and y=−0.2231x−1.2847, respectively (Fig. 2a–d). The TONOVET Plus most closely agreed with manometric IOP and did not diverge much from manometric IOP until around 50 mmHg, whereas the TONOVET®, Tono-Pen Vet™ and Tono-Pen AVIA Vet™ all began underestimating IOP at pressures within the low to physiological IOP range (5 – 15 mmHg).
Figure 2.
All tonometers tended to underestimate IOP, particularly as manometric pressure increased. (A) Measurements taken with the Icare® TONOVET Plus, most closely agreed with manometric IOP, especially in the clinically relevant range of 5 – 50 mmHg, whereas the (B) TONOVET, (C) Tono-Pen Vet™, and (D) Tono-Pen AVIA™ all began underestimating IOP between 5 and 15 mmHg. Upper and lower red dotted lines indicate 95% Limits of Agreement. Some data points are super-imposed.
This study demonstrated that the Icare® TONOVET Plus (in ‘lapine’ mode), obtained significantly more accurate IOP readings in rabbit eyes, compared to IOP readings obtained with the TV01, Tono-Pen Vet™ and Tono-Pen AVIA Vet™. Although the Icare® TONOVET Plus still tended to underestimate true IOP, particularly at high pressures, readings were very close to true IOP in the clinically relevant range of 5 – 50 mmHg. One major benefit of both the TV01 and TONOVET Plus is the ease of use and ability to acquire readings without application of topical anesthetic. In the case of the TONOVET Plus, obtaining accurate and reproducible IOP measurements is facilitated by several features that provide feedback to the user regarding distance and position of the tonometer probe relative to the ocular surface. In addition, each IOP measurement can be acquired more quickly as the TONOVET Plus allows for six measurements (which are then average by the instrument software) to be taken with a single press of the button and does not require repeatedly pressing the acquisition button to obtain each measurement.
The TONOVET Plus, TV01, Tono-Pen Vet™, and Tono-Pen AVIA Vet™ all had high levels of precision, or repeatability. Although the TONOVET Plus and TV01 were both significantly more precise than the Tono-Pen AVIA Vet™, precision was still adequate with the Tono-Pen AVIA Vet™ (R2 =0.916). The IOP data obtained from all of the tonometers tested were linearly correlated with manometric IOP, thus, accurate estimates of actual IOP can be derived from the linear regressions and equations reported in previous studies (McLellan, 2013; Zhang, 2014).
Both applanation tonometry and rebound tonometry do have their limitations and it is important for researchers and veterinary clinicians to be aware of these to account for discrepancies in the data obtained with different devices. Previous studies have indicated that corneal thickness, along with other corneal biomechanical properties, such as hysteresis, can influence the accuracy of TONOVET readings (Iliev et al, 2006; Martinez-de-la-Casa et al, 2006; Kalesnykas and Uusitalo, 2007; Chui et al, 2008; Jorge et al, 2008; Poostchi et al, 2009). In addition, corneal edema, scarring, or pigmentation can affect readings taken with both applanation and rebound tonometers (von Spiessen et. al., 2015). In humans, as corneal thickness and corneal rigidity increase, IOP readings taken with both the TONOVET and TonoPen tend to overestimate IOP (Chihara, 2008).
There were some limitations to the current study, not least that tonometers were evaluated in rabbit eyes post-mortem and were not compared in rabbits in vivo. Previous studies have shown that biomechanical properties, such as corneal thickness and hysteresis can significantly affect the accuracy of TONOVET readings (Chui et al., 2008; Iliev et al., 2006; Rusanen et al., 2010; Zhang et al., 2014). Furthermore, post-mortem changes in central corneal thickness (CCT) and biomechanical properties may have impacted tonometry values obtained in this ex vivo study. Using optical coherence tomography to measure CCT in sheep eyes ex vivo, significant changes were identified that were dependent on post-mortem time over a range of ambient room temperature and humidity (Napoli et al., 2016). In contrast, in a previous study done in our lab, no profound changes in CCT or corneal edema were observed over time or with increasing manometric pressure in the eyes of rabbits that had been euthanized ~16 hours prior to testing, but maintained at 4°C in a humid environment (McLellan, unpublished data). In addition, the mean CCT values in those eyes all fell within the previously published normal range for this species, as measured by ultrasonic pachymetry (Li et al, 1997; McLellan, unpublished data). In the current study, the eyes were tested within 5 hours of euthanasia, were maintained at 4°C until testing and the testing procedure only took ~45 minutes. No corneal edema or cataract was observed prior to, during, or after the procedure in any of the eyes tested. Nevertheless, the authors acknowledge that accuracy and precision could be different when using this device in live animals and in animals with corneal defects and/or disease. Further studies evaluating the TONOVET Plus in rabbits in vivo and in rabbits with ocular abnormalities are warranted. In addition, breed or age-related differences in ocular structure could also impact estimation of IOP by rebound tonometry (Zhang et al., 2017). Thus, evaluation of rebound tonometry in rabbits of different ages and breeds should be considered.
In conclusion, the Icare® TONOVET Plus rebound tonometer provides IOP readings in normal rabbit eyes which are highly precise, and are significantly more accurate than readings obtained with the TONOVET, Tono-Pen Vet™, and Tono-Pen AVIA™. Based on the above findings, the Icare® TONOVET Plus appears to be the most appropriate choice currently available for measuring IOP in rabbits in a clinical or research setting.
Acknowledgements
The authors would like to thank ICare Finland Oy for providing the TONVET Plus tonometer used in this study.
This work was supported in part by the National Institutes of Health [National Eye Institute grant number P30 EY16665] and unrestricted funds to the Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chui WS, Lam A, Chen D, Chiu R. The influence of corneal properties on rebound tonometry. Ophthalmol. 2008: 115; 80–84. DOI: 10.1016/j.ophtha.2007.03.Q61 [DOI] [PubMed] [Google Scholar]
- 2.Elsmo EJ, Kiland JA, Kaufman PL, McLellan GJ. Evaluation of rebound tonometry in non-human primates. Exp Eye Res 2011; 92: 268–273. DOI: 10.1016/j.exer.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliev MA, Goldblum D, Katsoulis K, Amstutz C, Frueh B. Comparison of rebound tonometry with Goldmann applanation tonometry and correlation with central corneal thickness. Br J Ophthalmol. 2006; 90: 833–835. DOI: 10.1136/bjo.2005.089870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Görig C, Coenen RTI, Stades FC, Djajadiningrat-Laanen SC, Boeve MH. Comparison of the use of new handheld tonometers and established applanation tonometers in dogs. AJVR 2006; 67: 134–144. DOI: 10.2460/ajvr.67.1.134 [DOI] [PubMed] [Google Scholar]
- 5.Kalesnykas G, Uusitalo H. Comparison of simultaneous readings of intraocular pressure in rabbits using Perkins handheld, Tono-Pen XL, and TonoVet tonometers. Graefe’s Arch Clin Exp Ophthalmol 2007; 245: 761–762. DOI: 10.1007/s00417-006-0470-8 [DOI] [PubMed] [Google Scholar]
- 6.Leiva M, Naranjo C, Pena MT. Comparison of the rebound tonometer (ICare®) to the applanation tonometer (Tonopen XL®) in normotensive dogs. Vet Ophthalmol 2006; 9: 17–21. DOI: 10.1111/j.1463-5224.2005.00429.x [DOI] [PubMed] [Google Scholar]
- 7.Ma D, Chen CB, Liang J, Lu Z, Chen H, Zhang M. Repeatability, reproducibility and agreement of intraocular pressure measurement in rabbits by the TonoVet and Tono-Pen. SciRep. 2016; 6: 35187 DOI: 10.1038/srep35187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorge JM, González-Méijome JM, Queirós A, Fernandes P, Parafita MA. Correlations between corneal biomechanical properties measured with the ocular response analyzer and ICare rebound tonometry. J Glaucoma. 2011; 20: 463 DOI: 10.1097/IJG.0b013e31815f52b8 [DOI] [PubMed] [Google Scholar]
- 9.Martinez-de-la-Casa JM, Garcia-Feijoo JG, Vico E, Fernandez-Vidal A, Benitez del Castillo JM, Wasfi M, Garcia-Sanchez J. Effect of corneal thickness on dynamic contour, rebound and Goldmann tonometry. Ophthalmol. 2006; 12; 2156–2162. DOI.org/ 10.1016/j.ophtha.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 10.McLellan GJ, Kemmerling JP, Kiland JA. Validation of the TONOVET R Rebound Tonometer in Normal and Glaucomatous Cats. Vet Ophthalmol. 2013; 16(2): 111–118. DOI: 10.1111/j.1463-5224.2012.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata N, Yuki M, Hasegawa T. 2011. In Vitro and In Vivo comparison of applanation tonometry and rebound tonometry in dogs. J Vet Med Sci. 2011; 73(12): 1585–1589. [DOI] [PubMed] [Google Scholar]
- 12.Napoli PE, Nioi M, d’Aloja E, Fossarello M. Post-Mortem corneal thickness measurements with a portable optical coherence tomography system: a reliability study. Scientific Rep. 2016; 6: 30428; doi: 10.1038/srep30428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poostchi A, Mitchell R, Nicholas S, Purdie G, Wells A. The iCare rebound tonometer: comparisons with Goldmann tonometry, and influence of central corneal thickness. Clin Exp Ophthalmol. 2009; 37: 687–691. DOI: 10.1111/j.1442-9071.2009.02109.x [DOI] [PubMed] [Google Scholar]
- 14.Rusanen E, Florin M, Hassig M, and Spiess BM. Evaluation of a rebound tonometer (TONOVET®) in clinically normal cat eyes. Vet Ophthalmol. 2010; 13: 31–36. DOI: 10.1111/j.1463-5224.2009.00752.x [DOI] [PubMed] [Google Scholar]
- 15.von Spiessen L, Karck J, Rohn K, Meyer-Lindenberg A. Clinical evaluation of the TONOVET (R) rebound tonometer in dogs and cats considering potential errors in handling. Tieraerztliche Praxis Ausgabe Kleintiere Heimtiere 2013; 41(4): 213–220. [PubMed] [Google Scholar]
- 16.Wilensky JT. Epidemiology of open-angle glaucoma, In: Textbook of Ophthalmology Volume 7 (Glaucoma) (ed. Kaufman PL, Mittag TW). Mosby: St. Louis, 1994; 29–33. [Google Scholar]
- 17.Zhang H,, Yang D, Ross CM, Wigg JP, Pandav S, Crowston JG. Validation of rebound tonometry for intraocular pressure measurement in the rabbit. Exp Eye Res. 2014; 121: 86–93. DOI: 10.1016/j.exer.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Qin X, Cao X, Zhang D, Li L. Age-related variations of rabbit corneal geometrical and clinical biomechanical parameters. Biomed Res Int. Volume 2017; Article ID 3684971, 11 pages. DOI: 10.1155/2017/3684971. [DOI] [PMC free article] [PubMed] [Google Scholar]