Abstract
Cortical iron has been shown to be elevated in Alzheimer’s disease (AD), but the impact of directly measured iron on the clinical syndrome has not been assessed. We investigated the association between post-mortem iron levels with the clinical and pathological diagnosis of AD, its severity, and the rate of cognitive decline in the 12 years prior to death in subjects from the Memory and Aging project (n=209). Iron was elevated (β[S.E.]=9.7 [2.6]; P=3.0×10−4) in the inferior temporal cortex only in subjects who were diagnosed with clinical AD during life and had a diagnosis of AD confirmed post mortem by standardized criteria. Whereas iron was weakly associated with the extent of proteinopathy in tissue with AD neuropathology, it was strongly associated with the rate of cognitive decline (e.g. Global Cognition: β[S.E.]=−0.040 [0.005], P=1.6 ×10−14). Thus, cortical iron might act to propel cognitive deterioration upon the underlying proteinopathy of AD, possibly by inducing oxidative stress or ferroptotic cell death, or may be related to an inflammatory response.
Neuritic β-amyloid plaques and neurofibrillary tangles (NFT) are the defining proteopathies of Alzheimer’s disease (AD), yet post mortem pathology studies1–3, β-amyloid PET imaging studies4, 5, and CSF biomarker studies6–8, have demonstrated that severe ‘AD’ pathology is present in ~30% of cognitively normal elders. In longitudinal studies, biomarker-confirmed pathology is predictive of future cognitive deterioration in cognitively normal cohorts7–9, but the variance in the extent of proteinopathy and clinical presentation may indicate that other pathologies, such as iron accumulation, could combine with NFT and β-amyloid plaque to advance cognitive decline.
Iron is elevated in cortical regions of AD brains. A meta-analysis involving 300 AD cases in 19 studies reported that iron is significantly elevated in multiple cortical regions of the brain although the elevations are variable among regions and studies10. Iron accumulation may be hazardous since elevated iron may itself cause neurodegeneration (e.g. in Neurodegeneration with Brain Iron Accumulation11), possibly by inducing oxidative stress and neurodegeneration by ferroptosis12. We previously found that biomarkers for high brain iron levels, CSF ferritin levels13, 14 and Quantitative Susceptibility Mapping-MRI15, predicted cognitive decline across the clinical severity spectrum of AD.
Whereas prior post mortem studies have reported elevated iron in AD10, various limitations in these studies hamper their interpretation, including small sample size, but importantly, none performed analyses stratified by clinical and pathological diagnoses. This is important because clinical AD is often misdiagnosed during life where post-mortem examination reveals the lack of significant AD neuropathology. Conversely, analysis based only on brain pathology criteria is problematic because ≈33% of elderly people have AD pathology without significant cognitive impairment. These seeming anomalies suggest that there are unrecognized brain changes involved in neurodegeneration. In the current study, we examined the relations of brain iron levels to neuropathological and clinical outcomes of AD using data from a community study of initially non-demented older adults who were cognitively assessed in the years prior to death and donated their brain for analyses upon death.
Materials and Methods
The Memory and Aging Project
The Memory and Aging Project (MAP) is an ongoing clinical-neuropathological cohort study of older adults that began in 1997 and includes Chicago residents of retirement communities and subsidized housing16. At enrollment, participants were dementia free, and they agreed to undergo annual clinical neurological evaluations and brain autopsy at death. Written informed consent was obtained from all study participants, and the institutional review board of Rush University approved the study. Brain samples from all available subjects at the time of analysis were used in the study.
Clinical evaluation procedures
All subjects underwent a uniform, structured, clinical evaluation that included self-reported medical history, a neurologic examination by a trained nurse, and cognitive testing by a trained neuropsychological test technician, as previously described3. Years of formal education, and history of change in memory and other cognitive abilities relative to 10 years earlier were documented. All medications used in the prior 2 weeks were directly inspected and recorded. A complete neurologic examination was performed by trained nurses, who documented evidence of stroke or Parkinsonian signs. AD clinical diagnosis required dementia and progressive loss of episodic memory based on the criteria of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA)17.
Cognitive performance tests
Cognitive performance tests have previously been described for this cohort3. A battery of 19 cognitive tests, including tests of the Consortium to Establish a Registry for Alzheimer Disease (CERAD), are administered annually to assess a broad range of cognitive abilities that appear to have different anatomic substrates commonly affected by AD and/or widely used for clinical classification of dementia. The battery includes multiple tests of each of 5 cognitive domains: 7 tests of episodic memory (Word List Memory, Word List Recall and Word List Recognition from the procedures established by the CERAD; immediate and delayed recall of Story A from the Logical Memory subtest of the Wechsler Memory Scale-Revised; and immediate and delayed recall of the East Boston Story), 3 tests of semantic memory (15-item Boston Naming Test, Verbal Fluency, 15-item word reading test), 3 tests of working memory (Digit Span Forward, Digit Span Backward, Digit Ordering), 4 tests of perceptual speed (symbol digits modality, Stroop color naming, Stroop word reading, number comparisons) and 2 tests of visuospatial ability (Judgement of Line Orientation and 16-item version of the Standard Progressive Matrices). Composite scores were computed for each of the cognitive domains and for global cognition by converting raw scores on each test to standardized scores using the mean and standard deviation from the baseline population evaluations, and then averaging the standardized scores to yield the composite z-scores. Summary measures have the advantage of minimizing floor and ceiling effects, and other sources of random variability. A valid summary score required that at least half of the component scores be present.
Brain Neuropathology
The methods for brain autopsies and pathologic evaluations are described in detail elsewhere3. A board-certified neuropathologist, blinded to participant ages and clinical data, determined the neuropathology diagnoses. Briefly, slabs from 1 cerebral hemisphere were placed in a −80OC freezer and used for metal analyses. Slabs from the contralateral hemisphere were fixed in 4% paraformaldehyde, and then dissected tissue samples from brain regions were embedded in paraffin blocks, cut into 6-micron sections, and mounted onto slides.
Alzheimer disease neuropathologies:
diffuse and neuritic amyloid plaques and neurofibrillary tangles, were identified using modified Bielschowsky silver-stained 6-micron sections in multiple cortical regions. Raw counts (greatest density in 1-mm2 area) of the neuritic and diffuse plaques and tangles were standardized in each region (entorhinal cortex, hippocampus, mid-temporal cortex, inferior parietal cortex, mid-frontal cortex) and averaged across regions as summary scores that reflect a global measure of Alzheimer disease pathology. Neuropathologic data was categorized based on: AD pathologic diagnosis criteria of the National Institute on Aging (NIA) and the Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association with criteria ranging from no tangle and plaque pathology, low pathology, moderate pathology and high pathology. CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) criteria assess the likelihood of AD pathological diagnosis using the extent of plaque pathology and assigns either rating of possible, probable or definiate. Braak criteria ranks the extent of tangle pathology ranging from 0 (no tangle pathology) to 6 (high tangle pathology).
Lewy body pathology (see18):
Lewy body pathology was assessed using α-synuclein immunostain (Zymed; 1:50) on paraffin-embedded brain-tissue sections (6 μm) from mid-frontal, mid-temporal, inferior parietal, anterior cingulate, entorhinal and hippocampal cortices, basal ganglia and midbrain. Immunohistochemistry was performed using the VECTASTAIN ABC method with alkaline phosphatase as the color developer. Nigral Lewy bodies were identified as round, intracytoplasmic structures with a darker halo. In the cortex, Lewy bodies were identified as round intracytoplasmic structures, often lacking any halo and with an eccentric nucleus. Only intracytoplasmic Lewy bodies were uses as an indicator of positive staining. The brain was considered positive for Lewy bodies if there were Lewy bodies present in one or more region.
Gross Vascular infarcts (see19):
The presence of one or more gross chronic cerebral infarctions was identified by the naked eye on fixed slabs. All grossly visualized and suspected macroscopic infarcts were dissected for histologic confirmation.
Microinfarcts (see19):
A minimum of 9 regions in 1 hemisphere that were examined for one or more microinfarcts on 6-μm paraffin-embedded sections stained with hematoxylin–eosin. 6 cortical regions (mid frontal, middle temporal, entorhinal, hippocampal, inferior parietal, and anterior cingulate cortices), 2 subcortical regions (anterior basal ganglia, thalamus), and mid brain. Locations of microinfarcts were recorded.
Iron Analyses
Brain iron concentrations were measured in the biopsy tissue from the inferior temporal cortex (an area markedly affected by Alzheimer disease neuropathology) and the cerebellum (a region relatively spared by Alzheimer disease neuropathology). Brain tissue was cut into 100 mg samples using a ceramic blade (to avoid metal contamination) and sent to the University of Missouri Research Reactor to be assessed for iron by instrumental neutron activation analysis20. Samples were loaded into cleaned, high-purity quartz vials, lyophilized, and then irradiated for 50 hours in a neutron flux of ~1013 n/cm2 s and allowed to decay for 20 to 40 days before being counted using a high-purity germanium detector system. Measured concentrations of the metals in reference materials were analyzed for consistency with accepted standards and agreed well with certified values.
Statistical Analysis
Statistical analysis was performed with R (version 3.3.2). All available subjects with complete data were included in the analysis (none were excluded). Multiple regression models of inferior temporal iron and cerebellum iron included the following covariates: age at death, sex, APOE ε4 allele, years of education, neuritic plaque counts, neurofibrillary tangles (NFT) counts, presence of gross infarcts, Lewy bodies, and clinical AD diagnosis. A Bonferroni adjustment was used to correct for analysis of 2 brain regions (α/2), therefore significance inferred when P<0.025. Multiple regression models of tangle score and plaque score included the following covariates: age of death, age of death, sex, APOE ε4 allele, neuritic plaque score (only for models where NFT score was the outcome variable), NFT score (only for models where neuritic plaque score was the outcome variable), clinical diagnosis and either inferior temporal iron or cerebellum iron. A Bonferroni adjustment was used to correct for analysis of 2 brain regions (α/2), therefore significance inferred when P<0.025. Mixed-effects linear models of cognitive composite scores (Global Cognition, Episodic Memory, Perceptual Organization, Perceptual Speed, Semantic Memory, and Working Memory) included the following covariates: age of death, sex, APOE ε4 allele, years of education, neuritic plaque score, and tangle score, inferior temporal iron or cerebellum iron, and time (years prior to death), and the interaction of all these variables with time. A Bonferroni adjustment was applied to P values generated from the cognitive modeling to address inflated type 1 error due to the testing of 6 cognitive composite scores (α/6), therefore significance inferred when P<0.0083 for the mixed-effects cognitive models (due to Bonferroni adjustment). Mediation analysis was performed in people with high AD pathology using the mediation package in R to assess the proportion of the effect of NFT on cognitive decline mediated by iron. The algorithm is based on the quasi-Bayesian Monte Carlo approximation (1000 simulations) in which the posterior distribution of quantities of interest is approximated by their sampling distribution. The proportion mediated is expressed as the Causal mediated effect divided by the total effect. All models were tested for normal distribution of the residuals and absence of multicollinearity. Hypothesis tests were 2-sided.
Results
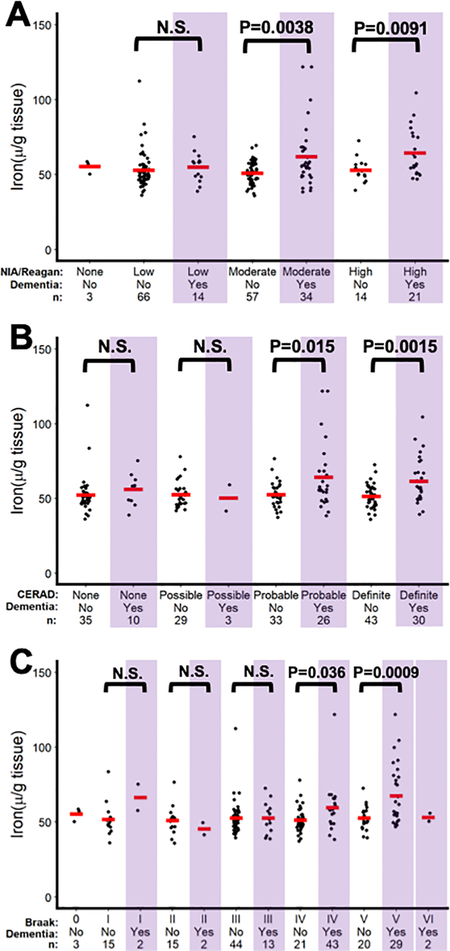
We measured iron in grey matter from the inferior temporal cortex (affected early in AD) and cerebellum (minimally affected) from 209 MAP cohort donors. The individuals were stratified by clinical diagnosis of AD during life (using NINCDS-ADRDA criteria) and pathological criteria at autopsy using either NIA/Reagan (based on plaque and tangle pathology severity), CERAD (plaque pathology), or Braak criteria (tangle pathology). With each type of pathology criteria applied, there were subjects identified with high pathology and without a clinical diagnosis, indicating pre-clinical disease burden, and conversely people with low pathology but with a clinical diagnosis of AD, indicating clinical miss-diagnosis.
When comparing iron levels in the inferior temporal cortex according to strata of clinical and pathology criteria, iron levels were increased in people only with a clinical diagnosis of dementia who also had high pathology according to NIA/Reagan criteria for moderate (P=0.0003) and high pathology 4 (P=0.0190; Figure 1A), CERAD criteria for probable (P=0.0066) and definiate pathology (P=0.0003; Figure 1B), and Braak criteria IV (P=0.0067) and V (P=0.0031; Figure 1C). Too few cases of Braak criteria VI were available to analyse. Iron levels in the cerebellum did not differ significantly by pathology severity or clinical classficiation (Supplementary Figure 1A–C).
Figure 1.
Iron levels in the inferior temporal cortex in people stratified by clinical AD diganosis and AD pathological criteria of (A) NIA/Reagan (B) CERAD and (C) Braak.
Given that iron was elevated in clinical dementia cases that also had high ratings of any criteria of pathology, we focused on simplified NIA/Reagan criteria of high AD pathology (1&2) and low AD pathology (3&4) to explore further the clinical variables that were associated with iron (Table 1). In a multiple regression model of inferior temporal iron in subjects with high AD pathology, iron was positively associated with AD clinical diagnosis β[S.E.]=9.7 [2.6]; P=3.0×10−4) and neurofibrillary tangles (NFT) counts (β[S.E.]=4.6 [1.9] P=0.0190) but not the other covariates in the analysis (age, sex, APOE ε4, neuritic plaque counts, presence of gross infarcts, and Lewy bodies).
Table 1. Subject characteristics.
High AD pathology indicates classification of moderate or high pathology on NIA/Reagan criteria, Low AD pathology indicates classification of no or low pathology using NIA/Reagan criteria. Low AD pathology post mortem in subjects with a diagnosis of AD in life indicates clinical misdiagnosis.
| AD Pathology (NIA/Reagan) | Low | Low | High | High |
|---|---|---|---|---|
| Clinical diagnosis | Cognitively Normal | Alzheimer’s | Cognitively Normal | Alzheimer’s |
| N | 69 | 14 | 71 | 55 |
| Age at death: mean (S.D.) | 87∙8 (6∙0) | 92∙9 (4∙5) | 89∙9 (6∙5) | 90∙7 (5∙1) |
| Female sex: n (%) | 23 (33%) | 7 (50%) | 20 (28%) | 19 (35%) |
| APOE ε4: n (%) | 5 (7%) | 2 (14%) | 23 (32%) | 15 (27%) |
| Education years: mean (S.D.) | 14∙5 (2∙6) | 14∙6 (2∙4) | 14∙5 (2∙4) | 15∙1 (2∙8) |
| Presence of Lewy bodies: n (%) | 9 (13%) | 6 (43%) | 9 (13%) | 15 (27%) |
| Presence of gross infarcts: n (%) | 19 (28%) | 8 (57%) | 30 (42%) | 25 (45%) |
| Presence of microinfarcts: n (%) | 15 (22%) | 4 (29%) | 23 (32%) | 20 (36%) |
No significant difference in iron levels was observed in demented subjects with low AD pathology (i.e. non-AD dementia), however there was a modest trend to elevation, and it is possible that the result would have reached significance if there were more cases in the dementia group with low AD pathology, as this is the lowest powered group. Power analysis reveals that 232 low AD pathology subjects would be required to achieve a significant result at this difference (P = 0.05; α = 0.8).
Iron was also not significantly elevated in the cerebellum regardless of clinical or pathological diagnosis (NIA/Reagan (Table 2). Cerebellum iron was elevated in males compared to females; a dimorphism that was not observed in the inferior temporal cortex.
Table 2. Association between clinical variables and iron levels in the Inferior Temporal Cortex or Cerebellum.
Data are from separate multiple regressions of iron in the inferior temporal cortex or cerebellum of cases with low- or high- pathology. High AD pathology indicates classification of moderate or high pathology on NIA/Reagan criteria, Low AD pathology indicates classification of no or low pathology using NIA/Reagan criteria. β-coefficients represent 1 μg/g change in iron for 1 unit difference in the case for the categorical variables (sex, APOE ε4, clinical diagnosis) or one standard deviation change for the continuous variables (age at death, neuritic plaque burden, and NFT burden). Alpha was set at 0.05, and a Bonferroni adjustment of 2 was applied to account for multiple tests. Therefore P values less than 0.025 were considered significant, and are italicized.
| Low Pathology | High Pathology | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inferior Temporal Iron | Cerebellum Iron | Inferior Temporal Iron | Cerebellum Iron | |||||||||
| β | (S.E.) | P | β | (S.E.) | P | β | (S.E.) | P | β | (S.E.) | P | |
| Age at death | −0∙31 | (0∙22) | 0∙168 | −0∙01 | (0∙45) | 0∙983 | −0∙25 | (0∙21) | 0∙244 | 0∙09 | (0∙34) | 0∙799 |
| Sex (male) | −1∙36 | (2∙69) | 0∙615 | 2∙53 | (5∙40) | 0∙642 | 4∙29 | (2∙62) | 0∙105 | 14∙9 | (4∙26) | 0∙001 |
| APOE ε4 | −5∙28 | (4∙55) | 0∙250 | −5∙83 | (9∙14) | 0∙526 | 1∙25 | (2∙87) | 0∙665 | 6∙72 | (4∙66) | 0∙152 |
| Plaque | 2∙85 | (6∙15) | 0∙645 | 1∙36 | (12∙3) | 0∙913 | −0∙92 | (1∙99) | 0∙884 | −2∙11 | (3∙22) | 0∙514 |
| NFT | 4∙03 | (6∙14) | 0∙514 | 3∙35 | (12∙3) | 0∙787 | 4∙60 | (1∙92) | 0∙019 | 2∙30 | (3∙11) | 0∙462 |
| Presence of Lewy bodies | 0∙09 | (3∙43) | 0∙980 | −10∙3 | (6∙88) | 0∙140 | 0∙84 | (3∙12) | 0∙793 | −2∙60 | (5∙16) | 0∙616 |
| Presence of Gross infarcts | 0∙71 | (2∙81) | 0∙801 | 0∙05 | (5∙64) | 0∙994 | 1∙61 | (2∙39) | 0∙504 | −9∙30 | (3∙88) | 0∙019 |
| Clinical AD diagnosis | 3∙72 | (3∙86) | 0∙338 | 12∙7 | (7∙74) | 0∙106 | 9∙73 | (2∙60) | 3∙0 × 10−4 | 4∙19 | (4∙48) | 0∙351 |
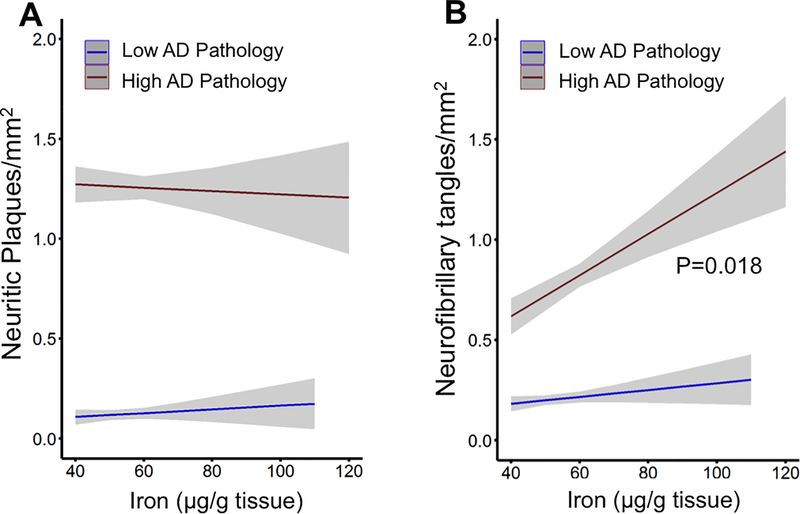
Iron is reportedly enriched in amyloid plaque21, 22, and iron levels are associated with amyloid severity23. CSF ferritin, a marker of brain iron load, has also been related to amyloid deposition in longitudinal studies24. Of note, in the current study, iron was not associated with increased AD proteinopathy in the absence of dementia (Figure 1). To discriminate the association between iron and the components of pathology, we investigated determinants of NFT and neuritic plaque counts in subjects with low or high AD pathology using separate multiple regression models, controlling for covariates: age at death, sex, APOE ε4 allele, neuritic plaque number (only for models where NFT count was the outcome variable), NFT number (only for models where neuritic plaque count was the outcome variable), presence of Lewy bodies, presence of gross infarcts, and clinical AD diagnosis. In subjects with high AD pathology, iron in the inferior temporal cortex was not associated with the extent of plaque burden (Figure 2A), and weakly associated with the extent of neurofibrillary tangles (β[S.E.] = 0.14 [0.06]; P = 0.018; Figure 2B).
Figure 2. Association between iron and pathology severity.
(A) Association between plaque load and inferior temporal iron in post mortem tissue with background low- or high- AD pathology (by NIA Reagan criteria). High AD pathology indicates classification of moderate or high pathology on NIA/Reagan criteria, Low AD pathology indicates classification of no or low pathology using NIA/Reagan criteria. Data are from multiple regression models of plaque burden with covariates: age, sex, APOE ε4, NFT burden, diagnosis, Lewy Bodies, gross vascular infarcts, and inferior temporal iron level. (B) Association between NFT burden and inferior temporal iron levels in post mortem cases of low- or high- AD pathology. Data are from multiple regression models of NFT burden with covariates: age, sex, APOE ε4, plaque burden, diagnosis, Lewy bodies, gross vascular infarcts, and inferior temporal iron level. Models represent means +/− standard error.
Since iron in the inferior temporal cortex was strongly associated with the clinical diagnosis, and only modestly with pathology, we hypothesized that iron does not merely accumulate with the proteinopathy but rather potentiates degeneration once the pathology is sufficiently severe.
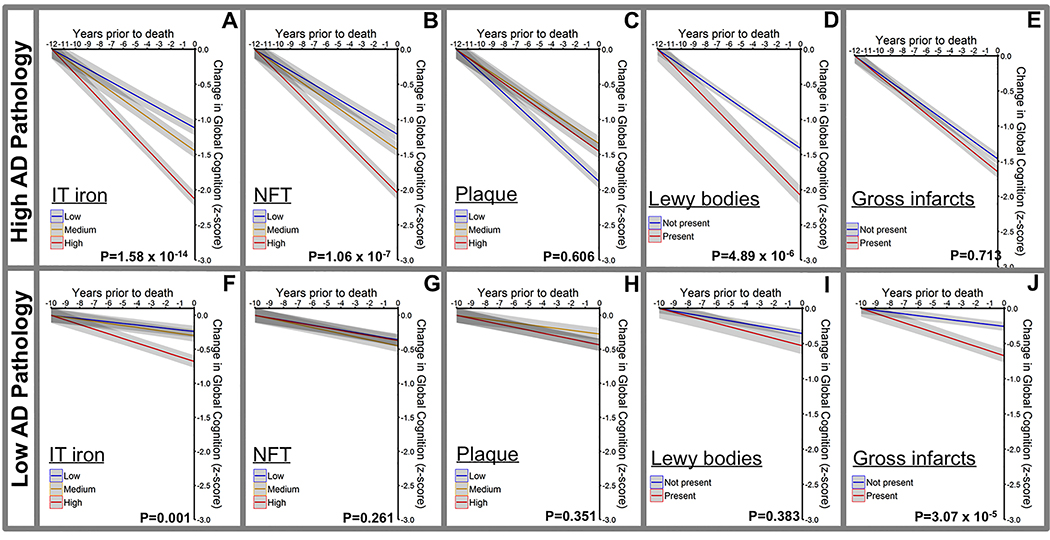
The MAP participants underwent annual cognitive assessment up to 12 years before death (5 cognitive domain composites generated from 19 tests performed during annual assessment) and were free of dementia when they enrolled in the study (Supplementary Table 1), enabling us to explore whether iron levels are related to the rate of cognitive decline. Indeed, in people with high AD pathology (NIA/Reagan criteria), iron was strongly associated (β(S.E.)= −0.040 (0.005), P=1.58×10−14) with the rate of decline in the Global Cognition composite in the years prior to death in mixed effects models controlling for other components of mixed pathology and clinical variables including APOE ε4, age, sex, years of education, abundance of NFTs, abundance of neuritic plaques, presence of Lewy bodies, and presence of gross vascular infarcts (Figure 3A–E). Similar results were observed for the other cognitive composites (Supplementary Figure 2). These results did not change when substituting gross vascular infarcts for microinfarcts (Supplementary Tables 4 & 5). The results were also upheld when the cohort was stratified by dichotomous vairables of CERAD or Braak criteria (Supplementary Figure 3).
Figure 3. Association of iron burden and other neuropathological features with antecedent cognitive decline.
Associations between plaque, NFT and iron (inferior temporal cortex) and change in the global cognition score in the years prior to death in post mortem cases stratified by NIA Reagan criteria into high AD pathology (moderate and high pathology; n=126; A-E) and low AD pathology (no or low pathology; n= 83; F-J). Data are from mixed effects linear models with covariates: age, sex, APOE ε4, plaque burden, NFT, Lewy bodies, gross vascular infarcts, years of education, and inferior temporal iron. Inferior temporal iron, NFT and plaque were included as continuous variables in modeling, but stratified in tertiles for visual display. Lewy bodies and gross infarcts were dichotomous variables (presence/absence). Data are means +/− standard error.
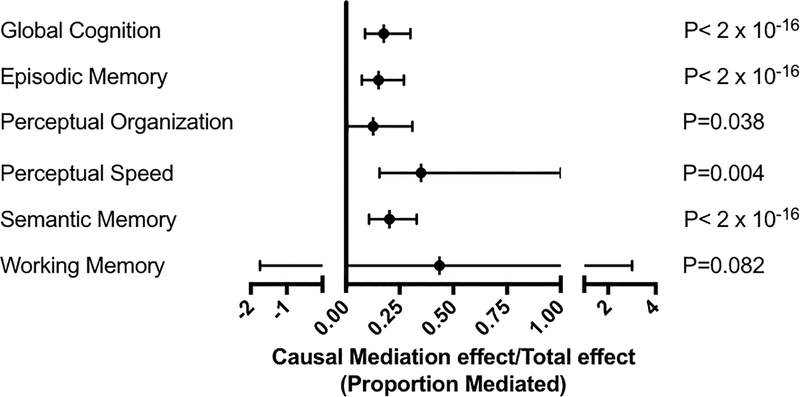
Previous studies have shown that NFT burden is a feature of AD pathology that is more closely associated than amyloid burden with cognitive and neuronal loss25. Since we observed an association between inferior temporal iron and extent of neurofibrillary tangle burden (Figure 2B), and both iron and tangle burden were associated with cognitive decline, we performed mediation analysis to determine whether iron mediates the effects of NFT burden on cognition in subjects with underlying Alzheimer’s pathology. We found that iron levels mediated 17% of the effect of NFTs on Global Cognition (Figure 4). Thus, while NFTs are confirmed to be associated with cognitive decline, as expected, among the high AD pathology cases iron burden emerges as a factor that acts largely independent of NFTs.
Figure 4. NFT burden effect on antecedent cognitive decline mediated by iron in subjects with high AD pathology.
Causal mediation analysis was modeled using a quasi-Bayesian Monte Carlo approximation (mediation package in R), where proportion mediated is calculated from the ratio of the causal mediation effect divided by the total effect.
In subjects with low AD pathology, elevated inferior temporal iron burden was also associated with decline in global cognitive score (β(S.E.) = −0.015 (0.004), P = 0.001), but not the individual cognitive domain scores in mixed effects models containing the other AD risk variables (Figure 3F–J, Supplementary Figure 4). Cerebellum iron was not consistently associated with cognitive decline, regardless of pathology severity (Supplementary Table 5).
Discussion
Here we report that the iron load of the inferior temporal lobe, a major affected brain region in AD, strongly corresponded to antecedent cognitive decline in those individuals who had underlying plaque and tangle pathology. Our results agree with a meta-analysis that revealed iron was elevated in the temporal lobe of AD cases10, but we extend upon these prior results by defining our cohort by pathological and clinical diagnostic criteria, which importantly revealed that cortical iron elevation discriminated the clinical diagnosis of AD from prodromal (non-symptomatic) subjects with high AD pathology. This finding is consistent with iron burden contributing to cognitive decline upon underlying AD pathology. We interrogated the inferior temporal cortex as a representative disease-affected region, but, as we have previously observed by QSM-MRI15, we expect that iron elevation would be observed in other affected cortical regions and could also contribute to impairment across cognitive domains. The temporal sequence of the spread of iron elevation through the cortex is yet to be determined.
The results of this study where historical clinical data were used are in accord with recent prospective studies using CSF ferritin and MRI measures of brain iron13–15, 24, and support the hypothesis that when AD pathology is present, brain iron differentiates the symptomatic progression of the disease. This study design has not previously been employed to investigate the impact of brain iron in AD, but it has for other proteinopathies25. The post mortem approach has the advantage of allowing direct measurements of brain iron, which is not currently possible in living people, although improvements in MRI-measures allow for more accurate measures of brain iron load15. This study design is limited because the only measure of iron is at the final time point, and the staging of iron elevation during the progression of AD cannot be directly assessed. However, given the various stages of pathology burden and cognitive presentation represented in this study, we were able to infer where iron elevation fits in the natural history of the disease. We expect that it is unlikely that iron increases during the pre-clinical stage of the disease when pathology emerges, since high pathology cases without clinical AD did not exhibit iron accumulation, and pathology load was only marginally associated with iron load. Rather, it is likely that iron elevation emerges with cognitive deterioration, since iron was strongly associated with cognitive decline, and also, iron elevation was only observed in demented subjects with high AD pathology but not in non-demented subjects with the same level of pathology.
Prior studies have shown iron enrichment in plaque22, 26–29 and tangles21, and biomarkers of iron have been shown to predict risk of plaque accumulation30. However, we observed no association between iron and plaque pathology, and only a modest association between iron and tangle pathology, indicating that the iron deposition within these pathologies might only be a small component of the disease-associated iron burden within the tissue.
It might be that biochemical changes secondary to the proteinopathies may contribute to iron burden. For example, loss of soluble tau protein due to deposition in neurofibrillary tangles could cause iron elevation, since tau promotes the stabilization of the iron-exporting protein, ferroportin, by trafficking APP to the neuronalsurface31. In mice, Aβ toxicity was shown to cause iron elevation that was dependent on tau32. So it is possible that the iron changes in AD occur as a consequence of protein accumulation and altered iron trafficking related to tau and Aβ.
We did not find evidence that iron was elevated due to the APOE ε4 risk allele, at variance with previous reports that APOE ε4 carriers express elevated biomarkers of brain iron, QSM-MRI33 and CSF ferritin13. Since the ε4 allele was not associated with iron levels in our cohort, the allele alone is unlikely to explain iron-related changes.
Our current results are correlative, and do not determine whether iron is causally involved in symptomatic decline, or whether iron elevation occurs as an epiphenomenon during this stage of AD. Neuroinflammation and activation of microglia are also characteristics of AD34, and could induce both neurodegeneration and iron elevation, since activated microglia in AD are also elevated in iron35. Tissue iron retention is characteristic of inflammation, where pro-inflammatory signals promote cellular iron capture so that invading pathogens are deprived of extracellular iron for their growth36. It is therefore possible that iron elevation in AD could occur as a consequence of neuroinflammation (Supplementary Figure 5).
Changes in iron might also be related to vascular pathology that occurs in ~80% of AD patients37. Vascular lesions may cause iron elevation from blood caused by microbleeds in the brain – indeed the paramagnetic properties of iron containing hemosiderin are exploited to detect microbleed using MRI38. However, we observed no correlation between micro or gross vascular infarcts and iron levels, so these sources of iron burden do not appear to contriute to the associations between cortical iron and clinical and pathological outcomes in this cohort.
Regardless of whether iron elevation is downstream of protein accumulation, AD genetics, vascular pathology, or an inflammation response, secondary iron toxicity may still propel the neurodegenerative process. Iron accumulation can induce neurodegeneration, e.g. in Neurodegeneration with Brain Iron Accumulation39, and given the iron elevation in AD observed in the current and previous studies, it is reasonable to hypothesize that the iron burden in AD contributes to neurotoxicity. How might iron contribute to neurodegeneration? Iron can directly induce oxidative damage, which is conspicuous in AD40. Iron is also the key mediator of the recently described regulated cell death pathway, ferroptosis, where Fe2+ catalyzes the formation of lipid hydroperoxides that cause catastrophic membrane damage12. Evidence for ferroptosis in AD include multiple studies that show increased lipid peroxidation40–45 and decreased glutathione levels46 (which is required to control lipid peroxides) in AD, changes that are also the signature of ferroptosis. The mechanism of neuronal death in AD is unproven, and we hypothesize that it is from ferroptotic cell death.
While iron changes in AD have been reported previously, the results of this study, which employed a large cohort and are stratified by both pathology as well as clinical diagnostic criteria, expands on previous work by providing evidence that iron elevation is concomitant with functional deterioration, during the neurodegeneration phase of the disease (Supplementary Figure 5). Like all the neuropathologies of AD, it remains to be determined whether iron drives neurodegeneration or is merely a marker of other pathogenic changes. While the cause of iron elevation and molecular mechanisms of how iron might contribute to AD continue to emerge, the observations that iron associates strongly with cognitive decline invites the hypothesis that iron is indeed causative of degeneration. It is likely that this hypothesis can only be tested by a clinical trial using a drug that lowers iron (or inhibits ferroptosis) to determine whether this treatment impacts on disease progression.
Supplementary Material
Acknowledgments
Funding
This study was supported by grants from the National Institute of Health (R01AG017917, R21E2021290, and RF1AG054057). Analysis was supported by funds from the Australian Research Council, the Australian National Health & Medical Research Council (NHMRC), the Cooperative Research Centre for Mental Health (the Cooperative Research Centre program is an Australian Government Initiative). The Florey Institute of Neuroscience and Mental Health acknowledges support from the Victorian Government, in particular funding from the Operational Infrastructure Support Grant. No funder of this study had any role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Abbreviations
- AD
Alzheimer’s disease
- CERAD
Consortium to Establish a Registry for Alzheimer Disease
- NFT
Neurofibrillary Tangles
- MAP
Memory and Aging Project
- NINCDS-ADRDA
National Institute of Neurologic and Communicative Disorders and Stroke and the AD and Related Disorders Association
Footnotes
Conflict of interest statement
AIB is a shareholder in Prana Biotechnology Ltd, Cogstate Ltd, Brighton Biotech LLC, Grunbiotics Pty Ltd, Eucalyptus Pty Ltd, and Mesoblast Ltd. He is a paid consultant for, and has a profit share interest in, Collaborative Medicinal Development Pty Ltd.
References
- 1.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Annals of neurology 1999; 45(3): 358–368. [DOI] [PubMed] [Google Scholar]
- 2.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of neurology 2008; 65(11): 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006; 66(12): 1837–1844. [DOI] [PubMed] [Google Scholar]
- 4.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006; 67(3): 446–452. [DOI] [PubMed] [Google Scholar]
- 5.Rowe CC, Bourgeat P, Ellis KA, Brown B, Lim YY, Mulligan R et al. Predicting Alzheimer disease with beta-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Annals of neurology 2013; 74(6): 905–913. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC et al. Cerebrospinal Fluid Biomarker Signature in Alzheimer’s Disease Neuroimaging Initiative Subjects. Annals of neurology 2009; 65(4): 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology 2007; 69(7): 631–639. [DOI] [PubMed] [Google Scholar]
- 8.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Archives of neurology 2007; 64(3): 343–349. [DOI] [PubMed] [Google Scholar]
- 9.Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain : a journal of neurology 2014; 137(Pt 1): 221–231. [DOI] [PubMed] [Google Scholar]
- 10.Tao Y, Wang Y, Rogers JT, Wang F. Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: a systematic review and meta-analysis. Journal of Alzheimer’s disease : JAD 2014; 42(2): 679–690. [DOI] [PubMed] [Google Scholar]
- 11.Schneider SA, Hardy J, Bhatia KP. Syndromes of neurodegeneration with brain iron accumulation (NBIA): an update on clinical presentations, histological and genetic underpinnings, and treatment considerations. Movement disorders : official journal of the Movement Disorder Society 2012; 27(1): 42–53. [DOI] [PubMed] [Google Scholar]
- 12.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017; 171(2): 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayton S, Faux NG, Bush AI, Alzheimer’s Disease Neuroimaging Initiative I. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nature communications 2015; 6: 6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayton S, Faux NG, Bush AI. Association of Cerebrospinal Fluid Ferritin Level With Preclinical Cognitive Decline in APOE-epsilon4 Carriers. JAMA neurology 2017; 74(1): 122–125. [DOI] [PubMed] [Google Scholar]
- 15.Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Lim YY et al. Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain : a journal of neurology 2017; 140(8): 2112–2119. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Current Alzheimer research 2012; 9(6): 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34(7): 939–944. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain : a journal of neurology 2012; 135(Pt 10): 3005–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke; a journal of cerebral circulation 2011; 42(3): 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samudralwar DL, Diprete CC, Ni BF, Ehmann WD, Markesbery WR. Elemental imbalances in the olfactory pathway in Alzheimer’s disease. Journal of the neurological sciences 1995; 130(2): 139–145. [DOI] [PubMed] [Google Scholar]
- 21.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proceedings of the National Academy of Sciences of the United States of America 1997; 94(18): 9866–9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plascencia-Villa G, Ponce A, Collingwood JF, Arellano-Jimenez MJ, Zhu X, Rogers JT et al. High-resolution analytical imaging and electron holography of magnetite particles in amyloid cores of Alzheimer’s disease. Sci Rep 2016; 6: 24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Duijn S, Bulk M, van Duinen SG, Nabuurs RJA, van Buchem MA, van der Weerd L et al. Cortical Iron Reflects Severity of Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD 2017; 60(4): 1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayton S, Diouf I, Bush AI, Alzheimer’s disease Neuroimaging I. Evidence that iron accelerates Alzheimer’s pathology: a CSF biomarker study. Journal of neurology, neurosurgery, and psychiatry 2017: doi: 10.1136/jnnp-2017-316551. [DOI] [PubMed] [Google Scholar]
- 25.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. Journal of neuropathology and experimental neurology 2012; 71(5): 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meadowcroft MD, Peters DG, Dewal RP, Connor JR, Yang QX. The effect of iron in MRI and transverse relaxation of amyloid-beta plaques in Alzheimer’s disease. NMR in biomedicine 2014; 28(3): 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayton S, James SA, Bush AI. Nanoscale Imaging Reveals Big Role for Iron in Alzheimer’s Disease. Cell chemical biology 2017; 24(10): 1192–1194. [DOI] [PubMed] [Google Scholar]
- 28.Telling ND, Everett J, Collingwood JF, Dobson J, van der Laan G, Gallagher JJ et al. Iron Biochemistry is Correlated with Amyloid Plaque Morphology in an Established Mouse Model of Alzheimer’s Disease. Cell chemical biology 2017: 10.1016/j.chembiol.2017.1007.1014. [DOI] [PubMed] [Google Scholar]
- 29.Everett J, Collingwood JF, Tjendana-Tjhin V, Brooks J, Lermyte F, Plascencia-Villa G et al. Nanoscale synchrotron X-ray speciation of iron and calcium compounds in amyloid plaque cores from Alzheimer’s disease subjects. Nanoscale 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayton S, Diouf I, Bush AI, Alzheimer’s disease Neuroimaging I. Evidence that iron accelerates Alzheimer’s pathology: a CSF biomarker study. Journal of neurology, neurosurgery, and psychiatry 2018; 89(5): 456–460. [DOI] [PubMed] [Google Scholar]
- 31.Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nature medicine 2012; 18(2): 291–295. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Lei P, Tuo Q, Ayton S, Li QX, Moon S et al. Enduring Elevations of Hippocampal Amyloid Precursor Protein and Iron Are Features of beta-Amyloid Toxicity and Are Mediated by Tau. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 2015; 12(4): 862–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Bergen JM, Li X, Hua J, Schreiner SJ, Steininger SC, Quevenco FC et al. Colocalization of cerebral iron with Amyloid beta in Mild Cognitive Impairment. Sci Rep 2016; 6: 35514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al. Neuroinflammation in Alzheimer’s disease. Lancet neurology 2015; 14(4): 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeineh MM, Chen Y, Kitzler HH, Hammond R, Vogel H, Rutt BK. Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiology of aging 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wessling-Resnick M Iron homeostasis and the inflammatory response. Annual review of nutrition 2010; 30: 105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain : a journal of neurology 2013; 136(Pt 9): 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T, Surapaneni K, Lou M, Cheng L, Spincemaille P, Wang Y. Cerebral microbleeds: burden assessment by using quantitative susceptibility mapping. Radiology 2012; 262(1): 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider SA, Dusek P, Hardy J, Westenberger A, Jankovic J, Bhatia KP. Genetics and Pathophysiology of Neurodegeneration with Brain Iron Accumulation (NBIA). Current neuropharmacology 2013; 11(1): 59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM et al. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiology of disease 2008; 30(1): 107–120. [DOI] [PubMed] [Google Scholar]
- 41.Hajimohammadreza I, Brammer M. Brain membrane fluidity and lipid peroxidation in Alzheimer’s disease. Neurosci Lett 1990; 112(2–3): 333–337. [DOI] [PubMed] [Google Scholar]
- 42.Bradley MA, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med 2010; 48(12): 1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging 2006; 27(8): 1094–1099. [DOI] [PubMed] [Google Scholar]
- 44.Montine TJ, Kaye JA, Montine KS, McFarland L, Morrow JD, Quinn JF. Cerebrospinal fluid abeta42, tau, and f2-isoprostane concentrations in patients with Alzheimer disease, other dementias, and in age-matched controls. Arch Pathol Lab Med 2001; 125(4): 510–512. [DOI] [PubMed] [Google Scholar]
- 45.Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol 2005; 58(5): 730–735. [DOI] [PubMed] [Google Scholar]
- 46.Mandal PK, Saharan S, Tripathi M, Murari G. Brain glutathione levels--a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biological psychiatry 2015; 78(10): 702–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.