Abstract
[Purpose] The neck and trunk play crucial roles in body movement and are extremely important areas of treatment for physical therapists. However, many aspects of the neural basis of this motor control remain unknown. Therefore, we investigated the distribution and electrophysiological properties of the neck and trunk in the primary motor cortex in rats. [Subjects and Methods] Using intracortical microstimulation, we investigated the somatotopic representation and movements induced by electrical stimulation of the neck and truck areas of the motor cortex in 8 Wistar rats. [Results] We determined that the neck and trunk areas are located separately on the rostral and caudal sides of the motor cortex, respectively. The neck area was significantly larger in size, while the threshold was significantly larger for the trunk area. Stimulation of the neck area with a current higher than the threshold induced movement of the forelimbs, jaw, trunk, and whiskers. However, stimulation of the trunk area did not result in movement in sites other than the trunk. [Conclusion] During movement, the respective activities of the neck and trunk are interdependent. However, due to the separate locations of these areas in the motor cortex, their properties differ greatly.
Key words: Motor cortex, Neck, Trunk
INTRODUCTION
The neck, which connects the head and the trunk, not only supports the head, but is also involved in a variety of functions, such as balance and movements related to vision1). Neck movement disorders change the motor output of the entire body, primarily the trunk; therefore, for physical therapists, the neck is the most important joint to consider in therapeutic strategies, and understanding its normal function is of utmost importance2). Many studies have examined human neck/trunk movement from a biomechanical standpoint. However, due to technical limitations, few studies have examined systems in the central nervous system that regulate neck/trunk movement; findings in these studies have been limited to identification of the approximate location of the neck area in the motor cortex3,4,5,6). Therefore, to understand the systems that regulate neck/trunk movement, we must rely on basic experiments using animals.
Previous basic studies have found that the cerebrum and brainstem are involved in regulation of neck/trunk movement, which is affected by the regulation of various descending tracts originating from the cerebrum and brainstem7, 8). However, the data cited earlier was obtained from cats; there is little such data related to rodents, which are widely used in basic studies today7). Therefore, in the present study, we electrically stimulated the primary motor cortex, which is considered to play a significant role in voluntary neck/trunk movement in rats. By doing so, we aimed to determine the locations of the neck and trunk areas, elucidate their electrophysiological properties, and obtain data that will serve as a foundation for research which uses animal models of disease.
SUBJECTS AND METHODS
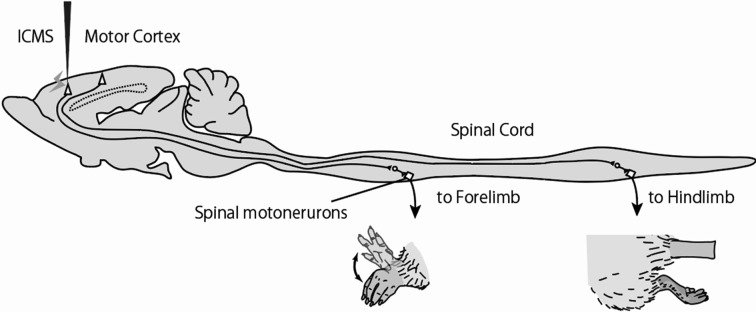
The present experiment used eight male Wistar rats (aged 13−16 weeks, weighing 241−298 grams). The motor cortex was mapped using intracortical microstimulation (ICMS, Fig. 1), a previously established method9). In brief, we maintained mixed anesthesia using ketamine hydrochloride (70 mg/kg, ip) and xylazine (5 mg/kg, ip), with additional injections of ketamine (20 mg/kg, ip) and acepromazine (0.02 mg/kg, ip) as necessary. Next, the rats’ heads were fixed with a head fixation device, and their body temperatures were maintained at 37 °C using a temperature management system. The right skull was removed to expose the right sensorimotor cortex; 40-kΩ tungsten microelectrodes (Unique Medical, Tokyo, Japan) were implanted every 500 μm from the surface of the cerebellum to a depth of 1,800 μm, which approximately corresponds to layers V and VI of the cortex; and an SEN-7130 stimulator (Nihon Kohden, Tokyo, Japan) and SS-04 J isolator (Nihon Kohden) were used to provide stimulation with 333-Hz, 300-μs square pulses for 30 ms. The stimulation current was gradually increased to an intensity at which body movement could be identified by sight and touch. The minimum stimulation current at which any body movement was induced, was defined as the threshold. If no body movement was induced despite an increase in stimulation current to 50 μA, the area in question was considered unresponsive. A total of 112 sites were mapped; responsive areas were converted to a value of 0.25 mm2 to determine the distribution and total area representing each part of the body. The present study was conducted with the approval of the Health Science University Institutional Animal Care and Use Committee (18-2). Data are presented as mean ± standard deviation. Differences in measurements were assessed using the Mann-Whitney U test.
Fig. 1.
Schematic diagram for intracortical microstimulation in the motor cortex.
Intracortical microstimulation (ICMS) of the motor cortex evokes somatic movement. In this case, it evoked movement in the forelimb, but not in the hindlimb. Based on such observations, we could identify the stimulation sites as forelimb area, i.e., the pyramidal cell of the stimulated site connects the cervical spinal cord and drives the spinal motoneurons innervating the forelimb muscles. By repeating this operation, a map of the entire motor cortex can be prepared.
RESULTS
The neck area was located rostral to the bregma such that it divided the rostral forelimb and caudal forelimb areas and expanded laterally from there (Fig. 2). The neck area was significantly larger than the trunk area (Table 1, p=0.0005). In all rats, the neck region was 2−4 mm lateral to and 0.5−2.5 mm rostral to the bregma. The threshold of the neck area was <10 μA in the area between the rostral and caudal forelimb areas but was ≥10 μA outside that area (Fig. 2; Fig. 3A, F). When the neck area was stimulated with a current between the threshold and 50 μA, movement occurred in the adjacent motor areas; specifically, those for the whiskers, forelimbs, and jaw. In the part of the neck area with a low threshold, movement was induced in the trunk, despite the trunk area not being adjacent to the neck area (Fig. 3F–J).
Fig. 2.
Typical representations of various body parts in the motor cortex.
The figure shows typical representations of various body parts in the motor cortex (Rat. No. 3). The location of the neck area in the murine primary motor cortex divides the rostral forelimb and caudal forelimb areas. The whisker area is medially adjacent, while the jaw area is laterally adjacent. The trunk area is located in the most caudal part of the motor cortex. The hindlimb area is rostrally adjacent, while the whisker area is laterally adjacent.
Table 1. Average areas and thresholds of the neck area and trunk area.
| Average area (mm2) | Average threshold (μA) | |
| Neck area | 1.8 ± 0.3 | 18.4 ± 3.2 |
| Trunk area | 0.9 ± 0.2 | 40.0 ± 3.6 |
| p value | 0.0005 | 0.0002 |
Fig. 3.
Body movements induced by stimulation of the neck and trunk areas with a current greater than the threshold.
The figure shows body movements that occur when the neck and trunk areas in the motor cortex are stimulated with a current greater than the threshold and less than 50 μA, as well as the stimulation currents applied. Stimulation greater than the threshold induced movement in other parts of the body only when applied to the neck region. A: Representations of various body parts in the motor cortex (Rat No. 6) and their thresholds (F). B: Sites in the neck and trunk areas at which stimulation induced forelimb movements, and the stimulation currents at which these movements occurred (G). C: Sites at which jaw movements were induced, and the stimulation currents at which these movements occurred (H). D: Sites at which whisker movements were induced, and the stimulation currents at which these movements occurred (I). E: Sites at which trunk movements were induced, and the stimulation currents at which these movements occurred (J).
The trunk area was 2.5−4 mm lateral to and 2.0−4.0 mm caudal to the bregma (Fig. 2, Fig. 3A). The threshold for the trunk area was generally higher than that for the neck area, even reaching as high as 40−50 μA (Fig. 3F, Table 1, p=0.0002). Unlike with the neck area, stimulation of the trunk area greater than the threshold did not induce movement anywhere else in the body (Fig. 3F–J).
DISCUSSION
The results of the present study showed that in most cases, the neck area in the motor cortex divided the rostral and caudal forelimb areas and expanded laterally from there, and the trunk area was located caudal to the hindlimb area. In the human motor area, somatic representation extended in the medio-lateral directions of the motor cortex, while the rat motor area extended in the anterior-posterior direction. These results were consistent with those of the previous study8). In contrast, electrophysiological characteristics of neck and trunk area, except for their location, were unclear8). In this study, however, we indicated such electrophysiological characteristics of both areas for the first time. The threshold for the neck area was the lowest between the rostral and caudal forelimb areas but was high in the surrounding area. Stimulation of the neck area with a current greater than the threshold but less than 50 μA induced movement in body parts represented by the adjacent areas. Although it is conceivable that stimulation current leaked into the adjacent areas, it is unlikely that this phenomenon would occur with a maximum current of 50 μA10). We also considered the effect of anesthetic depths because it is known that the difference in the anesthetic depths affects the electric thresholds and representations of the neck motor area11), although the individual differences in electric thresholds and representations of neck motor area was small. Thus, it was unlikely that, at least, the differences in the depth of anesthesia between individuals contributed to the evoke movement in other body parts. Therefore, it is thought that the neck area contains not only corticospinal tract cells that regulate neck movement, but also a considerable number of those corticospinal tract cells that control the adjacent areas. In addition, stimulation of low-threshold sectors of the neck area resulted in movement of not only body parts represented by adjacent areas, but also of the trunk. However, the trunk area is located more than 3 mm caudal to the neck area and is not adjacent to the trunk area in the primary motor cortex; therefore, it is unlikely that neck-regulating and trunk-regulating corticospinal tract cells would have coexisted in the same stimulation site. The most conceivable explanations are the following: there are intracortical fiber pathways from the low-threshold neck area to the trunk area; or a descending tract originating from the neck area excites motor neurons that control the trunk in the lower brain, such as in the brain stem or the spinal cord. However, we cannot prove any of the above possible explanations from the results of the present study.
We learnt the following characteristics of the trunk area: it is located caudal to the hindlimb area, it has a higher threshold than the neck area, and stimulation above the threshold induces almost no movement in any body parts represented by the adjacent areas. This last finding clearly distinguishes the trunk area from the neck area, as stimulation of the neck area greater than its threshold induces movement in other parts of the body. These results suggest that the trunk area has less effect on movement of other parts of the body than does the neck area. It is unknown how these characteristics affect the close, complex interaction between neck and trunk activity12).
In conclusion, the present study demonstrated that in the murine motor cortex, the neck area is located rostrally, while the trunk area is located caudally. We also learnt that the neck and trunk area differ in the following regard: stimulation of the neck area above its threshold induces movement in other parts of the body, primarily in parts represented by adjacent areas; whereas this effect is not observed with the trunk area. Considering the clinical aspect, it is well known that alterations in the representation of motor cortex are induced by not only brain damage but also peripheral nerve injury and diabetes9, 13). Therefore, the relationship between the neck and trunk motor area and movement disorders using disease model animals must be investigated in future studies.
Funding and Conflicts of interest
We have no conflicts of interest to declare.
REFERENCES
- 1.Treleaven J: Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man Ther, 2008, 13: 2–11. [DOI] [PubMed] [Google Scholar]
- 2.Cheng CH, Chien A, Hsu WL, et al. : Changes of postural control and muscle activation pattern in response to external perturbations after neck flexor fatigue in young subjects with and without chronic neck pain. Gait Posture, 2015, 41: 801–807. [DOI] [PubMed] [Google Scholar]
- 3.Bogduk N, Mercer S: Biomechanics of the cervical spine. I: Normal kinematics. Clin Biomech (Bristol, Avon), 2000, 15: 633–648. [DOI] [PubMed] [Google Scholar]
- 4.Bogduk N, Yoganandan N: Biomechanics of the cervical spine Part 3: minor injuries. Clin Biomech (Bristol, Avon), 2001, 16: 267–275. [DOI] [PubMed] [Google Scholar]
- 5.Cusick JF, Yoganandan N: Biomechanics of the cervical spine 4: major injuries. Clin Biomech (Bristol, Avon), 2002, 17: 1–20. [DOI] [PubMed] [Google Scholar]
- 6.Gandevia SC, Applegate C: Activation of neck muscles from the human motor cortex. Brain, 1988, 111: 801–813. [DOI] [PubMed] [Google Scholar]
- 7.Shinoda Y, Sugiuchi Y, Izawa Y, et al. : Long descending motor tract axons and their control of neck and axial muscles. Prog Brain Res, 2006, 151: 527–563. [DOI] [PubMed] [Google Scholar]
- 8.Neafsey EJ, Bold EL, Haas G, et al. : The organization of the rat motor cortex: a microstimulation mapping study. Brain Res, 1986, 396: 77–96. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu K, Ikutomo M, Tamaki T, et al. : Effect of streptozotocin-induced diabetes on motor representations in the motor cortex and corticospinal tract in rats. Brain Res, 2018, 1680: 115–126. [DOI] [PubMed] [Google Scholar]
- 10.Bagshaw EV, Evans MH: Measurement of current spread from microelectrodes when stimulating within the nervous system. Exp Brain Res, 1976, 25: 391–400. [DOI] [PubMed] [Google Scholar]
- 11.Tandon S, Kambi N, Jain N: Overlapping representations of the neck and whiskers in the rat motor cortex revealed by mapping at different anaesthetic depths. Eur J Neurosci, 2008, 27: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgerton VR, Wolf SL, Levendowski DJ, et al. : EMG activity in neck and back muscles during selected static postures in adult males and females. Physiother Theory Pract, 1997, 13: 179–195. [Google Scholar]
- 13.Donoghue JP, Sanes JN: Organization of adult motor cortex representation patterns following neonatal forelimb nerve injury in rats. J Neurosci, 1988, 8: 3221–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]