Abstract
This investigation was undertaken to find out whether the positive charges in the Extracellular Loops A (ELA) and C (ELC) of Aquaporin 0 (AQP0) are involved in lens fiber cell-to-cell adhesion (CTCA), and the possible mechanism of CTCA. AQP0 ELA or ELC was substituted with the corresponding AQP1 loop via Polymerase Chain Reaction. Positively charged arginine (R) and histidine (H) of mouse AQP0 ELA and ELC were substituted individually with glutamine (Q) to create R33Q, H40Q, R113Q and H122Q by mutagenesis. cRNA expression, immunostaining, Forster Resonance Energy Transfer (FRET) studies and protein analyses showed localization of all mutants except AQP0-AQP1ELC chimera (AQP0 ELC substituted with AQP1 ELC) at the plasma membrane. Osmotic Swelling Assay revealed comparable water permeability (Pf) among AQP0-AQP1ELA, R33Q, R113Q, and WT. CTCA assay demonstrated a significant reduction in adhesion in all mutants compared to the WT (14 to 73%) suggesting the importance of the conserved positively charged residues of ELA and ELC for adhesion. Studies involving AQP0-transfected L-cells, and lipid vesicles indicated that CTCA was due to the electrostatic interaction between the positively charged amino acids of AQP0 extracellular loops and the negative charges of the plasma membrane. Schematic models are provided to illustrate the mechanism.
Graphical Abstract

1. Introduction
Aquaporin 0 (AQP0) is the most abundant membrane protein in the multilayered fiber cells of the mammalian lens. Among the thirteen aquaporin homologs (AQP0–12) identified in mammals, AQP0, AQP1 and AQP5 are expressed in the lens (Varadaraj et al., 2005, 2007; Kumari et al., 2012; 2013; Grey et al., 2013) Structurally, AQPs contain six transmembrane domains (helices (H) 1–6), two half-membrane-spanning domains (HB, HE), three extracellular loops (ELA, ELC and ELE), two intracellular loops (LB and LD), and cytoplasmic N- and C-termini (Fig. 1; mouse AQP0). The pore of the channel is formed by folding back the two half helices (HB and HE) and loops B and E into the membrane.
Fig. 1.
Schematic representation of mouse AQP0. The monomer structure shows folds, helix assignment, and the location at the membrane. The membrane-spanning α-helices are shown as H1–H6, the three extracellular loops as ELA, ELC and ELE and the two intracellular loops as LB, LD. The two water pore-lining helices are labeled as HB and HE. Two highly conserved asparagine–proline–alanine (NPA) motifs in HB and HE that line the water pore of aquaporin and considered responsible for water-selectivity are shaded in yellow. N, amino terminus; C, carboxyl terminus. ‘+’ and ‘−’ represent amino acid charges in the extracellular and cytoplasmic domains.
AQP0 is the major transmembrane protein with important functions in the lens, which plays a critical role in focusing images on the retina and providing visual acuity. By functioning as a water channel AQP0 contributes toward maintaining lens transparency and homeostasis. It also functions as a cell-to-cell adhesion (CTCA) molecule (Zampighi et al., 1989; Costello et al., 1985, 1989; FitzGerald, 1987; Michea et al., 1994, 1995; Fotiadis et al., 2000; Gonen et al., 2005; Kumari and Varadaraj, 2009; 2014a; Kumari et al., 2011; Varadaraj et al., 2010) to maintain lens refractive index gradient (Kumari and Varadaraj, 2014b) and biomechanics (Kumari et al., 2015). The expression level of AQP0 protein in the lens fiber cell membranes modulates gap junction coupling (Kumari et al., 2017). The significance of the role played by AQP0 in the lens is underlined by its contribution of ~45% of the membrane proteins in the fiber cells. However, the single-channel water permeability (Pf) of mammalian AQP0 is much lower than that of AQP1 and AQP5 (Chandy et al., 1997; Yang and Verkman, 1997). Attempts to understand the reason behind the prolific expression of AQP0 at the fiber cell membranes have included gene knockout approach. Irrespective of the loss of one copy (heterozygous) or both (homozygous), lack of AQP0 in mouse resulted in severe lens cataract (Shiels et al., 2001; Al-Ghoul et al., 2003; Kumari et al 2011; Lo et al., 2014; Zhou et al., 2016).
Mutations of AQP0 caused autosomal dominant lens cataract (Shiels et al., 1996, 2000; Okamura et al., 2003; Zhou et al., 2016) signifying the role of AQP0 for lens transparency and homeostasis. Replacing AQP0 with AQP1 in the fiber cells resulted in the loss of CTCA in a transgenic mouse model (Kumari et al., 2011) indicating that fulfillment of water channel requirements alone is insufficient to achieve lens cellular integrity. Therefore, CTCA must be an important physiological role of AQP0. Lens expresses several adhesion proteins (Bassnett et al., 2009); cadherins are well characterized in this regard (Ferreira-Cornwell et al., 2000; Xu et al., 2002; Leonard et al., 2008; Pontoriero et al., 2009; Scheiblin et al., 2014; Logan et al., 2017). In vitro (Kumari and Varadaraj 2009, 2014a; Liu et al., 2011; Kumari et al., 2013; Chauvigné et al., 2016; Nakazawa et al., 2017) ex vivo and in vivo studies have shown the capability of AQP0 to perform CTCA (Costello et al 1989; Michea et al., 1995; Fotiadis et al., 2000; Zampighi et al., 2002; Kumari et al., 2011). Gu et. al. (2007) identified a point mutation that caused autosomal dominant congenital lens cataract in a five-generation Chinese family. This mutation at codon 33 that showed the substitution of cysteine (C) for arginine (R) is in the ELA of human AQP0 (Fig. 1). Functional characterization of R33C mutation revealed that protein trafficking and Pf remained unaffected in contrast to CTCA and gap junction coupling (Kumari et al., 2013).
Through the present investigation, we sought to identify the functional consequences of mutating the positively charged amino acid residues in ELA and ELC of AQP0 to a neutral residue individually or substituting ELA or ELC with the corresponding loops from AQP1. ELA and ELC have been predicted by biochemical (Michea et al., 1994, 1995; Kumari et al., 2011) and structural studies (Gonen et al., 2004; Harries et al., 2004; Jensen et al., 2008) to play a significant role(s) in CTCA. We tested WT, and loop-substituted or charge-altered mutant AQP0 using heterologous expression systems for protein trafficking, and Pf and CTCA functions.
2. Materials and Methods
2.1. Animals
Xenopus laevis female frogs were purchased from Nasco (Fort Atkinson, WI, USA) and acclimatized to laboratory conditions for collecting oocytes to conduct expression studies and AQP water permeability measurements. Details of frog oocyte collection are given in detail in the Supplementary Section. All animal procedures were performed according to the ‘ARVO Statement for the Use of Animals in Ophthalmic and Vision Research’, ‘Guide for the Care and Use of Laboratory Animals’ by the National Institutes of Health (NIH; Bethesda, MD) and the protocols approved by Stony Brook University Animal Care and Use Committee (IACUC Protocol #205778).
2.2. Construction of plasmids and cRNA expression
An expression plasmid that encodes mouse WT AQP0 was constructed with a fluorescent tag (EGFP, Clontech, Mountain View, CA) at the C-terminal end. pcDNA 3.1 myc-His vector (Invitrogen, CA) containing CMV and T7 promoters was used for cloning (Varadaraj et al., 2008). In short, the coding sequence of WT mouse AQP0 was amplified by PCR, gel purified and cloned into the vector with a C-terminal EGFP tag. This AQP0 construct was used for creating point mutations R33Q and H40Q in ELA, and R113Q, H122Q in ELC. QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and specific oligonucleotides were used (Table S1, given in Supporting Information Section) for creating the mutants as described (Varadaraj et al., 2008; Kumari et al., 2013). We have selected the amino acid glutamine (Q) as the substituent amino acid based on its polar nature and close similarity in molecular size to arginine and histidine; also, the 3-D structural analysis after the substitution did not show any significant structural alteration. Additionally, using specific oligonucleotide primers and overlap polymerase chain reaction, ELA and ELC of mouse AQP1 were amplified separately and substituted for corresponding loops in AQP0 and cloned into the vector. Our previous in vitro study has shown that under the normal physiological conditions present in the lens cortex, attachment of the tag does not interfere with the functions (Pf and CTCA) of AQP0 (Kumari et al., 2013). Bidirectional automated sequencing was performed using our University Sequencing Facility to ascertain introduced mutations and substitutions, as well as the entire insert sequences. WT-AQP1 and E-Cadherin expression constructs previously generated (Kumari and Varadaraj., 2009) were included in experiments as necessary. Using T7 RNA polymerase and mMESSAGE mMACHINE T7 Ultra Kit (Ambion, USA), capped complementary RNAs (cRNAs) were synthesized in vitro and quantified. aliquots were stored at −80°C. Ovarian lobes with stage V and VI oocytes from Xenopus laevis frog were surgically removed, defolliculated with Collagenase Type II (Sigma-Aldrich, St. Louis, MO) and the oocytes were maintained at 18°C. cRNA of the respective expression construct (5 or 25 ng in 25 nl/oocyte) was injected into healthy oocytes (Varadaraj et al., 2008)]. Control oocytes received an equal volume of distilled water.
2.3. Immunostaining of AQP0 proteins expressed in oocytes
Xenopus laevis oocytes injected with distilled water (control) or the corresponding cRNA to express WT AQP0, R33Q, H40Q, R113Q, H122Q, AQP0-AQP1ELA, or AQP0-AQP1ELC protein were fixed in 4% paraformaldehyde and cryosectioned at 12–18μm thickness. The sections were immunostained with polyclonal rabbit antibody raised against human AQP0 (Catalog # sc-99059, epitope 220–263; Santa Cruz Biotechnology, Inc., Dallas, TX), further processed and mounted in anti-fade Vectamount (Vector Laboratories, Inc., Burlingame, CA). Using Zeiss Axiovert 200M motorized inverted fluorescence microscope, optimized Z-sectional digital images were acquired as described (Kumari et al., 2013; Varadaraj et al., 2008).
2.4. Oocyte membrane Pf measurement
The rate of swelling of control (distilled water-injected) or cRNA of WT- or mutant AQP0-injected oocytes in response to hypo-osmotic shock was recorded, measured and used to calculate oocyte Pf (μm/s), as described previously (Varadaraj et al., 2005, 2008). Details of Pf measurement are given in detail in the Supplementary Section.
2.5. Expression and localization studies of WT and mutant proteins in cultured cells
Membrane-specific localization was tested using Madin Darby Canine Kidney (MDCK) cells (ATCC (Manassas, VA). Using Effectene reagent (Qiagen, USA) or X-tremeGENE HP DNA transfection reagent (Roche Diagnostics, Indianapolis, IN) WT AQP0, R33Q, H40Q, R113Q, H122Q, AQP0-AQP1 ELA or AQP0-AQP1ELC expression constructs with EGFP tag were transfected separately, following the company protocol, into MDCK cells and cultured in a 37°C incubator with 5% CO2. WT-AQP0-EGFP or AQP0-mutant-EGFP expressing MDCK cells were seeded onto square coverslips in 35 mm culture dishes and grown to 100% confluence. These cells were transduced with Organelle Light Plasma Membrane Targeted Red Fluorescent Protein (PM-RFP; CellLight® Reagent BacMam 2.0, Life Technologies) which localizes at the plasma membrane, following the manufacturer’s protocols. After washing the cells twice with pre-warmed normal growth medium and replacing with fresh medium, cells were incubated for 36 h at 37°C as above. The cells were washed in warm 1X PBS, fixed in 4% paraformaldehyde, washed again, mounted with anti-fade Vectamount containing DAPI nuclear stain and optimized Z-sectional digital images were acquired using a Carl Zeiss AxioVision Confocal microscope.
Colocalization of AQP0 (WT or mutant) and PM-RFP was tested using FRET technique, to find out plasma membrane localization of the tested protein. AQP0-mutant-EGFP served as the donor (Ex 488 and Em 507) and PM-RFP as the acceptor (Ex 587 Em 610). Images were acquired using a Zeiss microscope which contained a 63x oil immersion lens and the following filters/dichroic sets (nm): (1) Texas Red cube, excitation (EX) 545/30, emission (EM) 620/60, beamsplitter 570 (longpass); (2) EGFP cube, excitation (EX) 470/40, emission (EM) 525/50, beamsplitter 495 (longpass); (3) FRET cube, EX 470/40, EM 640/50, beamsplitter 495 (longpass) (Chroma Technology Corp, USA). As a control, WT-AQP0-EGFP and PM-RFP were co-transfected and subjected to FRET analysis.
2.6. CTCA studies
2.6.1. Characterization and quantification of L-cell clones expressing WT or mutant AQP0 protein at the plasma membrane
L-cell clones of WT and mutants with comparable levels of EGFP fluorescence and FRET signals were selected. An equivalent amount of plasma membrane expression of WT and mutant AQP0 was confirmed by protein biotinylation using Pierce Cell Surface Protein Isolation Kit following the manufacturer’s instructions (Thermo Scientific, Waltham, MA, USA) followed by Western blotting. In brief, cells were cultured in 75 cm2 culture flasks (four flasks/clone) and incubated for 45 min at 4 °C with Sulfo-NHS-SS-Biotin, quenched using 100mM glycine in PBS and washed to remove excess biotin and byproducts. The cells were harvested by gentle scraping and lysed using the lysis buffer with a protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). The supernatants of the lysates were incubated with Neutravidin Agarose beads for 2 h in a column. The biotinylated proteins were separated from the unbound intracellular proteins by centrifugation of the column, eluted using dithiothreitol (DTT) and concentrated using a Microcon YM-10 filter (Millipore, Billerica, MA, USA) for gelelectrophoresis. Proteins were quantified using the BCA Protein Assay Kit (Pierce, ThermoScientific) and stored at −80 °C. Western blotting was performed as given in the Supplementary section. Clones expressing equal amounts of protein as in the WT AQP0 clone were selected for cell adhesion studies.
2.6.2. Cell aggregation assay using a rotary gyratory shaker
Cell aggregation assay was performed to test the CTCA property as described (Hiroaki et al., 2006; Kumari and Varadaraj, 2009; Kumari et al., 2014a). This assay allows a semi-quantitative measurement of CTCA and does not quantify the adhesive force involved. More cell clustering and firm adhesion are exhibited by a protein that promotes comparatively stronger adhesion at a given time and vice versa. Clones of mouse fibroblast L-cells (CCL-1.3; ATCC, Manassas, VA) stably expressing empty vector, AQP1 (negative controls), E-cadherin (positive control), WT-AQP0, each of the created charge mutants of ELA or ELC, or loop-substituted chimera were grown separately to 75–85% confluence. L-cells expressing WT or mutant AQP0 were prepared gently and the assay was done as described in the Supplementary section. The mean and standard deviations were calculated from six repetitive aggregation assays (for each sample) and plotted as a bar graph.
2.6.3. CTCA assay using an epifluorescent microscope
A fluorescent imaging technique was used in this assay as described by Kumari and Varadaraj (2009). Adhesion-deficient L-cells stably expressing empty vector, AQP1, E-cadherin, WT-AQP0 or mutant AQP0 were grown to 80% confluence. The cells were loaded with membrane permeable CellTracker™ Red CMPTX dye (Kumari and Varadaraj 2009, 2014a) and CTCA assay was performed as given in the Supplementary section. Mean and standard deviations were calculated from the values of six repetitions and represented as a bar graph. Student’s t-test was used to evaluate the statistical significance of the difference between the values for two samples, using the data from the six repetitions.
2.6.4. CTCA assay to test the mechanism of AQP0-induced adhesion using an epifluorescent microscope
Dye-loaded cells were prepared as described above using the same expression clones. The cells were seeded over a monolayer of untransfected L-cells cultured in 24-well microplates and incubated for 1h at 37°C. CTCA assay and imaging using a Zeiss epifluorescent microscope were done as described above. Mean and standard deviations were calculated from the values of eight repetitions. Statistical significance of the difference between the values for two samples was evaluated using Student’s t-test. The data were also compared with those obtained in the above section, ‘CTCA assay using an epifluorescent microscope’.
2.6.5. CTCA assay using unilamellar lipid vesicles and AQP0-expressing cells
To test whether CTCA was facilitated by the interaction of the positively charged amino acids in the extracellular loops of AQP0 with the negative charges of the neighboring fiber cell plasma membrane or those in the proteins of the fiber cell plasma membrane or extracellular matrix, we conducted CTCA experiments involving pure lipids such as negative charged phosphatidylserine (PS) and neutral phosphatidylcholine (PC). The assay was done following the fluorescent imaging technique (Varadaraj and Kumari (2018). Briefly, adhesion deficient L-cells stably expressing empty vector, WT-AQP0 or mutants of AQP0 (AQP0-AQP1ELA chimera [AQP0 ELA substituted with AQP1 ELA], AQP0-AQP1ELC [AQP0 ELC substituted with AQP1 ELC] and missense mutants [R33Q, H40Q, R113Q, and H122Q]) were grown to 100% confluence. Large unilamellar vesicles (LUVs; Avanti Polar Lipids.Inc., AL.) of negatively charged L-α-phosphatidylserine (PS) and neutral phosphatidylcholine (PC) were prepared for experiments as described (Varadaraj et al., 1999). LUVs were loaded with membrane permeable CellTracker™ Red CMPTX dye and seeded over a monolayer of 100% confluent cells expressing empty vector, WT-AQP0 or mutant AQP0 and incubated for 1h at room temperature. The cells were washed with serum-free MEM culture medium containing 1% BSA for 5 min each (3 times) at room temperature to eliminate loose and weakly adhered LUVs. Cells were fixed in 2% glutaraldehyde and imaged using a Zeiss epifluorescent microscope. For each group, the number of fluorescent LUVs that remained attached to the cell lawn was counted. Mean and standard deviations were calculated from five repetitions. Student’s t-test was used to evaluate the statistical significance.
2.7. Statistics
SigmaPlot 2000 software Version 6.10 was used to perform the Student’s t-tests. A P-value of ≤ 0.05 was considered significant.
3. Results
3.1. Expression systems and mutants
Using heterologous expression systems, we sought to characterize the impact of the mutations in membrane trafficking and functions of AQP0. We used two such expression systems, Xenopus laevis oocytes, and mammalian cells, to conduct experiments.
Figure 2 (a–d) shows the topology of WT and mutant AQP0 with relevant information. In the present study, we generated and tested extracellular loop substitution mutants and missense mutants of charged residues in the ELA or ELC of AQP0; for creating loop substitution mutants, ELA and ELC of AQP0 were substituted separately with the corresponding loop of AQP1. Note, AQP1 is normally expressed in lens epithelial cells and does not possess CTCA property (Kumari and Varadaraj, 2009, 2014a; Hiroaki et al., 2006; Nakazawa et al., 2017). The substitution mutants were designated as AQP0-AQP1ELA (Fig. 2b) and AQP0-AQP1ELC (Fig. 2c). Analysis of the length of ELA of AQP0 and AQP1 reveals that the former has a shorter loop than the latter (Fig. 2b). The net charge of the loop after the substitution turned negative and the length of the protein increased by 7 amino acids to a total of 270 and the mass increased from 28.2 to 29 kDa. At the position of histidine, another positively charged residue arginine was incorporated. However, the substitution introduced two negatively charged residues in the loop. ELC substitution did not change the total number of amino acids of the protein (Fig. 2c, the ELC of both AQP0 and AQP1 are each 21 amino acids long). The substitution resulted in net charge zero for this loop since there were two positively (arginine (R) and histidine (H)) and two negatively (Aspartic acids (D)) charged residues in the ELC of AQP1. By site-directed mutagenesis, we mutated the charged residues, arginine and histidine (denoted with ‘+’ signs in the extracellular region in Fig. 1), individually in the ELA and ELC of AQP0. As indicated in Figure 2, WT AQP0 has 263 amino acids with a calculated molecular mass of 28.2 kDa.
Fig. 2.
Schematics of AQP0 showing WT and the sites targeted for site-directed mutagenesis and extracellular loop (EL) domain substitutions. (a) WT; (b) AQP0-AQP1ELA chimera (ELA of AQP0 is replaced with AQP1ELA (indicated in red)); (c) AQP0-AQP1ELC chimera (ELC of AQP0 is replaced with AQP1ELC (indicated in red)); (d) Site-directed mutants of ELA (R33Q, H40Q; indicated with asterisks on the loop) or ELC (R113Q, H122Q; shown by asterisks on the loop) had a charged residue (arginine, R or histidine, H) replaced with a neutral amino acid residue, glutamine (Q).
3.2. Expression and localization of WT and mutant AQP0 in Xenopus laevis oocytes
Proper trafficking and expression of protein at the plasma membrane are required for normal functioning of AQP0. As anticipated, water-injected oocytes (Fig. 3Aa) did not show any immunostaining at the plasma membrane. WT AQP0 readily localized at the plasma membrane (Fig. 3Ab). AQP0-AQP1ELA protein was able to traffic and localize at the plasma membrane (Fig. 3Ac). Even though ELC substitution did not change the total number of amino acids of the protein (Fig. 2c), it hindered the trafficking of AQP0-AQP1ELC chimeric protein and caused cytoplasmic retention (Fig. 3Ad). The missense mutants (Fig 3A e–h) localized at the oocyte plasma membrane like WT AQP0 (Fig. 3Ab). All mutant AQP0 expressed comparable amounts of protein except AQP0-AQP1ELC when total membrane proteins from oocytes for each sample were tested by immunoblotting using AQP0 antibody (data not shown).
Fig. 3.
Expression of wild type and mutant AQP0, and characterization of water channel function. (A). Expression of WT and mutants of AQP0 in the Xenopus oocytes. Cryosections of oocytes injected with distilled water or complementary RNAs (cRNAs) of WT or mutant AQP0 were immunostained using a polyclonal AQP0 antibody; the secondary antibody was conjugated to FITC: (a) Water-injected oocyte; (b-h) cRNA-injected: (b) WT AQP0; (c) AQP0-AQP1ELA; (d) AQP0-AQP1ELC; (e) R33Q; (f) H40Q; (g) R113Q; (h) H122Q. White solid arrows pointing to the plasma membrane indicate the absence or expression of AQP0. (B). Functional expression of WT AQP0, extracellular loop domain chimeras (AQP0-AQP1ELA and AQP0-AQP1ELC) and missense mutants (R33Q, H40Q, R113Q, and H122Q) in Xenopus laevis oocytes. For the study, the oocytes were injected with either distilled water or cRNA of WT or mutant AQP0 (25 ng/oocyte). Average Pf of twelve oocytes (mean ± SD) of WT or mutant AQP0 is shown. Star represents lack of Pf in AQP0-AQP1ELC, which is similar to dH2O-injected control. ‘+’ denotes the significant increase in Pf compared to WT AQP0.
3.3. Oocyte plasma membrane Pf
Figure 3B shows that Pf of all mutants except AQP0-AQP1ELC was comparable to that of WT AQP0, indicating that the introduced mutation/substitution did not affect protein folding or trafficking characteristics and did not hinder Pf function significantly (Table 1). Pf of the histidine mutants, H40Q and H122Q, increased significantly (indicated by ‘+’ in Fig. 3B) compared to that of WT. AQP0-AQP1ELC did not cause any increase in oocyte volume in response to the hypo-osmotic shock; the level of Pf stayed low (indicated by a star in Fig. 3B) and was comparable to that of the distilled water-injected oocytes. The difference observed in water channel function between WT and different mutants (Fig. 3B) could be due to the difference in the channel opening efficiency caused by alteration/s in mutation-induced protein folding. Experiments using Xenopus laevis oocytes were intended to test whether the mutants of AQP0 can traffic to the plasma membrane and form functional water channels.
Table 1.
Plasma membrane water permeability of Xenopus laevis oocytes microinjected with cRNA of WT or mutant AQP0
| cRNA | Plasma Membrane Water Permeability (μm/s) | Fold Increase Compared to Distilled Water Injected Oocytes |
|---|---|---|
| Control (distilled water injected) | 10.9 ± 2.47 | |
| WT AQP0 | 46.4 ± 3.92 | 4.26 |
| AQP0-AQP1ELA | 42.8 ± 6.61 | 3.93 |
| AQP0-AQP1ELC | 9.5 ± 2.07 | 0.00 |
| R33Q | 45.8 ± 6.96 | 4.20 |
| H40Q | 55.2 ± 6.32 | 5.06 |
| R113Q | 45.1 ± 6.90 | 4.14 |
| H122Q | 53.4 ± 6.95 | 4.90 |
3.4. Expression and localization studies of WT and mutant proteins in cultured mammalian cells
To verify plasma membrane localization of WT or mutant protein in mammalian cells, FRET method was followed. WT or each of the mutants was transfected separately into MDCK cells. Plasma Membrane marker PM-RFP was transduced into each cell group. Captured FRET signals (Fig. 4A) showed plasma membrane localization of all the mutants except AQP0-AQP1ELC and corroborated the immunostaining results obtained using oocyte expression system (Fig. 3A). FRET signal above background occurs when WT or mutant AQP0-EGFP and plasma membrane protein-RFP colocalize within 100Å. The signals observed by conducting FRET on WT AQP0 served as a positive control for plasma membrane localization of AQP0.
Fig. 4.
Verification of WT and mutant AQP0 expression and localization in MDCK cells. (A). Plasma membrane localization of WT and mutant AQP0 by FRET analysis. Colocalization of EGFP tagged (a-c) WT AQP0, (d-f) AQP0-AQP1ELA, (g-i) AQP0-AQP1ELC, (j-l) R33Q, (m-o) H40Q, (p-r) R113Q, (s-u) H122Q coexpressed with plasma membrane (PM-RFP (CellLight® Red Fluorescent Protein, PM-RFP). Coexpressing cells were viewed under an EGFP fluorescent filter (column 1) and the same cells were viewed under Texas Red fluorescent filter to see protein localization at the plasma membrane (column 2). Plasma membrane localization was assessed using Forster Resonance Energy Transfer (FRET) signal intensity, shown in red (column 3). FRET studies were conducted using MDCK cells expressing WT AQP0-EGFP (a) or mutant AQP0-EGFP (d, g, j, m, p, s) and PM-RFP (b, e, h, k, n, q, t). Column 1 (a, d, g, j, m, p, s), cells excited at 488 nm and emission recorded at 507 nm; Column 2 (b, e, h, k, n, q, t), cells excited at 587 nm and emission recorded at 610 nm; Column 3 (c, f, i, l, o, r, u), cells excited at 470 nm and emission recorded at 640 nm; fluorescence due to FRET indicates colocalization of WT AQP0-EGFP or mutant AQP0-EGFP with PM-RFP within 100Å. Nuclei were stained with DAPI (blue). Compared to other AQP0 mutants and WT AQP0, AQP0-AQP1ELC (g-i) did not exhibit considerable FRET signal. (B). WT and mutant AQP0 protein expression levels at the plasma membranes of adhesion-deficient mouse fibroblast L-cells. Expression levels of the proteins were tested by Western blotting using a C-terminal-specific AQP0 antibody. Top panel: Western blotting. Immunoreactivity of EGFP tagged WT AQP0 (lane 1), AQP0-AQP1ELC (lane 2), AQP0-AQP1ELA (lane 3), mutants (R33Q lane 4), H40Q (lane 5), R113Q (lane 6) and H122Q (lane7). A ~55 kDa band reacted intensely in all samples except in AQP0-AQP1ELC. Immunoreactive band intensities were quantified and represented in the bottom panel.
3.5. CTCA Studies
With the observations made using Xenopus laevis oocytes and eukaryotic mammalian MDCK cell expression systems for proper protein trafficking and Pf in all the mutants except AQP0-AQP1ELC, we tested the CTCA function. Mouse L-fibroblast cells were used for CTCA studies since these cells lack endogenous adhesion molecules. The L-cells stably expressing vector (negative control), AQP1 (negative control), E-cadherin (positive control), WT-AQP0 or each one of the mutants separately was studied. The stably expressing L-cell clones of WT and mutant AQP0 were tested to ascertain comparable levels of protein expression at the plasma membrane, using the biotinylation technique. Expression levels of the proteins were confirmed by Western blotting using C-terminal-specific AQP0 antibody (Fig. 4B, top). Quantification of the band intensity (Fig. 4B, the bar graph on the bottom left) showed that except AQP0-AQP1ELC, all the mutant AQP0 clones selected expressed a relatively equal amount of protein at the plasma membrane. Also, both WT and mutant AQP0 clones were tested for the housekeeping gene Na/K-ATPase as loading control; Figure 4B bottom panel of the Western blot shows comparable levels of loading control protein. The bar graph on the bottom right in Figure 4B shows no significant difference in the loading of the WT and mutant samples. Before conducting CTCA experiments, we performed Western blotting to ensure that our trypsinization procedure did not cleave the proteins of interest (provided in the Supplementary section; Figure S1).
The adhesive property was tested by means of cell aggregation assay at different time points (30, 60, 90 min). Figure 5 shows that cells expressing the positive control E-cadherin exhibited the highest cell aggregation which was manifest by the reduction in the number of independent particles remaining as a function of time, followed by WT AQP0, H122Q, H40Q, R33Q, R113Q, and AQP0-AQP1ELA in descending order. AQP0-AQP1ELC was not tested since it did not traffic to the plasma membrane. AQP0 mutants AQP0-AQP1ELA, R33Q and R113Q showed a statistically significant decrease in aggregation compared to the WT at 30, 60, and 90 min time points. H40Q and H122Q mutants showed a decrease in aggregation at all the tested time points; however, H40Q showed a statistically significant decrease in aggregation only at 90 min time point compared to the WT. H122Q showed a decreasing trend in aggregation as a function of time. We did not test for a longer time because the cells are not suitable for studies for extended periods. All the mutants showed a decrease in aggregation compared to the WT.
Fig. 5.
Cell aggregation assay. Cell aggregation assays were performed for WT or mutant AQP0-transfected mouse fibroblast L-cells using a rotary gyratory shaker. Cell aggregation (%) exhibited by the adhesion-deficient L-cells expressing empty vector, AQP1, E-cadherin, WT AQP0 or mutant AQP0 (AQP0-AQP1ELA, R33Q, H40Q, R113Q or H122Q) in relation to incubation time is shown in bar graphs: A, 30 min; B, 60 min and C, 90 min. As cells aggregate, the number of particles decreases. (Nt - total number of particles at time ‘t’ of incubation; N0 - initial number of particles). Compared to WT AQP0, the mutants (AQP0-AQP1ELA, R33Q, H40Q, R113Q) exhibited significantly low (P < 0.001) cell aggregation. E-cadherin - positive control. Empty vector and AQP1 - negative controls. The significant (P < 0.05) reduction in cell to cell aggregation in mutant AQP0 compared to WT is indicated by an asterisk. Eight repetitive aggregation assays were conducted for each sample.
To investigate the mechanism(s) of CTCA, a fluorescence imaging assay developed in our laboratory (Kumari and Varadaraj, 2009, 2014a) was followed. Mouse fibroblast L-cell groups stably expressing WT AQP0, each one of the mutants as separate cultures, empty vector, AQP1 or E-cadherin were tested (Fig. 6A). Empty vector and AQP1 served as negative controls and E-Cadherin as a positive control. CTCA exerted by all the mutants was significantly less compared to that of WT-AQP0 (Fig. 6A, B; Table 2). Adhesion data collected for AQP0-AQP1ELC was comparable only to that of the negative controls as the chimeric protein failed to traffic and localize at the plasma membrane (Figs. 3A, Bd; 4A g–i). The trend seen with the cell aggregation assay was true with the results obtained using the fluorescence method. Among those mutants that trafficked and localized at the plasma membrane, AQP0-AQP1ELA exhibited the least CTCA property, 36% less compared to WT AQP0 (Table 2), indicating an important role for the ELA of AQP0 in CTCA. Among the missense mutants, the substitution of the positively charged arginine with neutral glutamine significantly affected CTCA more than the positively charged histidine replaced with glutamine (Table 2; P< 0.001). Histidine mutation in either of the loops tested exerted comparable CTCA between them, so did the arginine mutants. Altogether, the results point out that the charged residues in the ELA and ELC are critical for the CTCA function of AQP0.
Fig. 6.
CTCA assay. (A). Over a monolayer of L-cells expressing empty vector, AQP1, E-cadherin, WT AQP0 or mutant AQP0 (AQP0-AQP1ELA, AQP0-AQP1ELC, R33Q, H40Q, R113Q or H122Q) corresponding cells loaded with CellTracker Red were plated. At the end of the procedure, the cells were imaged under an epifluorescent microscope. Cells/aggregates were counted and plotted. (B). Bar graph represents the number of dye-loaded L-cells expressing empty vector, AQP1, E-cadherin, WT AQP0 or mutant AQP0 (AQP0-AQP1ELA, AQP0-AQP1ELC, R33Q, H40Q, R113Q or H122Q) that remained attached to the matching cDNA construct-transfected L-cells (without dye) due to CTCA. Samples were tested using the fluorescence assay and incubated for 1h for CTCA. Compared to WT-AQP0, mutant AQP0 exhibited significantly low (P < 0.05) CTCA, denoted with a star for each sample. E-cadherin - positive control. (C). CTCA assay testing the possible mechanism of CTCA. The number of dye-loaded cells expressing empty vector, AQP1, E-cadherin, WT AQP0 or mutant AQP0 (AQP0-AQP1ELA, AQP0-AQP1ELC, R33Q, H40Q, R113Q or H122Q) that remained attached to untransfected L-cells, due to CTCA in the samples tested using the fluorescence assay with 1h of incubation are represented. Star on the mutants denotes a reduction in CTCA in comparison with WT AQP0. Note: Number of E-cadherin transfected cells that remained attached was much less than that of WT AQP0 transfected cells and represented with two stars.
Table 2.
Cell-to-cell adhesion
| Expression construct in L-cells | Number of Dye Loaded Cells Attached | Decrease in Cell-to-cell Adhesion Compared to WT AQP0 (%) |
|---|---|---|
| Vector | 36.17 ± 6.55 | |
| AQP1 | 119.53 ± 13.70 | |
| E-cadherin | 725.17 ± 26.58 | |
| WT AQP0 | 554.57 ± 49.59 | |
| AQP0-AQP1ELA | 357.97 ± 37.53 | 36* |
| AQP0-AQP1ELC | 147.35 ± 20.76 | 73* |
| R33Q | 419.12 ± 23.12 | 24* |
| H40Q | 465.37 ± 25.72 | 16* |
| R113Q | 395.30 ± 16.26 | 29* |
| H122Q | 476.48 ± 24.18 | 14* |
P <0.05
To test whether CTCA was promoted by the interaction of positively charged amino acids in the extracellular loops of AQP0 and negative charges in the plasma membrane, we plated WT or mutant AQP0 expressing L-cells over untransfected, adhesion-deficient L-cells (parental L-cells). CTCA was significantly reduced (to ~50%) both in the WT and the different mutants of AQP0 (Fig. 6C; Table 3) compared to the number of cells attached when cells in the culture plate and overlaid cells both expressed matching transfected AQP0 (Fig. 6B; Table 2). The increased CTCA (Fig. 6B) could be due to the interaction of AQP0 present in the cells of the dish and in the overlaid cells, with the opposing cell’s plasma membrane (bidirectional). Decreased cell adhesion as seen in Fig. 6C could be due to lack of AQP0 in the parental L-cells in the culture dish causing only unidirectional adhesion i.e., reduction in electrostatically-driven protein-plasma membrane interaction. The reduction in adhesion among the mutants followed a trend similar to that observed in Figure 6B (Table 2); however, E-cadherin exhibited the most significant reduction in CTCA (to ~90%; Fig. 6C; Table 3) compared to E-cadherin adhesion seen in Figure 6B. This could be due to lack of E-cadherin in the opposing untransfected L-cells grown in the plates since E-cadherin exerts CTCA by the interaction between its EC loops in opposing cell membranes.
Table 3.
Cell-to-cell adhesion of expression construct transfected, dye loaded L-cells that were plated over parental L-cells.
| Expression construct in L-cells | Number of Dye Loaded Cells Attached | Decrease in CTCA Compared to WT AQP0 (%) | Decrease in CTCA compared to data in column 2 of Table 2 (%) |
|---|---|---|---|
| Vector | 37.63 ± 6.48 | ||
| AQP1 | 118.25 ± 20.43 | ||
| E-cadherin | 89.25 ± 15.32 | 88* | |
| WT-AQP0 | 273.75 ± 26.48 | 51* | |
| AQP0-AQP1ELA | 179.63 ± 20.08 | 34* | 50* |
| AQP0-AQP1ELC | 75.13 ± 9.75 | 73* | 49* |
| R33Q | 187.88 ± 17.12 | 31* | 55* |
| H40Q | 212.75 ± 26.00 | 22* | 54* |
| R113Q | 187.00 ± 20.93 | 32* | 53* |
| H122Q | 232.75 ± 22.00 | 15* | 51* |
P <0.05
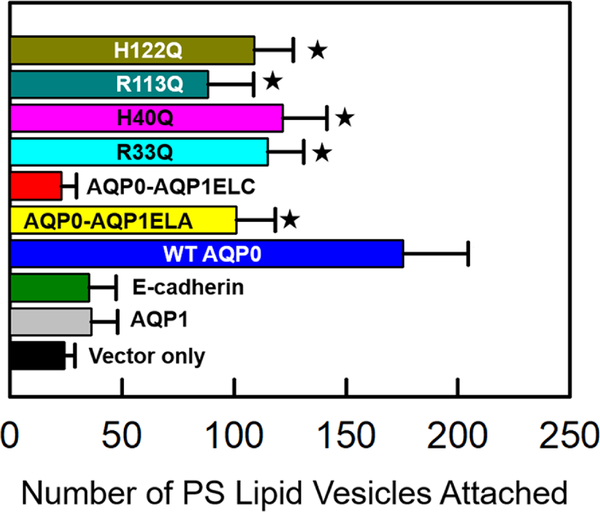
To further test whether CTCA was promoted by the interaction of the positively charged amino acids in the extracellular loops of AQP0 with the negative charges in the plasma membrane, we plated negatively charged phosphatidylserine (PS) or neutral phosphatidylcholine (PC) lipid large unilamellar vesicles (LUVs) over 100% confluent L-cells expressing vector, AQP1, E-cadherin, WT or mutant AQP0 and performed the adhesion assay. The data show a significant increase in the adhesion of PS LUVs to the cells expressing WT and mutants of AQP0 in contrast to the cells that express AQP1 ELC loop chimera (AQP0-AQP1ELA), vector, AQP1 or E-cadherin (Fig. 7A). Another important observation is a significant reduction in the adhesion of PS LUVs to cells expressing mutant AQP0 containing fewer positive charges in the extracellular loops compared to the adhesion of PS LUVs to WT AQP0. Adhesion of PS LUVs to the cells expressing AQP0-AQP1ELA showed a significant (P<0.05) reduction, compared to the WT, possibly due to the loss of two positive charges as a result of the substitution of AQP1 ELA loop. Neutral PC LUVs did not show any significant adhesion to vector, AQP1, E-cadherin, WT or mutant AQP0-expressing cells (Fig. 7B).
Fig. 7.
Cell-to-Lipid vesicle adhesion assay. (A). Bar graph representing the number of dye-loaded unilamellar phosphatidylserine (PS) lipid vesicles (negatively charged) attached to L-cells expressing empty vector, AQP1, E-cadherin, WT AQP0 or mutant AQP0 (AQP0-AQP1ELA, AQP0-AQP1ELC, R33Q, H40Q, R113Q, H122Q) without dye. Samples were incubated for 1h. PS lipid vesicles exhibited increased adhesion to WT-AQP0 and most of the mutants compared to vector-transfected cells (P < 0.05), denoted with a star for each sample. (B). Histogram representing the number of dye-loaded unilamellar phosphatidylcholine (PC) neutral lipid vesicles attached to L-cells expressing empty vector, AQP1, E-cadherin, WT AQP0 or mutant AQP0 (AQP0-AQP1ELA, AQP0-AQP1ELC, R33Q, H40Q, R113Q, H122Q) without dye. Samples were incubated for 1h. In contrast to PS vesicles, PC lipid vesicles exhibited no significant adhesion to WT-AQP0 or mutant AQP0 (P > 0.05).
4. Discussion
In this investigation, we sought to find out the domain(s) in AQP0 responsible for its CTCA function and the molecular mechanism behind it. Several studies using different methods have pointed out the possible participation of ELA and ELC in CTCA: electron microscopy (Costello et al., 1985, 1989; Zampighi et al., 2002; Scheuring et al., 2007), liposome studies (Michea et al., 1994, 1995), electron diffraction microscopy (Gonen et al., 2004), X-ray crystallography (Harries et al., 2004) and molecular dynamics simulation (Jensen et al., 2008). Gu et. al. (2007) reported a human AQP0 mutation in which the positively charged polar amino acid arginine (R) was replaced with neutral cysteine (C) in the ELA (R33C) that caused a severe lens cataract. Functional characterization of R33C showed that while Pf remained unaffected, CTCA reduced significantly (Kumari et al., 2013). In the current study, the missense mutants in which a positively charged residue was replaced with a neutral residue trafficked to the plasma membrane and performed Pf. However, when domain swapping was performed, in contrast to AQP0-AQP1ELA chimera, AQP0-AQP1ELC was unable to traffic and localize at the plasma membrane even though the substitution consisted of an equal number of residues and preserved the total length as 263 amino acids as in the WT. The substitution might have caused constraints in protein folding and trapped it in the cytoplasm (Figs. 3B, 4A).
The missense mutants and AQP0-AQP1ELA exhibited a decrease in CTCA compared to WT AQP0. AQP0 forms tetramer units and each subunit functions independently. In mouse, WT AQP0 tetramer has 24-positive charges, two in each extracellular loop, thus six positive charges are contributed by each monomer. These positive charges could electrostatically interact with negative charges of the plasma membrane, causing strong CTCA. In AQP0-AQP1ELA chimera, the substitution of AQP1 ELA introduced one positive charge, two negative charges and an additional seven residues (Fig. 2b). The charge composition of ELA changed from being net positive to net negative. Therefore, instead of 24 positive charges in a tetramer, AQP0-AQP1ELA chimera had only twelve. In the missense mutants, the total number of positive charges became 20, since the charge reduction was four per tetramer. It is noteworthy that none of the extracellular loops of WT AQP0 contains a negative charge. Considering our result that loss of positive charge/s or gain of negative charges in the extracellular loop results in decreased fiber cell adhesion, extracellular loop positive charges are critical for CTCA. Since each WT tetramer contributes 24 positive charges, there could be substantial charge density in square array clusters, which result from natural N- and C-terminal truncations of AQP0 (Kumari et al., 2014a). These positive charges could attract negatively charged moieties of the opposing plasma membrane (Michea et al., 1994; Zampighi et al., 1989; Harries et al., 2004). Such electrostatic attraction and interaction could lead to the creation of thin junctions with extremely narrow extracellular spaces (0.5–0.7 nm; Zampighi et al., 1989). The R33C mutation in human (Gu et al., 2007; Kumari et al., 2013) that caused lens cataract phenotype due to the loss of one positive charge per monomer, further strengthens our hypothesis that the positive charges in the AQP0 extracellular loops are necessary to facilitate electrostatic interaction for narrowing of the extracellular space. The CTCA property of AQP0-R33C was also significantly reduced (P < 0.001) compared to that of intact (WT) AQP0 (Kumari et al., 2013).
The mechanism of AQP0-facilitated CTCA was investigated in vitro in the present study by seeding dye-loaded cells expressing WT, mutant AQP0 or control cDNAs, over untransfected adhesion-deficient (parental) L-cells. There was ~50% reduction (Fig. 6C; Table 3) in CTCA compared to when cells expressing AQP0 were plated over AQP0 expressing L-cells (Fig. 6B; Table 2); lack of AQP0 in the parental L-cells could be leaving the negative charges in the plasma membranes of the overlaid AQP0-transfected L-cells free of any interaction. CTCA was reduced to ~90% when E-cadherin expressing cells were overlaid on parental L-cells (Fig. 6C; Table 3). E-cadherins promote CTCA by homophilic interaction between the extracellular loops present in two opposing cell membranes. Since there was no E-cadherin in the parental L-cells to bring about this interaction, CTCA was reduced drastically. These results corroborate the notion that AQP0 could be promoting CTCA by charge interaction with the negative charges of the opposing fiber cell plasma membrane, as suggested previously (Michea et al., 1994, 1995; Zampighi et al., 1989; Harries et al., 2004). L-cells expressing lens aquaporin, AQP1 or AQP0 and LUV adhesion studies strongly suggest that positively charged amino acids in the AQP0 extracellular loop domains electrostatically interact with the negative charges in the plasma membrane to promote fiber cell-to-cell adhesion.
Even though the missense mutants showed a statistically significant reduction in CTCA compared to WT AQP0, the arginine to glutamine mutation caused more reduction than the histidine to glutamine change. A possible reason for the difference in the decrease in CTCA could be the respective amino acid pKa. The net charge of AQP0 monomer could be dictated by the pKa of the amino acid side chain based on its environmental pH. Fifty percent of the arginines remain charged at its pKa of 13.5; below this, the percentage of arginine that becomes charged, increases. Similarly, 50% of histidine is charged at its pKa of 6.8 (Hass and Mulder, 2015). The lens has a pH gradient of 7.2 to 6.8 from the cortex to the nucleus (Mathias et al., 1991). We tested the adhesion properties at pH 7.2. Due to the replacement of arginine with glutamine, the amino acid that normally stays positively charged at pH 7.2, is lost; the charged residue that remains in the tested extracellular loop is histidine, which is poorly charged at pH 7.2 due to its pKa of 6.8. This could have ultimately brought down the CTCA capability of the arginine-mutant AQP0. However, the low pKa of histidine could be advantageous in the lens nucleus (for exerting CTCA) that has characteristically low pH (6.8), and more histidine could remain charged compared to those in the cortex. The presence of charged amino acids R and H in each of the extracellular loops could be an adaptive strategy of the lens for modulating fiber cell to fiber cell adhesion. It appears that the pH gradient in the lens could facilitate CTCA gradient formation from the outer cortex to the inner nucleus to regulate lens accommodation. Testing of pH-dependent adhesion as a function of depth into the lens will be pursued as future work.
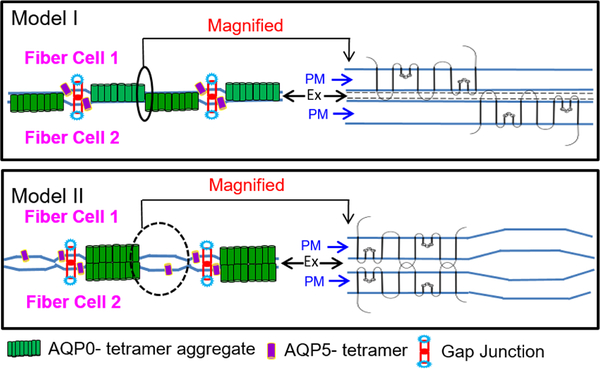
Currently, there are two proposals on the mechanism of AQP0-induced CTCA, either an AQP0-plasma membrane interaction (Michea et al., 1994; Zampighi, 1989, 2002) or an AQP0-AQP0 interaction (Gonen et al., 2004). Considering the current results, and theories put forth by others, we present models indicating the mechanism of CTCA involving the extracellular loop positive charges of AQP0 (Fig. 8, Models I and II). Model I, AQP0-plasma membrane interaction: Positive charges in the extracellular domains of large patches of AQP0 square arrays electrostatically interact with negatively charged opposing fiber cell plasma membrane to provide strong adhesion and draw the membranes closer. Model II shows AQP0 head-to-head interaction involving the loops 1 (Loop A) and 3 (Loop C) with the corresponding loops of AQP0 in the opposing fiber cell membrane. In the models, the black ovals are enlarged and drawn to the right to show the envisioned details of the interaction of two AQP0 monomers from tetramers in the opposing membranes. Comparison of the two models shows that in Model I, the same amount of AQP0 can cover more area and provide CTCA to reduce extracellular space and water. In Model II, N- and C- terminal cleaved AQP0 establish head-to-head interaction to promote CTCA; compared to Model I, only 50% of the area is covered by AQP0 with thin junctions (~0.5–0.7 nm extracellular space). The other half contains wider extracellular space and presumably more water content. Areas without thin junctions but with larger extracellular space (3–30 nm) could significantly affect CTCA and the refractive index gradient of the lens and lead to spherical aberration while focusing. Supporting indirect evidence for Model I can be drawn from other studies in the scientific literature. Electron microscopy in combination with immunolocalization studies has shown that connexin 50 antibody binds to both sides of gap junctions (Zampighi et al., 1989). This is expected because connexins form head-to-head interaction and form 16–17 nm junctions to promote gap junctional coupling. In the same study, as opposed to the head-to-head binding of connexin antibody, AQP0 antibody specifically bound to one side of the membrane at thin junctions. This could be due to the interaction of AQP0 with the negatively charged plasma membrane rather than with the opposing AQP0 of the neighboring fiber cell plasma membrane. The extracellular space in thin junctions are about 0.5 to 0.7 nm wide; if the interaction occurred between AQPs of the opposing membranes, the thin junctions formed would possibly be larger than the recorded width of 0.5 to 0.7 nm but smaller than 16–17 nm gap junctions. However, no data for such a dimension is available in the literature. Therefore, it is logical to suggest that the positive charges in the extracellular loops of AQP0 interact electrostatically with the negatively charged opposing plasma membrane as proposed by several investigators (Costello et al., 1985; Michea et al.,1994, 1995; Zampighi et al., 1989; Harries et al., 2004; Jensen et al., 2008). In vitro liposome experiments by Michea et al. (1994) indicate that AQP0 adheres to negatively charged membrane vesicles, indicating the electrostatic nature of the adhesion. The current data on AQP0 expressing cells adhering to the untransfected parental L-cells in contrast to E-cadherin expressing cells, whose adhesion capability dropped below that of the negative control (Fig. 6C; Table 3), also support Model I (Fig. 8). In a recent investigation (Varadaraj and Kumari, 2018), we used intact and C-terminal end cleaved AQP0 to study the mechanism of AQP0-induced CTCA. Separate assays involving negatively charged lipid vesicles or lens fiber cell membrane vesicles (FCMVs) of wild type and AQP0 knockout mice demonstrated AQP0-membrane interaction. Constantly changing lipid composition in the mature fiber cells could regulate AQP0 protein-lipid electrostatic interactions and modulate fiber cell-to-fiber cell adhesion process from the outer cortex (fiber cells adhere to each other less firmly) to the center (fiber cells adhere to each other more firmly) of the lens. At present, we do not know whether specific negatively charged lipids or negative charges of other proteins in the outer leaflet of the membrane bilayer and/or specific negative charges in the proteins in the extracellular matrix electrostatically interact with the positively charged AQP0 extracellular loop amino acids.
Fig. 8.
Schematic models. Models to illustrate the possible mechanism of AQP0-mediated fiber cell-to-fiber cell adhesion in the lens. Based on the current data, our previous ultrastructural studies and available literature, two models are depicted to show how WT AQP0 exerts CTCA. Model I: Positively charged extracellular domains of AQP0 interact with negatively charged opposing fiber cell plasma membrane to draw the membranes closer. Model II: Extracellular domains of AQP0 from opposing fiber cell plasma membranes interact with each other to draw the membranes closer. In each Model, the area indicated by a black filled or dashed oval is drawn to the right to show a clear, magnified version of the interaction of two AQP0 monomers in the opposing plasma membranes. A comparison of the two models shows that in Model I, the same amount of AQP0 occupies more area and provides strong CTCA to reduce extracellular space and water. In Model II, the head-to-head interaction could cover only 50% of the area occupied by AQP0 in Model I; the other half has wider extracellular space and probably retains more water. Reduction in extracellular space and water is necessary for creating a refractive index gradient in the lens to avoid spherical aberration during focusing, for which Model I would be fitting. Ex - Extracellular space, PM - plasma membrane.
5. Conclusions
Positive charges in the extracellular loops A and C of AQP0 are critical for the electrostatic interaction with the negative charges in the plasma membrane through charge complementarity for firm CTCA. Mutation of a charged amino acid(s) interferes with this type of interaction and leads to weakening or loss of CTCA.
Supplementary Material
Highlights.
Extracellular loops A and C of AQP0 facilitate cell-to-cell adhesion
Electrostatic interaction is involved in cell-to-cell adhesion
ELA and ELC positive residues of AQP0 interact with plasma membrane negative charges
Acknowledgments
This work was supported by NIH-NEI grants R01: EY20506 and EY026155.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski R, Shiels A, Kuszak JR. Lens structure in MIP-deficient mice. Anat. Rec. A Discov. Mol. Cell Evol. Biol 2003; 273: 714–730. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol. Vis 2009; 15: 2448–2463. [PMC free article] [PubMed] [Google Scholar]
- Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J. Membr. Biol 1997; 159: 29–39. [DOI] [PubMed] [Google Scholar]
- Chauvigné F, Fjelldal P, Cerdà J, Finn RN. Auto-adhesion potential of extraocular Aqp0 during teleost development. PLoS One. 2016; 11: e0154592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MJ, McIntosh TJ, Robertson JD. Membrane specializations in mammalian lens fiber cells: distribution of square arrays. Curr. Eye Res 1985; 4:1183–1201. [DOI] [PubMed] [Google Scholar]
- Costello MJ, McIntosh TJ, Robertson JD. Distribution of gap junctions and square array junctions in the mammalian lens. Invest Ophthalmol. Vis. Sci 1989; 30:975–989. [PubMed] [Google Scholar]
- Ferreira-Cornwell MC, Veneziale RW, Grunwald GB, Menko AS. N-cadherin function is required for differentiation-dependent cytoskeletal reorganization in lens cells in vitro. Exp. Cell Res 2000; 256: 237–247. [DOI] [PubMed] [Google Scholar]
- FitzGerald PG. Main intrinsic polypeptide proteolysis and fiber cell membrane domains. Invest. Ophthalmol. Vis. Sci. 1987; 28: 795–805. [PubMed] [Google Scholar]
- Fotiadis D, Hasler L, Müller DJ, Stahlberg H, Kistler J, Engel A. Surface tongue-andgroove contours on lens MIP facilitate cell-to-cell adherence. J. Mol. Biol 2000; 300: 779–789. [DOI] [PubMed] [Google Scholar]
- Gonen T, Sliz P, Kistler J, Cheng Y, Walz T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 2004; 429: 193–197. [DOI] [PubMed] [Google Scholar]
- Grey AC, Walker KL, Petrova RS, Han J, Wilmarth PA, David LL, Donaldson PJ, Schey KL. Verification and spatial localization of aquaporin-5 in the ocular lens. Exp. Eye Res 2013; 108: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Zhai H, Li D, Zhao L, Li C, Huang S, Ma X. A novel mutation in major intrinsic protein of the lens gene (MIP) underlies autosomal dominant cataract in a Chinese family. Mol. Vis 2007; 13: 1651–1656. [PubMed] [Google Scholar]
- Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-A resolution. Proc. Natl. Acad. Sci. USA, 2004; 101: 14045–14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass MA, Mulder FA. Contemporary NMR Studies of Protein Electrostatics. Annu Rev Biophys. 2015; 44: 53–75. [DOI] [PubMed] [Google Scholar]
- Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, Mizoguchi A, Fujiyoshi AY. Implications of the aquaporin-4 structure on array formation and cell adhesion. J. Mol. Biol 2006; 355: 628–639. [DOI] [PubMed] [Google Scholar]
- Jensen M, Dror R, Xu H, Borhani D, Arkin I, Eastwood M, Shaw D. Dynamic control of slow water transport by aquaporin 0: implications for hydration and junction stability in the eye lens. Proc. Natl. Acad. Sci. USA 2008; 105: 14430–14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Eswaramoorthy S, Mathias RT, Varadaraj K. Unique and analogous functions of aquaporin 0 for fiber cell architecture and ocular lens transparency. Biochim. Biophys. Acta 2011; 1812: 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Gandhi J, Mustehsan MH, Eren S, Varadaraj K. Functional characterization of an AQP0 missense mutation, R33C, that causes dominant congenital lens cataract, reveals impaired cell-to-cell adhesion. Exp. Eye Res 2013; 116: 371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Gao J, Mathias RT, Sun X, Eswaramoorthy A, Browne N, Zhang N, Varadaraj K. Aquaporin 0 Modulates Lens Gap Junctions in the presence of Lens-Specific Beaded Filament Proteins. Invest. Ophthalmol. Vis. Sci 2017; 58: 6006–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Gupta N, Shiels A, FitzGerald PG, Menon AG, Mathias RT, Varadaraj K. Role of Aquaporin 0 in lens biomechanics. Biochem. Biophys. Res. Commun 2015; 62: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K. Intact AQP0 performs cell-to-cell adhesion. Biochem. Biophys. Res. Commun. 2009; 390: 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Varadaraj K. Intact and N- or C-terminal end truncated AQP0 function as open water channels and cell-to-cell adhesion proteins: End truncation could be a prelude for adjusting the refractive index of the lens to prevent spherical aberration. Biochim. Biophys. Acta 2014a; 1840: 2862–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K. Aquaporin 0 plays a pivotal role in refractive index gradient development in mammalian eye lens to prevent spherical aberration. Biochem. Biophys. Res. Commun 2014b; 45: 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj M, Yerramilli VS, Menon AG, Varadaraj K. Spatial expression of aquaporin 5 in mammalian cornea and lens, and regulation of its localization by phosphokinase A. Mol. Vis 2012; 18: 957–967. [PMC free article] [PubMed] [Google Scholar]
- Leonard M, Chan Y, Menko AS. Identification of a novel intermediate filament-linked N-cadherin/gamma-catenin complex involved in the establishment of the cytoarchitecture of differentiated lens fiber cells. Dev. Biol 2008; 319: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xu J, Gu S, Nicholson BJ, Jiang JX. Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50. J. Cell Sci 2011; 124: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WK, Biswas SK, Brako L, Shiels A, Gu S, Jiang JX. Aquaporin-0 targets interlocking domains to control the integrity and transparency of the eye lens. Invest. Ophthalmol. Vis. Sci 2014; 55: 1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CM, Rajakaruna S, Bowen C, Radice GL, Robinson ML, Menko AS. N-cadherin regulates signaling mechanisms required for lens fiber cell elongation and lens morphogenesis. Dev. Biol 2017; 428: 118–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RT, Riquelme G, Rae JL. Cell to cell communication and pH in the frog lens. J Gen Physiol. 1991; 98: 1085–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michea LF, Andrinolo D, Ceppi H, Lagos N. Biochemical evidence for adhesion-promoting role of major intrinsic protein isolated from both normal and cataractous human lenses. Exp. Eye Res 1995; 61: 293–301. [DOI] [PubMed] [Google Scholar]
- Michea LF, de la Fuente M, Lagos N. Lens major intrinsic protein (MIP) promotes adhesion when reconstituted into large unilamellar liposomes. Biochemistry 1994; 33: 7663–7669. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y, Oka M, Funakoshi-Tago M, Tamura H, Takehana M. The extracellular C-loop domain plays an important role in the cell adhesion function of Aquaporin 0. Curr. Eye Res 2017; 42: 617–624. [DOI] [PubMed] [Google Scholar]
- Okamura T, Miyoshi I, Takahashi K, Mototani Y, Ishigaki S, Kon Y, Kasai, N. Bilateral congenital cataracts result from a gain-of-function mutation in the gene for aquaporin-0 in mice. Genomics 2003; 81: 361–368. [DOI] [PubMed] [Google Scholar]
- Pontoriero GF, Smith AN, Miller LA, Radice GL, West-Mays JA, Lang RA. Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival. Dev. Biol 2009; 326: 403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiblin DA, Gao J, Caplan JL, Simirskii VN, Czymmek KJ, Mathias RT, Duncan MK. Beta-1 integrin is important for the structural maintenance and homeostasis of differentiating fiber cells. Int. J. Biochem. Cell Biol 2014; 50: 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuring S, Buzhynskyy N, Jaroslawski S, Gonçalves RP, Hite RK, Walz T. Structural models of the supramolecular organization of AQP0 and connexons in junctional microdomains. J. Struct. Biol 2007; 160: 385–394. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bassnett S. Mutations in the founder of the MIP gene family underlie cataract development in the mouse. Nat. Genet 1996; 12: 212–215. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bassnett S, Varadaraj K, Mathias RT, Al-Ghoul K, Kuszak J, Donoviel D, Lilleberg S, Friedrich G, Zambrowicz B. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol. Genomics 2001; 7: 179–186. [DOI] [PubMed] [Google Scholar]
- Shiels A, Mackay D, Bassnett S, Al-Ghoul K, Kuszak J. Disruption of lens fiber cell architecture in mice expressing a chimeric AQP0-LTR protein. FASEB J. 2000; 14: 2207–2212. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS. Molecular mechanism of Aquaporin 0-induced fiber cell to fiber cell adhesion in the eye lens. Biochem. Biophys. Res. Commun 2018; 506: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Mathias RT. Functional expression of aquaporins in embryonic, postnatal, and adult mouse lenses. Dev. Dyn 2007; 236: 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Mathias RT. Transgenic expression of AQP1 in the fiber cells of AQP0 knockout mouse: effects on lens transparency. Exp. Eye Res 2010; 91: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Patil R, Wax MB, Mathias RT. Functional characterization of a human aquaporin 0 mutation that leads to a congenital dominant lens cataract. Exp. Eye Res 2008; 87: 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest. Ophthalmol. Vis. Sci 2005; 46: 1393–1402. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kushmerick C, Baldo GL, Bassnett S, Shiels A, Mathias RT. The role of MIP in lens fiber cell membrane transport. J. Membr. Biol 1999; 170: 191–203. [DOI] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp. Eye Res 2002; 74: 753–760. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem 1997; 272: 16140–16146. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Hall JE, Zampighi L, Kreman M. Microdomains of AQP0 in lens equatorial fibers. Exp. Eye Res 2002; 75: 505–519. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Hall JE, Ehring GR, Simon SA. The structural organization and protein-composition of lens fiber junctions. J. Cell Biol 1989; 108: 2255–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bennett TM, Shiels A. Lens ER-stress response during cataract development in Mip-mutant mice. Biochim. Biophys. Acta 2016; 1862: 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.