Abstract
Mechanical failure of the ciliary zonule characterizes several ocular and systemic diseases. The mouse has emerged as a useful model system to investigate the composition and structure/function relationships of the zonule. However, visualizing the organization of the diaphanous fibers that comprise the zonule is technically challenging because the fibers do not take up conventional histological stains and are disrupted easily during processing. Here, we describe a simple method for maintaining physiological pressure within the mouse eye during fixation, and a gel-embedding technique for stabilizing the zonular fibers during subsequent tissue processing and imaging steps. This approach facilitates quantitative measurements of fiber number and crosssectional dimensions and will allow the effects of targeted disruption of zonule components to be assessed systematically.
1. Introduction
The ciliary zonule is the system of extracellular fibers that suspends the lens in the eye (Streeten, 1982). In humans, zonular fibers facilitate accommodation by transmitting the forces that flatten the lens, bringing distant objects into focus. Although mice do not accommodate, a well-developed zonule is present in that species also, projecting from the pars plana region of the non-pigmented ciliary epithelium (NPCE) and attaching to the lens at its equator (Shi et al., 2013).
The composition of the human and bovine zonule has been elucidated in recent proteomic studies (Cain et al., 2006; De Maria et al., 2017; Eckersley et al., 2018). Collectively, these analyses have confirmed that the most abundant component is fibrillin-1 (FBN1). The zonule proteome is also enriched in other glycoproteins (notably, latent TGFβ-binding protein-2 (LTBP2) and microfibril-associated protein-2 (MFAP2)).
Mutations in FBN1 underlie Marfan syndrome (Dietz et al., 1991; Lee et al., 1991) which, in the eye, is characterized by zonule fragility and lens dislocation (ectopia lentis). Mutations in FBN1 or LTBP2 can cause Weill-Marchesani syndrome (types 2 and type 3, respectively), where ectopia lentis and microspherophakia are prominent manifestations (Faivre et al., 2003; Haji-Seyed-Javadi et al., 2012). Ectopia lentis may also be transmitted as an isolated trait, linked to mutations in FBN1 (Ades et al., 2004), LTBP2 (Alias et al., 2018), or ADAMTSL4 (Ahram et al., 2009). To better understand the pathophysiological processes that culminate in zonule failure and ectopia lentis, mouse models have been developed. For example, targeted deletion of Fbn1 in the mouse NPCE results in ectopia lentis by three months of age (Jones et al., 2019). Ectopia lentis similarly develops in mice harboring a null mutation in Ltbp2 (Inoue et al., 2014) or a nonsense mutation in Adamtsl4 (Collin et al., 2015). The mouse thus serves as a useful model system; providing insights into connective tissue disorders, such as Marfan syndrome, that adversely affect the eye.
The structure of the mouse zonule has proved challenging to visualize. The constituent fibers are generally < 1 μm in diameter (Jones et al., 2019), transparent, and absorb conventional histological stains poorly. We previously used confocal microscopy to visualize the three-dimensional organization of the zonule in the semi-intact mouse eye (Jones et al., 2019; Shi et al., 2013). This technique offered several advantages over traditional histological approaches but the resulting images were of relatively low spatial resolution. In the current paper, we describe a method to preserve and visualize the delicate zonular fibers at higher resolution, in three dimensions, and in a near native configuration. This approach should facilitate the analysis of the zonule in mouse models of human connective tissue disease, helping define the structural deficits that contribute to mechanical failure of the zonule
2. Materials and supplies
2.1. Supplies
Primary antibodies
Goat anti-MFAP2 (Cat # AF4977; R&D Systems) or chicken anti-LTBP2 (gift of Dr. Nakamura, Kansai Medical University, Osaka, Japan).
Secondary antibodies
Alexa Fluor Plus 488-conjugated goat anti-chicken (Cat # A32931, Thermo Fisher Scientific) or Alexa Fluor Plus 647—conjugated donkey anti-goat (Cat#A32814).
Nuclear counterstain
DAPI (4′,6-diamidino-2-phenylindole; NucBlue™ Thermo Fisher).
Pressurized Fixation Apparatus
31-gauge x 8 mm 1 ml tuberculin syringe (Walgreens).
Silicone tubing (OD: 4mm, ID: 2.5 mm).
15 ml Falcon tubes (with holes in the cap to accommodate syringe barrel or tubing).
10 ml syringe
3-way stopcock with Luer connections.
Tissue processing
4% Paraformaldehyde (from 16% stock; Electron Microscopy Sciences, Hatfield PA).
Vectashield mounting medium (Vector Labs, Burlingame CA).
Low melting point agarose (LMP agarose, Fisher Scientific).
Phosphate buffered saline (PBS; Fisher Scientific).
Deconvolution
175 nm-diameter fluorescent microspheres (PS-Speck, Thermofisher Scientific).
Huygens Essential deconvolution software (confocal module) and PSF distiller (Scientific Volume Imaging, Hilversum, The Netherlands).
2.2. Equipment
Confocal microscope (FV 1000; Olympus).
Objective lenses: 10x UPLSAPO (numerical aperture (NA): 0.4), 25x XLPLN (1.05 NA) and 60x PLAPON (1.42 NA).
Tissue slicer (Leica VT1000S)
Hybridization oven (Problot 12, Labnet International)
Orbital shaker (belly button shaker, IBI Scientific)
3. Detailed methods
3.1. Fixation under pressure
To maintain lens centration in vivo, zonular fibers are under tension. Zonular tension is maintained, in part, by intraocular pressure (IOP), which acts on the inner wall of the eye to keep the globe inflated. If the globe is punctured, IOP is lost and elastic energy stored in the zonule is released as kinetic energy, drawing the eye wall closer to the lens and collapsing the circumlental space (the gap between the lens equator and the ciliary body). To preserve the ciliary zonule in a near-native configuration, therefore, it is necessary to maintain IOP during the fixation process.
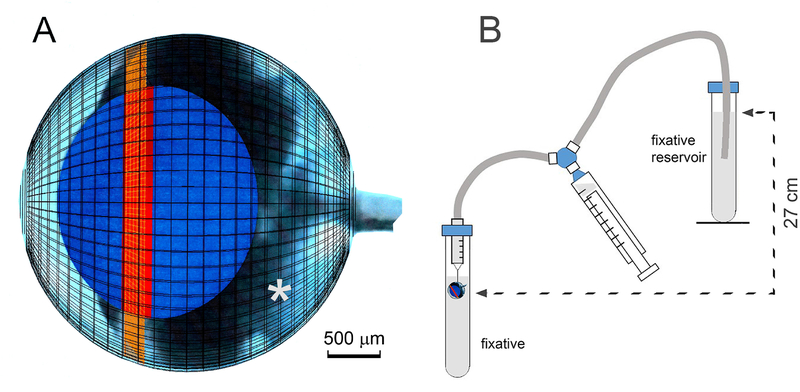
In mice, IOP varies from 10 to 20 mmHg (depending on strain, age of animal, and time of day (Savinova et al., 2001)). To maintain IOP during the fixation process, we developed a simple pressurized fixation system. In procedures approved by the Washington University Animal Studies Committee, one-month-old mice were euthanized by CO2 inhalation and their eyes were enucleated into PBS. The tip of a 31-gauge needle was inserted carefully through the posterior sclera and into the vitreous space (Fig. 1A). The needle was attached to a 1 ml syringe barrel, which in turn was connected to a length of silicon tubing. The syringe and tubing were pre-filled with fixative solution (4% paraformaldehyde in PBS), drawn from a reservoir by a means of a 3-way tap (see Fig. 1B). Once impaled on the needle tip, the eye was moved to a 15 ml tube filled with fixative (4% paraformaldehyde in PBS). The globe was submerged just beneath the surface of the liquid and the fixative reservoir was then raised to a position 27 cm above the impaled globe (27 cm of H2O being approximately equivalent to 20 mmHg). In this arrangement, fixative in the tube bathed the outer layers of the eye, while the interior tissues were exposed to fixative diffusing from the needle tip. The 27 cm pressure head ensured that the eye remained inflated throughout. Preliminary experiments demonstrated that fixative did not leak from impaled eyes, indicating that the seal around the needle was tight enough to resist an internal pressure of 27 cm H2O. The pressure fixation apparatus was set up in a chemical fume hood, to avoid exposure to paraformaldehyde vapor.
Figure 1.
Apparatus for pressurized fixation of the mouse eye A. Scale model of the eye at one month of age. The positions of the lens (blue), germinative zone of the lens epithelium (red) and ciliary body (orange) are indicated. The tip of the fixation needle is inserted in the narrow space (*) between the posterior surface of the lens and the anterior surface of the retina. B. Diagram of the fixation apparatus. The eye is first impaled on the tip of a 31-gauge needle and then moved to a 15 ml fixative-filled Falcon tube where it is submerged just beneath the surface of the fixative. The injection needle is connected via fixative-filled tubing to a fixative reservoir positioned 27 cm above the eye. A 10 ml syringe connected to the needle and the fixative reservoir via a three-way stopcock is used to prime the system. Four such assemblies were set up in parallel, allowing ocular tissue from two mice to be processed simultaneously.
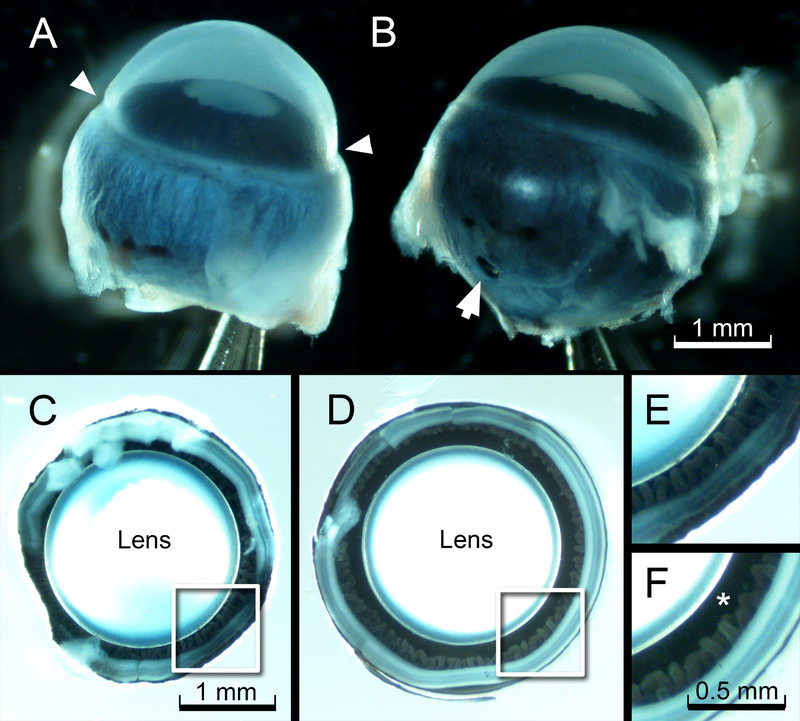
After 3 h of fixation at room temperature, the eye was removed from the fixative and the needle tip was withdrawn carefully. Significantly, the globes remained inflated (Fig. 2A,B). Thus, once fixed, tissues maintained their in vivo configuration in the absence of supportive internal pressure. To complete the fixation process, a small hole (≈1 mm diameter) was made in the posterior sclera and globes were incubated overnight in fixative at 4°C. The following day, globes were transferred to PBS and the posterior portion was removed by dissecting the sclera/retina to a level just above the ciliary folds. The interiors of the fixed and dissected eyes were photographed from the posterior aspect to visualize the circumlental space (Fig. 2 C–F). In pressure fixed eyes, the circumlental space was approximately 150 μm wide. In contrast, in eyes fixed in the absence of IOP, the gap was rarely greater than 50 μm and often non-existent.
Figure 2.
Effect of pressurized fixation on circumlental space. A. Eye of a one-month-old mouse after conventional immersion fixation. Note the constriction at the limbus (arrowheads). B. Appearance of the contralateral globe, fixed under an internal pressure of 27 cm H20, as described in the text. Note that the eye retains its smooth, spherical contours. The puncture wound caused by the fixation needle is visible in the posterior sclera (arrow). C. The deflated eye (from A) photographed from the posterior aspect, after removal of the sclera. D. The appearance of the eye (from B) fixed under pressure. E and F. Higher magnification views of the boxed regions from C and D, respectively. Note that the circumlental space (*) collapses in the eye prepared using conventional immersion fixation (E) but remains patent in the pressure-fixed eye (F).
3.2. Gel Embedding
To support the zonular fibers during tissue processing, we embedded the dissected anterior segments in low melting point (LMP) agarose. The embedding gel was prepared by melting LMP agarose (5% weight/volume in PBS) in a microwave and then allowing it to cool to 45° C in a hybridization oven. A small volume of molten agarose was poured into the base of a 35 mm Petri dish and left for a few minutes on the benchtop to set. The remainder of the dish was then filled with liquid agarose in which one or more dissected anterior segments were quickly immersed. The Petri dish was covered with a lid and returned to the 45°C oven, where it was placed on an oribital platform. The globes were incubated in molten agarose for 30 minutes with gentle agitation, allowing the agarose to infiltrate the ocular tissues. The dish was then removed from the oven, the eyes oriented under a dissecting microscope, and the gel allowed to set.
3.3. Tissue slicing
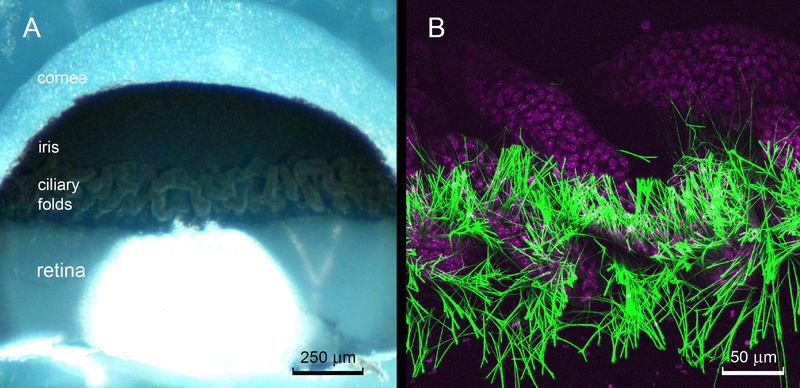
A block of gelled agarose containing the eye tissue was trimmed to size and attached to the sample holder of a tissue slicer (Leica VT1000S) with superglue. The tissue was oriented such that tissue slices were cut parallel to the optical axis. The sectioning plane was positioned above the tissue initially and advanced until the sclera was encountered. Sections (50 μm thick) were collected individually and examined under the dissecting scope. The initial sections contained sclera and choroid and then the proximal and finally, distal regions of the ciliary processes. Once the section plane had cleared the inner tips of the ciliary folds, a single 270 μm-thick section was collected. This section contained gel-embedded zonular fibers and the superficial region of the lens equator (see Fig.3). The slices were fragile and transferred between solutions supported on a metal spatula.
Figure 3.
The ciliary folds. A. A 270 μm-thick, sagittal slice of the eye wall visualized from the inner aspect contains sclera, iris, retina and ciliary folds. The ciliary folds are formed from an irregular arrangement of tubular elements. Note that the zonular fibers are not visible in this bright field image. B. Maximum intensity projection of a confocal image stack of the central region of the ciliary folds. The nuclei of the non-pigmented ciliary epithelial cells (magenta) are stained with DAPI. Zonular fibers (green) are visualized using anti-LTBP2 immunofluorescence. The fibers project from the posterior portion of the ciliary body (i.e., the region closest to the retina). Most of the fibers originate in the narrow clefts between the folds.
3.4. Immunofluorescence and Imaging
Gel slices were incubated in 0.1% Triton-X 100/PBS for 30 min at room temperature on an orbital shaker. Slices were then immersed in blocking solution (5% BSA in PBS, preserved with 0.02% sodium azide) for one hour. The blocking solution was replaced by the primary antibody solution. To visualize zonular structure, antibodies against any of the major structural components worked well. Here, we used anti-MFAP2 or anti-LTBP2 (each diluted 1:500 in blocking solution). Primary antibody incubation was carried out overnight at 4°C. The following day, slices were washed extensively in PBS for 1 h and then incubated for 3 h at room temperature in an appropriate fluorescently-conjugated secondary antibody (1:500 in blocking solution). Nuclei were counterstained with DAPI, which was included in the secondary antibody solution. Finally, samples were washed for 1 h in PBS and mounted in custom-made chamber slides. To construct the chamber slides, two parallel strips of double-sided tape (1 cm apart) were laid across a glass microscope slide. The tape was 90 μm thick, so three layers of tape were required to produce a chamber 270 μm deep. Tissue slices were mounted in the space between the multilayered tape strips using Vectashield mounting medium (Vector Laboratories). The chamber was then coverslipped and sealed with clear nail polish.
Slices were viewed on an upright FV1000 confocal microscope (Olympus). To visualize the full length of the zonule, 10x UPLSAPO (0.4 NA) or 25x XLPLN (1.05 NA) objective lenses were used. For high resolution imaging, a 60x PLAPON (1.42 NA) lens was used and the resulting images were deconvolved using Huygens Essential software (version 18.10, Scientific Volume Imaging, The Netherlands) and a measured point spread function (PSF). The PSF of the optical path was obtained by collecting a through-focus image series of 175 nm diameter fluorescent microspheres at high zoom, as described (Shi et al., 2011). Image analysis was performed on segmented images using ImageJ (Schneider et al., 2012) software (Ver. 1.52n). For measurements of fiber cross sectional area, a circularity object classifier (0.8–1.0) was applied, ensuring that only fibers oriented at 90° to the plane of the optical section were included in the analysis.
3.5. Origin of the zonule
Slices of the eye wall containing the ciliary folds and adjacent retina were used to visualize the proximal portions of the zonular fibers (Fig. 3). The ciliary body was ≈ 200 μm wide, interposed between the border of the retina and the root of the iris. In mice, the ciliary folds have an irregular, tubular appearance (Fig. 3A). The zonular fibers were associated specifically with the posterior region of the ciliary body. Most of the fibers originated in the narrow valleys between the tubular folds.
3.6. Fibers at midspan
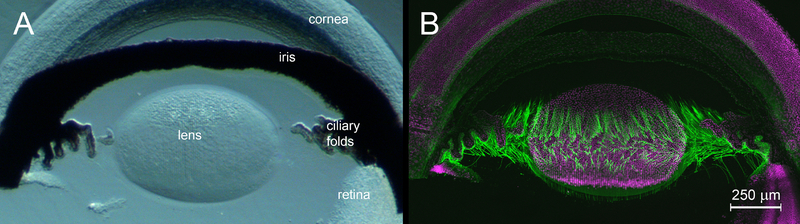
Zonular fibers were not visible in brightfield (Fig. 2F, 3A, 4A) but were readily visualized by confocal immunofluorescence (Fig. 4B). The fibers diverged as they projected towards the lens equator, eventually spanning a region ≈ 300 μm wide of the lens surface (Fig. 4B). The complex, three-dimensional arrangement of the zonular fibers was best appreciated in animated reconstructions (see Supplemental data video).
Figure 4.
Three-dimensional organization of the ciliary zonule in the anterior segment of a one-month-old wild type mouse. A. Bright field image of an agarose-embedded tissue slice. B. Matching immunofluorescence image. The zonule (green) is visualized using an antibody against LTBP2. Cell nuclei (magenta) are counterstained with DAPI. Zonular fibers project from the posterior surface of the ciliary folds and span a region ≈ 300 μm wide on the equatorial lens surface.
At high magnification, individual optical sections revealed zonular fibers in cross section (Fig. 5). Such images were used to estimate the total number of fibers in the zonule and the individual and total cross-sectional areas of the fibers. Image analysis of optical sections from three samples indicated that there were 2561 ±231 (mean ± SD) fibers per 1 mm segment of the NPCE. Measured on images such as that shown in Fig. 2D, the circumlental space had a circumference of 7.05 ± 0.14 mm (mean ± SD; n=4), suggesting that, in total, 18,055 fibers projected from the surface of the NPCE toward the lens in one-month-old wild type mice. The number of fibers attaching to the lens surface likely surpassed this number significantly because most fibers branched several times as they coursed toward the lens (see below).
Figure 5.
Optical section through the zonule near the surface of the NPCE (≈ 100 μm above the lens). The section passes through all zonular fibers projecting into a 0.18 mm-wide segment of the circumlental space. Anterior zonular fibers are located at the top of the image.
As shown in Figure 5, individual optical sections contained fibers with a wide range of cross-sectional areas (see Fig. 5) although few fibers (<1%) exceeded 1 μm2 in area. The average cross-sectional area of zonular fibers determined in three independent samples was 0.39 ± 0.39 μm2 (mean ± SD; n=777 fibers).
3.7. Branching properties
The use of image deconvolution techniques allowed the three-dimensional structure of individual fibers to be visualized at high resolution (Fig. 6). Reconstructions revealed that most fibers branched multiple times. The cross-sectional areas of the fibers were measured immediately above and below branching nodal points (see Figure 6 inset). In measurements of 52 randomly selected nodes, the area of fiber “trunks” was indistinguishable from the total area of the subjacent “branches” (98.6 ± 19.6% (mean ± SD)).
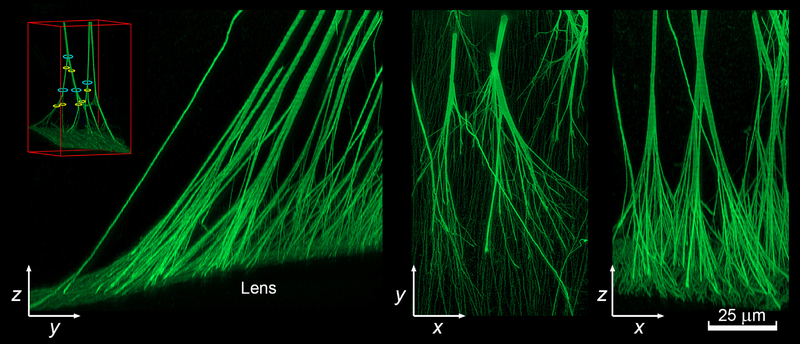
Figure 6.
High resolution reconstruction of the attachment of the posterior zonular fibers to the lens surface visualized in yz, xy, and xz projections. Fibers were visualized using anti-LTBP2 immunofluorescence. The image stack was deconvolved before rendering. Note that the fibers branch multiple times as they approach the lens. The inset shows an example of the trunks (blue) and branches (yellow) on either side of a nodal point (see text for details).
4. Potential pitfalls and trouble shooting
Beyond the difficulties usually encountered when preparing tissue for immunofluorescence analysis, a couple of steps in this procedure require particular attention. In mice, the volume of the lens is large compared to the overall volume of the globe. As a consequence, the vitreous chamber is small (see Fig. 1A). Care should therefore be taken, to ensure that the tip of the fixation needle is angled away from the lens when piercing the globe, so as not to accidentally snag the lens capsule. With practice, the fixation needle can be inserted successfully more than 90% of the time.
LMP agarose begins to gel at temperatures below 37°C. Once gelled, it will not melt unless the temperature is raised above 70°C. Therefore, it is important to move quickly when immersing the anterior segments in the molten gel or when orienting the tissue prior to allowing the gel to set.
When preparing the agarose slices, it is essential not to cut too deeply into the lens. The outer layers of the lens (to a depth of ≈ 200 μm) are relatively soft and easy to section. In contrast, the center is dense and tough. Should the section plane pass through the lens core, the cutting blade will lodge in the tissue, ripping the lens from the gel and ruining the slice. The recommended section thickness (270 μm) is sufficient to encompass the zonule and the outer 100 μm of lens tissue.
Finally, imaging the zonule by confocal microscopy poses a number of challenges. The zonular fibers are relatively small (cross sectional area <1 μm) but quite long (>100 μm) and are contained within a 270 μm-thick agarose slice. Further, the fibers diverge as they approach the lens, eventually spanning a 300 μm-wide swath of the lens equator. To image the entire structure in x, y, and z at sufficient resolution requires the judicious selection of objective lenses and imaging parameters. It is necessary to balance the need for a sufficient field of view, adequate working distance and high NA. We have utilized several lenses for visualizing the zonule but, in our hands, the objective lens offering the best combination of features for this particular application was the 25x XLPLN (1.05 NA) lens. This is a water immersion lens with a long (2 mm) working distance and a field of view sufficient to encompass the full span of the zonule.
Even with the highest resolution objectives available (NA >1.4), accurately measuring the cross-sectional area of individual zonular fibers is problematic because the smaller fibers approach the limits of optical resolution. As a result, their area is likely to be overestimated. There are also significant challenges involved in imaging the full length of a zonular fiber because the immunofluorescence signal of the fine processes near the lens surface is substantially weaker than the thick process near the NPCE. To visualize simultaneously the thick trunks of the zonular fibers near their origin points at the eye wall and the fine ramifications near the lens surface, it is important to collect images with sufficient dynamic range. In the current study, we collected 12 bit images, allowing us to use non-linear lookup tables to display the images. Adjusting the gamma values in this fashion allowed the entirety of the zonule to be imaged.
5. Conclusions
In this study, we presented a simple method for preserving the structure of the zonule in a near native form (i.e., in an extended rather than relaxed configuration). The method involved fixing the eye globe under physiological pressure and infiltrating it subsequently with LMP agarose, to support the fibers during tissue sectioning. In contrast to traditional immersion fixation, this approach successfully preserved the circumlental space, allowing the three-dimensional organization of the zonule to be visualized by confocal microscopy. Here, we utilized onemonth-old animals, but we have used this technique successfully on much older mice, so the approach seems to be broadly applicable.
Zonular fibers originated in the posterior portion of the NPCE, near the anterior border of the retina, as noted previously (Shi et al., 2013). In situ hybridization analysis has shown that cells in that region express strongly all the major structural components of the zonule (Jones et al., 2019). Quantitative measurements indicated that ≈18,000 fibers projected from the surface of the NPCE. The number of attachment points on the lens surface was presumably greater than this because fibers branched many times as they coursed toward the lens. We found that the cross sectional area of fibers above and below a given branch node was conserved. Thus, fibers in the ciliary zonule appear to follow Leonardo Da Vinci’s classic branching rule, a fundamental pattern of nature that describes the ramifications of tree branches and river tributaries. In his notebooks Leonardo observed that “All the branches of trees at every stage of their height, united together, are equal to the thickness of their trunk” (Maccurdy, 1938). Applied to the zonule, this suggests that the total cross sectional area is independent of where along the fiber length it is measured. This has implications for the measurement of biomechanical properties (see below).
When considering the mechanical properties of a zonular fiber, it is useful to compute the elastic modulus (Young’s modulus). The modulus is a fundamental material property that describes the deformation of a system in response to a given stress. With regard to the zonule, the most challenging aspect of the modulus calculation is determining the total cross-sectional area of the zonule. We found that measured close to the NPCE, the mean cross-sectional area of an individual zonular fiber was 0.39 μm2. Multiplying this value by 18,055 (the total number of fibers projecting from the ciliary zonule) provides an estimate of 7,041 μm2 for the total cross-sectional area of the zonule in a one-month-old mouse. Combining this value with stress/strain data obtained previously (Jones et al., 2019) provides an estimate of 1.3 MPa for the modulus of the mouse zonule. This is approximately four-fold greater than values of 0.2–0.3 MPa reported for pig or human (Bocskai et al., 2014; Michael et al., 2012), suggesting that the zonule in the mouse is stiffer than in those species. Whether this reflects a difference in the composition or organization of the component microfibrils is currently unclear.
In conclusion, we describe a straightforward method for preserving and visualizing the diaphanous fibers of the mouse zonule. The technique lends itself to 3-D image analysis and allows the elastic modulus of the zonule to be calculated. In combination with targeted gene disruption approaches, this should allow us to assess the contributions of specific proteins to the biomechanical properties of the zonule and provide insights into the pathophysiological changes culminating in ectopia lentis.
Supplementary Material
Supplemental data video. Pressurized fixation preserves the complex, three-dimensional organization of the zonule. The video shows an animated reconstruction of the zonule and its attachment site at the lens surface. The nuclei (light blue) of the underlying lens epithelial cells are counterstained with DAPI and visible between the zonular fibers. Fibers are labeled with antibodies against two zonular proteins, LTBP2 (green) and MAGP1 (red). The reconstructed volume was visualized using the SFP volume renderer (Huygens, SVI) and had dimensions (x,y,z) of 529×529×150 μm.
Acknowledgements
The author thanks Drs. Wendell Jones and Juan Rodriquez for their assistance. Dr. Tomoyuki Nakamura kindly provided the anti-mouse LTBP2 antibody. Supported by National Institute of Health grants R01 EY029130, P30 EY02687, the National Marfan Foundation, and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ades LC, Holman KJ, Brett MS, Edwards MJ, Bennetts B, 2004. Ectopia lentis phenotypes and the FBN1 gene. Am J Med Genet A 126A, 284–289. [DOI] [PubMed] [Google Scholar]
- Ahram D, Sato TS, Kohilan A, Tayeh M, Chen S, Leal S, Al-Salem M, El-Shanti H, 2009. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am J Hum Genet 84, 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alias L, Crespi J, Gonzalez-Quereda L, Tellez J, Martinez E, Bernal S, Gallano MP, 2018. Next-generation sequencing reveals a new mutation in the LTBP2 gene associated with microspherophakia in a Spanish family. BMC Med Genet 19, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocskai ZI, Sandor GL, Kiss Z, Bojtar I, Nagy ZZ, 2014. Evaluation of the mechanical behaviour and estimation of the elastic properties of porcine zonular fibres. J Biomech 47, 3264–3271. [DOI] [PubMed] [Google Scholar]
- Cain SA, Morgan A, Sherratt MJ, Ball SG, Shuttleworth CA, Kielty CM, 2006. Proteomic analysis of fibrillin-rich microfibrils. Proteomics 6, 111–122. [DOI] [PubMed] [Google Scholar]
- Collin GB, Hubmacher D, Charette JR, Hicks WL, Stone L, Yu M, Naggert JK, Krebs MP, Peachey NS, Apte SS, Nishina PM, 2015. Disruption of murine Adamtsl4 results in zonular fiber detachment from the lens and in retinal pigment epithelium dedifferentiation. Hum Mol Genet 24, 6958–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria A, Wilmarth PA, David LL, Bassnett S, 2017. Proteomic Analysis of the Bovine and Human Ciliary Zonule. Invest Ophthalmol Vis Sci 58, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. , 1991. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352, 337–339. [DOI] [PubMed] [Google Scholar]
- Eckersley A, Mellody KT, Pilkington S, Griffiths CEM, Watson REB, O’Cualain R, Baldock C, Knight D, Sherratt MJ, 2018. Structural and compositional diversity of fibrillin microfibrils in human tissues. J Biol Chem 293, 5117–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le Merrer M, Collod-Beroud G, Boileau C, Munnich A, Cormier-Daire V, 2003. In frame fibrillin-1 gene deletion in autosomal dominant WeillMarchesani syndrome. J Med Genet 40, 34–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji-Seyed-Javadi R, Jelodari-Mamaghani S, Paylakhi SH, Yazdani S, Nilforushan N, Fan JB, Klotzle B, Mahmoudi MJ, Ebrahimian MJ, Chelich N, Taghiabadi E, Kamyab K, Boileau C, Paisan-Ruiz C, Ronaghi M, Elahi E, 2012. LTBP2 mutations cause Weill-Marchesani and Weill-Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum Mutat 33, 1182–1187. [DOI] [PubMed] [Google Scholar]
- Inoue T, Ohbayashi T, Fujikawa Y, Yoshida H, Akama TO, Noda K, Horiguchi M, Kameyama K, Hata Y, Takahashi K, Kusumoto K, Nakamura T, 2014. Latent TGF-beta binding protein-2 is essential for the development of ciliary zonule microfibrils. Hum Mol Genet 23, 5672–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W Jr., Rodriguez J, Bassnett S, 2019. Targeted deletion of fibrillin-1 in the mouse eye results in ectopia lentis and other ocular phenotypes associated with Marfan syndrome. Dis Model Mech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Godfrey M, Vitale E, Hori H, Mattei MG, Sarfarazi M, Tsipouras P, Ramirez F, Hollister DW, 1991. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature 352, 330–334. [DOI] [PubMed] [Google Scholar]
- Maccurdy E, 1938. The Notebooks of Leonardo Da Vinci. Jonathan Cape Ltd., London. [Google Scholar]
- Michael R, Mikielewicz M, Gordillo C, Montenegro GA, Pinilla Cortes L, Barraquer RI, 2012. Elastic properties of human lens zonules as a function of age in presbyopes. Invest Ophthalmol Vis Sci 53, 6109–6114. [DOI] [PubMed] [Google Scholar]
- Savinova OV, Sugiyama F, Martin JE, Tomarev SI, Paigen BJ, Smith RS, John SW, 2001. Intraocular pressure in genetically distinct mice: an update and strain survey. BMC Genet 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW, 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, De Maria AB, Wang H, Mathias RT, FitzGerald PG, Bassnett S, 2011. Further analysis of the lens phenotype in Lim2-deficient mice. Invest Ophthalmol Vis Sci 52, 7332–7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tu Y, De Maria A, Mecham RP, Bassnett S, 2013. Development, composition, and structural arrangements of the ciliary zonule of the mouse. Invest Ophthalmol Vis Sci 54, 2504–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeten BW, 1982. The nature of the ocular zonule. Trans Am Ophthalmol Soc 80, 823–854. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data video. Pressurized fixation preserves the complex, three-dimensional organization of the zonule. The video shows an animated reconstruction of the zonule and its attachment site at the lens surface. The nuclei (light blue) of the underlying lens epithelial cells are counterstained with DAPI and visible between the zonular fibers. Fibers are labeled with antibodies against two zonular proteins, LTBP2 (green) and MAGP1 (red). The reconstructed volume was visualized using the SFP volume renderer (Huygens, SVI) and had dimensions (x,y,z) of 529×529×150 μm.