Abstract
Purpose:
Among eyes with proliferative diabetic retinopathy (PDR), identify whether baseline characteristics impact the benefit of ranibizumab over panretinal photocoagulation (PRP) in DRCR.net Protocol S.
Methods:
Participants had PDR, visual acuity (VA) of 20/320 or better, and no prior PRP. Eyes were randomized to PRP or intravitreous 0.5-mg ranibizumab.
Results:
Ranibizumab was superior to PRP for change in VA and development of vision-impairing central-involved diabetic macular edema (CI-DME) over 2 years (P < .001). Among 25 characteristics, there were none in which participants assigned to PRP had superior outcomes relative to ranibizumab-assigned participants. The relative benefit of ranibizumab over PRP for change in VA appeared greater in participants with higher mean arterial pressure (P = .03), without prior focal/grid laser (P = .03), with neovascularization of the disc and elsewhere on clinical exam (P = .04), and with more advanced PDR on photographs (P = .02). For development of vision-impairing CI-DME, the relative benefit of ranibizumab over PRP appeared greater among non-white participants (P = .01) and those with higher mean arterial pressure (P = .01).
Conclusion:
There were no characteristics identified in which outcomes were superior with PRP compared with ranibizumab. These exploratory analyses provide additional support that ranibizumab may be a reasonable alternative to PRP for PDR over a two-year period.
INTRODUCTION
Proliferative diabetic retinopathy (PDR) remains a common and potentially vision-impairing complication of diabetes, particularly among individuals who are unable to control glycemia, have longer duration of disease, or have other systemic and ocular comorbidities.1,2 Panretinal photocoagulation (PRP) has greatly reduced the frequency with which patients suffer severe vision loss from PDR and has been the standard therapy for several decades.3
The Diabetic Retinopathy Clinical Research Network (DRCR.net) conducted a randomized multicenter trial, Protocol S, comparing intravitreous ranibizumab injections versus PRP for management of PDR. At 2 years, ranibizumab was non-inferior to PRP for the primary outcome of change in visual acuity from baseline (5-letter non-inferiority margin).4 Furthermore, ranibizumab was superior to PRP for change in visual acuity over 2 years (area under the curve).4 In addition, development of vision-impairing (20/32 or worse) central-involved diabetic macular edema (CI-DME) over 2 years was significantly less likely in the ranibizumab group.4 At 2 years, ranibizumab also was superior to PRP for preserving visual field, avoiding vitrectomy, and lowering rates of PDR-worsening events (manifested as vitreous hemorrhage, retinal detachment, or anterior segment neovascularization).4,5 Despite these clinical benefits, an economic analysis suggested that ranibizumab was not cost effective through 2 years for the treatment of PDR relative to PRP, unless DME was simultaneously present (in which case anti-VEGF is indicated to simultaneously treat DME).6 Additional follow up will determine if these differences persist through 5 years and further our understanding of potential long-term treatment burdens.
Pre-planned analyses from Protocol S evaluated whether baseline factors affected the relative treatment group difference for change in visual acuity from baseline at two years. Treatment group interactions were considered for the presence of the following baseline characteristics: vision-impairing CI-DME, CI-DME irrespective of visual acuity, visual acuity, prior DME treatment, and diabetic retinopathy severity.4 The one and only possible interaction identified involved prior DME treatment and suggested that ranibizumab was superior to PRP among treatment-naïve eyes but that PRP was perhaps favored among eyes with prior DME treatment. Note, however, that ranibizumab was non-inferior (but not superior) to PRP in the primary analysis (change in visual acuity at 2 years). As these pre-planned analyses were limited in scope, many unanswered questions remain as to whether other ocular and systemic factors may affect the relative efficacy of ranibizumab compared with PRP in the management of PDR.
Using data from Protocol S, this report addresses whether there are any baseline characteristics that impact the relative treatment benefit of ranibizumab versus PRP for outcomes that were not included in the pre-planned analysis described above. The outcomes evaluated here are change in visual acuity over 2 years and development of vision-impairing CI-DME, two outcomes for which ranibizumab was superior to PRP in the overall cohort.
METHODS
Study procedures were reported previously and are briefly summarized here.4 The protocol is available on the DRCR.net website (www.drcr.net; accessed October 10, 2017). The study adhered to the tenets of the Declaration of Helsinki and was approved by multiple institutional review boards. Study participants provided written informed consent. Fifty-five clinical sites enrolled 305 participants (394 study eyes) with PDR in at least 1 eye, no prior PRP, and best corrected visual acuity letter score at least 24 (Snellen equivalent 20/320 or better) using the Electronic-Early Treatment Diabetic Retinopathy Study (E-ETDRS) test.4,7 Participants were randomly assigned to prompt PRP or intravitreous ranibizumab injections with a structured retreatment algorithm. In both treatment groups, all eyes with baseline vision-impairing CI-DME received ranibizumab at baseline followed by as-needed retreatment for DME. Vision-impairing DME was defined as visual acuity letter score 78 or less (Snellen equivalent 20/32 or worse) and OCT central subfield thickness (CST) greater than 2 standard deviations above the sex- and instrument-specific norm for the population (Heidelberg Spectralis ≥ 320 µm for men and ≥ 305 µm for women, Zeiss Cirrus and Optovue RTVue ≥ 305 µm for men and ≥ 290 µm for women, Zeiss Stratus ≥ 250 µm for both sexes). Eyes without vision-impairing CI-DME at baseline in each treatment group could receive ranibizumab at investigator discretion at any time to treat DME.
All participants had assessment visits every 4 months (16, 32, 52, 68, 84, and 104 weeks). Participants assigned to ranibizumab had additional study visits every 4 weeks throughout the first year to determine the need for ranibizumab to treat PDR. Visits were extended up to 16 weeks in the second year if injections were continually deferred based on the structured retreatment algorithm. All participants receiving ranibizumab for DME at the investigator’s discretion may have had additional study visits to assess and treat DME. In the ranibizumab group, PRP was allowed if pre-specified failure criteria were met. In the PRP group, supplemental PRP was permitted if the size or amount of neovascularization increased. Best-corrected visual acuity using the E-ETDRS test was obtained at all study visits and standardized OCT images of the macula were obtained at all treatment and annual visits.
Outcomes and Analysis Cohorts
Change in visual acuity over 2 years (area under the curve of time versus change in visual acuity) was evaluated for eyes that completed the 2-year visit (328 of 394 [83%]) and was calculated using the trapezoidal rule with data from assessment visits, which were common to both treatment groups.4 This is analogous to taking a weighted average of the change in visual acuity from each assessment visit with the weights proportional to the time between visits.
Development of vision-impairing CI-DME was evaluated only in eyes without vision-impairing CI-DME at baseline (302 of 394 [77%]). Data from eyes that did not complete the 2-year visit were censored on the date of their last visit if vision-impairing CI-DME had not developed.
Both change in visual acuity over 2 years and development of vision-impairing CI-DME over 2 years were pre-specified secondary outcomes in Protocol S.
Statistical Analyses
All analyses were adjusted for baseline visual acuity (a component of each outcome) and CST (a pre-randomization factor that determined whether ranibizumab for DME was required at baseline). Twenty-five baseline characteristics were initially considered for analysis (Supplemental Table 1). To increase statistical precision, a minimum sample size of 20 eyes per treatment group was required for a characteristic to be analyzed.
To identify factors that may have affected the treatment group effect, a treatment group by baseline characteristic interaction was tested for each characteristic. To account for the correlation within participants having both eyes enrolled in the study, change in visual acuity over 2 years was analyzed with a linear mixed effects model with a random effect for participant. Likewise, development of vision-impairing CI-DME was analyzed using a marginal Cox proportional hazards regression model with a robust sandwich estimate of the covariance matrix.8
For the purpose of tabulation, continuous characteristics were dichotomized using clinically relevant cut points that approximated the median values of the full, randomized cohort. However, P values and tests of significance were based on continuous variables for continuous characteristics (i.e., retinopathy severity level, mean arterial blood pressure, etc.; Supplemental Table 1). In these exploratory analyses, there was no formal adjustment for multiple hypothesis testing and therefore P <.05 was considered suggestive, rather than definitive, evidence of a true difference. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
The distribution of baseline characteristics according to treatment group for each analysis cohort is in Supplemental Table 2. There were no meaningful differences between treatment groups.
Change in Visual Acuity Over 2 Years
Mean baseline visual acuity was 76 letters (approximate Snellen equivalent 20/32), and mean baseline CST was 255 µm (Stratus equivalent) in the cohort that completed the 2-year visit. The overall ranibizumab-PRP treatment group difference for change in visual acuity over 2 years was 4.5 letters (95% CI: 3.2 to 5.8, P <.001).
Tables 1 and 2 show the ranibizumab-PRP treatment group difference for change in visual acuity over 2 years after adjustment for baseline visual acuity and CST by baseline participant and ocular characteristics within each treatment group (unadjusted means provided in Supplemental Table 3). There were no characteristics identified in which the change in visual acuity over 2 years was superior with PRP compared with ranibizumab. However, there were several possible quantitative interactions. Among participant characteristics, the relative benefit of ranibizumab over PRP appeared to increase as mean arterial pressure increased, with a ranibizumab-PRP treatment group difference of 3.8 letters for participants with mean arterial pressure < 100 mmHg and 5.4 letters for participants with mean arterial pressure ≥ 100 mmHg (continuous P = .03). Among ocular characteristics, greater relative benefit of ranibizumab compared with PRP was potentially seen for three baseline factors. First, in eyes without vs. with prior focal/grid laser (5.1- vs 1.0-letter ranibizumab-PRP difference; P = .03). Second, in eyes with neovascularization of the disc (NVD) and elsewhere (NVE) vs. NVD or NVE only (6.7- vs. 3.6-letter ranibizumab-PRP difference; P = .04). Third, in eyes with high-risk PDR or worse (≥ ETDRS level 71) vs. moderate PDR or better (≤ ETDRS level 65) (6.1- vs. 3.8-letter ranibizumab-PRP difference; continuous P = .02, Figure 1).
Table 1.
Change in Visual Acuity Over 2 Years (Area Under the Curve) by Baseline Participant Characteristics
| Characteristic | Ranibizumab N | PRP N | Ranibizumab-PRP Adjusted Difference (95% CI) | Interaction P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 71 | 77 | 3.2 (1.2, 5.1) | .07 |
| Male | 89 | 91 | 5.6 (3.8, 7.4) | |
| Age (y) | ||||
| < 50 | 71 | 78 | 5.7 (3.8, 7.6) | .25 |
| ≥ 50 | 89 | 90 | 3.5 (1.6, 5.3) | |
| Race/ethnicity* | ||||
| White | 88 | 84 | 3.8 (1.9, 5.6) | .28 |
| Non-White | 71 | 82 | 5.2 (3.3, 7.2) | |
| Diabetes type† | ||||
| Type 1 | 38 | 30 | 4.0 (1.1, 6.9) | .63 |
| Type 2 | 117 | 131 | 4.8 (3.3, 6.3) | |
| Duration of diabetes (y) | ||||
| < 20 | 85 | 100 | 4.8 (3.1, 6.6) | .15 |
| ≥ 20 | 75 | 68 | 3.9 (1.9, 5.9) | |
| HbA1c (%)‡ | ||||
| < 9 | 90 | 86 | 3.9 (2.1, 5.7) | .30 |
| ≥ 9 | 65 | 77 | 5.1 (3.1, 7.1) | |
| Mean arterial pressure (mmHg) | ||||
| < 100 | 88 | 93 | 3.8 (2.0, 5.6) | .03 |
| ≥ 100 | 72 | 75 | 5.4 (3.4, 7.4) | |
| Hypertension | ||||
| No | 53 | 52 | 4.9 (2.7, 7.1) | .63 |
| Yes | 107 | 116 | 4.3 (2.6, 5.9) |
CI=confidence interval; PRP=panretinal photocoagulation.
Race/ethnicity unknown or not reported for 1 ranibizumab and 2 PRP eyes
Diabetes type unknown for 5 ranibizumab and 7 PRP eyes
HbA1c unavailable for 5 ranibizumab and 5 PRP eyes
Table 2.
Change in Visual Acuity Over 2 Years (Area Under the Curve) by Baseline Ocular Characteristics
| Characteristic | Ranibizumab N | PRP N | Ranibizumab-PRP Adjusted Difference (95% CI) | Interaction P Value |
|---|---|---|---|---|
| Prior treatment for DME | ||||
| No | 122 | 137 | 5.1 (3.6, 6.5) | .11 |
| Yes | 38 | 31 | 2.4 (−0.6, 5.3) | |
| Prior focal/grid laser treatment for DME | ||||
| No | 131 | 143 | 5.1 (3.7, 6.5) | .03 |
| Yes | 29 | 25 | 1.0 (−2.3, 4.4) | |
| Vitreous hemorrhage at baseline | ||||
| No | 113 | 111 | 3.5 (1.9, 5.1) | .07 |
| Yes | 47 | 57 | 6.3 (3.8, 8.8) | |
| Visual acuity (letter score) | ||||
| ≥ 79 (20/25 or better) | 79 | 82 | 4.5 (2.3, 6.7) | .77 |
| < 79 (20/32 or worse) | 81 | 86 | 4.6 (2.4, 6.7) | |
| Neovascularization on clinical examination* | ||||
| NVD or NVE only | 103 | 107 | 3.6 (1.9, 5.2) | .04 |
| NVD and NVE | 51 | 57 | 6.7 (4.4, 9.1) | |
| OCT central subfield thickness (Stratus equivalent, µm)† | ||||
| < 250 | 109 | 115 | 4.6 (3.0, 6.2) | .59 |
| ≥ 250 | 50 | 52 | 4.6 (2.3, 7.0) | |
| OCT retinal volume (Stratus equivalent, µL)‡ | ||||
| < 8 | 93 | 95 | 4.9 (3.2, 6.7) | .88 |
| ≥ 8 | 41 | 40 | 3.7 (1.1, 6.4) | |
| Epiretinal membrane within 500 µm of the macula center§ | ||||
| No | 133 | 138 | 4.4 (3.0, 5.8) | .76 |
| Yes | 20 | 24 | 5.0 (1.3, 8.8) | |
| Cystoid abnormalities within 500 µm of the macula center ║ | ||||
| No | 74 | 78 | 4.3 (2.4, 6.2) | .87 |
| Yes | 77 | 83 | 4.5 (2.7, 6.4) | |
| Diabetic retinopathy severity (ETDRS)¶ | ||||
| Moderate PDR (level 65) or better | 99 | 106 | 3.8 (2.1, 5.5) | .02 |
| High-risk PDR (level 71) or worse | 59 | 59 | 6.1 (3.9, 8.4) | |
| Hemorrhages or microaneurysms within 1800 µm of the macula center#16 | ||||
| None, questionable, or mild (<Standard 1) | 53 | 48 | 3.1 (0.8, 5.5) | .25 |
| Moderate (<Standard 2a), severe (<Standard 2b), or very severe (≥Standard 2b) | 102 | 114 | 4.8 (3.2, 6.4) | |
| Hard exudates within 1800 µm of the macula center** | ||||
| None | 49 | 60 | 5.2 (2.9, 7.6) | .51 |
| Questionable or definite | 108 | 102 | 4.2 (2.6, 5.9) |
CI=confidence interval; DME=diabetic macular edema; ETDRS=Early Treatment Diabetic Retinopathy Study; NVD=neovascularization of the disc; NVE=neovascularization elsewhere; OCT=optical coherence tomography; PC IOL= Posterior Chamber Intraocular Lens; PDR=proliferative diabetic retinopathy; PRP=panretinal photocoagulation; VEGF=vascular endothelial growth factor.
Neovascularization type unavailable for 6 ranibizumab and 4 PRP eyes
Central subfield thickness unavailable for 1 ranibizumab and 1 PRP eye
Retinal volume unavailable for 26 ranibizumab and 33 PRP eyes
Epiretinal membrane presence unavailable for 7 ranibizumab and 6 PRP eyes
Cystoid abnormalities presence unavailable for 9 ranibizumab and 7 PRP eyes
Diabetic retinopathy severity level unavailable for 2 ranibizumab and 3 PRP eyes
Presence of hemorrhages or microaneurysms unavailable for 5 ranibizumab and 6 PRP eyes
Presence of hard exudates unavailable for 3 ranibizumab and 6 PRP eyes
Figure 1. Change in Visual Acuity Over 2 Years (Area Under the Curve) by Baseline Diabetic Retinopathy Severity Level.
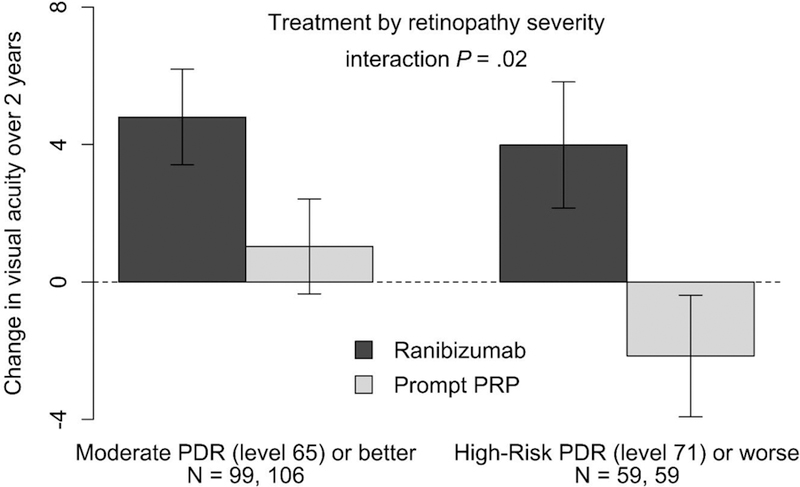
Mean change in visual acuity over 2 years (area under the curve) by treatment group and diabetic retinopathy severity (ETDRS) adjusted for baseline visual acuity and central subfield thickness. Error bars represent 95% confidence intervals. Abbreviations: ETDRS, Early Treatment Diabetic Retinopathy Study; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation.
A secondary analysis limited the cohort to eyes without vision-impairing CI-DME at baseline (N = 256), i.e., excluding eyes in the PRP group that required ranibizumab at baseline. In this cohort, the ranibizumab-PRP treatment group difference for change in visual acuity over 2 years was 4.9 letters (95% CI: 3.4 to 6.4, P <.001). The factors that remained P <.05 were prior focal/grid laser for DME (ranibizumab-PRP difference: no, 5.7 vs. yes, 0.8 letters; P = .02) and ETDRS diabetic retinopathy severity level (ranibizumab-PRP difference: high-risk PDR [level 71] or worse, 6.9 vs. moderate PDR [level 65] or better, 4.0 letters; continuous P = .04).
Development of Vision-Impairing Central-Involved DME
Among eyes without vision-impairing CI-DME at baseline, mean visual acuity was 78 letters (approximate Snellen equivalent 20/32) and mean CST was 218 µm (Stratus equivalent). Through 2 years, 10% (15 of 147) of ranibizumab eyes developed vision-impairing CI-DME compared with 27% (42 of 155) of PRP eyes (hazard ratio = 0.26, 95% CI: 0.14 to 0.46, P < .001).4
Tables 3 and 4 show the ranibizumab/PRP hazard ratio for developing vision-impairing CI-DME through 2 years after adjustment for baseline visual acuity and CST by baseline participant and ocular characteristics (unadjusted percentages provided in Supplemental Table 4). There were no characteristics in which PRP reduced the development of vision-impairing CI-DME compared with ranibizumab. However, there were several possible quantitative interactions. Among participant characteristics, the relative benefit of ranibizumab over PRP appeared to increase as mean arterial pressure increased with a ranibizumab/PRP hazard ratio of 0.39 for participants with mean arterial pressure < 100 mmHg versus 0.16 for participants with mean arterial pressure ≥ 100 mmHg (continuous P = .01). In addition, the relative benefit of ranibizumab over PRP appeared greater among non-white participants vs. white participants (ranibizumab/PRP hazard ratio: non-white, 0.10 vs. white, 0.50; P = .01). Of the 302 eyes without vison-impairing CI-DME at baseline (the cohort used for this analysis), the most frequent races/ethnicities were white (49%), Hispanic or Latino (25%), and black/African American (22%). The percentages of eyes developing vision-impairing CI-DME with ranibizumab vs. PRP were 15% (12 of 79) vs. 24% (17 of 70) for white participants, 3% (1 of 35) vs. 24% (10 of 41) for Hispanic or Latino participants, and 7% (2 of 29) vs. 35% (13 of 37) for black/African American participants. There were no interactions with P < .05 among ocular characteristics.
Table 3.
Development of Vision-Impairing Central-Involved Diabetic Macular Edema* Through 2 Years by Baseline Participant Characteristics
| Characteristic | Ranibizumab N | PRP N | Ranibizumab/PRP Adjusted Hazard Ratio (95% CI) | Interaction P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 66 | 67 | 0.37 (0.17, 0.81) | .25 |
| Male | 81 | 88 | 0.17 (0.07, 0.46) | |
| Age (y) | ||||
| < 50 | 71 | 80 | 0.18 (0.07, 0.48) | .92 |
| ≥ 50 | 76 | 75 | 0.35 (0.17, 0.75) | |
| Race/ethnicity† | ||||
| White | 79 | 70 | 0.50 (0.24, 1.03) | .01 |
| Non-White | 67 | 82 | 0.10 (0.03, 0.30) | |
| Diabetes type‡ | ||||
| Type 1 | 37 | 33 | 0.49 (0.15, 1.57) | .23 |
| Type 2 | 105 | 119 | 0.21 (0.10, 0.43) | |
| Duration of diabetes (y) | ||||
| < 20 | 76 | 90 | 0.25 (0.11, 0.56) | .60 |
| ≥ 20 | 71 | 65 | 0.27 (0.11, 0.64) | |
| HbA1c (%)§ | ||||
| < 9 | 80 | 71 | 0.36 (0.16, 0.80) | .37 |
| ≥ 9 | 61 | 79 | 0.17 (0.07, 0.40) | |
| Mean arterial pressure (mmHg) | ||||
| < 100 | 84 | 83 | 0.39 (0.17, 0.87) | .01 |
| ≥ 100 | 63 | 72 | 0.16 (0.07, 0.41) | |
| Hypertension | ||||
| No | 48 | 46 | 0.38 (0.15, 0.94) | .31 |
| Yes | 99 | 109 | 0.20 (0.09, 0.44) |
CI=confidence interval; PRP=panretinal photocoagulation.
Visual acuity letter score ≤ 78 (Snellen equivalent 20/32 or worse) and OCT central subfield thickness greater than 2 standard deviations above the sex- and instrument-specific norm for the population (Heidelberg Spectralis ≥ 320 µm for men and ≥ 305 µm for women, Zeiss Cirrus and Optovue RTVue ≥ 305 µm for men and ≥ 290 µm for women, Zeiss Stratus ≥ 250 µm for both sexes)
Race/ethnicity was unknown/not reported for 1 ranibizumab and 3 PRP eyes
Diabetes type unknown for 5 ranibizumab and 3 PRP eyes
HbA1c unavailable for 6 ranibizumab and 5 PRP eyes
Table 4.
Development of Vision-Impairing Central-Involved Diabetic Macular Edema* Through 2 Years by Baseline Ocular Characteristics
| Characteristic | Ranibizumab N | PRP N | Ranibizumab/PRP Adjusted Hazard Ratio (95% CI) | Interaction P Value |
|---|---|---|---|---|
| Prior treatment for DME | ||||
| No | 115 | 130 | 0.26 (0.14, 0.47) | .80 |
| Yes | 32 | 25 | 0.33 (0.06, 1.95) | |
| Prior focal/grid laser treatment for DME | ||||
| No | 122 | 135 | 0.27 (0.15, 0.49) | .87 |
| Yes | 25 | 20 | 0.22 (0.02, 2.29) | |
| Vitreous hemorrhage at baseline | ||||
| No | 102 | 103 | 0.34 (0.16, 0.69) | .29 |
| Yes | 45 | 52 | 0.16 (0.06, 0.48) | |
| Visual acuity (letter score) | ||||
| ≥ 79 (20/25 or better) | 87 | 93 | 0.43 (0.18, 1.04) | .10 |
| < 79 (20/32 or worse) | 60 | 62 | 0.17 (0.08, 0.38) | |
| Neovascularization on clinical examination† | ||||
| NVD or NVE only | 95 | 97 | 0.32 (0.15, 0.68) | .47 |
| NVD and NVE | 49 | 55 | 0.21 (0.08, 0.55) | |
| OCT central subfield thickness (Stratus equivalent, µm) | ||||
| < 250 | 125 | 133 | 0.23 (0.11, 0.46) | .83 |
| ≥ 250 | 22 | 22 | 0.50 (0.18, 1.40) | |
| OCT retinal volume (Stratus equivalent, µL)‡ | ||||
| < 8 | 102 | 101 | 0.22 (0.10, 0.48) | .82 |
| ≥ 8 | 22 | 29 | 0.08 (0.01, 0.78) | |
| Cystoid abnormalities within 500 µm of the macula center § | ||||
| No | 83 | 85 | 0.48 (0.18, 1.23) | .18 |
| Yes | 54 | 63 | 0.19 (0.08, 0.45) | |
| Diabetic retinopathy severity (ETDRS)║ | ||||
| Moderate PDR (level 65) or better | 97 | 97 | 0.26 (0.11, 0.59) | .16 |
| High-risk PDR (level 71) or worse | 49 | 55 | 0.25 (0.11, 0.60) | |
| Hemorrhages or microaneurysms within 1800 µm of the macula center¶16 | ||||
| None, questionable, or mild (<Standard 1) | 56 | 47 | 0.26 (0.07, 1.03) | .85 |
| Moderate (<Standard 2a), severe (<Standard 2b), or very severe (≥Standard 2b) | 86 | 102 | 0.30 (0.16, 0.58) | |
| Hard exudates within 1800 µm of the macula center# | ||||
| None | 53 | 59 | 0.36 (0.11, 1.18) | .54 |
| Questionable or definite | 91 | 91 | 0.23 (0.12, 0.47) |
CI=confidence interval; DME=diabetic macular edema; ETDRS=Early Treatment Diabetic Retinopathy Study; NVD=neovascularization of the disc; NVE=neovascularization elsewhere; OCT=optical coherence tomography; PC IOL= Posterior Chamber Intraocular Lens; PDR=proliferative diabetic retinopathy; PRP=panretinal photocoagulation; VEGF=vascular endothelial growth factor.
Visual acuity letter score ≤ 78 (Snellen equivalent 20/32 or worse) and OCT central subfield thickness greater than 2 standard deviations above the sex- and instrument-specific norm for the population (Heidelberg Spectralis ≥ 320 µm for men and ≥ 305 µm for women, Zeiss Cirrus and Optovue RTVue ≥ 305 µm for men and ≥ 290 µm for women, Zeiss Stratus ≥ 250 µm for both sexes)
Neovascularization type unavailable for 3 ranibizumab and 3 PRP eyes
Retinal volume unavailable for 23 ranibizumab and 25 PRP eyes
Cystoid abnormalities presence unavailable for 10 ranibizumab and 7 PRP eyes
Diabetic retinopathy severity level unavailable for 1 ranibizumab and 3 PRP eyes
Presence of hemorrhages or microaneurysms in grid unavailable for 5 ranibizumab and 6 PRP eyes
Presence of hard exudates unavailable for 3 ranibizumab and 5 PRP eyes
DISCUSSION
A clinician’s decision to recommend a particular intervention to manage PDR may be affected by many factors, such as the presence of features that are associated with clinically relevant outcomes when using one treatment modality versus another. In this post hoc analysis, across all baseline characteristics analyzed, there was no baseline characteristic in which PRP was clearly superior to ranibizumab for the treatment of PDR with respect to change in visual acuity over 2 years or development of vision-impairing CI-DME through 2 years. The possible qualitative interaction with prior DME treatment previously noted for the primary outcome (change in VA at 2 years) was not supported in these additional analyses.4 However, Protocol S was not specifically powered to detect treatment by baseline characteristic interactions and the analyses presented here were evaluated post hoc and are intended to generate, rather than confirm, hypotheses. Nonetheless, we did identify several possible quantitative interactions in which the relative benefit of ranibizumab over PRP varied according to certain baseline participant or ocular characteristics.
The treatment group effects stratified by baseline diabetic retinopathy severity may be of particular interest since eyes with high-risk PDR face the greatest risk of severe vision loss without intervention.9,10 This analysis suggests that eyes with the most advanced forms of PDR have the largest relative benefit of ranibizumab compared with PRP when managing PDR, regardless of whether retinopathy severity was assessed by the clinician (NVD and NVE together vs. either alone) or by masked reading center review of color fundus photographs (ETDRS grading). However, such results may depend on patient retention and good adherence to the follow-up treatment regimen, which may be challenging in this patient population.
There are several possible causes for a greater vision benefit of ranibizumab in eyes with more advanced retinopathy. First, eyes with more advanced levels of PDR are at greater risk of vision loss. This is often due to progression of PDR owing to vitreous hemorrhage, retinal detachment, neovascular glaucoma, or complications of procedures to manage these events (e.g., vitrectomy). The previously reported lower rates of these PDR-worsening events in the ranibizumab group compared with the PRP group may be responsible for the quantitative interactions we observed.5 Finally, eyes with more advanced retinopathy may be more prone to vision-impairing CI-DME due to higher levels of VEGF.11 The lower rate of new, vision-impairing CI-DME in eyes with advanced retinopathy managed with ranibizumab may have resulted in greater improvement in vision compared with the PRP group.4
A possible quantitative interaction involving mean arterial pressure, in which there was greater relative benefit of ranibizumab among eyes of participants with higher pressure, was found for both the visual acuity and vision impairing CI-DME outcomes. The hyperpermeability of retinal capillaries caused by hyperglycemia may lead to greater fluid extravasation and vision loss in the setting of higher levels of blood pressure.12–14 The anti-permeability effects of anti-VEGF therapy may be able to blunt this extravasation even in eyes of participants with higher blood pressure whereas PRP may contribute to the development of DME. However, a larger treatment benefit of ranibizumab over PRP specifically in eyes with higher mean arterial pressure was not a pre-specified hypothesis and this finding may be due to chance. Confirmation of this association in future studies would be desirable.
Among eyes without prior focal/grid laser treatment for DME, there was a greater relative treatment benefit in the ranibizumab group compared to the PRP group for the vision outcome. Eyes with prior focal/grid laser may have irreversible anatomic and functional damage such that limited vision improvement can be attained in these eyes irrespective of management modality. Conversely, eyes that have not had laser treatment may retain greater potential for vision improvement. In addition, some eyes without prior laser may have had subclinical or early DME (central or para-central thickening with minimal or no vision impairment). The anti-permeability properties of anti-VEGF therapy may have thinned the macula in these eyes, possibly resulting in vision improvement whereas eyes with similar anatomy assigned to PRP may have developed macular edema resulting in a negative effect on visual acuity.
Similar to Protocol S, the CLARITY trial compared an anti-VEGF agent (aflibercept) versus PRP for the treatment of PDR. In the CLARITY trial, aflibercept was superior to PRP for the primary outcome of change in visual acuity from baseline at 1 year. 15 The CLARITY trial differed from Protocol S in that it excluded eyes with baseline DME and included eyes with prior PRP. Further analyses from the CLARITY trial may provide comparative information for this post hoc analysis of DRCR.net Protocol S data.
Limitations of this study include its post hoc design and the small number of eyes manifesting certain characteristics such that some factors could not be adequately explored (e.g., surface wrinkling retinopathy). The study was not specifically powered to detect associations between baseline factors and the relative treatment effect; therefore, the absence of a significant interaction cannot be interpreted as definitive evidence that an interaction does not exist. In addition, possible quantitative interactions identified will require confirmation in future studies because a large number of analyses were performed without pre-specified hypotheses and some associations could have been identified due to chance. This report was intended to be exploratory and suggest hypotheses for future investigations, rather than provide definitive findings. Finally, the number of ranibizumab injections received may have differed by baseline factors, and it is unknown what effect this would have on possible interactions.
In summary, ranibizumab was superior to PRP with respect to change in visual acuity over 2 years and prevention of vision-impairing CI-DME over 2 years, regardless of baseline characteristics. These analyses provide additional support that ranibizumab may be a reasonable alternative treatment to PRP for PDR over a 2-year period, especially among participants with high blood pressure and eyes with more advanced grades of PDR.4
Supplementary Material
Acknowledgments
Financial support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY23207, EY18817. Genentech provided ranibizumab for the study and funds to DRCR.net to defray the study’s clinical site costs.
The National Institutes of Health participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study nor in the collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Per the DRCR.net Industry Collaboration Guidelines (available at http://www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol.
Footnotes
Financial Disclosures: A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
REFERENCES
- 1.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984;102:527–532. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001;44(2):156–163. [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL 3rd, Davis MD, Aiello LM. Treatment of diabetic retinopathy. N Engl J Med 1999;341(9):667–678. [DOI] [PubMed] [Google Scholar]
- 4.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA 2015;314(20):2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bressler SB, Beaulieu WT, Glassman AR, et al. Factors associated with worsening proliferative diabetic retinopathy in eyes treated with panretinal photocoagulation or ranibizumab. Ophthalmology 2017;124(4):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutton DW, Stein JD, Bressler NM, Jampol LM, Browning D, Glassman AR. Incremental cost-effectiveness of intravitreous ranibizumab compared with panretinal photocoagulation for proliferative diabetic retinopathy. JAMA Ophthalmol 2017;In-Press. [DOI] [PMC free article] [PubMed]
- 7.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol 2003;135(2):194–205. [DOI] [PubMed] [Google Scholar]
- 8.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association 1989;84(408):1074–1078. [Google Scholar]
- 9.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98:823–833. [PubMed] [Google Scholar]
- 10.The Diabetic Retinopathy Study Research Group. Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study. Arch Ophthalmol 1979;97:654–655. [DOI] [PubMed] [Google Scholar]
- 11.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331(22):1480–1487. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmalogy 1998;105(10):1801–1815. [DOI] [PubMed] [Google Scholar]
- 13.Lopes de Faria JM, Jalkh AE, Trempe CL, McMeel JW. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand 1999;77(2):170–175. [DOI] [PubMed] [Google Scholar]
- 14.Suzuma I, Hata Y, Clermont A, et al. Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes 2001;50(2):444–454. [DOI] [PubMed] [Google Scholar]
- 15.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017;389(10085):2193–2203. [DOI] [PubMed] [Google Scholar]
- 16.Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


