Abstract
Thiourethane (TU) additives and difunctional, polymerizable crosslinking agents have been demonstrated to increase toughness in methacrylate-based materials. The aim of this study was to evaluate the potential reinforcement of acrylic denture bases by combining thiourethane additives and 1,6 hexanediol dimethacrylate (HDDMA) as an additional crosslinking agent. One commercial acrylic resin (Nature-Cryl MC; GC America) was tested by adding 0 (control) or 10 wt% TU, each of them combined with 0 (control), 10, 20 and 30 wt% HDDMA, for a total of 8 experimental groups. Materials were processed using microwave energy (500 W for 3 min) using microwave-safe molds and flasks. Flexural strength, modulus and toughness were obtained in 3-point bending (ISO 4049) using bars measuring 2×2×25 mm (n=6). Dynamic mechanical analysis was used to determine glass transition temperature (Tg), breadth of tan delta (as a measure of polymer heterogeneity) and crosslinking density in 1×3×15 mm bars (n=6) tested in tension, using a 3 °C/min heating rate (−30 to 180 °C). Viscosity samples were evaluated in a parallel plate reometer. Data were analyzed by two-way ANOVA and Tukey’s test (α=0.05). Results showed that on the samples not containing TU, HDDMA up to 20 wt% increased the flexural strength and thoughness (and up to 30 wt% HDDMA increased the modulus). The addition of TU did not affect those properties (except for the increase in elastic modulus), but the combination TU+ HDDMA led to decreased properties overall. The addition of HDDMA decreased the viscosity for all materials, and the presence of TU did not affect viscosity. The Tg increased linearly with the concentration of HDDMA, except in the groups containing TU – in general, the addition of TU reduced Tg. The crosslinking density increased with the addition of HDDMA for all materials, regardless of the presence of TU. The addition of TU significantly decreased crosslinking density. The breadth of tan delta was not affected by the addition of HDDMA, but significantly increased with the addition of TU. In conclusion, the chain-breaking effect of TU on polymerizing methacrylates was deleterious in the case of methyl methacrylate, since it forms a linear polymer. The addition of HDDMA up to 20 wt% and not combined with TU significantly improved the tested properties.
Keywords: Acrylic resin, Thiourethane oligomers, Crosslinking agent, flexural strength, dynamic mechanical analysis
Introduction
Acrylic resins based on poly (methyl methacrylate) – PMMA, have been used as denture base materials for many years. These materials are low cost and relatively simple to process, but have notorious drawbacks including polymerization shrinkage, high water sorption and solubility, are prone to bacterial and fungal colonization (Aguayo et al., 2017), and present low impact resistance (Praveen et al., 2014). In addition, stresses generated during polymerization and cooling are the main causes of the linear dimensional change and distortion, in addition to induction of defects, which compromise conventional denture’s longevity (Consani et al., 2002). The linear dimensional change of the acrylic resin is influenced by several factors during the denture base processing, such as inner stresses caused by different coefficients of thermal expansion of the materials (Becker et al., 1977; Savirmath and Mishra, 2016; Takamata et al., 1989), base thickness (Chen et al., 1988), locations of the model inside the flask (Wolfaardt et al., 1986), differences in composition between commercial brands of acrylic resins (Consani et al., 2002), and molecular weight of the polymer beads (Kawaguchi et al., 2011). The processing itself has been a matter of debate, with the relatively recent introduction of microwave energy processing for commerncial denture bases. Microwave energy has the advantage of faster processing times, but the effects on mechanical properties, porosity and longevity are highly dependent on polymerization conditions (Figuerôa et al., 2018; Ozkir et al., 2018). These factors may negatively combine and lead to poor adaptation and retention of the denture base and displacement of the artificial teeth, as well as cause weakening of the structure, ultimately leading to the need to replace the denture.
PMMA is a linear polymer, prone to solvent absorption and, in general, mechanical properties such as rigidity, hardness, flexural strength, and elastic modulus and impact resistance are also relatively low (Beyli and von Fraunhofer, 1981; Darbar et al., 1994). Therefore, to increase mechanical properties and the resistance to crazing, difunctional crosslinkers such as ethylene glycol dimethacrylate (EGDMA) are added to the formulation. Relatively high concentrations of that crosslinking agent are necessary, however, for any reinforcement effect to be observed (Harrison et al., 1978). In fact, some studies have failed to find a correlation between EGDMA concentrations and improvement on impact and tensile resistance (Harrison et al., 1978). Several other cross-linking agents with different chain lengths and backbone rigidity have been studied, with varied results in terms of flexural and tensile strength, and impact resistance (Caycik and Jagger, 1992; Price, 1986). In terms of glass transition temperature (Tg) measured by thermomechanical analysis, increasing EGDMA concentrations (0–40%) led to Tg increase in all concentrations. The use of TEGDMA (tetra ethylene glycol dimethacrylate) did not affect Tg up to 20% in concentrations, but significantly decreased the Tg at 30 and 40% (Jerolimov et al., 1994). Longer chains, such as PEG 600 DMA (poly ethylene glycol dimethacrylate) significantly reduced the Tg in all concentrations. Therefore, the interconnection of PMMA (poly methyl methacrylate) with crosslink agents significantly affects the Tg (Jerolimov et al., 1994). All of the crosslinking agents cited so far are relatively hydrophilic, and it is conceivable that this affects the denture base material interaction with water and saliva in the oral environment. Other additives, such as fibers (nylon, polyethylene, polyamide) and inorganic fillers (alumina, zirconia, silver, titanium, nanogold, carbon, silica and hybrid fillers) have been tested, with some evidence that silanized nanoparticles and hybrid systems can offer some reinforcement (Gad et al., 2017).
One alternative organic modifier that has shown positive results in terms of mechanical reinforcement and stress reduction in dental composites and cements are thiourethane-based oligomers (TU). They have been shown to delay gelation and vitrification of photocured crosslinked polymer networks by means of chain transfer events of pending thiol groups to the vinyls of the free-radical polymerizing systems. The presence of thiourethane bonds have been shown to increase toughness (Senyurt et al., 2007), while the delayed gelation and vitrification brought by the presence of thiol functionalities lead to greater degree of conversion (Berchtold et al., 2002), reduction of the volumetric contraction and polymerization stress, increase of the rigidity, flexural strength and fracture thoughness of photoactivated resin cements (Bacchi et al., 2015; Pfeifer et al., 2011), and restorative composites (Bacchi et al., 2016) based on methacrylates. Thiourethanes are multifunctional by nature, and may provide additional crosslinking in denture base materials. Because of the increase in toughness, the use of these materials to increase impact resistance can be envisioned for applications in Dentistry such as mouthguards and denture base materials. Those materials are usually processed via heat-curing, which intrinsically leads to slower polymerizatino rates compared to photocuring (Dean and Cook, 2002). Thiourethanes usually also decrease the rate of polymerization to some extent (Bacchi et al., 2015), so it is possible that the use of this additive with a heat-cured material will allow for additional stress relaxation opportunity by increasing the time scale component. The combination of the thiourethane with hydrophobic difunctional crosslinkers is envisioned to contribute to a reduction in water sorption and improvement of properties. Therefore, the aim of this study was to assess how the addition of thiourethane oligomers in combination with a hydrophobic crosslinking agent (1,6-hexanediol dimethacrylate - HDDMA) affects flexural strength, elastic modulus, toughness, viscosity, and dynamic mechanical properties of PMMA-based denture materials processed with the use of microwave energy. The hypotheses studied were: 1- The addition of thiourethane oligomers will improve the properties tested; 2- The addition of HDDMA as the crosslinker will improve the properties tested.
Materials and methods
1. Experimental acrylic resin:
The experimental material was developed based on a two-part commercial acrylic resin (Nature-Cryl MC; GC America, Also, IL, USA). The powder component consists of pre-polymerized polymethyl methacrylate spheres and a small amount of benzoyl peroxide (initiador). The liquid component consists of unpolymerized methyl methacrylate and small amounts of hydroquinone (inhibitor). A crosslinking agent (glycol dimethacrylate) also is added to the liquid. The control material was prepared according to the manufacturer’s instructions (powder: liquid ratio of 3:1 vol). Thiourethane oligomers were synthesized as previously described (Bacchi et al., 2018), based on the combination of trimethylol-tris-3-mercaptopropionate (TMP) and 1,3-bis(1-isocyanato-1-methylethyl) benzene (BDI) at a 2:1 molar ratio. In the groups containing TU (0 – control, or 10 wt%), the oligomer concentration was calculated in relation to the weight of the powder (in other words, part of the linear polymethyl methacrylate polymer was replaced by the crosslinked thiourethane oligomer). The overall thiourethane concentration (when considering the monomer + polymer mass) was 3 wt%. The thiourethane concentration was determined in a pilot study which evaluated 5, 10 and 20 wt% - 10 wt% presented the best compromise between handling characteristics and screened flexural properties. When used, the thiourethane was mixed with and dissolved in the liquid, which was then mixed with the polymethyl methacrylate beads (powder). Hexanediol dimethacrylate (HDDMA, Sigma Aldrich, St. Louis, MO, USA) was used as a crosslinking agent. This difunctional monomer was added to the liquid at 0 (control) 10, 20 or 30 % by weight, calculated as a percentage of the weight of the liquid (in other words, part of the mono-functional methyl methacrylate monomer was replaced by the difunctional HDDMA). The liquid was then mixed at 3:1 powder:liquid ratio with the control (not containg TU) or the TU-modified powder. The final experimental design resulted in a total of 8 groups to be tested.
2. Sample preparation:
The bar samples for flexural strength, elastic modulus and toughness (n=6) were prepared according to ISO 4049 (Standardization, 2009) in the dimensions of 25 mm × 2 mm × 2 mm. Since the intention of this study was to screen properties of materials, rather than to provide standardize values for comparison among labs, the 4049 standard was selected instead of ISO 20795, which requires the use of much larger and material-consuming specimens. For dynamic mechanical analysis (n=6) dimensions were 15 mm × 3 mm × 1 mm. For the microwave processing of the denture base material, the unpolymerized material was packed into silicone molds with dimensions appropriate for each test, then placed inside microwavable flasks, previously prepared to accommodate the specimen+mold assembly in set type II gypsum isolated with an alginate-based solution (Aislar, Kulzer LCC, South Bend, IN, USA). The flasks were trial packed at 1000 psi, then final closure was achieved with 2000 psi of pressure for 1 min using a hydraulic press (VH Press, EssenceDental, Araraquara, SP, Brazil) and then polymerized in conventional microwave oven (Sharp Carousel; Mahwah, NJ, USA) with 500 W for 3 mim. Samples were deflasked after flask cooling at room temperature and finished and polished. The initial polishing was carried out in dental bench lathe with pumice/water paste (Laboratory Pumice, Keystone, Gibson, NJ, USA), followed by paste of calcium carbonate/water paste (Asfer, Dental Parameter, Santos, SP, Brazil). The final polishing was done with aluminum oxide paste (Universal Polishing Paste; Kota Dental Products, Sao Paulo, SP, Brazil).
3. Flexural strength:
Flexural strength (MPa) was evaluated in 3-point bending, carried out in a universal test machine (Instron; Canton, MA, USA) at a cross-head speed of 0.5 mm/min until fracture. Flexural strength values were calculated according to the formula:
where: FS = flexural strength; F = load at fracture (N); L = distance between the supports of the sample (25 mm); b = sample width (2 mm); h = sample thickness (2 mm).
4. Flexural modulus:
Flexural modulus (GPa) was determined from the slope of the initial linear part of the stress-strain curve, calculated according to the formula:
where: E= Elastic modulus; F= load in some point of the linear region of the stress-strain curve (N); L= distance between the supports of the sample (25 mm); b= sample width (2 mm); h= sample thickness (2 mm); d= slack compensated deflection at load F.
5. Toughness:
Toughness (MPa) was calculated from the integration of the area under the stress-strain curve (Origin 9.1, OriginLab Corporation, Northampton, MA, USA).
6. Viscosity:
The viscosity (Pa.s) of the methyl methacrylate liquid component modified with HDDMA and/or thiourethane was evaluated in a parallel plate rheometer (DH-R1; TA Instruments, New Castle, DE, USA). Approximately 0.5 mL of each acrylic resin liquid was placed between 40-mm diameter plates using a gap of 300 μm, and evaluated in flow sweep mode (strain rate range: 0.1 to 1000 Hz, n=3).
7. Dynamic mechanical analysis:
Dynamic mechanical analysis of the samples was evaluated with DMA Q800 (TA Instruments, New Castle, DE, USA) using temperature scan from −25 to 180°C at 3°C/min, in tension mode. The peak value of tan delta curve was used to define the glass transition temperature (Tg in °C), and the homogeneity of the polymer network was defined by the width at half-height of the Tan delta curve. Crosslinking density was calculated based on the storage modulus at the rubbery plateau (Bacchi and Pfeifer, 2016), using the equation:
Where: ν is the concentration of active strands (or crosslinking density, in mol/kg); d is the density (kg/m3); R is the gas constant (8.314 J·K−1·mol−1) and T is the temperature (K).
8. Statistical analysis:
Data were analyzed with two-way ANOVA (HDDMA x TU concentration). Multiple comparisons were done using Tukey’s test. The level of significance was set at 95%.
Results
Table 1 shows the results for flexural strength, flexural modulus and toughness for all tested groups. As for flexural strength, the factors TU concentration (p=0.001), HDDMA concentration (p=0.001), and the interaction between them (p=0.003) were statistically significant. The highest value was shown for the group where the acrylic resin was reinforced by 10% HDDMA, statistically similar to 20% HDDMA. The addition of 30 % HDDMA led to values statistically lower than both. The addition of TU did not affect the FS of the neat acrylic resin, nor of the groups where HDDMA was added up to 20%. At 30% HDDMA, the addition of TU led to the smallest values found on this study.
Table 1 -.
Means (SD) of the flexural strength (FS, MPa), flexural modulus (FM, GPa) and toughness (MPa) for all groups tested. Different HDDMA concentrations were added to materials without (0 wt%) or with (10 wt%) thiourethane oligomer. Values followed by the same letters in each column are statistically similar (α=0.05).
| TU concentration (wt%) | HDDMA concentration (wt%) | Flexural strength (FS, MPa) | Flexural modulus (FM, GPa) | Toughness (MPa) |
|---|---|---|---|---|
| 0 | 0 | 29.63 (5.46)bc | 1.70 (0.47)ab | 1.38 (0.78)ab |
| 10 | 50.92 (15.21)a | 2.05 (0.32)a | 1.91 (0.69)a | |
| 20 | 40.18 (2.86)ab | 2.13 (0.18)a | 1.31 (0.30)ab | |
| 30 | 18.38 (4.71)d | 2.10 (0.18)a | 0.48 (0.26)de | |
| 10 | 0 | 31.48 (6.83)b | 2.08 (0.17)a | 0.99 (0.24)bc |
| 10 | 19.08 (3.78)d | 1.81 (0.20)ab | 0.55 (0.13)d | |
| 20 | 21.11 (3.05)cd | 1.63 (0.20)b | 0.77 (0.21)cd | |
| 30 | 13.25 (4.58)d | 1.75 (0.35)ab | 0.36 (0.16)e |
TU (Thio-urethane).
HDDMA (1.6-Hexanediol dimethacrylate).
As for flexural modulus, the factor TU concentration (p=0.033) and the interaction (p=0.003) were significant, but the factor HDDMA concentration (p=0.965) was not. The addition of HDDMA led to increased modulus for the materials not containing TU, though not statistically different. The addition of TU by itself did not affect the modulus, but the combination with HDDMA led to statistically reduced modulus results. As for toughness, the factors TU concentration (p=0.001), HDDMA concentration (p=0.001), and the interaction between them (p=0.005) were statistically significant. Similar to the trend observed for FS, the highest value of toughness was presented by the group where the acrylic resin was reinforced by 10% HDDMA, statistically similar do 20% HDDMA. The addition of 30% HDDMA led to values statistically lower than both. The addition of TU did not affect the toughness of the neat acrylic resin, but reduced the toughness for the groups where HDDMA was added at any concentration.
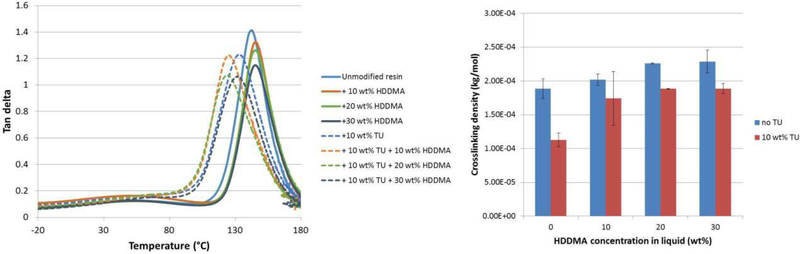
Table 2 shows the viscosity, glass transition temperature (Tg), breadth of tan delta (width at half height - WHH) and crosslinking values for all groups tested. In terms of viscosity, both factors and the interaction were statistically significant (p<0.05). The addition of TU did not affect the viscosity of the neat resin, whereas the addition of HDDMA led to reduced viscosity values at all concentrations, and regardless of the presence of TU. In terms of Tg, both factors were significant, but the interaction was not (p=0.138). The addition of the TU reduced the Tg of the neat resin, as well as the Tg of resins modified with 10 or 20% HDDMA. The addition of HDDMA did not affect the Tg of the material. In terms of WHH, both factors were significant (p<0.001), but the interaction was not (p>0.05). The breadth of tan delta increased for all groups where TU was added, and was not affected by the concentration of HDDMA. In terms of crosslinking density, both factors were significant (p<0.001), but the interaction was not (p>0.05). The crosslinking density increased with the concentration of HDDMA for materials with or without TU. The presence of TU led to lower values of crosslinking density at all HDDMA concentrations. Figure 1 shows representative tan delta curves obtained for all the groups, as well as the calculated crosslinking density.
Table 2 -.
Means (SD) of the viscosity (η, Pa.s) for the liquid component modified with the HDDMA and/or thiourethane, and dynamic properties of polymerized bars: glass transition temperature (Tg, °C), breadth of tan delta (width at half-height, °C) and crosslinking density (mog/kg). Different HDDMA concentrations were added to materials without (0 wt%) or with (10 wt%) thiourethane oligomer. Values followed by the same letters in each column are statistically similar (α=0.05).
| TU wt% | HDDMA wt% | Viscosity (η, Pa.s× 10−4) | Glass transition temperature (Tg, °C) | Width at half-height of tan delta peak (°C) | Crosslinking density at 180°C (mol/kg × 10−4) |
|---|---|---|---|---|---|
| 0 | 0 | 10.6 (8.6)a | 138.5 (8.8)ab | 29.94 (0.61)b | 1.89 (0.15)b |
| 10 | 3.4 (0.0)b | 144.5 (1.0)a | 29.94 (1.25)b | 2.02 (0.09)ab | |
| 20 | 3.5 (0.0)b | 146.8 (1.4)a | 32.40 (0.26)b | 2.26 (0.11)a | |
| 30 | 3.5 (0.0)b | 145.7 (0.5)a | 31.69 (0.5l)b | 2.29 (0.17)a | |
| 10 | 0 | 9.4 (1.0)a | 121.9 (1.42)c | 45.89 (1.14)a | 1.13 (0.09)d |
| 10 | 3.5 (0.0)b | 128.2 (2.6)bc | 43.49 (0.02)a | 1.74 (0.40)c | |
| 20 | 4.8 (2.2)b | 125.5 (0.8)c | 47.32 (0.1 l)a | 1.88 (0.01)bc | |
| 30 | 3.5 (0.0)b | 134.6 (2.2)b | 45.04 (0.67)a | 1.89 (0.07)bc |
TU (Thio-urethane).
HDDMA (1.6-Hexanediol dimethacrylate).
Figure 1-.
Tan delta curves for acrylic resin modified by the addition of HDDMA (A) or HDDMA + TU (B). The width at half-height was used as an estimate of the polymer homogeneity, with wider ranges of temperature indicating a more heterogeneous polymer. Samples were tested in temperature sweep from −25 to 180°C.
Discussion
The addition of thiourethane oligomers has proven efficient at improving mechanical properties in crosslinked dimethacrylate networks (Bacchi et al., 2015; Bacchi et al., 2016; Bacchi et al., 2018) as well as in other applications where impact resistance and higher toughness are required (Hoyle and Bowman, 2010). In this study, one thiourethane oligomer was combined with a denture base resin in the presence of increasing concentrations of a dimethacryalte crosslinker, in an attempt to increase mechanical properties of those materials. The effects of adding hexanediol dimethacrylate (HDMMA) as the crosslinker were complex. For the materials not containing TU, the addition of HDDMA up to 10 wt% increased the flexural strength and toughness, but subsequent increases in the HDDMA concentration did not provide the same benefit. The flexural modulus of the materials not containing TU also increased with the addition of HDDMA, up to 30 wt%, though the increase was not statistically significant. The addition of HDDMA was indeed expected to improve mechanical properties, as demonstrated in several other studies evaluating the addition of crosslinkers to linear polymers (Caycik and Jagger, 1992; Harrison et al., 1978; Price, 1986).
Linear polymers rely on chain entanglement and secondary intermolecular interactions to improve mechanical properties below Tg (Odian, 2004). The addition of multifunctional monomers as crosslinkers provides covalent interactions between the linear chains, in theory stabilizing the structure to reduce water sorption and solubility and increase flexural properties. Glycol dimethacrylate is commonly used as crosslinking agent in denture base resins since is chemically and structurally similar to methyl methacrylate and may also be incorporated into polymer chains. One possible explanation for the results found in this study is that the molecular structure of selected crosslinker, with a six-carbon, flexible spacer between methacrylates, was not capable of improving the packing between the existing linear polymer chains. Other crosslinkers normally used in commercial PMMA-based materials, such as ethylene glycol dimethacrylate (EGDMA), are much shorter and provide some intermolecular interactions in the form of hydrogen bonding, thus reinforcing the linear polymer when added up to 12% (Jerolimov et al., 1993). EGDMA is added to improve crazing resistance, although no correlation was observed between the impact resistance and EGDMA concentrations (Harrison et al., 1978). Crosslinkers with longer and more flexible led to decreased flexural and tensile strength, and increased impact strength (Caycik and Jagger, 1992). In some cases, higher EGDMA concentrations lead to decrease in mechanical properties (Jerolimov et al., 1993). Therefore, there is a threshold in crosslinker concentration above which no benefit to the mechanical properties of PMMA is observed. This is due to the fact that the methyl groups on the methacrylate work to impede the sliding of polymer chains – in fact, in the absence of the methyl groups, as is the case for polymethyl acrylate (PMA), the Tg drops from about 100°C (in PMMA) to 5°C (in PMA) (Brandup et al., 1999). It has also been demonstrated that as the length of side chain increases in linear polymers (for example, from methyl to ethyl to butyl) the Tg of the resulting polymer also decreases (Brandup et al., 1999). For the commercial material used here, an unknown concentration of crosslinker is also added by the manufacturer - therefore, the addition of extra crosslinker, and especially of a relatively long and flexible one such as HDMMA, likely fell above the threshold in crosslinker concentration where benefits to the mechanical properties are observed. Therefore, the first hypothesis is rejected.
In general, the addition of TU by itself did not affect the flexural strength/modulus, the toughness or the viscosity of the materials, while leading to lower glass transition temperature and crosslinking density. The drop in Tg was expected – thiourethane oligomers have been shown to present Tg ranging from −50 to 250 °C (Bacchi and Pfeifer, 2016; Carioscia et al., 2005). This is done to prevent macrogelantion during the synthesis of the oligomer, according to the Flory-Stockmeyer theory (Carioscia et al., 2005), and also to provide stress relief during polymerization (Bacchi et al., 2018), as well as to improve toughness (Bacchi et al., 2016). Indeed, other studies have demonstrated that the addition of thiourethane improves toughness and fracture toughness in crosslinked networks (Bacchi and Pfeifer, 2016). The thiols pending from the additive are available to undergo chain-transfer reactions with the methacrylate groups in the secondary matrix they are added to (Pfeifer et al., 2011). Chain transfer is a chain-breaking mechanisms, which naturally leads to shorter chains, and also potentially less crosslinking in the cases when the chain-transfer agent (in this case, a thiol) are monofunctional (Berchtold et al., 2002). In highly crosslinked networks, the addition of multi-functional thiols is expected to decrease chain length, but the loss in crosslinking from the di-functional monomers is somewhat compensated by the crosslinking provided by the multifunctional thiols (Bacchi et al., 2018). In the case of methyl methacrylate, the addition of thiol-containing additives was expected to reduce the chain length of the linear PMMA, while increasing crosslinking, since the oligomer contains multiple pendant thiols avaliable for the reaction. However, this is contrary to what was observed here: the addition of TU systematically decreased crosslinking density and led to the formation of more heterogeneous networks. It is likely that the addition of the oligomer interfered with the packing of the linear polymer chains, reducing secondary intermolecular interactions and increasing the free volume of the material. In addition, the crosslinks provided by the thiourethane are much longer than for the multi-functional, small molecule monomers normally used, and present much lower Tg, which jeopardized properties in this study. These effects, in turn, likely annulled any positive effect the addition of tough thio-carbamates might have had on properties, explaining the absence of effect on mechanical properties.
When HDDMA was combined with TU oligomers, properties either did not increase (modulus), or were reduced (flexural strength, toughness and Tg), with complex concentration-dependent relationships. In these cases, it is likely that the deleterious effects on polymer packing (increased free volume) were potentiated. The free volume of the resulting networks was not measured in this study, but some insight into the polymeric structure can be gained by the breadth of the tan delta curves (Dean et al., 2006). Wider tan delta curves correlated with more heterogeneous networks (Bacchi and Pfeifer, 2016). In the case of the present study, the breadth of tan delta was analyzed by comparing the width at half height (WHH) of the curves for all materials tested. The results show that the materials containing HDDMA as the crosslinker presented lower WHH values than the materials not containing crosslinker, regardless of the presence of the TU additive. This shows that the addition of HDDMA led to the formation of more homogeneous polymers. Moreover, a previous study has shown that the Tg resulting of the thiourethane/thiol−ene hybrid network progressively increases as a function of the TU content due to higher extent of hydrogen bonding, leading to enhanced mechanical properties (Bacchi et al., 2018). In this study, the same beneficial effect observed in those hybrid networks was not observed when TU was combined with HDDMA as the crosslinking agent.
The viscosity decreased for all HDDMA concentrations, combined or not with TU, while no change was observed when TU alone was added. Previous studies with the addition of pre-polymerized particles have reported exponential increase in viscosity (Morães et al., 2012) due to increased particle-particle interaction. In the case of the present study, the amount of TU added was 10% by mass of the polymer beads. Therefore, the oligomers were replacing another pre-polymerized particle. This was done exactly to avoid viscosity increases that could potentially jeopardize the adaptation of the denture-base material to the molds in the flask. The decrease in viscosity was expected with the addition of increased concentrations of HDMMA, because it replaced a percentage by weight of the monomer methyl methacrylate. Methyl methacrylate has a much lower molecular weight for almost the same viscosity (Mw=100.1 g/mol, η=6 mPa.s) compared to HDDMA (Mw=254.3 g/mol, η=4 mPa.). Therefore, roughly 2.5 mols of methyl methacrylate were replaced by each molecule of HDDMA, which at least partially explains the decreased viscosity. Compounded with that is the fact that the much larger and flexible HDDMA might have decreased the opportunity for intermolecular interactions (Odian, 2004), decreasing the viscosity even further. Other than material adaptation to the mold, the viscosity has influence on the kinetics of polymerization, but it is unlikely that this was a concern in this study since the material was polymerized at a temperature very close to the Tg of the cured material, where molecular mobility is intrinsically high anyway. A literature review with many in vitro studies has shown that the ideal material to be used as an additive to improve the mechanical properties of the acrylic resin for denture base has yet to be developed (Gad et al., 2017). In addition, mechanical properties of denture base polymers vary depending on other factors related to the composition of the products, as well as processing and testing conditions, such as the storage conditions (wet or dry) (Hoyle and Bowman, 2010).
Conclusion
Based on the results and within the limitations of this study, it can be concluded that the addition of up to 20 wt% of HDDMA as a crosslinking agent improved selected properties of denture base resins (not combined with thiourethanes). The addition of a low Tg additive based on thiourethanes (TU) did not affect mechanical properties in flexure when used alone, but led to a decrease in Tg both due to the low Tg of the additive itself and also due to the decrease in molecular weight of the individual PMMA strands. The combination of TU with HDDMA jeopardized all properties tested.
Acknowledgements
Financial support from NIH-NIDCR (R15-DE023211 and U01-DE023756 to CSP) is greatly appreciated. The authors thank FAPESP (Foundation for Research Support of the State of Sao Paulo, Brazil - Grant 16/14217–0) for the financial support for the postdoctoral study provided to Rafael Leonardo Xediek Consani at Oregon Health & Science University. Special thanks to Dr. Jack F. Ferracane by the relevant assistance. The authors declare no potential conflicts of interest with respect to the material developed in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguayo S, Marshall H, Pratten J, Bradshaw D, Brown JS, Porter SR, Spratt D, Bozec L, 2017. Early Adhesion of Candida albicans onto Dental Acrylic Surfaces. Journal of Dental Research 96, 917–923. [DOI] [PubMed] [Google Scholar]
- Bacchi A, Consani RL, Martim GC, Pfeifer CS, 2015. Thio-urethane oligomers improve the properties of light-cured resin cements. Dental Materials 31, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi A, Nelson M, Pfeifer CS, 2016. Characterization of methacrylate-based composites containing thio-urethane oligomers. Dental Materials 32, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi A, Pfeifer CS, 2016. Rheological and mechanical properties and interfacial stress development of composite cements modified with thio-urethane oligomers. Dental Materials 32, 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi A, Yih JA, Platta J, Knight J, Pfeifer CS, 2018. Shrinkage / stress reduction and mechanical properties improvement in restorative composites formulated with thio-urethane oligomers. Journal of the mechanical behavior of biomedical materials 78, 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Smith DE, Nicholls JI, 1977. The comparison of denture-base processing techniques. Part II. Dimensional changes due to processing. The Journal of prosthetic dentistry 37, 450–459. [DOI] [PubMed] [Google Scholar]
- Berchtold KA, Lovestead TM, Bowman CN, 2002. Coupling chain length dependent and reaction diffusion controlled termination in the free radical polymerization of multivinyl (meth)acrylates. Macromolecules 35, 7968–7975. [Google Scholar]
- Beyli MS, von Fraunhofer JA, 1981. An analysis of causes of fracture of acrylic resin dentures. The Journal of prosthetic dentistry 46, 238–241. [DOI] [PubMed] [Google Scholar]
- Brandup J, Immergut EH, Gulke EA, 1999. Polymer Handbook, 4th ed. Wiley Interscience, New York. [Google Scholar]
- Carioscia JA, Lu H, Stanbury JW, Bowman CN, 2005. Thiol-ene oligomers as dental restorative materials. Dental Materials 21, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Caycik S, Jagger RG, 1992. The effect of cross-linking chain length on mechanical properties of a dough-molded poly(methylmethacrylate) resin. Dental materials : official publication of the Academy of Dental Materials 8, 153–157. [DOI] [PubMed] [Google Scholar]
- Chen JC, Lacefield WR, Castleberry DJ, 1988. Effect of denture thickness and curing cycle on the dimensional stability of acrylic resin denture bases. Dental materials : official publication of the Academy of Dental Materials 4, 20–24. [DOI] [PubMed] [Google Scholar]
- Consani RL, Domitti SS, Rizzatti Barbosa CM, Consani S, 2002. Effect of commercial acrylic resins on dimensional accuracy of the maxillary denture base. Brazilian dental journal 13, 57–60. [PubMed] [Google Scholar]
- Darbar UR, Huggett R, Harrison A, 1994. Denture fracture--a survey. British dental journal 176, 342–345. [DOI] [PubMed] [Google Scholar]
- Dean K, Cook WD, 2002. Effect of Curing Sequence on the Photopolymerization and Thermal Curing Kinetics of Dimethacrylate/Epoxy Interpenetrating Polymer Networks. Macromolecules 35, 7942–7954. [Google Scholar]
- Dean KM, Cook WD, Lin MY, 2006. Small angle neutron scattering and dynamic mechanical thermal analysis of dimethacrylate/epoxy IPNs. European Polymer Journal 42, 2872–2887. [Google Scholar]
- Figuerôa RMS, Conterno B, Arrais CAG, Sugio CYC, Urban VM, Neppelenbroek KH, 2018. Porosity, water sorption and solubility of denture base acrylic resins polymerized conventionally or in microwave. Journal of Applied Oral Science 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad MM, Fouda SM, Al-Harbi FA, Napankangas R, Raustia A, 2017. PMMA denture base material enhancement: a review of fiber, filler, and nanofiller addition. International journal of nanomedicine 12, 3801–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Huggett R, Jagger RC, 1978. The effect of a cross-linking agent on the abrasion resistance and impact strength of an acrylic resin denture base material. Journal of dentistry 6, 299–304. [DOI] [PubMed] [Google Scholar]
- Hoyle CE, Bowman CN, 2010. Thiol-ene click chemistry. Angewandte Chemie -International Edition 49, 1540–1573. [DOI] [PubMed] [Google Scholar]
- Jerolimov V, Jagger RG, Hugget R, 1993. Effect of butadiene acrylate crosslinking on impact strength of poly methyl methacrylate. Acta Stomatologica Croatia 27, 11–15. [Google Scholar]
- Jerolimov V, Jagger RG, Millward PJ, 1994. Effect of crosslinking chain length on mechanical properties of dough-molded poly methyl methacrylate resins. Acta Stomatologica Croatia 28, 3–9. [Google Scholar]
- Kawaguchi T, Lassila LV, Tokue A, Takahashi Y, Vallittu PK, 2011. Influence of molecular weight of polymethyl(methacrylate) beads on the properties and structure of cross-linked denture base polymer. Journal of the mechanical behavior of biomedical materials 4, 1846–1851. [DOI] [PubMed] [Google Scholar]
- Morães RR, Garcia JW, Wilson ND, Lewis SH, Barros MD, Yang B, Pfeifer CS, Stansbury JW, 2012. Improved dental adhesive formulations based on reactive nanogel additives. Journal of Dental Research 91, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odian G, 2004. Principles of Polymerization, 4th ed. Wiley Interscience, New Yori. [Google Scholar]
- Ozkir SE, Yilmaz B, Unal SM, Culhaoglu A, Kurkcuoglu I, 2018. Effect of heat polymerization conditions and microwave on the flexural strength of polymethyl methacrylate. European Journal of Dentistry 12, 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW, 2011. Delayed gelation through chain-transfer reactions: Mechanism for stress reduction in methacrylate networks. Polymer 52, 3295–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveen B, Babaji HV, Prasanna BG, Rajalbandi SK, Shreeharsha TV, Prashant GM, 2014. Comparison of Impact Strength and Fracture Morphology of Different Heat Cure Denture Acrylic Resins: An In vitro Study. Journal of international oral health : JIOH 6, 12–16. [PMC free article] [PubMed] [Google Scholar]
- Price CA, 1986. The effect of cross-linking agents on the impact resistance of a linear poly(methyl methacrylate) denture-base polymer. J Dent Res 65, 987–992. [DOI] [PubMed] [Google Scholar]
- Savirmath A, Mishra V, 2016. A Comparative Evaluation of the Linear Dimensional Changes of Two Different Commercially Available Heat Cure Acrylic Resins during Three Different Cooling Regimens. Journal of clinical and diagnostic research : JCDR 10, ZC50–ZC54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyurt AF, Hoyle CE, Wei H, Piland SG, Gould TE, 2007. Thermal and mechanical properties of cross-linked photopolymers based on multifunctional thiol-urethane ene monomers. Macromolecules 40, 3174–3182. [Google Scholar]
- Standardization, I.O.f., 2009. ISO 4049:2009 - Dentistry -- Polymer-based restorative materials.
- Takamata T, Setcos JC, Phillips RW, Boone ME, 1989. Adaptation of acrylic resin dentures as influenced by the activation mode of polymerization. Journal of the American Dental Association (1939) 119, 271–276. [DOI] [PubMed] [Google Scholar]
- Wolfaardt J, Cleaton-Jones P, Fatti P, 1986. The influence of processing variables on dimensional changes of heat-cured poly(methyl methacrylate). The Journal of prosthetic dentistry 55, 518–525. [DOI] [PubMed] [Google Scholar]