Abstract
Purpose of review:
This review provides information regarding how exosomes may contribute to environmental chemical-induced pathogenesis of chronic diseases. In connecting exosome biology to environmental toxicology and disease pathogenesis, we address vital questions regarding what constitute exosomal cargo, how toxicants influence exosomal cargo, and how environmental stimuli influence exosomal physiological and pathological functions.
Recent findings:
Recent studies in the field demonstrate that exosomal cargo changes depending on external stimuli, which has consequences for the microenvironment of recipient cells.
Summary:
Based on recent findings, it is evident that exosomal cargo comprises various biological molecules including proteins, nucleic acids, and lipid molecules. Misfolded proteins and miRNA are examples of exosomal cargo molecules that can be altered by toxicants, ultimately changing the microenvironment of recipient cells in ways that are conducive to pathological processes. It will be crucial to map out the key signaling pathways that toxicants targets to modulate exosomal cargo and their release.
Keywords: Environmental toxicants, Exosomes, Metals, Pesticides, Toxicity, Misfolded proteins, Neurotoxicity
Introduction
In recent years, extracellular vesicles (EVs) have gained substantial importance in understanding not only various disease processes, but also how to target certain diseases therapeutically. EVs are small membrane-bound vesicles released from various cells of organisms ranging from prokaryotes to higher, multicellular eukaryotes, including plants, which are proficient at performing the intra- and intercellular communications vital to the normal functioning of tissues and multiple organ systems. Apoptotic bodies, macro-vesicles and exosomes are three main subclasses of EVs. Each of these subclasses are defined based on size, biogenesis, cellular origin, and composition. The largest EVs are apoptotic bodies (500–4000 nm), which are produced during programmed cell death by the outward budding of the plasma membrane (1). Macro-vesicles, which are smaller than apoptotic bodies, range in size from 50–1000 nm and are also formed by outer budding of the cell membrane (1). Exosomes, the smallest of EVs ranging in size from approximately 50–150 nm (2) were first discovered in 1985 by Pan et al. (3). Exosomes have an endocytic origin, they are called intraluminal vesicles while they are inside multivesicular bodies. They are released into the extracellular space by the merging of multivesicular bodies to the plasma membrane. Multiple published review articles (4–6) have covered biogenesis of exosomes in-depth therefore it will not be discussed in this review.
Exosomes were once thought to just be garbage bags carrying away unwanted lipids and proteins and other metabolites. Accumulating evidence now supports crucial roles for exosomes in cellular communication and molecular transport with wide-ranging human health implications linked to oncogenesis, immune activation as well as neurotoxicity (7, 8). This review will summarize how exposure to environmental toxicants modifies exosomal cargo and its significance to different disease conditions.
Exosomal composition
In this section, we review the structural features and biochemical contents of exosome cargo. Multiple techniques, such as trypsin digestion and mass spectrometry, FACS (fluorescence-activated cell sorting), and Western blotting, have been used to identify the composition of exosomes. Such efforts have identified a wide range of proteins found to be specific to exosomal cargo. Interestingly, one study found that 80% of the proteins composing dendritic cell-derived exosomes is conserved across both mice and humans (6). A complete list of the proteins composing exosomes is difficult to obtain as exosomal composition depends on their cell type of origin. For example, exosomes from antigen-presenting cells contain MHC class I and II proteins (9). CD86, which is a key T-cell co-stimulatory protein, is found in exosomes derived from dendritic cells (2). Exosomes from T-cells are particularly enriched with T-cell receptors. Exosomes also contain specific cytosolic and membrane proteins that can serve as exosomal markers. Such proteins include integrins, flotillins, heat shock proteins (HSC90, HSC70), tetraspanins (CD9, CD81, CD81 and CD63), as well as most other abundantly found protein families such as membrane transport and fusion proteins (annexins and RABs), signal transduction molecules (14–3-3, protein kinases and heterotrimeric G proteins), and metabolic enzymes (pyruvate kinases, peroxidases, enolase-1 and lipid kinases) are also found in exosomes (6). Immunoglobulins (ICAM1/CD54, A33 antigen, P-selectin), cytoskeletal proteins (actin, tubulin, actin-binding protein), cell surface peptidases (CD26, CD13) have also been reported in exosomal cargo (6). Saccharide groups along with polylactosamine, mannose, complex N-linked glycans, α−2,6 sialic acid can be found on the exosomal surface (2). Exosomes also carries pathological protein molecules that aids in diseases progression (7). Importantly, cell surface proteins likely serve another putative role, that of determining the specific recipient cell type to be targeted by the exosome (10).
In addition to proteins, exosomes are also found to contain substantial amounts of miRNA, mRNA, other non-coding RNAs (11). These molecules not only get released through exosomes but can also be transferred between cells where they can potentially regulate gene expression in recipient cells. It’s been suggested that the miRNA packing of exosomes can be induced by extracellular stimuli. Our lab found that exposure to the environmental toxicant manganese (Mn) changes the total exosomal load of small RNAs as well as the expression of certain miRNAs relative to controls (11). So far, researchers have reported approximately 2800 miRNAs, 9700 proteins, 3400 mRNAs, and about 1000 lipids in the exosomal cargos isolated from various organisms (12).
Exosomal functions
Biological roles
Exosomes are suspected of playing important roles in regulating normal biological functions such as tissue repair, maintenance of stem cells, blood coagulation, and immune surveillance (2). An immune response may depend in part on dendritic cell-derived exosomes, which express MHC class I and II molecules that activate T-cells such as CD4+ and CD8+ (13). Their regulatory functions stem from their ability to transport and deliver cargo intercellularly including mRNA, miRNA, transcription factors, infectious particles, as well as oncogenes. Exosome-mediated transmission occurs by their merging with the plasma membranes of recipient cells or by directly stimulating cell-surface receptors of neighboring cells (2, 14). Exosomes are capable of overpowering natural killer cells and the activity of CD8+ cells, stimulating or obstructing regulatory T-cell function and monocyte activation (15).
Exosomes are also proficient immune modulatory particles. Indeed, in 1996, Dr. Raposo was the first to report on the involvement of exosomes in cell-to-cell communication in an in vivo system by showing that exosomes released from B lymphoblastoid cells contain MHC class II particles that can constrain the T-cell response (16). Only after this discovery of exosomes functioning as antigen presentation in immune cells, other researchers started looking into their role in the pathogenesis and progression of multiple disease conditions such as carcinomas as well as infectious, neurodegenerative, and cardiovascular diseases (8, 17, 18).
Pathological roles
As mentioned above, exosomes contain a diverse array of particles as cargo, including miRNA, RNA, proteins, etc. Here in this section, we will review the emerging connections between particle-loaded exosomes and the pathogenesis and progression of various diseases.
Carcinogenesis
Cancer research has paved the way in understanding the vital role of exosome signaling in health and disease. Crucial questions in cancer research include not only how cancer cells grow, but also how they escape immune detection and metastasize? Exosomes from cancer cells contain many oncogenic nucleic acids and proteins. These molecules can not only diminish the immune response, but can also modulate recipient cell activity, proliferation, and gene expression in ways that can promote tumorigenesis, carcinogenesis, and metastasis. Exosomes originating from prostate cancer cells contain mRNA (K-ras, H-ras), oncogenic proteins, and certain miRNAs (miR-155b, 125b and 130b) capable of inducing the neoplastic transformation of ASCs (adipose-derived stem cells) (19, 20). When exposed to tumor-derived exosomes, endothelial cells can be activated to promote angiogenesis as well as thrombosis. Ohyashiki et al. (21) reported that exosomes derived from K562 (leukemia) cells contain miR-92a, a gene regulatory element that, by targeting integrin α5, improves tube formation and the migration of endothelial cells. Exosomes containing miR-210 released from hypoxic K562 cells stimulate angiogenesis in endothelial cells (22). Granger et al. (23) demonstrated that exosomes released from renal cancer CD105+ stem cells contain about 57 miRNAs involved in regulating metastatic as well as angiogenic activities.
Exosomes play a role in generating an immunosuppressive microenvironment by weakening the function of natural killer cells and effector T cells, hindering the differentiation of dendritic cells, stimulating the activity of regulatory T cells, and intensifying myeloid-derived suppressor cells (2). Tumor-derived exosomes promote tumor progression by assembling neutrophils and distorting the M2 polarization of macrophages (2). Moreover, exosomes from tumor cells can help other tumor cells develop resistance against drugs through multiple pathways, including the exosomal delivery of certain miRNAs and multidrug-resistant proteins, or by nullifying antibody-based drugs and transferring anticancer drugs.
Neurological diseases
One of the crucial characteristics of neurological diseases includes accumulation of misfolded proteins in specific areas of brain. Exosomes play a crucial role in brain and nervous system development by transmitting donor cell information throughout the body. On the other hand, due to their capacity to carry pathogenic molecules, exosomes have also been labeled the “Trojan horse of neurodegeneration” (24) given their potential role in disease pathogenesis. Neurological disorders such as Alzheimer’s diseases (AD), Huntington’s Disease (HD), Parkinson’s Disease (PD), prion diseases and amyotrophic lateral sclerosis (ALS) are characterized by the accumulation of misfolded disease-specific proteins for example, β-amyloid (Aβ), tau, Huntingtin, α-synuclein (αSyn), prion protein (PrP). Pathogenic forms of such proteins have been found in exosomes, thus implicating them as a mechanism for disease transmission between cells (7, 25, 26). In PD, pathogenic αSyn can be secreted through exosomes and can be taken up by neighboring cells (27). Sardar Sinha et al. (7) showed that exosomes transported between neurons in AD patients contain high levels of toxic amyloid-β oligomers. They also demonstrated that blocking the development, release and/or uptake of such exosomes decreased the spread of these oligomers and their toxic effects. Fevrier et al. (28) found that the conversion of normal prion proteins to infectious prion protein is facilitated by the exosomal spreading of contagious prion proteins. Misfolded forms of TDP-43 (TAR-DNA-binding protein-43) and SOD1 (Superoxide Dismutase 1) are associated with ALS, and similar to other proteopathic proteins, have been found in exosomes, including those derived from the CSF (cerebrospinal fluid) of ALS patients (29–31).
Traumatic brain injury (TBI) is frequently incurred during military combat and in contact sports, like boxing and football (32), and such head injuries can be repetitive in nature and have the potential to promote chronic, life-long neurological conditions. Several studies have shown that TBI increases the risk of Lewy body accumulation, PD, and AD (33, 34). Taylor and Gercel-Taylor (35) reported an increase in exosomes circulating in the blood of TBI patients. In a cell model of mild TBI, Ko et al. (36) showed that cultured neurons subjected to stretch injury also exhibit an increase in exosomes. These studies suggest that exosomes may serve as a biomarker for diagnosing TBI.
Toxicants, Exosomes and related Diseases
In this section, we will discuss studies showing how environmental toxicants alter exosome cargo, their release and to what extent these effects contribute to toxicant-mediated disease pathogenesis. One such study done by Xu et al. (37) demonstrated how arsenite acts as an environmental carcinogen capable of turning healthy cells neoplastic. By utilizing a non-contact co-culture system that physically separated arsenite-transformed human bronchial epithelial (HBE) cells from normal HBE cells in the same growth media, the authors determined that the arsenite-transformed HBE cells released exosomes rich in miR-21 into the shared media, which transformed the normal HBE cells in to malignant cells. Similar findings were reported by Chen et al. (38), who conducted experiments on human hepatic epithelial cells known as L-02 cells. Chen and colleagues found that exosomes released from arsenite-transformed cells contain more miR-155 than the normal neighboring cells that absorb these exosomes. The miR-155 upregulates levels of the proinflammatory cytokines IL-8 and IL-6 and the resulting chronic inflammation has been linked to DNA damage and carcinogenesis.
Exosomes have been suggested as the drivers behind ionizing radiation-mediated carcinogenesis. In a study using BHY and FaDu cells to explore how radiation influences the progression of squamous head and neck carcinoma, Mutschelknaus et al. (39) reported that ionizing radiation promotes the transmission of exosomal cargo that induces AKT signaling-mediated migration in neighboring recipient cells. Proteomics analysis of the study found that 36 proteins were down-regulated, and 39 proteins upregulated when comparing exosomes isolated from irradiated and non-exposed cells. Cigarette smoke (CS) is another potential toxicant that can promote carcinogenesis. Exposure to CS can stimulate lung carcinoma by influencing miRNAs that are linked to the EGFR function (40). Cordazzo et al. (41) reported that persistent exposure to CS can lead human mononuclear cells to release macrovesicles and exosomes that are capable of stimulating the epithelia to trigger a proinflammatory response.
Heavy metals, such as lead, cadmium, mercury, manganese (Mn), copper (Cu), and arsenic as well as neurotoxic pesticides, are considered neurotoxic elements and significant resources have been expended toward understanding their role in neurological disorders (8, 42–52). The protein α-synuclein (αSyn), is a major constituent of Lewy bodies in the pathogenesis of PD, and we previously reported that Mn and dieldrin interactions with αSyn modulate their neurotoxicity. Our laboratory recently discovered that Mn induces the exosomal release of misfolded αSyn and that exposure to misfolded αSyn-rich exosomes isolated from Mn-exposed hosts can trigger a neuroinflammatory as well as neurotoxic response in both cell and animal models (53). In another study from our laboratory, we found that Mn stimulates an inflammatory response by activating the NLRP3 inflammasome as well as by triggering the release of ASC through exosomes (54). Moreover, αSyn-containing exosomes are known to promote αSyn aggregation in recipient cells, thereby promoting disease progression and pathogenesis (55). We also detected misfolded αSyn and ASC in the serum samples of welders exposed to fumes containing Mn (54), demonstrating the translational relevance of our mechanistic studies in cell and animal models.
Rotenone is one of the neurotoxic pesticides capable of inducing the expression and accumulation of αSyn in the brain where it can lead to nigral degeneration in neurological diseases (56). In 2010, Pan-Montojo et al. (57) showed that chronic intragastric injection of rotenone in wild-type mice promotes the accumulation of pathogenic αSyn in the enteric nervous system, the dorsal motor nucleus of the vagus nerve, and the substantia nigra. In a follow-up study (58), the same group showed that exposure to rotenone triggers enteric neurons to release αSyn through exosomes, which are then taken up by presynaptic sympathetic neurites leading to αSyn accumulation and the spread of PD pathology. The herbicide paraquat is another environmental toxicant shown to trigger the accumulation of cytotoxic levels of TDP-43 (59). Similar to other studies reporting TDP-43 to be enriched in exosomes (30), these results suggest that agrochemicals like paraquat can promote disease pathogenesis, including ALS, by stimulating the release of misfolded TDP-43-enriched exosomes.
Given the role of viruses and bacterial particles in diseases pathogenesis, it is important to look into the role that they play in the formation and release of extracellular vesicles including exosomes. Human pathogens including the Ebola virus, the HIV (Human Immunodeficiency Virus), HSV1 (herpes simplex virus 1) and the rabies virus have been found to hijack ESCRT (Endosomal sorting complexes required for the transport) pathway, which is involved in endosomal vesicles biogenesis (60, 61). Exosomes have been reported to play various different roles following viral infection including, spread of viral infection, manipulation of the microenvironment as well as modulation of immunity (61). Moreover, exosomes derived from plants are reported to shape Gut Microbiota (62).
Conclusion
Results from studies reviewed here indicate that environmental toxicants are capable of triggering disease pathogenesis through exosomes as mediators by altering both their cargo and release (Fig. 1 and Table 1). Because of their ability to transfer biologically active molecules such as proteins, nucleic acids, and lipids, exosomes play a major role in influencing numerous physiological as well as pathological functions. Ultra-sensitive assays are now available with the capacity to detect exosomes in most biofluids, such as saliva, blood (serum, plasma), cerebrospinal fluid, and urine, thereby making them attractive candidates for biomarkers of disease progression. In this regard, we demonstrated that the αSyn RT-QuIC (real-time quaking-induced conversion) assay can be used to detect low pg concentrations of misfolded samples in cell, animal, and human samples (63). Meanwhile, efforts are also underway to explore the use of exosomes as a platform for delivery of therapeutic agents (64). In addition to their nanoscale size, other reasons why exosomes hold magnificent therapeutic potential include: 1) ability to cross the blood-brain barrier, 2) reduced threat of immune response against self-exosomes, and 3) a stable membrane that facilitates their uptake in delivering cargo from cell-to-cell. Future studies should be devoted to understanding the underlying molecular signaling pathways that, when intervened by environmental toxicants, alter exosomal cargo and/or their release, and once taken up, how exosomes influence the microenvironments of recipient cells to favor disease pathogenesis.
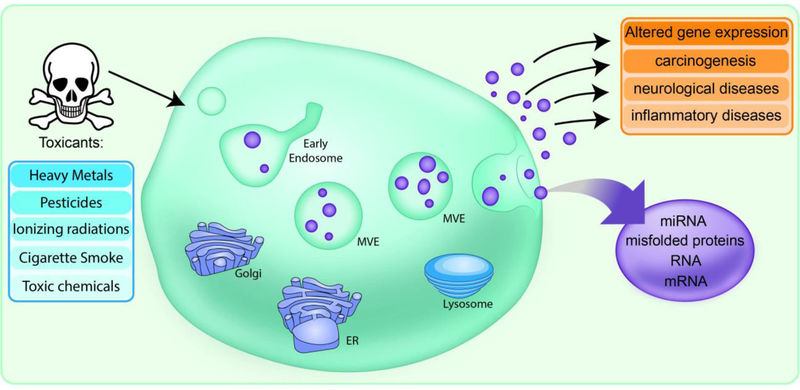
Figure 1. Schematic representation of toxicant-induced exosomal release and the role of exosomes as mediators of toxicity.
Exposure to toxicants including heavy metals, pesticides, ionizing radiations, cigarette smoke, and other toxic chemicals can induce the alteration of exosomal content, such as miRNA, mRNA, and misfolded proteins. These toxicants are also capable of influencing the exosomal release, which leads to altered gene expression, carcinogenesis and ultimately influencing various disease conditions.
Table 1.
Toxicant-influenced exosomal cargo and related disease pathogenesis.
| Toxicants | Type of model systems used | Exosomes Isolation method | Exosomal Cargo | Disease Pathogenesis | References |
|---|---|---|---|---|---|
| Manganese | MN9D cells, Primary microglial cells, LUHMES cells, The immortalized WT (C57BL/6) murine microglial cell line (WTMC), C57BL/6 mice, Human αSyn-A53T–overexpressing BAC transgenic rats | Ultracentrifugation, ExoQuick precipitation. | αSyn, ASC | Parkinson’s disease, synucleopathies, neurodegenerative disease | 53, 54 |
| Ionizing radiation | Head & neck cancer cell lines BHY & FaDu. | Ultracentrifugation | miRNAs, integrins and chemokines | Tracheal carcinoma | 39 |
| Cigarette smoke | Human mononuclear cells, airway epithelial cells | Ultracentrifugation | miRNAs | Lung carcinoma | 40, 41 |
| Arsenite | human bronchial epithelial (HBE) cells, human hepatic epithelial (L-02) cells | ExoQuick precipitation | miR-21, miR- 155 | Lung carcinogenesis, liver carcinogenesis | 37, 38 |
| Rotenone | C57BL/6J mice | Ultracentrifugation | αSyn | Synucleopathies, Parkinson’s disease, other neurodegenerative disease | 57,58 |
| Paraquat | Human neuroblastoma SH- SY5Y cells | ExoQuick precipitation | TDP-43, Cu/Zn SOD | Amyotrophic lateral sclerosis | 30, 59 |
Acknowledgement
This review was supported by National Institutes of Health R01 grants [ES026892, ES019267, and ES025991] to A.G.K. and NS088206 to A.K. The W. E. Lloyd Endowed Chair and Eminent Scholar in Veterinary Medicine and Armbrust Endowment to A.G.K. and the Salisbury Endowed Chair to A.K. are also acknowledged. We also thank Gary Zenitsky for assistance in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Dharmin Rokad, Huajun Jin, and Arthi Kanthasamy each declare no potential conflicts of interest.
Vellareddy Anantharam reports a startup company established by the PI of PK Biosciences.
Anumantha G. Kanthasamy reports a startup company established by the PI of PK Biosciences.
Competing interests
A.G.K. and V.A. have an equity interest in PK Biosciences Corporation located in Ames, IA. The terms of this arrangement have been reviewed and approved by Iowa State University in accordance with its conflict of interest policies. Other authors declare no competing financial interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References:
- 1.Revenfeld AL, Baek R, Nielsen MH, Stensballe A, Varming K, Jorgensen M. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin Ther 2014;36(6):830–46. [DOI] [PubMed] [Google Scholar]
- 2.Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacol Sin 2018;39(4):501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 1985;101(3):942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013;126(Pt 24):5553–65. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255–89. [DOI] [PubMed] [Google Scholar]
- 6.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2(8):569–79. [DOI] [PubMed] [Google Scholar]
- 7.••Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjo C, Larsson M, Lannfelt L, et al. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 2018;136(1):41–56.This study reports that exosomes isolated from the post-mortem brains of Alzheimer’s disease patients contain elevated levels of amyloid-beta oiligomers, thereby promoting neuron-to-neuron transmission of toxic protein species.
- 8.•Harischandra DS, Ghaisas S, Rokad D, Kanthasamy AG. Exosomes in Toxicology: Relevance to Chemical Exposure and Pathogenesis of Environmentally Linked Diseases. Toxicol Sci 2017;158(1):3–13.This article provides thorough description of the role of chemicals and exosomes in disease pathogenesis.
- 9.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014;14(3):195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays 2011;33(10):737–41. [DOI] [PubMed] [Google Scholar]
- 11.•Harischandra DS, Ghaisas S, Rokad D, Zamanian M, Jin H, Anantharam V, et al. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: Relevance to alpha-synuclein misfolding in metal neurotoxicity. Neurotoxicology 2018;64:267–77.This study reports that manganese alters exosomal cargo and highlights the differential expression of miRNA in a cell culture model of Parkinson’s disease.
- 12.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol 2016;428(4):688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998;4(5):594–600. [DOI] [PubMed] [Google Scholar]
- 14.Gonda A, Kabagwira J, Senthil GN, Wall NR. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol Cancer Res 2019;17(2):337–47. [DOI] [PubMed] [Google Scholar]
- 15.ELA S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013;12(5):347–57. [DOI] [PubMed] [Google Scholar]
- 16.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183(3):1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 2006;103(30):11172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527(7578):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol 2015;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells 2014;32(4):983–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013;32(22):2747–55. [DOI] [PubMed] [Google Scholar]
- 22.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem 2013;288(48):34343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res 2011;71(15):5346–56. [DOI] [PubMed] [Google Scholar]
- 24.Ghidoni R, Benussi L, Binetti G. Exosomes: the Trojan horses of neurodegeneration. Med Hypotheses 2008;70(6):1226–7. [DOI] [PubMed] [Google Scholar]
- 25.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 2012;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo BB, Bellingham SA, Hill AF. Stimulating the Release of Exosomes Increases the Intercellular Transfer of Prions. J Biol Chem 2016;291(10):5128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol 2015;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A 2004;101(26):9683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson AG, Gray E, Heman-Ackah SM, Mager I, Talbot K, Andaloussi SE, et al. Extracellular vesicles in neurodegenerative disease - pathogenesis to biomarkers. Nat Rev Neurol 2016;12(6):346–57. [DOI] [PubMed] [Google Scholar]
- 30.Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep 2013;4(1):124–34. [DOI] [PubMed] [Google Scholar]
- 31.Grad LI, Pokrishevsky E, Silverman JM, Cashman NR. Exosome-dependent and independent mechanisms are involved in prion-like transmission of propagated Cu/Zn superoxide dismutase misfolding. Prion 2014;8(5):331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahrami N, Sharma D, Rosenthal S, Davenport EM, Urban JE, Wagner B, et al. Subconcussive Head Impact Exposure and White Matter Tract Changes over a Single Season of Youth Football. Radiology 2016;281(3):919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, et al. Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol 2016;73(9):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 2015;77(6):987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DD, Gercel-Taylor C. Exosome platform for diagnosis and monitoring of traumatic brain injury. Philos Trans R Soc Lond B Biol Sci 2014;369(1652). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko J, Hemphill MA, Gabrieli D, Wu L, Yelleswarapu V, Lawrence G, et al. Smartphone-enabled optofluidic exosome diagnostic for concussion recovery. Sci Rep 2016;6:31215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Luo F, Liu Y, Shi L, Lu X, Xu W, et al. Exosomal miR-21 derived from arsenite-transformed human bronchial epithelial cells promotes cell proliferation associated with arsenite carcinogenesis. Arch Toxicol 2015;89(7):1071–82. [DOI] [PubMed] [Google Scholar]
- 38.Chen C, Luo F, Liu X, Lu L, Xu H, Yang Q, et al. NF-kB-regulated exosomal miR-155 promotes the inflammation associated with arsenite carcinogenesis. Cancer Lett 2017;388:21–33. [DOI] [PubMed] [Google Scholar]
- 39.Mutschelknaus L, Peters C, Winkler K, Yentrapalli R, Heider T, Atkinson MJ, et al. Exosomes Derived from Squamous Head and Neck Cancer Promote Cell Survival after Ionizing Radiation. PLoS One 2016;11(3):e0152213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldkorn T, Filosto S. Lung injury and cancer: Mechanistic insights into ceramide and EGFR signaling under cigarette smoke. Am J Respir Cell Mol Biol 2010;43(3):259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordazzo C, Petrini S, Neri T, Lombardi S, Carmazzi Y, Pedrinelli R, et al. Rapid shedding of proinflammatory microparticles by human mononuclear cells exposed to cigarette smoke is dependent on Ca2+ mobilization. Inflamm Res 2014;63(7):539–47. [DOI] [PubMed] [Google Scholar]
- 42.Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol 2014;54:141–64. [DOI] [PubMed] [Google Scholar]
- 43.Rokad D, Ghaisas S, Harischandra DS, Jin H, Anantharam V, Kanthasamy A, et al. Role of neurotoxicants and traumatic brain injury in alpha-synuclein protein misfolding and aggregation. Brain Res Bull 2017;133:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology 2005;26(4):701–19. [DOI] [PubMed] [Google Scholar]
- 45.Afeseh Ngwa H, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol 2011;256(3):227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langley MR, Ghaisas S, Ay M, Luo J, Palanisamy BN, Jin H, et al. Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson’s disease: Relevance to gene and environment interactions in metal neurotoxicity. Neurotoxicology 2018;64:240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi CJ, Kanthasamy A, Anantharam V, Kanthasamy AG. Interaction of metals with prion protein: possible role of divalent cations in the pathogenesis of prion diseases. Neurotoxicology 2006;27(5):777–87. [DOI] [PubMed] [Google Scholar]
- 48.Song C, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG. Paraquat induces epigenetic changes by promoting histone acetylation in cell culture models of dopaminergic degeneration. Neurotoxicology 2011;32(5):586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarkar S, Malovic E, Harischandra DS, Ngwa HA, Ghosh A, Hogan C, et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology 2018;64:204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DS, Jin H, Anantharam V, Gordon R, Kanthasamy A, Kanthasamy AG. p73 gene in dopaminergic neurons is highly susceptible to manganese neurotoxicity. Neurotoxicology 2017;59:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.•Kanthasamy AG, Choi C, Jin H, Harischandra DS, Anantharam V, Kanthasamy A. Effect of divalent metals on the neuronal proteasomal system, prion protein ubiquitination and aggregation. Toxicol Lett 2012;214(3):288–95.This study reports on the role of the divalent metal cadmium in proteosomal function, prion protein aggregation and neurotoxicity.
- 52.Choi CJ, Anantharam V, Martin DP, Nicholson EM, Richt JA, Kanthasamy A, et al. Manganese upregulates cellular prion protein and contributes to altered stabilization and proteolysis: relevance to role of metals in pathogenesis of prion disease. Toxicol Sci 2010;115(2):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.••Harischandra DS, Rokad D, Neal ML, Ghaisas S, Manne S, Sarkar S, et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci Signal 2019;12(572).This study is the first to establish the cell-to-cell transmission of misfolded alpha-synuclein protein as a possible mechanism underlying manganese-induced neurodegenerative disease.
- 54.••Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal 2019;12(563).This study is the first to report NLRP3 inflammasome activation and exosomal release of ASC as possible mechanisms underlying manganese-induced neurotoxicity.
- 55.Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, et al. Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem 2015;290(5):2969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 2000;3(12):1301–6. [DOI] [PubMed] [Google Scholar]
- 57.Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 2010;5(1):e8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, Pal A, et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2012;2:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A 2009;106(18):7607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Votteler J, Sundquist WI. Virus budding and the ESCRT pathway. Cell Host Microbe 2013;14(3):232–41.This review provides key information on how virus particle hijack ESCRT pathway for virus budding.
- 61.Alenquer M, Amorim MJ. Exosome Biogenesis, Regulation, and Function in Viral Infection. Viruses 2015;7(9):5066–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmadi Badi S, Moshiri A, Fateh A, Rahimi Jamnani F, Sarshar M, Vaziri F, et al. Microbiota-Derived Extracellular Vesicles as New Systemic Regulators. Front Microbiol 2017;8:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.•Manne S, Kondru N, Hepker M, Jin H, Anantharam V, Lewis M, et al. Ultrasensitive Detection of Aggregated alpha-Synuclein in Glial Cells, Human Cerebrospinal Fluid, and Brain Tissue Using the RT-QuIC Assay: New High-Throughput Neuroimmune Biomarker Assay for Parkinsonian Disorders. J Neuroimmune Pharmacol 2019.This study validates new diagnostic tool for detecting ultralow concentrations of protein aggregates in protein misfolding diseases.
- 64.Yamashita T, Takahashi Y, Takakura Y. Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol Pharm Bull 2018;41(6):835–42. [DOI] [PubMed] [Google Scholar]