Abstract
Purpose:
Fatty liver disease (FLD) affects over 25% of the global population and may lead to liver-related mortality due to cirrhosis and liver cancer. FLD caused by occupational and environmental chemical exposures is termed ‘toxicant associated steatohepatitis’ (TASH). The current review addresses the scientific progress made in the mechanistic understanding of TASH since its initial description in 2010.
Recent Findings:
Recently discovered modes of actions for volatile organic compounds and persistent organic pollutants include: (i) the endocrine, metabolism, and signaling disrupting chemical hypotheses; (ii) chemical-nutrient interactions and the two ‘hit’ hypothesis. These key hypotheses were then reviewed in the context of the steatosis adverse outcome pathway (AOP) proposed by the US Environmental Protection Agency.
Summary:
The conceptual understanding of the contribution of environmental exposures to FLD has progressed significantly. However, because this is a new research area, more studies including mechanistic human data are required to address current knowledge gaps.
Keywords: toxicant associated steatohepatitis, TASH, signaling disruption, endocrine disruption, polychlorinated biphenyls, vinyl chloride
INTRODUCTION
Chronic liver diseases may increase mortality due to liver-related causes (e.g., cirrhosis and hepatocellular carcinoma) and also due to increased risk for cardiovascular and infectious diseases. In fact, liver-related mortality has increased world-wide. Recent data demonstrate that between 1999 and 2016, the cirrhosis annual death rate increased by 65%, and the liver cancer annual death rate doubled in the United States [1]. The most common histologic form of liver pathology is fatty liver disease (FLD). FLD encompasses a progressive pathologic spectrum ranging from steatosis, to steatohepatitis with or without fibrosis, to cirrhosis, and hepatocellular carcinoma. Steatosis results from altered lipid metabolic pathways and complex changes in overall energy metabolism [2]. FLDs are named according to the etiologic exposure associated with their development. Alcoholic fatty liver disease was initially described followed by nonalcoholic fatty liver disease (NAFLD). NAFLD has been associated with diet-induced obesity, insulin resistance, and metabolic syndrome; but not all subjects with NAFLD have obesity or diabetes [3].
FLD is common, and the global prevalence of NAFLD alone is 25.2% [3]. More recently, it was recognized that occupational and environmental chemical exposures may be associated with the development of FLD. The Cave laboratory coined the term, toxicant associated steatohepatitis (TASH), to describe FLD occurring in highly-exposed polyvinyl chloride (PVC) production workers which was associated with increased pro-inflammatory cytokines, insulin resistance, and decreased antioxidants [4]. Numerous environmental chemicals have subsequently been associated with TASH in animal and/or epidemiological studies, including volatile organic chemicals (VOCs), persistent organic pollutants (POPs), metals, particulate matter, pesticides, and others (Table 1). These studies implicate both adult and developmental chemical exposures in the pathogenesis of FLD. While the exact number of environmental pollutants that cause fatty liver is unknown, one-third of chemicals in the National Institute for Occupational Safety and Health’s (NIOSH) Pocket Guide are associated with hepatotoxicity [5]. Moreover, the liver appears to be the most common target organ for chemical toxicity; and this is probably due to liver’s central role in xenobiotic detoxification [6].
Table 1.
Selected examples of chemicals associated with fatty liver disease.
| Category | Chemical / Chemical Group | Laboratory animals | Epidemiology/Clinical Evidence |
|---|---|---|---|
| POPs | Dioxins | Adult Exposure [92–95, 62, 96, 63] | [97–99] |
| Polychlorinated biphenyls | Adult Exposure [12, 23, 56, 65, 92, 59, 58, 67]. | [66, 97, 98,100–104] | |
| Perfluorooctanoic acid | Adult Exposure [80, 105, 74, 106] | [83–85, 107–109]. | |
| Perfluorooctanesulfonic acid | Adult Exposure [110–112, 78] | [108, 109, 84, 107] | |
| Developmental Exposure [113] | |||
| Polybrominated diphenyl ethers | Adult Exposure [114] | ||
| Diethylhexyl phthalate | Adult Exposure [115, 116] | ||
| Developmental Exposure [117] | |||
| Organochlorine Insecticides | [118–120] | [101, 97, 99] | |
| Atrazine | Adult Exposure [121–123] | ||
| VOCs | Vinyl Chloride/Metabolites | Adult Exposure [27, 28, 14] | [4, 29]. |
| Smoking/nicotine | Adult Exposure [124, 125] | [126, 127] | |
| Tributyltin | Adult Exposure [128] | ||
| Developmental Exposure [129–131] | |||
| Air Pollution / Particulate Matter | Adult Exposure [132–135] | [136] | |
| Benzo[a]pyrene | Adult Exposure [137] | ||
| Developmental Exposure [138] | |||
| Metals | Arsenic | Adult Exposure [139–143] | [145] |
| Developmental Exposure [144] | |||
| Lead | [100, 146] | ||
| Mercury | Adult Exposure [147, 148] | [100] | |
| Cadmium | Adult Exposure [149–151] | [152, 153] | |
| Others | Bisphenol A | Developmental Exposure [154–157] | |
| Fungicides | Adult Exposure [92, 158] | ||
| Developmental Exposure [92] | |||
| Glyphosate-based herbicides | Adult Exposure [159] | [160] | |
| Dinoseb | Adult Exposure [161] |
The mechanistic similarities and differences between alcoholic steatohepatitis (ASH), nonalcoholic steatohepatitis (NASH), and TASH were recently reviewed [7]. While ASH, NASH, and TASH are pathologically similar, disease mechanisms vary by etiologic exposure. Since its initial description nearly a decade ago, significant progress has been made in the scientific understanding of TASH, as previously reviewed [7, 6, 8–11]. This manuscript extends these prior review articles by examining: (i) key hypotheses currently of interest to the field; (ii) newly discovered modes of action for VOCs and POPs in the context of these hypotheses; and (iii) current limitations and suggested future research directions.
KEY HYPOTHESES CURRENTLY OF INTEREST TO THE FIELD
Several hypotheses contextualize TASH as a part of a systemic disease and provide the framework to evaluate recently described modes of action for environmental pollutants in TASH. These hypotheses include the endocrine, metabolism, and signaling disrupting chemical hypotheses, as well as chemical-nutrient interactions impacting the two ‘hit’ hypothesis.
The endocrine and metabolism disrupting chemical hypotheses were recently reviewed [11]. Endocrine disrupting chemicals (EDCs) interfere with any aspect of hormone action [11]. Metabolism disrupting chemicals (MDCs) promote metabolic changes that can result in obesity, type 2 diabetes, or fatty liver in animals and humans alike [11]. These metabolic changes can be independent of chemical effects on hormone action [11]. Thus, FLD can be considered as the hepatic manifestation of systemic endocrine and metabolic disruption. However, it was recently demonstrated that chemical exposures can also alter hepatokine production, demonstrating that TASH can also be a cause, and not just an effect, of systemic endocrine disruption [12]. MDCs may also cause hormone-independent alterations in hepatic metabolism through diverse mechanisms including receptor-based modes of action [13, 11, 8] and mitochondrial toxicity [14]. In fact, nuclear receptor ‘crosstalk’-based modes of action were proposed to be molecular initiating events (MIEs) in the hepatic steatosis adverse outcome pathway (AOP) proposed by the U.S. Environmental Protection Agency (EPA) [13]. These and other MIEs regulated the four identified apical key events impacting steatosis (e.g., fatty acid uptake, efflux, synthesis, and oxidative metabolism) [13]. EPA’s AOP were recently validated in vitro [15], and expanded to demonstrate the downstream systemic impact of the receptor-based MIE’s on diabetes and cardiovascular disease [16].
Signaling disrupting chemicals (SDCs) can disrupt the normal hepatic intracellular signaling that regulates metabolism, inflammation, and fibrosis. SDCs may ligand activate transcription factors implicated in TASH (such as dioxin and the aryl hydrocarbon receptor, AhR); antagonize these receptors; or indirectly impact receptor function. Recently, it was demonstrated that some POPs and pesticides may antagonize the epidermal growth factor receptor (EGFR) reducing signal transduction leading to altered transcription factor (including nuclear receptor) phosphorylation and function in TASH [17–20]. The EDC, MDC, and SDC hypotheses provide a framework to evaluate the mechanisms responsible for the observed transcriptional reprogramming in TASH. The AOP framework appears to be a useful new tool to evaluate mechanisms of EDCs, MDCs, and SDCs in TASH.
New understanding about how interactions between environmental chemicals and nutrition impact FLD extends Day’s two ‘hit’ hypothesis in new directions [21]. The two ‘hit’ hypothesis proposed that a second ‘hit’ is required for patients with steatosis to progress to more histologically advanced liver disease. Classically proposed second ‘hits’ include oxidative stress, insulin resistance, organelle dysfunction, and pro-inflammatory cytokines. Some exposures such as high-dose vinyl chloride were associated with steatohepatitis and fibrosis in humans through the simultaneous induction of multiple hit mechanisms [4, 22]. In animal models, a polychlorinated biphenyl (PCB) mixture caused steatohepatitis only in mice fed a high fat diet [23]. It was thus proposed that differential exposures to environmental chemicals could serve as a second ‘hit’ in the progression of diet-induced steatosis to steatohepatitis, again via upregulation of deleterious mechanisms like pro-inflammatory cytokines [23]. More recently, hepatic proteomics analysis of PCB-exposed mice demonstrated that PCB exposures were associated with the attenuation of liver’s protective responses against diet-induced obesity including the antioxidant response and nuclear receptor function (e.g., the farnesoid x receptor, FXR) [20]. Likewise, a recent mouse study demonstrated that low-dose vinyl chloride exposures caused steatohepatitis only in mice fed high fat diet [14], although vinyl chloride induced mitochondrial dysfunction in mice fed either a control or high fat diet. Over-nutrition likely exceeded the reserve capacity of mitochondria damaged by reactive vinyl chloride metabolites to worsen high fat diet-induced steatosis and cause steatohepatitis. Based, in part, on these observations, it was recently proposed that environmental pollutant exposures may be the first ‘hit’ which compromise the liver’s protective responses against over-nutrition to promote steatohepatitis following the second ‘hit’ of hypercaloric diets [20]. Thus, complex interactions between environmental chemicals and nutrients appear to be important determinants of liver disease. These interactions are being increasingly understood in the context of the two ‘hit’ hypothesis. Such exposure biology approaches take into consideration more than one biology factor in the analysis of liver disease susceptibility.
NEWLY DISCOVERED MODES OF ACTION FOR VOLATILE ORGANIC POLLUTANTS AND PERSISTENT ORGANIC POLLUTANTS IN THE CONTEXT OF KEY HYPOTHESES
Significant advances have been in the mechanistic understanding of TASH, particularly following VOC and POP exposures (Table 2).
Table 2.
Main targets and modes of action for volatile organic compounds and persistent organic pollutants in fatty liver disease.
| Target Category | Target/Receptor | Outcomes/Processes | Chemical Group | References |
|---|---|---|---|---|
| Hepatic Nuclear Receptors | PPARs | -steatosis -immuno-toxicity -apoptosis |
PFOA/PFOS VOCs |
[73, 162, 163] [33] |
| PXR, CAR, FXR | -lipogenesis & decreased fatty acid oxidation -decreased gluconeogenesis -hepatokine dysregulation -altered cholesterol/bile acid metabolism |
PCBs | [23, 67, 12] | |
| Sex Steroid Receptors | ERα, ERβ, AR | -endocrine-metabolic disruption | PCBs, PFAS | [164, 165] |
| Other Receptors | AhR | -lipid accumulation -inflammation and oxidative stress -hepatokine dysregulation -gut microbiome alterations |
PCBs, TCDD | [62, 12, 166, 93, 167, 63, 60] |
| EGFR | -EGFR signaling disruption -diminished HNF4A -altered insulin production |
PCBs, OCPs | [19, 17] | |
| Energy Sensors/Regulators | CREB | -disruption of hepatic energy ‘sensing’ | Dioxin-like PCBs | [57, 168] |
| AMPK, mTOR, | -glycogen depletion -lipid accumulation |
VOCs | [27, 29] | |
| Organelles/Protein Complex | Endoplasmic reticulum, Mitochondria, Inflammasome | -ER and oxidative stress -carbonyl stress -inflammation -enhanced neutrophil extracellular trap formation |
VOCs | [4, 14, 28, 43, 44, 54]. |
| Antioxidant Responses | NRF2 | -Reactive oxygen species generation -oxidative stress |
PCBs, VOCs | [4, 20] |
A. VOLATILE ORGANIC COMPOUNDS
VOCs are often present in household products such as paints/varnishes, cleaning supplies, gasoline, and dry-cleaned clothing [24]. VOCs also contaminate ground water and are frequently present at National Priority List (NPL) Superfund sites. As such, many VOCs rank highly on the Agency for Toxic Substances and Disease Registry’s (ATSDR) Hazardous Substance Priority List [24]. Ambient VOC levels are often higher indoors and can be significantly affected by the building’s proximity to contaminated sites and its ventilation [25, 26]. The VOC, vinyl chloride, is a direct hepatotoxicant at high exposures [4]. Low-dose vinyl chloride exposures that are not hepatotoxic per se, can enhance underlying liver injury due to another factor [27, 28, 14], consistent with the ‘two hit’ hypothesis. The toxicity of VOCs in TASH may be mediated by reactive VOC metabolites [27].
Several VOCs have been shown to be MDCs, by disrupting normal hepatic carbohydrate and lipid metabolism to induce steatosis. While highly-exposed vinyl chloride workers had insulin resistance (IR) [4]; low-dose vinyl chloride exposures caused IR in mice fed a high fat diet [14]. A recent serum metabolomics analysis of occupationally exposed vinyl chloride workers described changes in several lipid metabolites and metabolism regulating enzymes, such as AMP-activated protein kinase (AMPK) [29]. These results were recapitulated in a mouse model of vinyl chloride metabolite exposure, demonstrating that mammalian target of rapamycin (mTOR) and AMPK, which are normally activated in opposition, were both activated causing a paradoxical state of lipid accumulation and glycogen depletion [27]. By impacting normal regulatory kinase function, vinyl chloride may also be considered to be a SDC. Repeated exposure to high levels of perchloroethylene (PCE) also induced hepatic steatosis [30]. Metabolomics analyses demonstrated that even low-dose PCE significantly altered lipid homeostasis in vivo, contributing to enhanced steatosis [31, 32]. This was due, at least in part, to altered activation of peroxisome proliferator-activated receptor α (PPARα) [33, 34].
The carbonyl stress imposed by reactive VOC metabolites damages organelles, thus contributing to TASH. Mitochondria are key to maintaining cellular energy homeostasis, and several VOCs have been demonstrated to impact mitochondrial integrity and function. vinyl chloride-induced mitochondrial damage serves as a canonical example of an environmental exposure limiting the capacity of mitochondria to adapt appropriately to the metabolic stress imposed by the second ‘hit’ of a hypercaloric diet. Vinyl chloride and its metabolites directly damage mitochondrial complex I and II, leading to uncoupling of the electron transport chain. This causes the cell to increase flux through anaerobic glycolysis to compensate for this loss of ATP yield [14], also rendering it more sensitive to cytotoxic second ‘hits’. Mitochondria are also a significant source of endogenous reactive oxygen species (ROS) via electron leakage during normal oxidative respiration [35, 36]. In human subjects, vinyl chloride exposures were associated with antioxidant depletion consistent with oxidative stress [4] and increased circulating lipid peroxidation products and decreased carnitine/carnitine esters consistent with mitochondrial dysfunction [29] validating the animal studies. Active VOC metabolites are often strongly electrophilic and therefore highly reactive. Specifically, oxidative damage occurs upon covalent adduct formation on major macromolecules in the hepatocyte, including proteins, lipids, and/or DNA (i.e., carbonyl stress). For example, vinyl chloride metabolite exposure significantly increased 4-hydroxynonenal adduct formation in high fat diet-fed mice [28], likely due to increased electron leakage by damaged mitochondria [27, 14]. Similarly, major metabolites of PCE including trichloroacetic acid (TCA) and dichloroacetic acid (DCA), caused oxidative stress through formation of lipid peroxidation adducts in vivo [37, 38]. Likewise, acrolein is a well-known propagator of oxidative stress by causing lipid peroxidation adducts [39, 40].
The endoplasmic reticulum (ER) is the cell’s hub for protein folding and synthesis [41, 42]. Upon detection of adducted or misfolded proteins, the ER prompts the unfolded protein response (UPR) to remove these proteins. The aldehydes and ROS generated by VOCs avidly react with proteins resulting in ER stress [43]. For example, vinyl chloride and its metabolites enhanced the accumulation of oxidatively damaged proteins caused by high fat diet, accompanied by a robust dilation of the ER [27, 28, 14]. Likewise, acrolein significantly increased expression of ER stress markers, without protective UPR activation in primary human hepatocytes [39] and in intestinal epithelial cells [44]. The latter changes caused gut barrier disruption, which contributes to fatty liver pathogenesis by increasing portal venous endotoxemia [45–48]. Thus, the ER and mitochondria are examples of organelles damaged by the carbonyl stress imposed by VOC metabolism contributing to TASH.
The transition from steatosis to the more severe steatohepatitis requires the development of superimposed hepatic inflammation. High-dose vinyl chloride exposures were associated with increased liver inflammatory infiltrate and increased serum pro-inflammatory cytokines in PVC production workers [4]. In mice, chronic vinyl chloride exposures (sub-OSHA dose) increased the hepatic neutrophil accumulation caused by high fat diet-feeding [14]. As was the case with vinyl chloride-induced organelle toxicity, vinyl chloride-induced inflammation appears to be due, at least in part, to vinyl chloride metabolites. In a mouse model, treatment with vinyl chloride metabolites significantly increased neutrophil infiltration, inflammasome activation, and pro-inflammatory cytokine expression in mice fed a diet enriched with saturated fat (the second ‘hit’) [28]. Interestingly, mice fed a diet rich in unsaturated fat were protected from liver injury and inflammasome activation [28]. This specific diet-dependence observed in mice was surprising because vinyl chloride exposures in humans were associated with marked upregulation of oxidized linoleic metabolites known to active the inflammasome while inducing liver mitochondrial dysfunction and apoptosis [29, 49]. In addition to inflammasome activation, the local and systemic inflammatory responses associated with VOC exposures can be induced and propagated by VOC-induced oxidative and ER stress [50]. Carbon tetrachloride has also been shown to enhance monocyte recruitment to the liver [51]. Regarding the developmental origins of FLD, trichloroethylene exposures during lactation and gestation increased expression of pro-inflammatory chemokines in offspring of mice [52]. A new field in the study of mechanisms of inflammation is the formation and release of neutrophil extracellular traps (NETs). NETs are an extensive meshwork of decondensed chromatin and hydrolytic enzymes, contributing to injury and necrosis [53]. The VOC, acrolein, has recently been shown to increase hepatic tissue damage after ischemia reperfusion. Increased expression of pro-inflammatory cytokines and enhanced NET formation were observed in isolated neutrophils [54]. Thus, VOCs induce hepatic inflammation though mechanisms including inflammasome activation, organelle stress, and enhanced NET formation.
B. PERSISTENT ORGANIC POLLUTANTS
Dioxins and dioxin-like PCBs
Dioxins (e.g., 2,3,7,8-tetrachlorodibenzodioxin, TCDD) and dioxin-like PCBs (e.g., PCB 126) activate the aryl hydrocarbon receptor (AhR), and that is their proposed mode of action. Exposures to dioxins have long been associated with wasting syndrome, steatosis, and hypoglycemia. Following PCB 126 exposures in rats, specific hepatic fatty acids incorporated into triglycerides were increased in a dose-response with adrenic acid (22:4) showing the greatest maximal increase [55]. Likewise, mice exposed to PCB 126 had increased hepatic triglycerides and free fatty acids [12]. In rodent models, PCB 126-induced steatosis was reproducibly associated with increased lipid influx (via upregulation of AhR target genes including CD36 and fatty acid binding protein-1); variably decreased fatty acid oxidation (via downregulation of PPARα); and variably decreased lipid efflux (via downregulation of apolipoprotein B100); despite decreased lipogenesis (via downregulation of fatty acid synthase) [12, 56–59]. In some cases, the increased liver lipids were associated with reduced serum lipids and trend toward reduced adiposity, consistent with the central redistribution of fat to the liver [12]. PCB 126 reduced hepatic gluconeogenesis and increased insulin sensitivity to promote fasting hypoglycemia despite decreased insulin production [12, 56–58].
New mechanisms for PCB 126 in FLD were recently identified. In mice, PCB 126 decreased production of the hepatokine, fibroblast growth factor-21 (FGF-21) [58, 12], and the enterokine, glucagon-like peptide-1 (GLP-1) [60]. Because FGF-21 and GLP-1 protect against metabolic syndrome, these data implicate liver and intestinal disease as a cause of PCB 126-mediated endocrine disruption. The reduction in GLP-1 was associated with PCB 126-induced dysbiosis including a reduction in bifidobacteria and a significantly increased Firmicutes to Bacteroidetes ratio [60]. Following PCB 126 exposures, the development of more severe liver injury, inflammation, and fibrosis (e.g., steatohepatitis) may require a nutritional second ‘hit’ [59, 58]. A recent liver metabolomics analysis of PCB 126 treated mice demonstrated that compared to mice fed a control diet, mice fed a methionine-choline deficient (MCD) diet had more metabolites associated with dysfunctional pathways and increased hepatic lipid peroxidation, mitochondrial dysfunction, and thiol depletion [61]. Regarding signaling disruption, PCB 126 was the most potent EGFR inhibitor tested [18], and it also inhibited protective hepatic AMPK and cyclic AMP responsive element binding protein 1 (CREB-1) signaling [57]. TCDD exposures also increased hepatic steatosis via fatty acid uptake [62] and appeared to require high fat diet co-exposures in order to increase fibrosis [63]. Metabolic reprogramming by TCDD in FLD was recently reviewed [64]. The endocrine and metabolic disruption accounting for TCDD-induced steatosis shares many similarities with PCB 126-induced steatosis, strongly implicating AhR’s role in TASH [64].
Non-dioxin-like PCBs
Non-dioxin-like PCBs (NDL PCBs) disrupt hepatic energy metabolism through other receptor-based mechanisms including constitutive androstane receptor (CAR) and pregnane X receptor (PXR) [8]. In diet-induced obesity mouse models, exposures to the NDL PCB 153 increased steatosis [65]; while Aroclor 1260 exposures caused steatohepatitis [23]. The latter data were recently confirmed in a cross-sectional analysis of the Anniston Community Health Survey (ACHS) [66]. Using serologic biomarkers, a high prevalence of TASH was observed in this cohort with increased PCB exposures and overweight/obesity. TASH was associated with increased PCB exposures, insulin resistance, dyslipidemia, pro-inflammatory cytokines, and liver necrosis. ΣPCBs was inversely associated with leptin and pancreatic insulin production. EPA’s steatosis AOP proposed pollutant-induced PXR/CAR activation to be MIEs for FLD [13, 16]. Thus, we hypothesized that PXR or CAR knockout mice would be protected against the steatohepatitis associated with Aroclor 1260 in a diet-induced obesity model [67]. While PXR and CAR clearly modulated several mechanisms implicated in FLD, knocking out these receptors did not prevent steatohepatitis [67]; thus implicating additional mechanisms.
CAR can either be directly activated by ligand binding or indirectly activated via altered receptor phosphorylation. Direct CAR ligands, such as TCPOBOP, protect against FLD [68], suggesting that the indirect activators may cause TASH. PCBs are indirect murine CAR activators, but may activate human CAR both directly and indirectly [69, 18, 17]. Recently, the mechanism for PCB-induced indirect CAR activation was elucidated. PCBs antagonized EGFR via high-affinity hydrophobic binding at the ligand binding domain to prevent ligand-induced endocytosis and tyrosine kinase activation leading to downstream CAR de-phosphorylation and consequently increased CAR activity [18, 17]. Perhaps because it shares similarities with the insulin receptor, the EGFR also regulates numerous pathways involved in metabolism, regeneration, and gene expression [70, 71]. Disruption of these pathways may promote TASH.
A recent in vivo hepatic phosphoproteomics analysis revealed that PCB-induced signaling disruption impacted many pathways and interacted with diet [19]. Aroclor 1260 reduced hepatic phosphoprotein levels by nearly 25%. Consistent with ACHS, PCBs impacted leptin and insulin signaling pathways while liver necrosis was a pathologic ontology: increased by the interaction between PCBs and high fat diet. Casein kinase 2 (CK2), extracellular regulated kinase, protein kinase B, and cyclin dependent kinase activities were downregulated by PCBs, and this downregulation was worsened by diet-induced obesity. PCB-induced alterations in CK2 subunit expression negatively regulated caspase-3 to promote secondary liver necrosis. More recently, it was demonstrated that nuclear factor erythroid 2-related factor (NRF2) and hepatocyte nuclear factor 4-alpha (HNF4α) were epidermal growth factor sensitive targets whose functions were reduced by NDL PCBs [20]. PCB-induced NRF2 down-regulation decreased hepatic glutathione levels, rendering the liver more susceptible to the oxidative stress imposed by the diet-induced obesity second ‘hit’ [20]. HNF4α is a critical identity gene regulating the expression of the liver’s specific metabolic genes [72] as well as pancreatic insulin production. Other recently described novel modes of action for PCBs in FLD include: (i) reduced expression of signal transducer and activator of transcription 3 (STAT3, a transcription factor implicated in interleukin-6 and leptin signaling) [17]; (ii) reduced function of protective nuclear receptors [e.g., PPARα, β, γ and FXR)] [20]; (iii) altered hepatokine expression impacting the liver:pancreas axis [12]; (iv) gene:environment interactions [e.g., patatin-like phospholipase domain-containing protein (PNPLA3)] [12]; and (v) increased production of pro-fibrotic cytokines like transforming growth factor β (TGF-β) [20]. Thus, NDL PCBs impact multiple FLD mechanisms including nuclear receptors, signaling molecules and pathways, cell death pathways and antioxidant defenses.
Perfluoroalkyl substances
PFAS disrupt hepatic lipid metabolism by interacting with PPARs and other receptors due to their structural similarities with fatty acids [73]. PFAS appear to cause steatosis by upregulating lipogenesis and lipid influx, while downregulating lipid efflux [74–81]. PFAS are potent immunotoxic chemicals suppressing innate immune function, partly through PPARα-dependent mechanisms [82]. In the C8 Health Study, blood PFAS levels were positively associated with liver enzymes, a liver apoptosis biomarker, and sex differences in adipocytokine levels; but were inversely associated with serum tumor necrosis factor α [83–85]. In the National Health and Nutrition Examination Survey, low-level PFAS exposures were associated with elevated liver enzymes only in obese participants [86], consistent with the FLD two ‘hit’ hypothesis. Thus, PFAS exposures seem to uniquely regulate liver lipid metabolism, cell death, and inflammation in diet-induced obesity.
CURRENT LIMITATIONS AND SUGGESTED FUTURE RESEARCH DIRECTIONS
Because TASH is a recently described disease, important knowledge gaps remain in the understanding of TASH mechanisms. These data gaps inform future research directions. Most importantly, more mechanistic human data are required. Although pathology is the gold standard for the diagnosis of steatohepatitis and fibrosis; the liver biopsy procedure is often associated with risk. Moreover, FLD is asymptomatic or has nonspecific symptoms until it has progressed to decompensated cirrhosis or liver cancer; and standard serologic biomarkers for liver injury, such as alanine aminotransferase (ALT) may be insensitive for the diagnosis of TASH [4]. Therefore, liver biopsy is not justified or available in most environmental exposure cohorts, because even subjects with liver disease may be asymptomatic or have normal liver enzymes. The lack of human liver tissue paired with exposure assessment data remains a major barrier to the field. Several alternative strategies could alleviate this research barrier. First, exposure assessment could be performed in previously biopsied NAFLD cohorts. Second, novel blood [(e.g., cytokeratin 18 [4, 66, 85] or “liquid liver biopsy” [87]] or imaging-based biomarkers (e.g., fibroscan) for FLD could be applied to existing exposure cohorts. These studies could potentially (i) identify environmental chemicals associated with TASH, (ii) determine dose responses for these chemicals, and (iii) determine mechanisms. NAFLD mortality is associated with fibrosis [88], and more studies evaluating fibrosis in TASH are required. Some studies (Table 1) suggest a role for environmental exposures in the developmental origins of FLD. This along with the possible contribution of epigenetic mechanisms require further investigation. Polymorphisms in several genes including PNPLA3 have been associated with NAFLD, and some animal studies suggest that environmental exposures may regulate PNPLA3 expression [12]. More data are needed on gene:environment interactions in TASH. The potential reversibility of TASH is unknown, and therapy studies are required. The gut:liver axis has become increasingly important in the pathogenesis of FLD; and it is profoundly impacted by the gut microbiome. The microbiome may have a role in TASH [60], but more data are required. Likewise, environmental exposures may influence the genesis and progression of alcoholic liver disease [89], but more data are needed to better understand the potential role of exposures in alcoholic liver disease. Finally, some POPs disrupt sex hormone signaling. For instance, some low molecular weight PCBs are known to activate estrogen receptors and to antagonize androgen receptor, whereas higher molecular weight PCB congeners may be anti-estrogenic [90, 91]. More data are needed to identify potential sex differences in TASH.
CONCLUSIONS
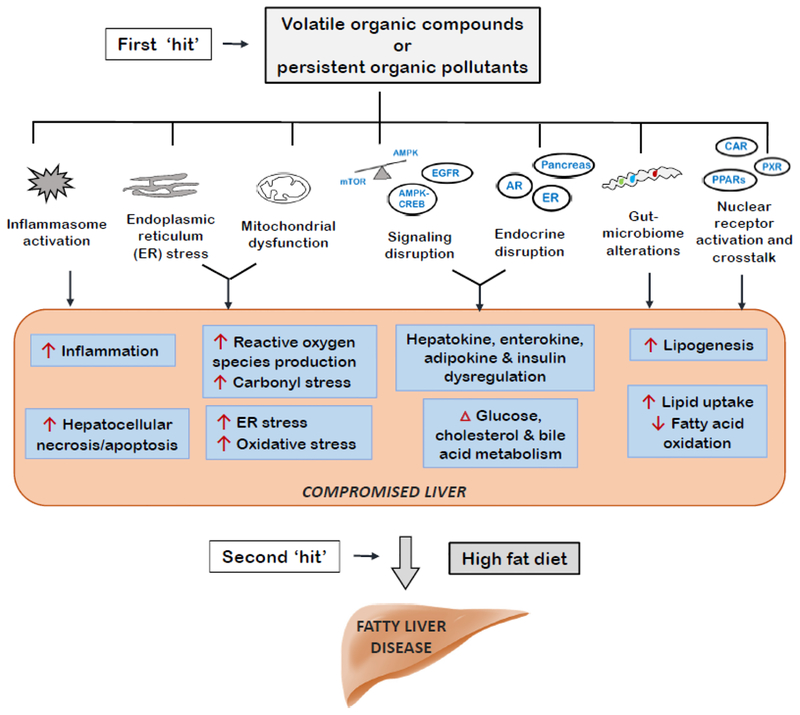
The conceptual understanding of the contribution of environmental exposures, particularly POPs and VOCs, to fatty liver disease has progressed significantly. Increasing numbers of environmental health studies now include liver endpoints, allowing for the further identification of chemicals implicated in TASH and their mechanisms. Several key hypothesis and the EPA’s proposed steatosis adverse outcome pathway have provided a better framework for understanding TASH mechanisms. Such key hypotheses include: the endocrine, metabolism, and signaling disrupting chemical hypotheses; and the chemical-nutrition interactions impacting the two “hit” hypothesis. Environmental exposures (first ‘hit’) may compromise the liver’s protective responses against over-nutrition (summarized in Figure 1) to promote steatohepatitis from hypercaloric diets (second ‘hit’). Finally, because this is a new research area, more studies are required to address current knowledge gaps.
Figure 1. Selected Modes of Action for Volatile Organic Compounds and Persistent Organic Pollutants in Fatty Liver Disease.
These modes of action are related to the endocrine, metabolism, and signaling disrupting hypotheses as well as nutritional interactions and the two ‘hit’ hypothesis.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institute of Environmental Health Sciences [R35ES028373, P42ES023716, T32ES011564, F31ES028982]; the National Institute of General Medical Sciences [P20GM113226]; the National Institute of Diabetes and Digestive and Kidney Diseases [K01DK096042, R03DK107912]; and the National Institute on Alcohol Abuse and Alcoholism [P50AA024337, R01AA024102].
LIST OF ABBREVIATIONS:
- ACHS
Anniston Community Health Survey
- AhR
aryl hyodrocarbon receptor
- ALT
alanine aminotransferase
- AMPK
AMP-activated protein kinase
- AOP
adverse outcome pathway
- ASH
alcoholic steatohepatitis
- ATSDR
Agency for Toxic Substances and Disease Registry
- CAR
constitutive androstane receptor
- CK2
casein kinase 2
- CREB-1
cyclic AMP responsive element binding protein 1
- EDCs
endocrine disrupting chemicals
- EGFR
epidermal growth factor receptor
- EPA
Environmental Protection Agency
- ER
endoplasmic reticulum
- FLD
fatty liver disease
- FXR
farnesoid x receptor
- FGF-21
fibroblast growth factor-21
- GLP-1
glucagon-like peptide-1
- HNF4α
hepatocyte nuclear factor 4-alpha
- IR
insulin resistance
- MCD
methionine-choline deficient
- MDCs
metabolism disrupting chemicals
- MIEs
molecular initiating events
- mTOR
mammalian target of rapamycin
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NDL
non-dioxin like
- NIOSH
National Institute for Occupational Safety and Health
- NAFLD
nonalcoholic fatty liver disease
- NPL
National Priority List
- NRF2
nuclear factor erythroid 2-related factor
- PCBs
polychlorinated biphenyls
- PCE
perchloroethylene
- PFAS
perfluoroalkyl substances
- POPs
persistent organic pollutants
- PNPLA3
patatin-like phospholipase domain-containing protein 3
- PPARα
peroxisome proliferator-activated receptor α
- PVC
polyvinyl chloride
- PXR
pregnane X receptor
- ROS
reactive oxygen species
- SDCs
signaling disrupting chemicals
- STAT3
signal transducer and activator of transcription 3
- TASH
toxicant associated steatohepatitis
- TCDD
2,3,7,8-tetrachlorodibenzodioxin
- TGF-β
transforming growth factor β
- UPR
unfolded protein response
- VOCs
volatile organic compounds
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
CONFLICT OF INTEREST
Banrida Wahlang, Jian Jin, Juliane I. Beier, Josiah E. Hardesty, Erica F. Daly, Regina D. Schnegelberger, K. Cameron Falkner, Russell A. Prough, Irina A Kirpich, and Matthew C. Cave each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
- 1.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbrini E, Magkos F. Hepatic Steatosis as a Marker of Metabolic Dysfunction. Nutrients. 2015;7(6):4995–5019. doi: 10.3390/nu7064995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md). 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Cave M, Falkner KC, Ray M, Joshi-Barve S, Brock G, Khan R et al. Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology (Baltimore, Md). 2010;51(2):474–81. doi: 10.1002/hep.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolman KG, Sirrine R. Occupational Hepatotoxicity. Clin Liver Dis. 1998;2(3):26. [Google Scholar]
- 6.Wahlang B, Beier JI, Clair HB, Bellis-Jones HJ, Falkner KC, McClain CJ et al. Toxicant-associated steatohepatitis. Toxicologic pathology. 2013;41(2):343–60. doi: 10.1177/0192623312468517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi-Barve S, Kirpich I, Cave MC, Marsano L, McClain CJ. Alcoholic, Non-alcoholic and Toxicant-Associated Steatohepatitis: Mechanistic Similarities and Differences. Cellular and Molecular Gastroenterology and Hepatology. 2015;1(4):356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta. 2016;9 1083–99. doi: 10.1016/j.bbagrm.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaunig JE, Li X, Wang Z. Role of xenobiotics in the induction and progression of fatty liver disease. Toxicol Res (Camb). 2018;7(4):664–80. doi: 10.1039/c7tx00326a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulds CE, Trevino LS, York B, Walker CL. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol. 2017;13(8):445–57. doi: 10.1038/nrendo.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA et al. Metabolism disrupting chemicals and metabolic disorders. Reproductive toxicology. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. *.Shi H, Jan J, Hardesty JE, Falkner KC, Prough RA, Balamurugan AN et al. Polychlorinated biphenyl exposures differentially regulate hepatic metabolism and pancreatic function: Implications for nonalcoholic steatohepatitis and diabetes. Toxicol Appl Pharmacol. 2019;363:22–33. doi: 10.1016/j.taap.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated how PCBs elicited varying effects on the liver and pancreas depending on their dioxin-like or non-dioxin-like nature.
- 13.Angrish MM, Kaiser JP, McQuene C, Chorley B. Tipping the Balance: Hepatotoxicity and the Four Apical Key Events of Hepatic Steatosis. Toxicological Sciences. 2016;150(2):261–8. [DOI] [PubMed] [Google Scholar]
- 14. **.Lang AL, Chen L, Poff GD, Ding WX, Barnett RA, Arteel GE et al. Vinyl chloride dysregulates metabolic homeostasis and enhances diet-induced liver injury in mice. Hepatol Commun. 2018;2(3):270–84. doi: 10.1002/hep4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated how vinyl chloride exacerbated liver injury caused by high fat diet feeding by inducing endoplasmic reticulum stress, leading to mitochondrial damage and altered metabolic homeostatis.
- 15.Angrish MM, McQueen CA, Cohen-Hubal E, Bruno M, Ge Y, Chorley BN. Editor’s Highlight: Mechanistic Toxicity Tests Based on an Adverse Outcome Pathway Network for Hepatic Steatosis. Toxicol Sci. 2017;159(1):159–69. doi: 10.1093/toxsci/kfx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knapen D, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L et al. Adverse outcome pathway networks I: Development and applications. Environ Toxicol Chem. 2018;37(6):1723–33. doi: 10.1002/etc.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardesty JE, Wahlang B, Falkner KC, Clair HB, Clark BJ, Ceresa BP et al. Polychlorinated biphenyls disrupt hepatic epidermal growth factor receptor signaling. Xenobiotica. 2017;47(9):807–20. doi: 10.1080/00498254.2016.1217572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. **.Hardesty JE, Al-Eryani L, Wahlang B, Falkner KC, Shi H, Jin J et al. Epidermal Growth Factor Receptor Signaling Disruption by Endocrine and Metabolic Disrupting Chemicals. Toxicol Sci. 2018;162(2):622–34. doi: 10.1093/toxsci/kfy004. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated mechanisms by which POPs antagonized the EGFR to cause signaling disruption.
- 19.Hardesty JE, Wahlang B, Falkner KC, Shi H, Jin J, Wilkey D et al. Hepatic signalling disruption by pollutant Polychlorinated biphenyls in steatohepatitis. Cell Signal. 2019;53:132–9. doi: 10.1016/j.cellsig.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. *.Hardesty JE, Wahlang B, Falkner KC, Shi H, Jin J, Zhou Y et al. Proteomic analysis reveals novel mechanisms by which polychlorinated biphenyls compromise the liver promoting diet-induced steatohepatitis. J Proteome Res. 2019;18(4):1582–1594. doi: 10.1021/acs.jproteome.8b00886. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated how PCBs acted as a first ‘hit’ to compromise the liver, making it suceptible to a second ‘hit’ such as high fat diet.
- 21.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–5. [DOI] [PubMed] [Google Scholar]
- 22.Fedeli U, Girardi P, Gardiman G, Zara D, Scoizzato L, Ballarin MN et al. Mortality from liver angiosarcoma, hepatocellular carcinoma, and cirrhosis among vinyl chloride workers. American journal of industrial medicine. 2019;62(1):14–20. doi: 10.1002/ajim.22922. [DOI] [PubMed] [Google Scholar]
- 23.Wahlang B, Song M, Beier JI, Cameron Falkner K, Al-Eryani L, Clair HB et al. Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol. 2014;279(3):380–90. doi: 10.1016/j.taap.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Registry AfTSD. ATSDR’s Substance Priority List 2017.
- 25.Agency USEP. Volatile organic compounds’ impact on indoor air quality 2017.
- 26.Cleary E, Asher M, Olawoyin R, Zhang K. Assessment of indoor air quality exposures and impacts on respiratory outcomes in River Rouge and Dearborn, Michigan. Chemosphere. 2017;187:320–9. doi: 10.1016/j.chemosphere.2017.08.091. [DOI] [PubMed] [Google Scholar]
- 27.Anders LC, Lang AL, Anwar-Mohamed A, Douglas AN, Bushau AM, Falkner KC et al. Vinyl Chloride Metabolites Potentiate Inflammatory Liver Injury Caused by LPS in Mice. Toxicol Sci. 2016;151(2):312–23. doi: 10.1093/toxsci/kfw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anders LC, Yeo H, Kaelin BR, Lang AL, Bushau AM, Douglas AN et al. Role of dietary fatty acids in liver injury caused by vinyl chloride metabolites in mice. Toxicol Appl Pharmacol. 2016;311:34–41. doi: 10.1016/j.taap.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. **.Guardiola JJ, Beier JI, Falkner KC, Wheeler B, McClain CJ, Cave M. Occupational exposures at a polyvinyl chloride production facility are associated with significant changes to the plasma metabolome. ToxicolApplPharmacol. 2016;313:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cohort study utilized plasma metabolomics to identify key protein targets that play a role in vinyl chloride toxicity including AMPK and Akt.
- 30.Philip BK, Mumtaz MM, Latendresse JR, Mehendale HM. Impact of repeated exposure on toxicity of perchloroethylene in Swiss Webster mice. Toxicology. 2007;232(1–2):1–14. doi: 10.1016/j.tox.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Cichocki JA, Furuya S, Luo YS, Iwata Y, Konganti K, Chiu WA et al. Nonalcoholic Fatty Liver Disease Is a Susceptibility Factor for Perchloroethylene-Induced Liver Effects in Mice. Toxicol Sci. 2017;159(1):102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cichocki JA, Furuya S, Konganti K, Luo YS, McDonald TJ, Iwata Y et al. Impact of Nonalcoholic Fatty Liver Disease on Toxicokinetics of Tetrachloroethylene in Mice. J Pharmacol Exp Ther. 2017;361(1):17–28. doi: 10.1124/jpet.116.238790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou YH, Cichocki JA, Soldatow VY, Scholl EH, Gallins PJ, Jima D et al. Editor’s Highlight: Comparative Dose-Response Analysis of Liver and Kidney Transcriptomic Effects of Trichloroethylene and Tetrachloroethylene in B6C3F1 Mouse. Toxicol Sci. 2017;160(1):95–110. doi: 10.1093/toxsci/kfx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–33. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 35.Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14(5):10497–538. doi: 10.3390/ijms140510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozlov AV, Lancaster JR Jr., Meszaros AT, Weidinger A. Mitochondria-meditated pathways of organ failure upon inflammation. RedoxBiol. 2017;13:170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassoun E, Mettling C. Dichloroacetate and Trichloroacetate Toxicity in AML12 Cells: Role of Oxidative Stress. J Biochem Mol Toxicol. 2015;29(11):508–12. doi: 10.1002/jbt.21720. [DOI] [PubMed] [Google Scholar]
- 38.Hassoun E, Cearfoss J, Mamada S, Al-Hassan N, Brown M, Heimberger K et al. The effects of mixtures of dichloroacetate and trichloroacetate on induction of oxidative stress in livers of mice after subchronic exposure. J Toxicol Environ Health A. 2014;77(6):313–23. doi: 10.1080/15287394.2013.864576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C et al. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265(1):73–82. doi: 10.1016/j.taap.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C et al. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143(2):242–55. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 2009;50 Suppl:S311–6. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, Gentile CL. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism. 2016;65(9):1238–46. doi: 10.1016/j.metabol.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vladykovskaya E, Sithu SD, Haberzettl P, Wickramasinghe NS, Merchant ML, Hill BG et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J Biol Chem. 2012;287(14):11398–409. doi: 10.1074/jbc.M111.320416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen WY, Wang M, Zhang J, Barve SS, McClain CJ, Joshi-Barve S. Acrolein disrupts tight junction proteins and causes ER stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am J Pathol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 46.Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10(11):637–44. doi: 10.1038/nrgastro.2013.146. [DOI] [PubMed] [Google Scholar]
- 47.Irene P, Chiara R, Laura A, Maria GD, Melania G, Cristina F et al. Lack of NLRP3-inflammasome leads to gut-liver axis derangement, gut dysbiosis and a worsened phenotype in a mouse model of NAFLD. Sci Rep. 2017;7(1):12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016;151(4):733–46 e12. doi: 10.1053/j.gastro.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuster S, Johnson CD, Hennebelle M, Holtmann T, Taha AY, Kirpich IA et al. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice. J Lipid Res. 2018;59(9):1597–609. doi: 10.1194/jlr.M083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology (Baltimore, Md). 2009;50(1):261–74. [DOI] [PubMed] [Google Scholar]
- 52.Blossom SJ, Fernandes L, Bai S, Khare S, Gokulan K, Yuan Y et al. Opposing Actions of Developmental Trichloroethylene and High-Fat Diet Coexposure on Markers of Lipogenesis and Inflammation in Autoimmune-Prone Mice. Toxicol Sci. 2018;164(1):313–27. doi: 10.1093/toxsci/kfy091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H, Biermann MH, Brauner JM, Liu Y, Zhao Y, Herrmann M. New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation. Front Immunol. 2016;7:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arumugam S, Girish Subbiah K, Kemparaju K, Thirunavukkarasu C. Neutrophil extracellular traps in acrolein promoted hepatic ischemia reperfusion injury: Therapeutic potential of NOX2 and p38MAPK inhibitors. J Cell Physiol. 2018;233(4):3244–61. doi: 10.1002/jcp.26167. [DOI] [PubMed] [Google Scholar]
- 55.Kania-Korwel I, Wu X, Wang K, Lehmler HJ. Identification of lipidomic markers of chronic 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) exposure in the male rat liver. Toxicology. 2017;390:124–34. doi: 10.1016/j.tox.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gadupudi GS, Klaren WD, Olivier AK, Klingelhutz AJ, Robertson LW. PCB126-Induced Disruption in Gluconeogenesis and Fatty Acid Oxidation Precedes Fatty Liver in Male Rats. Toxicol Sci. 2016;149(1):98–110. doi: 10.1093/toxsci/kfv215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gadupudi GS, Elser BA, Sandgruber FA, Li X, Gibson-Corley KN, Robertson LW. PCB126 Inhibits the Activation of AMPK-CREB Signal Transduction Required for Energy Sensing in Liver. Toxicol Sci. 2018;163(2):440–53. doi: 10.1093/toxsci/kfy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wahlang B, Perkins JT, Petriello MC, Hoffman JB, Stromberg AJ, Hennig B. A compromised liver alters polychlorinated biphenyl-mediated toxicity. Toxicology. 2017;380:11–22. doi: 10.1016/j.tox.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wahlang B, Barney J, Thompson B, Wang C, Hamad OM, Hoffman JB et al. Editor’s Highlight: PCB126 Exposure Increases Risk for Peripheral Vascular Diseases in a Liver Injury Mouse Model. Toxicol Sci. 2017;160(2):256–67. doi: 10.1093/toxsci/kfx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. *.Petriello MC, Hoffman JB, Vsevolozhskaya O, Morris AJ, Hennig B. Dioxin-like PCB 126 increases intestinal inflammation and disrupts gut microbiota and metabolic homeostasis. Environ Pollut. 2018;242(Pt A):1022–32. doi: 10.1016/j.envpol.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstarted how PCBs can alter the gut microbiome and promote metabolic toxicity through the gut:liver axis.
- 61.Deng P, Barney J, Petriello MC, Morris AJ, Wahlang B, Hennig B. Hepatic metabolomics reveals that liver injury increases PCB 126-induced oxidative stress and metabolic dysfunction. Chemosphere. 2019;217:140–9. doi: 10.1016/j.chemosphere.2018.10.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Angrish MM, Mets BD, Jones AD, Zacharewski TR. Dietary fat is a lipid source in 2,3,7,8-tetrachlorodibenzo-rho-dioxin (TCDD)-elicited hepatic steatosis in C57BL/6 mice. Toxicol Sci. 2012;128(2):377–86. doi: 10.1093/toxsci/kfs155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duval C, Teixeira-Clerc F, Leblanc AF, Touch S, Emond C, Guerre-Millo M et al. Chronic Exposure to Low Doses of Dioxin Promotes Liver Fibrosis Development in the C57BL/6J Diet-Induced Obesity Mouse Model. Environ Health Perspect. 2017;125(3):428–36. doi: 10.1289/EHP316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fader KA, Zacharewski TR. Beyond the Aryl Hydrocarbon Receptor: Pathway Interactions in the Hepatotoxicity of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Related Compounds. Curr Opin Toxicol. 2017;2:36–41. doi: 10.1016/j.cotox.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ et al. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem. 2013;24(9):1587–95. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. **.Clair HB, Pinkston CM, Rai SN, Pavuk M, Dutton ND, Brock GN et al. Liver Disease in a Residential Cohort With Elevated Polychlorinated Biphenyl Exposures. Toxicol Sci. 2018;164(1):39–49. doi: 10.1093/toxsci/kfy076. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cohort study reported that PCB exposures were asscoiated with a high prevelence of necrotic liver disease, insulin resistance, and pro-inflammatory cytokines, consistent with TASH.
- 67. **.Wahlang B, Prough RA, Falkner KC, Hardesty JE, Song M, Clair HB et al. Polychlorinated Biphenyl-Xenobiotic Nuclear Receptor Interactions Regulate Energy Metabolism, Behavior, and Inflammation in Non-alcoholic-Steatohepatitis. Toxicol Sci. 2016;149(2):396–410. doi: 10.1093/toxsci/kfv250. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript demonstrated that CAR and PXR knockout mice were not protected against non-dioxin-like PCB exposures, suggesting other PCB-mediated targets.
- 68.Baskin-Bey ES, Anan A, Isomoto H, Bronk SF, Gores GJ. Constitutive androstane receptor agonist, TCPOBOP, attenuates steatohepatitis in the methionine choline-deficient diet-fed mouse. World journal of gastroenterology. 2007;13(42):5635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wahlang B, Falkner KC, Clair HB, Al-Eryani L, Prough RA, States JC et al. Human receptor activation by aroclor 1260, a polychlorinated biphenyl mixture. Toxicol Sci. 2014;140(2):283–97. doi: 10.1093/toxsci/kfu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104(43):17081–6. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robles MS, Humphrey SJ, Mann M. Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell metabolism. 2017;25(1):118–27. doi: 10.1016/j.cmet.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Gunewardena S, Walesky C, Apte U. Global Gene Expression Changes in Liver Following Hepatocyte Nuclear Factor 4 alpha deletion in Adult Mice. Genomics data. 2015;5:126–8. doi: 10.1016/j.gdata.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobsen AV, Norden M, Engwall M, Scherbak N. Effects of perfluorooctane sulfonate on genes controlling hepatic fatty acid metabolism in livers of chicken embryos. Environ Sci Pollut Res Int. 2018;25(23):23074–81. doi: 10.1007/s11356-018-2358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB et al. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology. 2017;378:37–52. doi: 10.1016/j.tox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tatum-Gibbs K, Wambaugh JF, Das KP, Zehr RD, Strynar MJ, Lindstrom AB et al. Comparative pharmacokinetics of perfluorononanoic acid in rat and mouse. Toxicology. 2011;281(1–3):48–55. doi: 10.1016/j.tox.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Olsen GW, Hansen KJ, Stevenson LA, Burris JM, Mandel JH. Human donor liver and serum concentrations of perfluorooctanesulfonate and other perfluorochemicals. Environmental science & technology. 2003;37(5):888–91. [DOI] [PubMed] [Google Scholar]
- 77.Bijland S, Rensen PC, Pieterman EJ, Maas AC, van der Hoorn JW, van Erk MJ et al. Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicol Sci. 2011;123(1):290–303. doi: 10.1093/toxsci/kfr142. [DOI] [PubMed] [Google Scholar]
- 78.Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CK. PFOS-induced hepatic steatosis, the mechanistic actions on beta-oxidation and lipid transport. Biochim Biophys Acta. 2012;1820(7):1092–101. doi: 10.1016/j.bbagen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 79.Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J et al. Specific accumulation of lipid droplets in hepatocyte nuclei of PFOA-exposed BALB/c mice. Scientific reports. 2013;3:2174. doi: 10.1038/srep02174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan X, Xie G, Sun X, Li Q, Zhong W, Qiao P et al. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One. 2013;8(4):e61409. doi: 10.1371/journal.pone.0061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rebholz SL, Jones T, Herrick RL, Xie C, Calafat AM, Pinney SM et al. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicology reports. 2016;3:46–54. doi: 10.1016/j.toxrep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicologic pathology. 2012;40(2):300–11. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- 83.Darrow LA, Groth AC, Winquist A, Shin HM, Bartell SM, Steenland K. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a Mid-Ohio Valley Community. Environmental health perspectives. 2016;124(8):1227–33. doi: 10.1289/ehp.1510391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L et al. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect. 2012;120(5):655–60. doi: 10.1289/ehp.1104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. **.Bassler J, Ducatman A, Elliton M, Wen S, Wahlang B, Barnett J et al. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut. 2019; 247:1055–1063. doi: 10.1016/j.envpol.2019.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cohort study reported that environmental PFAS exposures were associated with the novel combination of increased biomarkers of hepatocyte apoptosis and decreased inflammation.
- 86.Jain RB, Ducatman A. Selective Associations of Recent Low Concentrations of Perfluoroalkyl Substances With Liver Function Biomarkers: NHANES 2011–2014 Data on US Adults Aged >/= 20 Years. J Occup Environ Med. 2018. doi: 10.1097/JOM.0000000000001532. [DOI] [PubMed] [Google Scholar]
- 87.Mann J, Reeves HL, Feldstein AE. Liquid biopsy for liver diseases. Gut. 2018;67(12):2204–12. doi: 10.1136/gutjnl-2017-315846. [DOI] [PubMed] [Google Scholar]
- 88.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–97 e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mailloux RJ, Florian M, Chen Q, Yan J, Petrov I, Coughlan MC et al. Exposure to a northern contaminant mixture (NCM) alters hepatic energy and lipid metabolism exacerbating hepatic steatosis in obese JCR rats. PLoS One. 2014;9(9):e106832. doi: 10.1371/journal.pone.0106832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pencikova K, Svrzkova L, Strapacova S, Neca J, Bartonkova I, Dvorak Z et al. In vitro profiling of toxic effects of prominent environmental lower-chlorinated PCB congeners linked with endocrine disruption and tumor promotion. Environ Pollut. 2018;237:473–86. doi: 10.1016/j.envpol.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Q, Lu M, Wang C, Du J, Zhou P, Zhao M. Characterization of estrogen receptor alpha activities in polychlorinated biphenyls by in vitro dual-luciferase reporter gene assay. Environ Pollut. 2014;189:169–75. doi: 10.1016/j.envpol.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Al-Eryani L, Wahlang B, Falkner KC, Guardiola JJ, Clair HB, Prough RA et al. Identification of Environmental Chemicals Associated with the Development of Toxicant-associated Fatty Liver Disease in Rodents. Toxicol Pathol. 2014. doi: 10.1177/0192623314549960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shan Q, Huang F, Wang J, Du Y. Effects of co-exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and polychlorinated biphenyls on nonalcoholic fatty liver disease in mice. Environ Toxicol. 2015;30(12):1364–74. doi: 10.1002/tox.22006. [DOI] [PubMed] [Google Scholar]
- 94.Kopec AK, Boverhof DR, Nault R, Harkema JR, Tashiro C, Potter D et al. Toxicogenomic evaluation of long-term hepatic effects of TCDD in immature, ovariectomized C57BL/6 mice. Toxicol Sci. 2013;135(2):465–75. doi: 10.1093/toxsci/kft156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsubara T, Tanaka N, Krausz KW, Manna SK, Kang DW, Anderson ER et al. Metabolomics identifies an inflammatory cascade involved in dioxin- and diet-induced steatohepatitis. Cell Metab. 2012;16(5):634–44. doi: 10.1016/j.cmet.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han M, Liu X, Liu S, Su G, Fan X, Chen J et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces hepatic stellate cell (HSC) activation and liver fibrosis in C57BL6 mouse via activating Akt and NF-kappaB signaling pathways. Toxicol Lett. 2017;273:10–9. doi: 10.1016/j.toxlet.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 97.Kumar J, Lind L, Salihovic S, van Bavel B, Ingelsson E, Lind PM. Persistent organic pollutants and liver dysfunction biomarkers in a population-based human sample of men and women. Environ Res. 2014;134:251–6. doi: 10.1016/j.envres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 98.Yorita Christensen KL, Carrico CK, Sanyal AJ, Gennings C. Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003–2004. Int J Hyg Environ Health. 2013;216(6):703–9. doi: 10.1016/j.ijheh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee DH, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and gamma glutamyltransferase: results from the National Health and Examination Survey 1999–2002. Clin Chem. 2006;52(9):1825–7. doi: 10.1373/clinchem.2006.071563. [DOI] [PubMed] [Google Scholar]
- 100.Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect. 2010;118(12):1735–42. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serdar B, LeBlanc WG, Norris JM, Dickinson LM. Potential effects of polychlorinated biphenyls (PCBs) and selected organochlorine pesticides (OCPs) on immune cells and blood biochemistry measures: a cross-sectional assessment of the NHANES 2003–2004 data. Environ Health. 2014;13:114. doi: 10.1186/1476-069X-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu ML, Guo YL, Hsu CC, Rogan WJ. Increased mortality from chronic liver disease and cirrhosis 13 years after the Taiwan “yucheng” (“oil disease”) incident. American journal of industrial medicine. 1997;31(2):172–5. [DOI] [PubMed] [Google Scholar]
- 103.Li MC, Tsai PC, Chen PC, Hsieh CJ, Leon Guo YL, Rogan WJ. Mortality after exposure to polychlorinated biphenyls and dibenzofurans: 30 years after the “Yucheng accident”. Environ Res. 2013;120:71–5. doi: 10.1016/j.envres.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li MC, Chen PC, Tsai PC, Furue M, Onozuka D, Hagihara A et al. Mortality after exposure to polychlorinated biphenyls and polychlorinated dibenzofurans: a meta-analysis of two highly exposed cohorts. Int J Cancer. 2015;137(6):1427–32. doi: 10.1002/ijc.29504. [DOI] [PubMed] [Google Scholar]
- 105.Beggs KM, McGreal SR, McCarthy A, Gunewardena S, Lampe JN, Lau C et al. The role of hepatocyte nuclear factor 4-alpha in perfluorooctanoic acid- and perfluorooctanesulfonic acid-induced hepatocellular dysfunction. Toxicology and applied pharmacology. 2016;304:18–29. doi: 10.1016/j.taap.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu X, Xie G, Xu X, Wu W, Yang B. Adverse bioeffect of perfluorooctanoic acid on liver metabolic function in mice. Environ Sci Pollut Res Int. 2018;25(5):4787–93. doi: 10.1007/s11356-017-0872-7. [DOI] [PubMed] [Google Scholar]
- 107.Yamaguchi M, Arisawa K, Uemura H, Katsuura-Kamano S, Takami H, Sawachika F et al. Consumption of seafood, serum liver enzymes, and blood levels of PFOS and PFOA in the Japanese population. J Occup Health. 2013;55(3):184–94. [DOI] [PubMed] [Google Scholar]
- 108.Gleason JA, Post GB, Fagliano JA. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environmental research. 2015;136:8–14. doi: 10.1016/j.envres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 109.Lin CY, Lin LY, Chiang CK, Wang WJ, Su YN, Hung KY et al. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. The American journal of gastroenterology. 2010;105(6):1354–63. doi: 10.1038/ajg.2009.707. [DOI] [PubMed] [Google Scholar]
- 110.Zhang L, Krishnan P, Ehresman DJ, Smith PB, Dutta M, Bagley BD et al. Perfluorooctane sulfonate-Choline Ion Pair Formation: A Potential Mechanism Modulating Hepatic Steatosis and Oxidative Stress in Mice. Toxicol Sci. 2016. doi: 10.1093/toxsci/kfw120. [DOI] [PubMed] [Google Scholar]
- 111.Fai Tse WK, Li JW, Kwan Tse AC, Chan TF, Hin Ho JC, Sun Wu RS et al. Fatty liver disease induced by perfluorooctane sulfonate: Novel insight from transcriptome analysis. Chemosphere. 2016;159:166–77. doi: 10.1016/j.chemosphere.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 112.Cheng J, Lv S, Nie S, Liu J, Tong S, Kang N et al. Chronic perfluorooctane sulfonate (PFOS) exposure induces hepatic steatosis in zebrafish. Aquatic toxicology. 2016;176:45–52. doi: 10.1016/j.aquatox.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 113.Lv Z, Li G, Li Y, Ying C, Chen J, Chen T et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol. 2013;28(9):532–42. doi: 10.1002/tox.20747. [DOI] [PubMed] [Google Scholar]
- 114.Bruchajzer E, Frydrych B, Sporny S, Szymanska JA. The effect of short-term intoxication of rats with pentabromodiphenyl ether (in mixture mimic commercial products). Hum Exp Toxicol. 2011;30(5):363–78. doi: 10.1177/0960327110371261. [DOI] [PubMed] [Google Scholar]
- 115.Chen H, Zhang W, Rui BB, Yang SM, Xu WP, Wei W. Di(2-ethylhexyl) phthalate exacerbates non-alcoholic fatty liver in rats and its potential mechanisms. Environ Toxicol Pharmacol. 2016;42:38–44. doi: 10.1016/j.etap.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 116.Ito Y, Nakamura T, Yanagiba Y, Ramdhan DH, Yamagishi N, Naito H et al. Plasticizers May Activate Human Hepatic Peroxisome Proliferator-Activated Receptor alpha Less Than That of a Mouse but May Activate Constitutive Androstane Receptor in Liver. PPAR research. 2012;2012:201284. doi: 10.1155/2012/201284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maranghi F, Lorenzetti S, Tassinari R, Moracci G, Tassinari V, Marcoccia D et al. In utero exposure to di-(2-ethylhexyl) phthalate affects liver morphology and metabolism in post-natal CD-1 mice. Reproductive toxicology. 2010;29(4):427–32. doi: 10.1016/j.reprotox.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 118.Kaiser JP, Lipscomb JC, Wesselkamper SC. Putative mechanisms of environmental chemical-induced steatosis. International journal of toxicology. 2012;31(6):551–63. doi: 10.1177/1091581812466418. [DOI] [PubMed] [Google Scholar]
- 119.Rodriguez-Alcala LM, Sa C, Pimentel LL, Pestana D, Teixeira D, Faria A et al. Endocrine Disruptor DDE Associated with a High-Fat Diet Enhances the Impairment of Liver Fatty Acid Composition in Rats. J Agric Food Chem. 2015;63(42):9341–8. doi: 10.1021/acs.jafc.5b03274. [DOI] [PubMed] [Google Scholar]
- 120.Liu Q, Wang Q, Xu C, Shao W, Zhang C, Liu H et al. Organochloride pesticides impaired mitochondrial function in hepatocytes and aggravated disorders of fatty acid metabolism. Sci Rep. 2017;7:46339. doi: 10.1038/srep46339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin Y, Lin X, Miao W, Wu T, Shen H, Chen S et al. Chronic exposure of mice to environmental endocrine-disrupting chemicals disturbs their energy metabolism. Toxicology letters. 2014;225(3):392–400. doi: 10.1016/j.toxlet.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 122.Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS et al. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009;4(4):e5186. doi: 10.1371/journal.pone.0005186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang XF, Zhang CH, Zheng J, Li LX, Geng TQ, Zhang Y. Potential biomarkers for monitoring the toxicity of long-term exposure to atrazine in rat by metabonomic analysis. Xenobiotica. 2018;48(3):241–9. doi: 10.1080/00498254.2017.1303221. [DOI] [PubMed] [Google Scholar]
- 124.Boue S, Tarasov K, Janis M, Lebrun S, Hurme R, Schlage W et al. Modulation of atherogenic lipidome by cigarette smoke in apolipoprotein E-deficient mice. Atherosclerosis. 2012;225(2):328–34. doi: 10.1016/j.atherosclerosis.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 125.Yuan H, Shyy JY, Martins-Green M. Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol. 2009;51(3):535–47. doi: 10.1016/j.jhep.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin C, Rountree CB, Methratta S, LaRusso S, Kunselman AR, Spanier AJ. Secondhand tobacco exposure is associated with nonalcoholic fatty liver disease in children. Environ Res. 2014;132:264–8. doi: 10.1016/j.envres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y, Dai M, Bi Y, Xu M, Xu Y, Li M et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): a population-based study in China. J Epidemiol. 2013;23(2):115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zuo Z, Chen S, Wu T, Zhang J, Su Y, Chen Y et al. Tributyltin causes obesity and hepatic steatosis in male mice. Environ Toxicol. 2011;26(1):79–85. doi: 10.1002/tox.20531. [DOI] [PubMed] [Google Scholar]
- 129.Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environmental health perspectives. 2013;121(3):359–66. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang J, Sun P, Kong T, Yang F, Guan W. Tributyltin promoted hepatic steatosis in zebrafish (Danio rerio) and the molecular pathogenesis involved. Aquatic toxicology. 2016;170:208–15. doi: 10.1016/j.aquatox.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 131.Lyssimachou A, Santos JG, Andre A, Soares J, Lima D, Guimaraes L et al. The Mammalian “Obesogen” Tributyltin Targets Hepatic Triglyceride Accumulation and the Transcriptional Regulation of Lipid Metabolism in the Liver and Brain of Zebrafish. PLoS One. 2015;10(12):e0143911. doi: 10.1371/journal.pone.0143911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng Z, Xu X, Zhang X, Wang A, Zhang C, Huttemann M et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol. 2013;58(1):148–54. doi: 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tomaru M, Takano H, Inoue K, Yanagisawa R, Osakabe N, Yasuda A et al. Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int J Mol Med. 2007;19(1):17–22. [PubMed] [Google Scholar]
- 134.Tan HH, Fiel MI, Sun Q, Guo J, Gordon RE, Chen LC et al. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J Immunotoxicol. 2009;6(4):266–75. doi: 10.1080/15476910903241704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vesterdal LK, Danielsen PH, Folkmann JK, Jespersen LF, Aguilar-Pelaez K, Roursgaard M et al. Accumulation of lipids and oxidatively damaged DNA in hepatocytes exposed to particles. Toxicology and applied pharmacology. 2014;274(2):350–60. doi: 10.1016/j.taap.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 136.Cho SJ, Echevarria GC, Lee YI, Kwon S, Park KY, Tsukiji J et al. YKL-40 is a Protective Biomarker for Fatty Liver in World Trade Center Particulate Matter-Exposed Firefighters. J Mol Biomark Diagn. 2014;5. doi: 10.4172/2155-9929.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Regnault C, Willison J, Veyrenc S, Airieau A, Meresse P, Fortier M et al. Metabolic and immune impairments induced by the endocrine disruptors benzo[a]pyrene and triclosan in Xenopus tropicalis. Chemosphere. 2016;155:519–27. doi: 10.1016/j.chemosphere.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 138.Ortiz L, Nakamura B, Li X, Blumberg B, Luderer U. In utero exposure to benzo[a]pyrene increases adiposity and causes hepatic steatosis in female mice, and glutathione deficiency is protective. Toxicology letters. 2013;223(2):260–7. doi: 10.1016/j.toxlet.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arteel GE, Guo L, Schlierf T, Beier JI, Kaiser JP, Chen TS et al. Subhepatotoxic exposure to arsenic enhances lipopolysaccharide-induced liver injury in mice. Toxicology and applied pharmacology. 2008;226(2):128–39. doi: 10.1016/j.taap.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tan M, Schmidt RH, Beier JI, Watson WH, Zhong H, States JC et al. Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicology and applied pharmacology. 2011;257(3):356–64. doi: 10.1016/j.taap.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shi X, Wei X, Koo I, Schmidt RH, Yin X, Kim SH et al. Metabolomic analysis of the effects of chronic arsenic exposure in a mouse model of diet-induced Fatty liver disease. J Proteome Res. 2014;13(2):547–54. doi: 10.1021/pr400719u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bambino K, Zhang C, Austin C, Amarasiriwardena C, Arora M, Chu J et al. Inorganic arsenic causes fatty liver and interacts with ethanol to cause alcoholic liver disease in zebrafish. Dis Model Mech. 2018;11(2). doi: 10.1242/dmm.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu Y, Yu C, Yao M, Wang L, Liang B, Zhang B et al. The PKCdelta-Nrf2-ARE signalling pathway may be involved in oxidative stress in arsenic-induced liver damage in rats. Environ Toxicol Pharmacol. 2018;62:79–87. doi: 10.1016/j.etap.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 144.Ditzel EJ, Nguyen T, Parker P, Camenisch TD. Effects of Arsenite Exposure during Fetal Development on Energy Metabolism and Susceptibility to Diet-Induced Fatty Liver Disease in Male Mice. Environmental health perspectives. 2016;124(2):201–9. doi: 10.1289/ehp.1409501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Islam K, Haque A, Karim R, Fajol A, Hossain E, Salam KA et al. Dose-response relationship between arsenic exposure and the serum enzymes for liver function tests in the individuals exposed to arsenic: a cross sectional study in Bangladesh. Environmental health : a global access science source. 2011;10:64. doi: 10.1186/1476-069x-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Poursafa P, Ataee E, Motlagh ME, Ardalan G, Tajadini MH, Yazdi M et al. Association of serum lead and mercury level with cardiometabolic risk factors and liver enzymes in a nationally representative sample of adolescents: the CASPIAN-III study. Environ Sci Pollut Res Int. 2014;21(23):13496–502. doi: 10.1007/s11356-014-3238-4. [DOI] [PubMed] [Google Scholar]
- 147.Chang LW, Yamaguchi S. Ultrastructural Changes of the Liver After Long-Term Diet of Mercury-Contaminated Tuna. Environ Res. 1974;7:16. [Google Scholar]
- 148.Hazelhoff MH, Torres AM. Gender differences in mercury-induced hepatotoxicity: Potential mechanisms. Chemosphere. 2018;202:330–8. doi: 10.1016/j.chemosphere.2018.03.106. [DOI] [PubMed] [Google Scholar]
- 149.Go YM, Sutliff RL, Chandler JD, Khalidur R, Kang BY, Anania FA et al. Low-Dose Cadmium Causes Metabolic and Genetic Dysregulation Associated With Fatty Liver Disease in Mice. Toxicol Sci. 2015;147(2):524–34. doi: 10.1093/toxsci/kfv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang S, Jin Y, Zeng Z, Liu Z, Fu Z. Subchronic Exposure of Mice to Cadmium Perturbs Their Hepatic Energy Metabolism and Gut Microbiome. Chem Res Toxicol. 2015;28(10):2000–9. doi: 10.1021/acs.chemrestox.5b00237. [DOI] [PubMed] [Google Scholar]
- 151.Sarmiento-Ortega VE, Trevino S, Flores-Hernandez JA, Aguilar-Alonso P, Moroni-Gonzalez D, Aburto-Luna V et al. Changes on serum and hepatic lipidome after a chronic cadmium exposure in Wistar rats. Arch Biochem Biophys. 2017;635:52–9. doi: 10.1016/j.abb.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 152.Kang MY, Cho SH, Lim YH, Seo JC, Hong YC. Effects of environmental cadmium exposure on liver function in adults. Occupational and environmental medicine. 2013;70(4):268–73. doi: 10.1136/oemed-2012-101063. [DOI] [PubMed] [Google Scholar]
- 153.Kelishadi R, Askarieh A, Motlagh ME, Tajadini M, Heshmat R, Ardalan G et al. Association of blood cadmium level with cardiometabolic risk factors and liver enzymes in a nationally representative sample of adolescents: the CASPIAN-III study. Journal of environmental and public health. 2013;2013:142856. doi: 10.1155/2013/142856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garcia-Arevalo M, Alonso-Magdalena P, Rebelo Dos Santos J, Quesada I, Carneiro EM, Nadal A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS One. 2014;9(6):e100214. doi: 10.1371/journal.pone.0100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiang Y, Xia W, Zhu Y, Li X, Wang D, Liu J et al. Mitochondrial dysfunction in early life resulted from perinatal bisphenol A exposure contributes to hepatic steatosis in rat offspring. Toxicology letters. 2014;228(2):85–92. doi: 10.1016/j.toxlet.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 156.Wei J, Sun X, Chen Y, Li Y, Song L, Zhou Z et al. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. J Endocrinol. 2014;222(3):313–25. doi: 10.1530/JOE-14-0356. [DOI] [PubMed] [Google Scholar]
- 157.Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan YX et al. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicology and applied pharmacology. 2015;284(2):101–12. doi: 10.1016/j.taap.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abdelhadya DH, El-Magd MA, Elbialy ZI, Saleh AA. Bromuconazole-induced hepatotoxicity is accompanied by upregulation of PXR/CYP3A1 and downregulation of CAR/CYP2B1 gene expression. Toxicol Mech Methods. 2017;27(7):544–50. doi: 10.1080/15376516.2017.1333555. [DOI] [PubMed] [Google Scholar]
- 159.Mesnage R, Renney G, Seralini GE, Ward M, Antoniou MN. Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of Roundup herbicide. Sci Rep. 2017;7:39328. doi: 10.1038/srep39328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vandenberg LN, Blumberg B, Antoniou MN, Benbrook CM, Carroll L, Colborn T et al. Is it time to reassess current safety standards for glyphosate-based herbicides? J Epidemiol Community Health. 2017;71(6):613–8. doi: 10.1136/jech-2016-208463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salla GBF, Bracht L, de Sa-Nakanishi AB, Parizotto AV, Bracht F, Peralta RM et al. Distribution, lipid-bilayer affinity and kinetics of the metabolic effects of dinoseb in the liver. Toxicol Appl Pharmacol. 2017;329:259–71. doi: 10.1016/j.taap.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 162.Frawley RP, Smith M, Cesta MF, Hayes-Bouknight S, Blystone C, Kissling GE et al. Immunotoxic and hepatotoxic effects of perfluoro-n-decanoic acid (PFDA) on female Harlan Sprague-Dawley rats and B6C3F1/N mice when administered by oral gavage for 28 days. J Immunotoxicol. 2018;15(1):41–52. doi: 10.1080/1547691X.2018.1445145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li K, Sun J, Yang J, Roberts SM, Zhang X, Cui X et al. Molecular Mechanisms of Perfluorooctanoate-Induced Hepatocyte Apoptosis in Mice Using Proteomic Techniques. Environ Sci Technol. 2017;51(19):11380–9. doi: 10.1021/acs.est.7b02690. [DOI] [PubMed] [Google Scholar]
- 164.Julien B, Pinteur C, Vega N, Labaronne E, Vidal H, Naville D et al. Evidence for estrogeno-mimetic effects of a mixture of low-dose pollutants in a model of ovariectomized mice. Environ Toxicol Pharmacol. 2018;57:34–40. doi: 10.1016/j.etap.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 165.Lin Y, Min L, Huang Q, Chen Y, Fang C, Sun X et al. The combined effects of DEHP and PCBs on phospholipase in the livers of mice. Environ Toxicol. 2015;30(2):197–204. doi: 10.1002/tox.21885. [DOI] [PubMed] [Google Scholar]
- 166.Liu D, Perkins JT, Petriello MC, Hennig B. Exposure to coplanar PCBs induces endothelial cell inflammation through epigenetic regulation of NF-kappaB subunit p65. Toxicol Appl Pharmacol. 2015;289(3):457–65. doi: 10.1016/j.taap.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bucher S, Le Guillou D, Allard J, Pinon G, Begriche K, Tete A et al. Possible Involvement of Mitochondrial Dysfunction and Oxidative Stress in a Cellular Model of NAFLD Progression Induced by Benzo[a]pyrene/Ethanol CoExposure. Oxid Med Cell Longev. 2018;2018:4396403. doi: 10.1155/2018/4396403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gadupudi GS, Klingelhutz AJ, Robertson LW. Diminished Phosphorylation of CREB Is a Key Event in the Dysregulation of Gluconeogenesis and Glycogenolysis in PCB126 Hepatotoxicity. Chem Res Toxicol. 2016;29(9):1504–9. doi: 10.1021/acs.chemrestox.6b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]