Abstract
Background
Meningococcal disease is a contagious bacterial infection caused by Neisseria meningitidis (N meningitidis). Household contacts have the highest risk of contracting the disease during the first week of a case being detected. Prophylaxis is considered for close contacts of people with a meningococcal infection and populations with known high carriage rates.
Objectives
To study the effectiveness, adverse events and development of drug resistance of different antibiotics as prophylactic treatment regimens for meningococcal infection.
Search methods
We searched CENTRAL 2013, Issue 6, MEDLINE (January 1966 to June week 1, 2013), Embase (1980 to June 2013) and LILACS (1982 to June 2013).
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs addressing the effectiveness of different antibiotics for: (a) prophylaxis against meningococcal disease; (b) eradication of N meningitidis.
Data collection and analysis
Two review authors independently appraised the quality and extracted data from the included trials. We analysed dichotomous data by calculating the risk ratio (RR) and 95% confidence interval (CI) for each trial.
Main results
No new trials were found for inclusion in this update. We included 24 studies; 19 including 2531 randomised participants and five including 4354 cluster‐randomised participants. There were no cases of meningococcal disease during follow‐up in the trials, thus effectiveness regarding prevention of future disease cannot be directly assessed.
Mortality that was reported in one study was not related to meningococcal disease or treatment. Ciprofloxacin (RR 0.04; 95% CI 0.01 to 0.12), rifampin (rifampicin) (RR 0.17; 95% CI 0.13 to 0.24), minocycline (RR 0.28; 95% CI 0.21 to 0.37) and penicillin (RR 0.47; 95% CI 0.24 to 0.94) proved effective at eradicating N meningitidis one week after treatment when compared with placebo. Rifampin (RR 0.20; 95% CI 0.14 to 0.29), ciprofloxacin (RR 0.03; 95% CI 0.00 to 0.42) and penicillin (RR 0.63; 95% CI 0.51 to 0.79) still proved effective at one to two weeks. Rifampin was effective compared to placebo up to four weeks after treatment but resistant isolates were seen following prophylactic treatment. No trials evaluated ceftriaxone against placebo but rifampin was less effective than ceftriaxone after one to two weeks of follow‐up (RR 5.93; 95% CI 1.22 to 28.68). Mild adverse events associated with treatment were observed.
Authors' conclusions
Using rifampin during an outbreak may lead to the circulation of resistant isolates. Use of ciprofloxacin, ceftriaxone or penicillin should be considered. All four agents were effective for up to two weeks follow‐up, though more trials comparing the effectiveness of these agents for eradicating N. meningitidis would provide important insights.
Plain language summary
Antibiotics for preventing meningococcal infections
Meningococcal disease is a contagious bacterial disease caused by the bacteria Neisseria meningitidis (N meningitidis) with high fatality rates: up to 15% for infection of the central nervous system (meningitis) and up to 50% to 60% among patients with blood stream infection and shock; up to 15% of survivors are left with severe neurological deficits. People who have had close contact with someone who has a meningococcal infection and populations with known high carriage rates are offered antibiotics in order to eradicate the bacteria and thus prevent disease.
Data from 24 studies, most of high quality, including 6885 participants found that rifampin (also known as rifampicin), ciprofloxacin, ceftriaxone and penicillin are effective agents for eradicating carriage of N meningitidis. However, the use of rifampin may have a disadvantage as development of resistance to the antibiotic has been noted following treatment. Mild adverse events are associated with the different antibiotics used. Disease prevention could not be evaluated directly in this review as only data for eradication of the bacteria were available. Different follow‐up periods were reported in the studies. Evidence in this review is current as of June 2013.
Background
Description of the condition
Meningococcal disease is a contagious bacterial disease caused by Neisseria meningitidis (N. meningitidis). It is spread by person‐to‐person contact through respiratory droplets. N meningitidis inhabits the mucosal membrane of the nose and throat where it usually causes no harm. Carriage rates vary from 10% among randomly sampled populations to 95% during epidemics. These carriers are crucial to the spread of the disease as most cases are acquired through exposure to asymptomatic carriers (WHO 2003a). The onset of symptoms of meningococcal disease is sudden and death can follow within hours. Case‐fatality rates from invasive meningococcal disease are 10% to 15%, rising as high as 50% to 60% among patients with meningococcaemia (blood stream infection) and shock. For survivors, there are persistent neurological defects including hearing loss, speech disorders, loss of limbs, mental retardation and paralysis in as many as 10% to 15% (Ferguson 2002).
Meningococcal disease occurs sporadically and in small clusters throughout the world. It accounts for a variable proportion of endemic bacterial meningitis with seasonal variations. In temperate regions the number of cases increases in winter and spring. Serogroups B and C together account for the large majority of cases in Europe, the Americas and Australasia (WHO 2003b).
Serogroup B meningococcal disease caused 68% of cases reported in Europe between 1993 and 1996 and has also caused outbreaks in other developed countries, with attack rates of 5 to 50 cases per 100,000 persons (Rosenstein 2001). Several local outbreaks due to serogroup C N meningitidis have also been reported in Canada and the USA (1992 to 1993) and in Spain (1995 to 1997). In New Zealand, meningococcal disease activity has increased in the past 10 years and an average of 500 cases occur every year. Most of these cases are due to serogroup B, while serogroup A is usually the cause of meningococcal disease in Asia (WHO 2003b).
In the African 'meningitis belt' that extends from Ethiopia in the east to Senegal in the west, serogroup A meningococcal disease poses a recurrent threat to public health and rates of meningococcal disease are several times higher than in industrialised countries. The reported mortality is usually around 10%, a rate similar to that in industrialised countries, but true mortality is probably much higher. Attack rates can be as high as 100 to 800 cases per 100,000 and individual communities have reported rates as high as 1000 per 100,000. In 1996, the largest outbreak ever reported occurred in the meningitis belt. The total number of cases was over 250,000 with 25,000 reported deaths. Between 1996 and 2002, 223,000 new cases of meningococcal disease were reported to the World Health Organization. The countries most affected were Burkina Faso, Chad, Ethiopia and Niger. In 2002, the outbreaks occurring in Burkina Faso, Ethiopia and Niger accounted for about 65% of the total cases reported in the African continent. In Burkina Faso alone, 13,000 cases caused by serogroup W135 were reported and 1500 deaths recorded. Furthermore, the meningitis belt appears to be extending further south. In 2002 the Great Lakes region was affected by outbreaks in villages and refugee camps, which caused more than 2200 cases and 200 deaths.
In addition, there is increasing evidence of serogroup W135 being associated with other outbreaks of considerable size. In 2000 and 2001 several hundred pilgrims attending the Hajj in Saudi Arabia were infected with N meningitidis W135. Outside Africa only Mongolia has reported a large epidemic in recent years (1994 to 1995) (WHO 2003b).
The relative frequency of disease caused by N. meningitidis has increased in recent years due to the widespread use of an effective vaccine for Haemophilus influenzae (H. influenzae) B and a vaccine for Streptococcus pneumoniae (S. pneumoniae). The successful use of these vaccines has left N. meningitidis as the most common cause of bacterial meningitis (Conterno 2006).
Description of the intervention
The aim of chemoprophylaxis is to reduce the risk of invasive disease by eradicating carriage. Individuals in close contact with cases of meningococcal disease are at increased risk of developing disease. The highest documented relative and absolute risk is for people living in the same household as a case of meningococcal disease, during the first seven days. If prophylaxis is not given, the absolute risk is about 1 in 300 (PHLS 2002), with the risk of contracting the disease increased by a factor of 400 to 800 (Rosenstein 2001). Prophylaxis is also considered in populations with known high carriage rates, such as military personnel. The carriers are at increased risk of contracting the disease themselves and may pose a risk of infection to others.
Post‐exposure immunisation with meningococcal polysaccharide vaccine can effectively decrease secondary cases of disease. However, given that the vaccine is not protective for at least 10 days after administration and no effective vaccine is currently available against serogroup B, N meningitidis chemoprophylaxis remains an important component in limiting disease spread (Girgis 1998).
Rifampin (rifampicin) given orally twice daily for two days in a 10 mg/kg dose (600 mg maximum) remains the drug of choice for meningococcal prophylaxis of high‐risk groups. Frequent side effects, contraindications during pregnancy and unavailability of a convenient suspension for paediatric usage limit the overall utility of rifampin. Rapid development of rifampin resistance by meningococcal isolates has also been indicated. Thus other systemic antibiotics that effectively eliminate nasopharyngeal carriage of N meningitidis, including ciprofloxacin and ceftriaxone, are also used as prophylactic agents. However, quinolones are not approved for routine paediatric usage and ceftriaxone requires parenteral administration (Girgis 1998).
How the intervention might work
People who had close contact with someone who has a meningococcal infection and populations with high carriage rates are offered antibiotics in order to eradicate the bacteria and thus prevent disease.
Why it is important to do this review
A systematic review comparing the effectiveness and adverse events of different antibiotics for preventing meningococcal infection should establish the best options for preventing further spread of this disease.
Objectives
To study the effectiveness, adverse events and development of drug resistance of different antibiotics as prophylactic treatment regimens for meningococcal infection, specifically:
preventing secondary cases of meningococcal disease after contact with a person with a meningococcal disease, both within and outside the household;
preventing cases of meningococcal disease in populations with a high rate of N meningitidis carriage; and
eradicating N meningitidis from the pharynx in healthy carriers of N meningitidis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs addressing the effectiveness of different antibiotic treatments for:
prophylaxis against meningococcal disease;
eradication of N meningitidis.
Types of participants
Healthy individuals:
exposed to someone with meningococcal disease, whether in the household or elsewhere;
exposed to N meningitidis carriers;
belonging to a population with a high rate of N meningitidis carriage, regardless of their carrier status.
Types of interventions
Antibiotic treatment versus placebo.
One antibiotic drug versus another.
Antibiotic treatment versus no intervention.
Types of outcome measures
Primary outcomes
Mortality.
Occurrence of meningococcal infection.
Secondary outcomes
Occurrence of any clinical adverse effects.
Proportion of meningococcal carriers and high‐risk persons who were culture‐negative at end of follow‐up.
Occurrence of relapse and re‐colonisation.
Occurrence of resistant strains subsequent to treatment.
Search methods for identification of studies
Electronic searches
For this 2013 review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 6, part of the Cochrane Library, www.thecochranelibrary.com (accessed 13 June 2013), which contains the Cochrane Acute Respiratory Infections Group Specialised Register, MEDLINE (April 2011 to June week 1, 2013), Embase (May 2011 to June 2013) and LILACS (May 2011 to June 2013). Details of previous searches are in Appendix 1.
We searched CENTRAL and MEDLINE using the following search strategy. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase (see Appendix 2) and LILACS (see Appendix 3). There were no language or publication restrictions.
MEDLINE (Ovid)
1 exp Meningitis, Meningococcal/ 2 Meningitis, Bacterial/ 3 exp Neisseria meningitidis/ 4 "N. meningitidis".tw. 5 ((neisseria or epidemic or meningococ*) adj2 mening*).tw. 6 meningococ*.tw. 7 or/1‐6 8 exp Chemoprevention/ 9 chemoprevent*.tw. 10 chemoprophyl*.tw. 11 Post‐Exposure Prophylaxis/ 12 (prophyla* or carri* or phary* or colon* or eradic* or prevent* or nasopharyn* or tonsillopharyng* or elimin*).tw. 13 or/8‐12 14 exp Anti‐Bacterial Agents/ 15 antibiotic*.tw,nm. 16 antibacterial*.tw,nm. 17 (oxytetracyc* or tetracyc* or penicilli* or erythromyci* or ampicilli* or sulfa* or ciprofloxacin* or norfloxaci* or ofloxaci* or quinol* or fluoroquinol* or fluoro‐quinolon* or ceftriaxon* or rifampi* or azithromyci* or coumermyci* or minocyclin* or macrolid* or cephalospori*).tw,nm. 18 or/14‐17 19 13 and 18 20 Antibiotic Prophylaxis/ 21 19 or 20 22 7 and 21
Searching other resources
We searched WHO ICTRP http://apps.who.int/trialsearch/Default.aspx and ClinicalTrials.gov http://clinicaltrials.gov/ct2/search/index for completed and ongoing trials (13 June 2013). We also searched references of all identified studies as well as major reviews for additional studies.
Data collection and analysis
Selection of studies
Two review authors (AF, AGG) independently inspected each reference identified by the search and applied the inclusion criteria. We obtained the full article for possibly relevant trials or in cases of disagreement and the two review authors inspected this independently. A third, independent review author (LL) was consulted where there was disagreement.
Data extraction and management
Two review authors (AF, AGG) independently extracted the data from included trials. In case of any disagreement, a third review author (MP) extracted the data. We discussed the data extraction, documented decisions and, where necessary, contacted the trial authors for clarification. We identified trials by the name of the first author and year in which the trial was first published and ordered these chronologically. We extracted, checked and recorded the following data.
Characteristics of trials
Date, location and setting of trial.
Publication status.
Concealment, randomisation method, blinding, drop‐outs.
Sponsor of trial (specified, known or unknown).
Duration of follow‐up.
Characteristics of participants
Contact status (household contact, outside household contact, no known contact).
Number of participants in each group, age, gender and nationality.
Characteristics of interventions
Type of antibiotic, dose, mode of administration, schedule, length of treatment and follow‐up.
Characteristics of outcome measures
We recorded the number of events previously listed under Types of outcome measures in each arm of the randomised trials whenever possible.
Assessment of risk of bias in included studies
Two review authors (AF, AGG) independently assessed trials fulfilling the review inclusion criteria for methodological quality. A third review author (MP) was consulted in case of disagreements. We extracted information about random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, sample size, exclusions after randomisation and different lengths of follow‐up. We did this using the criteria described in Higgins 2011.
Measures of treatment effect
We analysed dichotomous data by calculating the risk ratio (RR) for each trial with the uncertainty in each result being expressed using 95% confidence intervals (CIs). We pooled trial results according to the type of antibiotic assessed and the duration of follow‐up at the time outcomes were assessed.
Unit of analysis issues
Five studies including 4354 participants used cluster‐randomisation. It should be noted that four studies that used cluster‐randomisation but reported data for individuals are included in the meta‐analysis (Cuevas 1995; Guttler 1971; Munford 1974; Schwartz 1988).
Dealing with missing data
We analysed the drop‐out rates between the antibiotic and placebo arms across the studies; no significant differences were observed (Analysis 7.1). For the cluster‐randomised trials, due to the lack of data, the intra‐cluster correlation coefficient could not be calculated or estimated from other sources. Thus we could not account for the cluster‐randomisation in the meta‐analysis. Despite this limitation these studies were included in the analysis as removing them did not substantially alter the results. Four of the six trials conducted on household contacts used cluster‐randomisation (see the Characteristics of included studies table). We performed intention‐to‐treat (ITT) analyses; for the ITT analysis we used the number of persons randomised as the denominator. Any persons lost to follow‐up, or persons who did not complete the treatment and thus were not included in the original analysis for any reason, were assumed to be eradication failures.
7.1. Analysis.
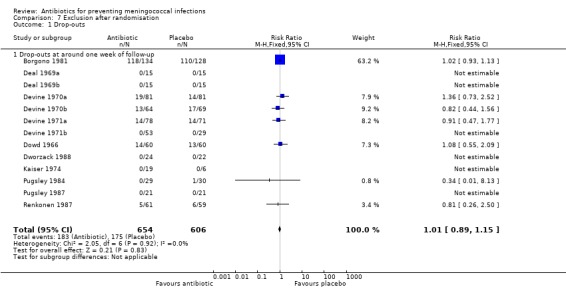
Comparison 7 Exclusion after randomisation, Outcome 1 Drop‐outs.
Assessment of heterogeneity
We initially assessed heterogeneity in the results of the trials by inspection of graphical presentations and then by calculating a test of heterogeneity (Chi2 test, I2 statistic). We performed sensitivity analyses in order to assess the impact of these possible sources of heterogeneity on the main results.
Assessment of reporting biases
We did not identify reporting bias across the studies; a small number of studies was available for each of the antibiotic regimens compared and funnel plots could not be performed to assess possible publication bias.
Data synthesis
We pooled trial results according to the type of antibiotic assessed. We anticipated between‐trial variation in the estimation of morbidity and mortality for trials comparing individuals at different risk levels (that is contact status). We used a fixed‐effect model throughout the review except in the event of significant heterogeneity between the trials (P < 0.10), when we chose the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to extract data separately for N. meningitidis carriers, different contact strata, N meningitidis serogroups and vaccination status.
Sensitivity analysis
We performed sensitivity analyses in order to assess the robustness of the findings to different aspects of the trials' methodology:
allocation concealment (adequate or unclear);
exclusions after randomisation (reported or not reported);
sample size; and
length of follow‐up.
Results
Description of studies
Results of the search
The computerised search strategies identified a large number of studies; not all were relevant for this present review. We screened these for randomised controlled trials (RCTs) and quasi‐RCTs, N meningitidis and prophylaxis or eradication. According to protocol, we searched their references in order to identify additional references. We considered 49 studies for this review.
Included studies
Study population
We included 24 trials, performed between the years 1966 to 2000, in the review (see Characteristics of included studies table). Six trials included household contacts of N meningitidis cases. Sixteen studies were eradication trials conducted on healthy individuals. Of these studies one included children exclusively, four included students, seven included army recruits, three included volunteers and in one study the population was unspecified. An additional eradication trial of N meningitidis included patients with extragenital gonorrhoea and another included patients with culture or smear‐positive tests for anogenital gonorrhoea or confirmed recent exposure to gonorrhoea. Children were included in five trials. In all, only six trials explicitly reported the trial population age (range or mean).
Carrier rates ranged from 6.7% to 72% (see Table 8) with a median of 22%.
1. Carrier rates.
| Study ID | Comparison | Study population | % carriers (N) |
| Blakebrough 1980 | Rifampin versus sulphadimidine | Household contacts | 17 (479) |
| Borgono 1981 | Rifampin versus placebo | Children | 12 (2132) |
| Cuevas 1995 | Rifampin versus ciprofloxacin versus ceftriaxone | Household contacts | 11 (1875) |
| Deal 1969b | Rifampin versus placebo | Students | 14.4 (270) |
| Deal 1969a | Cephalexin versus placebo | Students | 9.4 (352) |
| Devine 1970b | Rifampin versus placebo | Military recruits | 64 (103) |
| Devine 1971a | Minocycline versus placebo | Military recruits | 72 (121) |
| Devine 1970a | Coumermycin A1 versus placebo | Military recruits | 55 (129) |
| Dworzack 1988 | Ciprofloxacin versus placebo | Volunteers | 6.7 (620) |
| Girgis 1998 | Azithromycin versus placebo | Students | 24 (500) |
| Guttler 1971 | Rifampin versus minocycline | Military recruits | 21 (587) |
| Kaiser 1974 | Rifampin versus placebo | Household contacts | 35 (54) |
| Kaya 1997 | Ciprofloxacin versus rifampin | Hospital staff | 18 (300) |
| Munford 1974 | Rifampin versus minocycline versus minocycline/rifampin versus sulphadiazine | Household contacts | 25 (1187) |
| Pugsley 1984 | Sch 29,482 versus placebo | Volunteers | 25 (555) |
| Pugsley 1987 | Ciprofloxacin versus placebo | Students | 7 (461) |
| Renkonen 1987 | Ciprofloxacin versus placebo | Military recruits | 38.6 (552) |
| Schwartz 1988 | Rifampin versus ceftriaxone | Household contacts | 33 (347) |
| Simmons 2000 | Rifampin versus ceftriaxone | Household contacts | 21 (864) |
In 10 trials, allocation generation was performed before carrier status was determined (see Characteristics of included studies table). In five of these trials data were presented for proven carriers only (Blakebrough 1980; Cuevas 1995; Kaiser 1974; Munford 1974; Simmons 2000).
In 19 studies, 2531 persons were randomised. Five studies used cluster‐randomisation (households and military units). These studies included 4354 persons.
Antibiotic regimens
Fifteen studies compared an antibiotic drug given orally or intramuscularly to placebo or no intervention. The antibiotics compared to placebo were: rifampin, cephalexin, minocycline, ciprofloxacin, coumermycin A1, ampicillin, penicillin G and an investigational compound Sch 29,482. Eleven studies compared different antibiotic drugs and two studies included more than two study arms, one of which was given placebo.
Pre‐treatment susceptibility of meningococci to antibiotics was reported in 21 trials. All isolates were susceptible to the antibiotics tested in these trials except for a single study comparing sulphadimidine to placebo, in which nine of 93 strains were resistant to sulphadimidine (Blakebrough 1980). Susceptibility to sulphur drugs was variable when tested in the other trials.
Excluded studies
We excluded 22 studies (see Characteristics of excluded studies table). The design of 20 studies was incompatible with the inclusion criteria, one study included only patients with homozygous deficiency of the sixth component of complement (C6) and recurrent meningococcal disease (Potter 1990) and another was a trial of post‐exposure prophylaxis to H influenzae and not N meningitidis (Band 1984). We identified three reports as duplicate publications and considered these under their primary reference (Cuevas 1995; Schwartz 1988; Simmons 2000).
Risk of bias in included studies
We performed an intention‐to‐treat (ITT) analysis on three trials (Deal 1969a; Dworzack 1988; Judson 1984). In two trials the number evaluated was the same as the number randomised, with no mention of loss to follow‐up (Blakebrough 1980; Cuevas 1995). In the remaining studies ITT analysis was not performed. The length of follow‐up ranged from five days to 130 days. Both the mode and the median length of follow‐up were two weeks.
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
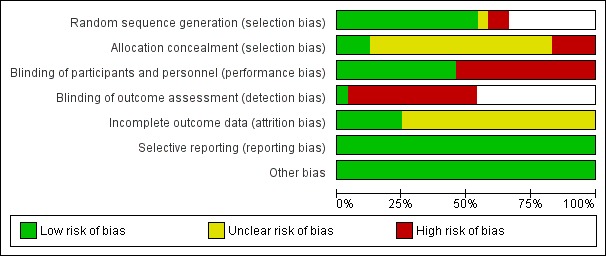
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
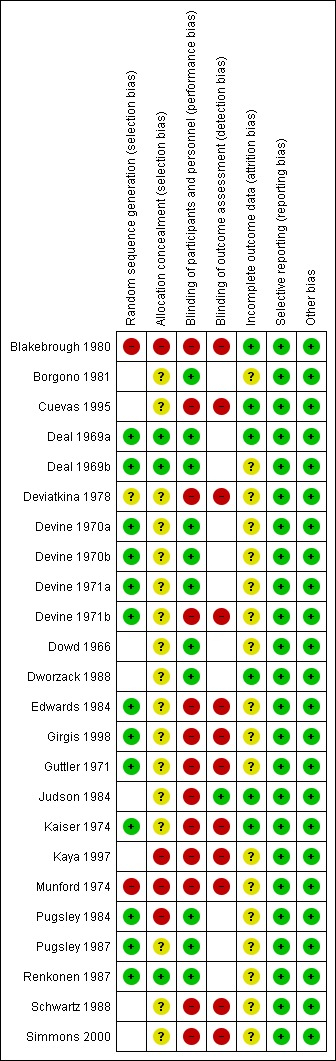
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequate allocation concealment, using central randomisation or numbered packages of antibiotic and identical‐looking placebo, was described in three trials (Deal 1969a; Deal 1969b; Renkonen 1987). These trials did not involve the same comparisons and when included in their respective analyses did not seem to differ from the other included studies. In the remaining trials allocation concealment was not described.
Adequate allocation generation was described in 13 trials (Deal 1969a; Deal 1969b; Devine 1970a; Devine 1970b; Devine 1971a; Devine 1971b; Edwards 1984; Girgis 1998; Guttler 1971; Kaiser 1974; Pugsley 1984; Pugsley 1987; Renkonen 1987). Two trials used quasi‐randomisation methods: one trial assigned households to their respective study arms 'alternately', following the order the index cases were admitted into hospital (Blakebrough 1980); another trial assigned households to their respective study arms 'serially', in the order they arrived at the study centre and with no knowledge of the index case's age, race or family size (Munford 1974). In one trial (Cuevas 1995) the study arm receiving ceftriaxone was not randomised, thus the data for this group were not included in the analysis.
Blinding
Eleven trials were conducted in a double‐blinded fashion (Borgono 1981; Deal 1969a; Deal 1969b; Devine 1970a; Devine 1970b; Devine 1971a; Dowd 1966; Dworzack 1988; Pugsley 1984; Pugsley 1987; Renkonen 1987); 12 others were open (see the Characteristics of included studies table) and in one the outcome assessor was blinded (Judson 1984).
Incomplete outcome data
We looked at the rate of drop‐outs after randomisation in the different study arms (see Data collection and analysis section). In one of the trials (Kaiser 1974) randomisation was performed before carrier status was determined, while data were presented for carriers only. There was no difference between the number of randomised carriers and the number evaluated. We thus entered this study as no drop‐outs. In another study (Guttler 1971) no information was provided regarding the number of individuals randomised as part of the cluster‐randomisation. Thus this trial was excluded from this analysis. No difference in loss to follow‐up was found between study arms.
Selective reporting
There was no evidence of selective reporting in the included studies.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
Primary outcomes
1. Mortality
Only one trial comparing rifampicin to ceftriaxone and to ciprofloxacin reported deaths during the study period (Cuevas 1995). These deaths (one in the rifampin group and two in the ceftriaxone group) were unrelated to meningococcal disease or the treatment.
2. Occurrence of meningococcal infection
Five studies provided information regarding meningococcal disease (see the Characteristics of included studies table). One trial reported a secondary case among study participants but the case was diagnosed before prophylaxis had begun (Blakebrough 1980). One case of meningococcal disease was reported in another trial but occurred 12 weeks after treatment with rifampin. This individual did not carry N meningitidis at any stage of the trial (Guttler 1971). No cases of meningococcal disease occurred in the other three trials thus the clinical effectiveness of chemoprophylaxis in disease prevention of meningococcal disease could not be assessed.
Secondary outcomes
1. Occurrence of any clinical adverse effects
Eighteen trials provided quantitative data regarding the occurrence of adverse effects. These were all mild in nature and included nausea, diarrhoea, abdominal pain, headaches, dizziness, skin rash and pain at injection site.
One study comparing rifampin to ceftriaxone yielded an overall risk ratio (RR) for any clinical adverse effects of 1.39 (95% confidence interval (CI) 1.10 to 1.75) (Analysis 1.1). Two studies comparing rifampin to ciprofloxacin yielded an overall non‐significant RR of 0.75 (95% CI 0.36 to 1.56) (Analysis 1.2).
1.1. Analysis.
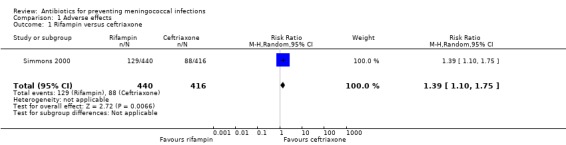
Comparison 1 Adverse effects, Outcome 1 Rifampin versus ceftriaxone.
1.2. Analysis.
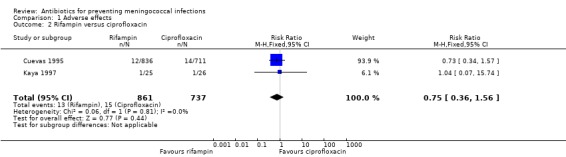
Comparison 1 Adverse effects, Outcome 2 Rifampin versus ciprofloxacin.
2. Proportion of meningococcal carriers and high‐risk persons who were culture‐negative at end of follow‐up
Up to one week of follow‐up
Failure to eradicate N meningitidis from the nasopharynx was the main outcome for all trials. Ciprofloxacin (RR 0.04; 95% CI 0.01 to 0.12), rifampin (RR 0.17; 95% CI 0.13 to 0.24), minocycline (RR 0.28; 95% CI 0.21 to 0.37) and penicillin (RR 0.47; 95% CI 0.24 to 0.94) all proved effective at eradicating N meningitidis one week after treatment when compared to placebo (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4).
2.1. Analysis.
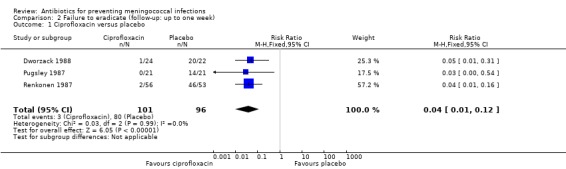
Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 1 Ciprofloxacin versus placebo.
2.2. Analysis.
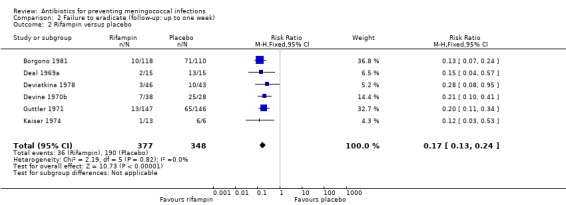
Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 2 Rifampin versus placebo.
2.3. Analysis.
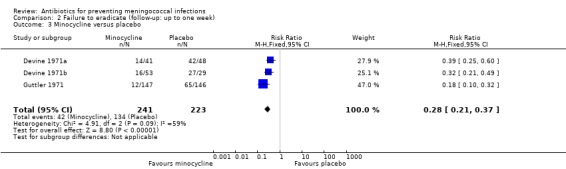
Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 3 Minocycline versus placebo.
2.4. Analysis.
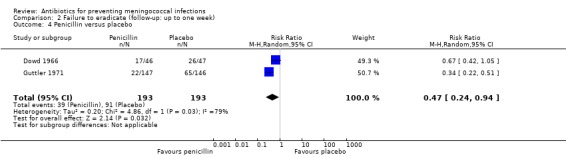
Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 4 Penicillin versus placebo.
Rifampin was more effective at eradicating N meningitidis when compared to ciprofloxacin but the difference did not reach statistical significance at the 95% confidence level (RR 0.34; 95% CI 0.11 to 1.02) (Analysis 2.6). No significant difference was found when rifampin was compared to ceftriaxone (RR 3.71; 95% CI 0.73 to 18.86) (Analysis 2.7).
2.6. Analysis.
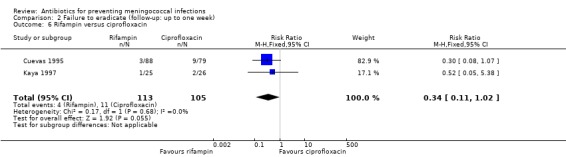
Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 6 Rifampin versus ciprofloxacin.
2.7. Analysis.
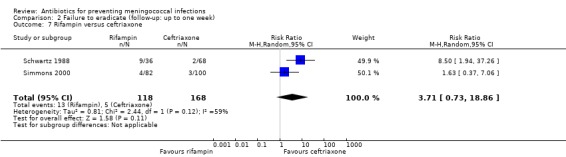
Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 7 Rifampin versus ceftriaxone.
Between one to two weeks of follow‐up
Ciprofloxacin (based on a single study: RR 0.03; 95% CI 0.00 to 0.42) (Pugsley 1987), rifampin (RR 0.20; 95% CI 0.14 to 0.29) and penicillin (RR 0.63; 95% CI 0.51 to 0.79) proved effective at eradicating N meningitidis between one and two weeks after treatment when compared to placebo (Analysis 3.1; Analysis 3.2; Analysis 3.4). Minocycline (RR 0.37; 95% CI 0.10 to 1.31) was not significantly effective compared to placebo (Analysis 3.3).
3.1. Analysis.
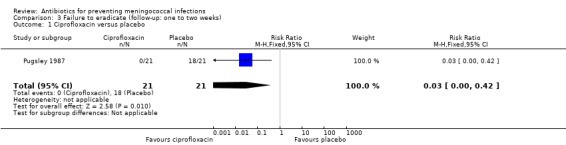
Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 1 Ciprofloxacin versus placebo.
3.2. Analysis.
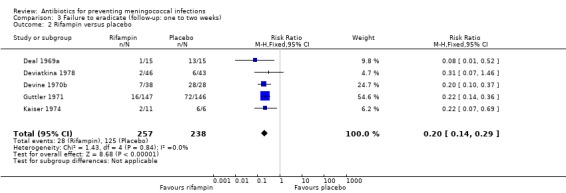
Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 2 Rifampin versus placebo.
3.4. Analysis.
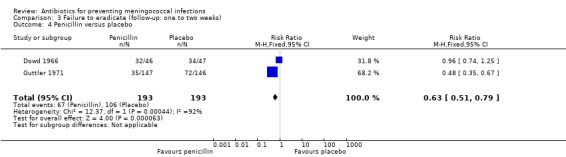
Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 4 Penicillin versus placebo.
3.3. Analysis.
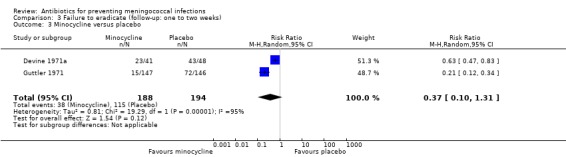
Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 3 Minocycline versus placebo.
When rifampin was compared with ciprofloxacin again, according to the point estimate, rifampin was more effective but not significantly (RR 0.31; 95% CI 0.09 to 1.11) (Analysis 3.6). Based on the point estimate of a single study (Schwartz 1988) rifampin proved less effective when compared to ceftriaxone (RR 5.93; 95% CI 1.22 to 28.68) (Analysis 3.7). No difference was found when rifampin was compared to minocycline (RR 1.01; 95% CI 0.57 to 1.77) (Analysis 3.8).
3.6. Analysis.
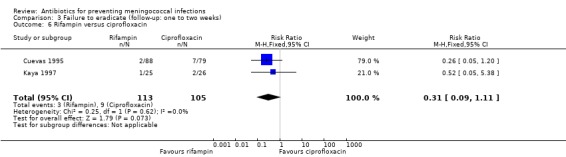
Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 6 Rifampin versus ciprofloxacin.
3.7. Analysis.
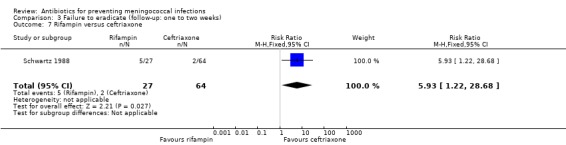
Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 7 Rifampin versus ceftriaxone.
3.8. Analysis.
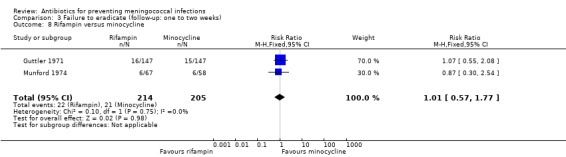
Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 8 Rifampin versus minocycline.
Follow‐up longer than two weeks
Six studies presented data for follow‐up periods of over two weeks (see Characteristics of included studies table). Of these studies three compared rifampin with placebo (Deal 1969a; Guttler 1971; Kaiser 1974). Rifampin proved effective when compared to placebo both at between two to three weeks (RR 0.25; 95% CI 0.16 to 0.37) and three to four weeks (RR 0.24; 95% CI 0.16 to 0.38) after treatment (Analysis 4.1; Analysis 5.1). In the study with the longest follow‐up (Kaiser 1974), at day 130 there were no positive cultures in the rifampin group (n = 7) while in the control group two of four individuals had a positive culture for N meningitidis. Two studies (Dowd 1966; Guttler 1971) compared penicillin to placebo after three to four weeks of follow‐up. Penicillin was found to be ineffective (RR 0.95; 95% CI 0.53 to 1.68) (Analysis 5.2).
4.1. Analysis.
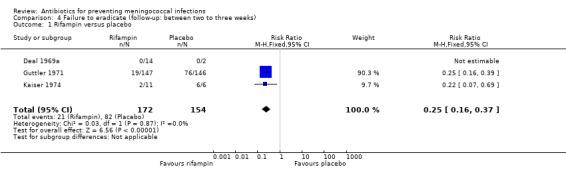
Comparison 4 Failure to eradicate (follow‐up: between two to three weeks), Outcome 1 Rifampin versus placebo.
5.1. Analysis.
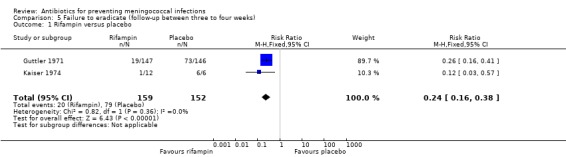
Comparison 5 Failure to eradicate (follow‐up between three to four weeks), Outcome 1 Rifampin versus placebo.
5.2. Analysis.
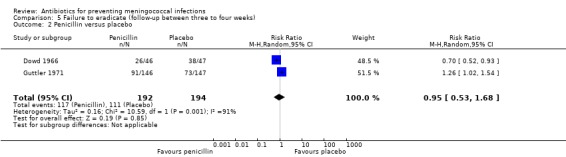
Comparison 5 Failure to eradicate (follow‐up between three to four weeks), Outcome 2 Penicillin versus placebo.
In a single study (Guttler 1971) comparing minocycline and rifampin after five weeks, no significant difference was seen between the two drugs' effectiveness for eradicating nasopharyngeal carriage of N meningitidis (RR 0.97; 95% CI 0.48 to 1.97) (Analysis 6.1).
6.1. Analysis.
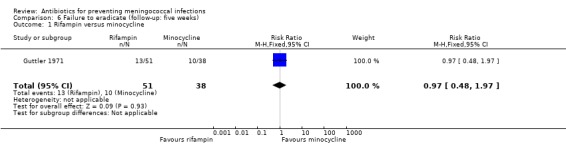
Comparison 6 Failure to eradicate (follow‐up: five weeks), Outcome 1 Rifampin versus minocycline.
Four trials were included in this review but not in the meta‐analysis. Blakebrough 1980 found that rifampin was more effective at eradicating carriage of N meningitidis than sulphadimidine at both two (RR 0.45; 95% CI 0.25 to 0.80) and seven weeks after treatment (RR 0.26; 95% CI 0.12 to 0.59). In Girgis 1998 no significant difference was found between the effectiveness of azythromycin and rifampin at one and two weeks post‐treatment (RR 0.30; 95% CI 0.30 to 5.54 and RR 0.81; 95% CI 0.23 to 2.88, respectively). Judson 1984 found that at day seven, spectinomycin eradicated carriage of only one out of nine initial N meningitidis carriers, while ceftriaxone eradicated carriage from 29 of 29 initial carriers. Edwards 1984 found no difference between amoxycillin and bacampicillin for eradicating N meningitidis (RR 1.4; 95% CI 0.16 to 12.60, favours bacampicillin).
3. Occurrence of relapse and re‐colonisation
Data on acquisition and re‐colonisation were too sparse to allow analysis.
4. Occurrence of resistant strains subsequent to treatment
Eleven trials reported the susceptibility of persistent isolates to at least one of the studied antibiotics (Blakebrough 1980; Deal 1969a; Deal 1969b; Devine 1971b; Dworzack 1988; Guttler 1971; Kaiser 1974; Munford 1974; Pugsley 1987; Renkonen 1987; Simmons 2000). No development of resistance was detected for any antibiotic drug other than rifampin.
Six trials assessed resistance development to rifampin (Blakebrough 1980; Deal 1969a; Guttler 1971; Kaiser 1974; Munford 1974; Simmons 2000). In Guttler 1971 rifampin‐resistant isolates requiring minimal inhibitory concentrations (MICs) of 100 to 200 µg/ml of rifampin were seen in 20 of 75 post‐treatment isolates, while MICs increased from pre‐treatment values of less than 0.25 µg/ml to 2 to 6 µg/ml in 37 additional isolates. All resistant isolates were detected among patients treated with rifampin. In Munford 1974, seven resistant isolates were detected out of 37 isolates among 67 patients treated with rifampin (MICs of 16 to 256 µg/ml). All pre‐treatment isolates were susceptible to rifampin and no resistance to rifampin developed among patients randomised to rifampin in addition to minocycline in this study. The meningococci identified in these two studies were serogroup B or C and all resistant isolates were identified as group C. One additional study assessing group A meningococci (Blakebrough 1980) found an increase in rifampin MICs from less than 0.1 µg/ml to 3.2 µg/ml (three isolates) and 6.4 µg/ml (one isolate) post‐treatment. In all trials seven eradication failures were assessed for resistance development, which was not found.
Subgroup analysis
Subgroup analysis could not be performed due to lack of data. For information regarding the main N. meningitidis serogroups for each trial see the Characteristics of included studies table.
Discussion
Summary of main results
No direct evidence was available in this review for meningococcal disease prevention. The results obtained for the eradication of bacteria outcome suggest ciprofloxacin, ceftriaxone and rifampin are the most effective immediate prevention measures with only minor adverse events reported. These options should be considered individually.
Overall completeness and applicability of evidence
Ciprofloxacin, rifampin, minocycline and penicillin proved effective at eradicating N. meningitidis one week after treatment, compared to placebo. However, after a longer follow‐up period of one to two weeks only rifampin, ciprofloxacin and penicillin still proved significantly effective when compared to placebo. No trials evaluated ceftriaxone against placebo but when compared to rifampin after one to two weeks of follow‐up, ceftriaxone was more effective in a single study. Rifampin continued to be effective, compared to placebo, for up to four weeks of post‐treatment follow‐up. However, a disadvantage may exist as isolates resistant to rifampin were identified following prophylactic treatment.
When minocycline was compared with placebo, it proved ineffective after one to two weeks of follow‐up; but when compared to rifampin after the same length of follow‐up (Guttler 1971; Munford 1974) and after five weeks (Guttler 1971) no significant difference was found between the two antibiotics. It should be noted that the comparison between rifampin and minocycline after five weeks of follow‐up is based on one study only (Guttler 1971).
Chemoprophylactic treatment to eradicate nasopharyngeal carriage of N meningitidis has been a key approach in the control of meningococcal disease for many decades (Samuelsson 2002), despite the fact that its effectiveness for disease prevention has never been demonstrated in experimental research.
In 1937, sulphonamide therapy radically altered the outcome of meningococcal infection and replaced serum in its treatment. Prophylaxis with sulphonamides eradicated the carrier state and provided a simple and safe method for the prevention of epidemics, particularly in the crowded environments of military barracks. Increasing sulphonamide resistance among meningococci was recognised by Schoenback and Phair in 1941 to 1943 but did not become a clinically significant problem until meningococcal epidemics occurred in 1963, in two military bases in California. Since then, many agents have been evaluated as agents of eradication under the assumption that eradicating carriage would prevent meningococcal disease (Mandell 2000).
Rifampin penetrates well into body tissues, achieving therapeutic concentrations in the mucosa. Resistance to rifampin among meningococci, as for other bacteria, has been attributed to mutations in the rpoB gene that encodes the beta unit of the RNA polymerase enzyme, which is rifampin's target (Carter 1994). Increased minimal inhibitory concentrations (MICs) for rifampin were described in three of six studies assessing pre‐ and post‐treatment rifampin susceptibilities, with frank resistance developing in 10% to 27% of isolates in these studies. Thus, despite rifampin's eradication efficacy, a note of caution is required. Induction of resistance may complicate further attempts at eradication.
A recent review that included non‐randomised studies, comparing treated and untreated groups, demonstrated that chemoprophylaxis (according to local guidelines) of household contacts reduced the risk of developing meningococcal disease (RR 0.11; 95% CI 0.02 to 0.58). No studies of non‐household contacts met the inclusion criteria. Based on four studies, the pooled meningococcal disease attack rate among non‐treated family contacts was estimated at five cases per 1000 household contacts (Purcell 2004).
Based on the median prevalence of carriers among household contacts of 230 per 1000 (see Table 8, 'Carrier rates') and the pooled RR obtained from the comparison of rifampin to placebo, the absolute risk reduction in such a population after a period of one week is 190 per 1000 (95% CI 177 per 1000 to 203 per 1000). Thus the number needed to treat (NNT) in order to eradicate carriage from one carrier is six (95% CI 5 to 20). As the risk of invasive disease following acquisition varies with environmental and host factors and strain characteristics we cannot estimate the NNT in order to prevent a case of meningococcal disease.
Quality of the evidence
Twenty‐four studies, most of high quality, were included in this review (see Characteristics of included studies table). There were similar results across the individual studies despite the different follow‐up periods.
Potential biases in the review process
No potential biases were identified in the review process.
Agreements and disagreements with other studies or reviews
The 24 studies included in this review and comparing different antibiotic regimens found similar effects in terms of efficacy for eradicating meningococcal infection and supported each others' findings. The use of the same antibiotics that were found effective in our review is supported by other existing evidence (Kimmel 2005; Manchanda 2006).
Authors' conclusions
Implications for practice.
Under the assumption that eradication of N meningitidis does reduce the risk of meningococcal infection, the most effective antibiotics to achieve eradication are ceftriaxone, rifampin and ciprofloxacin. Rifampin is usually the drug of choice in clinical practice (Hart 1993). However, given the facts that rifampin is contraindicated in pregnancy, liver disease and alcoholism, and long‐term therapy causes orange discolouration of urine, staining of contact lenses and induction of hepatic microsomal enzymes which might render contraceptive pills ineffective, the use of ciprofloxacin and ceftriaxone is recommended (Rosenstein 2001). In addition, despite rifampin's eradication efficacy a note of caution is required. Induction of resistance, seen as circulation of isolates with reduced sensitivity to rifampin, could complicate further attempts at prophylaxis, especially in an outbreak setting.
Although ceftriaxone is administered in a single intramuscular dose, resulting in more frequent adverse effects when compared to rifampin, intramuscular administration ensures adherence to prophylaxis (as opposed to oral administration) and the adverse effects recorded are mild. In addition, it can be given to young children and pregnant women. Ciprofloxacin is given in a single dose thus ensuring compliance and has minimal side effects but is contraindicated in pregnancy and is not recommended for use in children. Finally, trials evaluating ciprofloxacin and ceftriaxone reported no development of resistance following treatment (Dworzack 1988; Pugsley 1987; Renkonen 1987; Simmons 2000).
Implications for research.
Although direct data on the effectiveness of eradication of the carrier state on disease prevention are missing, placebo‐controlled trials do not seem ethical.
Trials comparing the effectiveness of ceftriaxone, ciprofloxacin and rifampin for eradicating N meningitidis could provide important insights as may trials evaluating oral third‐generation cephalosporins (cefixime, cefdinir, cefditoren‐pivoxil, cefpodoxime‐proxetil and ceftibuten). Trials should evaluate short (after one to two weeks) and long‐term (more than two weeks) eradication rates, separating cases of persistent infection from cases of re‐infection. Minimal inhibitory concentrations of isolates before and after treatment should be reported to allow for the assessment of the ecological impact of prophylactic treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 19 August 2019 | Amended | Lead author's contact details have been updated. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 13 June 2013 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 13 June 2013 | New search has been performed | Searches updated. No new studies were identified for inclusion or exclusion. |
| 5 August 2010 | Amended | Contact details updated. |
| 12 November 2008 | New search has been performed | Searches conducted. No new trials identified. |
| 27 July 2008 | Amended | Converted to new review format. |
| 5 June 2006 | New search has been performed | Searches conducted. No new trials identified. |
| 9 July 2004 | New search has been performed | Searches conducted. |
Acknowledgements
This research was supported through a project grant from the Israel National Institute for Health Policy and Health Services Research. Thanks to Liz Dooley for ongoing support and Sarah Thorning for the new searches for this update.
Appendices
Appendix 1. Details of previous search
For the 2011 review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 2, part of The Cochrane Library, www.thecochranelibrary.com (accessed 31 May 2011), which contains the Cochrane Acute Respiratory Infections Group Specialised Register, MEDLINE (from November 2008 to May Week 3, 2011), EMBASE (from November 2008 to May 2011) and LILACS (from November 2008 to May 2011). Details of the previous search are in Appendix 1.
In the 2008 review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, issue 4), which contains the Acute Respiratory Infections Group Specialised Register, MEDLINE (1950 to November week 3 2008), EMBASE (1980 to November 2008) and LILACS (1982 to November 2008). We also searched the references of all identified studies.
We searched CENTRAL and MEDLINE using the following strategy combined with the highly sensitive search strategy used by The Cochrane Collaboration (Lefebvre 2011).
MEDLINE (Ovid)
1 exp MENINGITIS, MENINGOCOCCAL/ 2 exp Neisseria meningitidis/ 3 (neisseria adj mening$).mp. 4 meningococ$.mp. 5 or/1‐4 6 exp CHEMOPREVENTION/ 7 exp Antibiotic Prophylaxis/ 8 chemoprophylaxis.mp. 9 (prophyla$ or carri$ or phary$ or colon$ or eradic$ or prevent$ or nasopharyn$ or tonsillopharyng$ or elimin$).mp. 10 or/6‐9 11 exp Anti‐Bacterial Agents/ 12 (antibiotic$ or oxytetracyc$ or tetracyc$ or penicilli$ or erythromyci$ or ampicilli$ or sulfa$ or ciprofloxacin$ or norfloxaci$ or ofloxaci$ or quinol$ or fluoroquinol$ or fluoro‐ quinolon$ or ceftriaxon$ or rifampi$ or azithromyci$ or coumermyci$ or minocyclin$ or macrolid$ or cephalospori$).mp 13 or/11‐12 14 5 and 10 and 13
We searched EMBASE and LILACS using adapted terms from the above search strategy. There were no language or publication restrictions.
Appendix 2. Embase.com search strategy
#24 #20 AND #239914 Dec 2010 #23 #21 OR #2282590714 Dec 2010 #22 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR allocat*:ab,ti OR assign*:ab,ti OR ((singl* OR doubl*) NEAR/2 (mask* OR blind*)):ab,ti AND [embase]/lim78719414 Dec 2010 #21 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim23351014 Dec 2010 #20 #7 AND #19187714 Dec 2010 #19 #17 OR #1816006514 Dec 2010 #18 'antibiotic prophylaxis'/de AND [embase]/lim1370014 Dec 2010 #17 #12 AND #1615403614 Dec 2010 #16 #13 OR #14 OR #1588484414 Dec 2010 #15 oxytetracyc*:ab,ti OR tetracyc*:ab,ti OR penicilli*:ab,ti OR erythromyci*:ab,ti OR ampicilli*:ab,ti OR sulfa*:ab,ti OR ciprofloxacin*:ab,ti OR norfloxaci*:ab,ti OR ofloxaci*:ab,ti OR quinol*:ab,ti OR fluoroquinol*:ab,ti OR ceftriaxon*:ab,ti OR rifampi*:ab,ti OR azithromyci*:ab,ti OR coumermyci*:ab,ti OR minocyclin*:ab,ti OR macrolid*:ab,ti OR cephalospori*:ab,ti AND [embase]/lim25683414 Dec 2010 #14 antibiotic*:ab,ti OR antibacterial*:ab,ti AND [embase]/lim19486614 Dec 2010 #13 'antibiotic agent'/exp AND [embase]/lim68511614 Dec 2010 #12 #8 OR #9 OR #10 OR #11157193614 Dec 2010 #11 prophyla*:ab,ti OR carri*:ab,ti OR phary*:ab,ti OR colon*:ab,ti OR eradic*:ab,ti OR prevent*:ab,ti OR nasopharyng*:ab,ti OR tonsillopharyng*:ab,ti OR elimin*:ab,ti AND [embase]/lim154702314 Dec 2010 #10 'prophylaxis'/de AND [embase]/lim4950814 Dec 2010 #9 chemoprevent*:ab,ti OR chemoprophyla*:ab,ti AND [embase]/lim1539014 Dec 2010 #8 'chemoprophylaxis'/de AND [embase]/lim1256514 Dec 2010 #7 #1 OR #2 OR #3 OR #4 OR #5 OR #62008214 Dec 2010 #6 meningococ*:ab,ti AND [embase]/lim742814 Dec 2010 #5 ((neisseria OR epidemic OR meningococ*) NEAR/2 mening*):ab,ti AND [embase]/lim999614 Dec 2010 #4 'n. meningitidis':ab,ti AND [embase]/lim171514 Dec 2010 #3 'neisseria meningitidis'/de AND [embase]/lim866614 Dec 2010 #2 'bacterial meningitis'/de AND [embase]/lim925614 Dec 2010 #1 'epidemic meningitis'/de AND [embase]/lim127514 Dec 2010
Appendix 3. LILACS search strategy
mh:"Meningitis, Meningococcal" OR mh:"Meningitis Meningocócica" OR "Meningitis Meningocóccica" OR "Meningite por Meningococos" OR mh:"Meningitis, Bacterial" OR mh:"Meningitis Bacterianas" OR mh:"Meningites Bacterianas" OR "bacterial meningitis" OR "Meningitis Bacteriana" OR "Meningitis por Bacterias" OR "Meningite Bacteriana" OR "Meningite por Bactéria" OR "Meningite por Bactérias" OR "Meningites por Bactérias" OR mh:"Neisseria meningitidis" OR mh:b03.440.400.425.550.550.641* OR mh:b03.660.075.525.520.500* OR meningococcus OR "N. meningitidis" OR meningococ* AND db:("LILACS") AND type_of_study:("clinical_trials")
Data and analyses
Comparison 1. Adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rifampin versus ceftriaxone | 1 | 856 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.10, 1.75] |
| 2 Rifampin versus ciprofloxacin | 2 | 1598 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.56] |
Comparison 2. Failure to eradicate (follow‐up: up to one week).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ciprofloxacin versus placebo | 3 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.12] |
| 2 Rifampin versus placebo | 6 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.13, 0.24] |
| 3 Minocycline versus placebo | 3 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.21, 0.37] |
| 4 Penicillin versus placebo | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.24, 0.94] |
| 5 Other antibiotics versus placebo | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Rifampin versus ciprofloxacin | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.11, 1.02] |
| 7 Rifampin versus ceftriaxone | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.73, 18.86] |
2.5. Analysis.
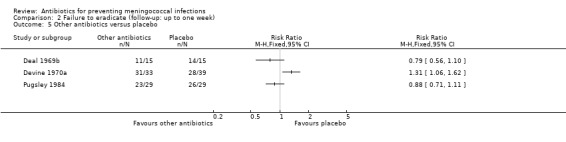
Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 5 Other antibiotics versus placebo.
Comparison 3. Failure to eradicate (follow‐up: one to two weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ciprofloxacin versus placebo | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.42] |
| 2 Rifampin versus placebo | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.14, 0.29] |
| 3 Minocycline versus placebo | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.31] |
| 4 Penicillin versus placebo | 2 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.51, 0.79] |
| 5 Other antibiotics versus placebo | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Rifampin versus ciprofloxacin | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.09, 1.11] |
| 7 Rifampin versus ceftriaxone | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.93 [1.22, 28.68] |
| 8 Rifampin versus minocycline | 2 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.57, 1.77] |
3.5. Analysis.
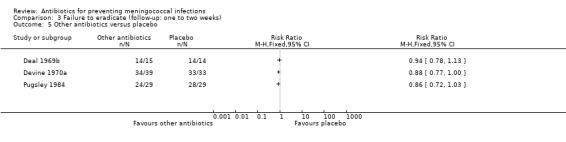
Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 5 Other antibiotics versus placebo.
Comparison 4. Failure to eradicate (follow‐up: between two to three weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rifampin versus placebo | 3 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.16, 0.37] |
Comparison 5. Failure to eradicate (follow‐up between three to four weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rifampin versus placebo | 2 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.16, 0.38] |
| 2 Penicillin versus placebo | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.53, 1.68] |
Comparison 6. Failure to eradicate (follow‐up: five weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rifampin versus minocycline | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.48, 1.97] |
Comparison 7. Exclusion after randomisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Drop‐outs | 13 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| 1.1 Drop‐outs at around one week of follow‐up | 13 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blakebrough 1980.
| Methods | Cluster‐randomisation by households, quasi‐randomisation by order of admission to hospital, open, no loss to f/u | |
| Participants | Household contacts, Nigeria | |
| Interventions | Rifampin: 0 to 2 years: 4 x 75 mg; 2 to 4 years 4 x 150 mg; 5 to 14 years 4 x 300 mg; 15+ years 4 x 600 mg versus sulphadimidine: 0 to 4 years 4 x 250 mg; 5 to 14 years 4 x 500 mg; 15+ years 4 x 1 G | |
| Outcomes | Morbidity, eradication, resistance developed Follow‐up: 6 to 7 weeks | |
| Notes | Data presented for carriers. Main serogroup: A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to f/u |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Borgono 1981.
| Methods | Double‐blind, no ITT | |
| Participants | Kindergarten and school children, Chile | |
| Interventions | Rifampin: 2 x 10 mg/kg versus placebo | |
| Outcomes | Eradication, adverse effects Follow‐up: 10 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Cuevas 1995.
| Methods | Cluster‐randomisation by households, open, no loss to f/u | |
| Participants | Household contacts, Malawi | |
| Interventions | Rifampicin: 2 to 18 years 4 x 20 mg/kg; > 18 years 4 x 600 mg versus single‐dose ciprofloxacin: 2 to 18 years 1 x 15 mg/kg for 2 to 18 years; > 18 years 1 x 750 mg versus IM ceftriaxone: pregnant women 2 g; < 2 years 50 mg/kg | |
| Outcomes | Morbidity, eradication, re‐acquisition, adverse effects Follow‐up: 2 weeks | |
| Notes | Data presented for carriers. Main serogroups: A W135. Ceftriaxone group not randomised and not included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to f/u |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Deal 1969a.
| Methods | Adequate allocation generation, double‐blind, ITT | |
| Participants | Students, USA | |
| Interventions | Rifampin: 4 x 600 mg versus placebo | |
| Outcomes | Eradication, failure serogroup, adverse effects Follow‐up: 30 days | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Deal 1969b.
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Students, USA | |
| Interventions | Cephalexin: 12 x 500 mg versus placebo | |
| Outcomes | Eradication, adverse effects, re‐acquisition Follow‐up: 2 weeks | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Deviatkina 1978.
| Methods | Open, no ITT | |
| Participants | USSR | |
| Interventions | Rifampin: 4 x 300 mg versus none | |
| Outcomes | Eradication, adverse effects Follow‐up: 10 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Devine 1970a.
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Coumermycin A1: 14 x 50 mg versus placebo | |
| Outcomes | Eradication, acquisition, failure serogroup Follow‐up: 10 days | |
| Notes | Data extracted for 1) all (regardless of carrier status), 2) carriers only, 3) non‐carriers only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Devine 1970b.
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Rifampin: 4 x 600 mg versus placebo | |
| Outcomes | Eradication, failure serogroup of eradication failure Follow‐up: 11 days | |
| Notes | Data extracted for 1) all (regardless of carrier status), 2) for carriers only, 3) for non‐carriers only. Main serogroup: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Devine 1971a.
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Minocycline 14 x 500 mg versus placebo | |
| Outcomes | Eradication | |
| Notes | Main serogroup: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Devine 1971b.
| Methods | Adequate allocation generation, open, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Minocycline: 14 x 500 mg versus none | |
| Outcomes | Eradication, adverse effects, resistance developed Follow‐up: 5 days | |
| Notes | Data extracted for 1) all (regardless of carrier status) and 2) carriers only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Dowd 1966.
| Methods | Double‐blind, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Ampicillin: 30 x 500 mg versus oral penicillin G: 30 x 462 mg versus placebo | |
| Outcomes | Eradication, serogroup of eradication failure, resistance developed Follow‐up: 26 days | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Dworzack 1988.
| Methods | Double‐blind, ITT | |
| Participants | Volunteers | |
| Interventions | Ciprofloxacin 1 x 750 mg versus placebo | |
| Outcomes | Eradication, resistance developed, adverse effects Follow‐up: 21 days | |
| Notes | Main serogroups: B, Z | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Edwards 1984.
| Methods | Adequate allocation generation, open, no ITT | |
| Participants | Patients with extragenital gonorrhoea, USA | |
| Interventions | Bacampicillin: 12 x 400 mg versus amoxycillin: 6 x 500 mg | |
| Outcomes | Eradication, adverse effects Follow‐up: 5 to 9 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Girgis 1998.
| Methods | Adequate allocation generation, open, no ITT | |
| Participants | Nursing students, Cairo | |
| Interventions | Azythromycin: 1 x 500 mg versus rifampin: 4 x 600 mg | |
| Outcomes | Eradication, reacquisition, resistance developed, adverse effects Follow‐up: 2 weeks | |
| Notes | Main serogroups: A, B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Guttler 1971.
| Methods | Cluster‐randomisation by companies, adequate generation of allocation, open, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Rifampin: 1 x 600 mg versus minocycline: 10 x 100 mg versus ampicillin: 10 x 500 mg versus placebo | |
| Outcomes | Morbidity, eradication, resistance developed Follow‐up: 26 days | |
| Notes | Separate data provided for rifampin and minocycline treatment arms Main serogroups: B, C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Judson 1984.
| Methods | Outcome assessor blinded, ITT | |
| Participants | Patients with culture or smear positive for anogenital gonorrhoea or confirmed recent exposure to gonorrhoea | |
| Interventions | IM ceftriaxone 1 x 125 mg versus IM spectinomycin 1 x 2 g | |
| Outcomes | Eradication, adverse effects Follow‐up: 7 days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Kaiser 1974.
| Methods | Adequate allocation generation, open, no ITT, no drop‐outs | |
| Participants | Household contacts, USA | |
| Interventions | Rifampin: > 66 lb weight 4 x 600 mg/day; < 66 lb 4 x 300 mg/day versus none | |
| Outcomes | Morbidity, eradication, failure serogroup Follow‐up: 130 days | |
| Notes | Data presented for carriers. Main serogroups: C, N | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Kaya 1997.
| Methods | Open, no ITT | |
| Participants | Volunteers, Turkey | |
| Interventions | Ciprofloxacin: 1 x 750 mg versus rifampin: 4 x 600 mg | |
| Outcomes | Eradication, adverse effects Follow‐up: 2 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Munford 1974.
| Methods | Cluster‐randomisation by households, quasi‐randomisation assigned by order of arrival at study centre, open, no ITT | |
| Participants | Household contacts, Brazil | |
| Interventions | Sulphadiazine: 4 x 1 g versus minocycline: 1 x 200 mg + 5 x 100 mg versus rifampin: 4 x 600 mg versus minocycline/rifampin: as above | |
| Outcomes | Eradication, serogroup of eradication failure, resistance developed, adverse effects Follow‐up: 2 weeks | |
| Notes | Data presented for carriers. Main serogroup: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Pugsley 1984.
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | College students | |
| Interventions | Sch 29,482: 4 x 250 mg versus placebo | |
| Outcomes | Eradication | |
| Notes | Main serogroups: B, Z | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Pugsley 1987.
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Volunteers | |
| Interventions | Ciprofloxacin: 10 x 500 mg versus placebo | |
| Outcomes | Eradication, resistance developed, failure serogroup adverse effects Follow‐up: 2 weeks | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Renkonen 1987.
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Army recruits | |
| Interventions | Ciprofloxacin: 4 x 250 mg versus placebo | |
| Outcomes | Eradication, serogroup of eradication failure, resistance developed, adverse effects Follow‐up: 6 days | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Schwartz 1988.
| Methods | Cluster‐randomisation by households, open, no ITT | |
| Participants | Household contacts, Saudi Arabia | |
| Interventions | IM ceftriaxone 1 x 250 mg or 125 mg for children versus rifampin: 4 x 600 mg or 10 mg/kg versus none | |
| Outcomes | Eradication, acquisition in non‐carriers Follow‐up: 2 weeks | |
| Notes | Data presented for carriers. Serogroup A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
Simmons 2000.
| Methods | Open, no ITT | |
| Participants | Household contacts, New Zealand | |
| Interventions | Rifampicin: 600 mg, children > 1 month, 10 mg/kg versus IM ceftriaxone: 250 mg < 12 years, 125 mg | |
| Outcomes | Morbidity, eradication, eradication of serogroup B, adverse effects Follow‐up: 6 days | |
| Notes | Data presented for carriers. Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
f/u: follow‐up IM: intramuscular ITT: intention‐to‐treat
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Artenstein 1967 | No control group |
| Band 1984 | Trial of post‐exposure prophylaxis for H. influenzae and not N. meningitidis |
| Beam 1971 | No randomisation |
| Beaty 1983 | Includes previously published data (Guttler 1971) included in this review. New data from a non‐randomised study also excluded |
| Cardenas 1995 | No control group |
| Chapalain 1992 | No randomisation |
| Cheever 1943 | No randomisation |
| Devine 1972 | No randomisation |
| Devine 1973 | No randomisation |
| Fairbrother 1940 | Not a controlled trial |
| Gaunt 1988 | Not a RCT. The type of publication was a letter to the editor and did not contain any relevant data |
| Gilja 1993 | Not a controlled trial |
| Gonzalez 2000 | No randomisation |
| Gray 1941 | No randomisation |
| Halstensen 1995 | No control group |
| Kuhns 1943 | No randomisation |
| Potter 1990 | Patients with homozygous deficiency of the sixth component of complement (C6) with recurrent meningococcal meningitis |
| Sanders 1967 | No control group |
| Sheehab 1991 | No control group |
| Sivonen 1978 | No randomisation |
| Wall 1982 | Not a RCT. The type of publication was a letter to the editor and did not contain any relevant data |
| Weidmer 1971 | No randomisation |
RCT: randomised controlled trial
Contributions of authors
Abigail Fraser (AF): responsible for the reference searches, article retrieval, study inclusion and exclusion, data extraction, analysis, interpretation of results and writing of the review. Mical Paul (MP): responsible for data extraction, study inclusion and exclusion, analysis, interpretation of results and writing of the review. Anat Gafter‐Gvili (AGG): responsible for data extraction, study inclusion and exclusion, interpretation of results and writing of the review. Leonard Leibovici: responsible for study inclusion and exclusion, analysis, interpretation of results and writing of the review. AF updated the electronic searches in June 2006. Anca Zalmanovici Trestioreanu (AZT) updated the review in April 2011.
Sources of support
Internal sources
No sources of support supplied
External sources
Israel National Institute for Health Policy and Health Services Research, Israel.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Blakebrough 1980 {published data only}
- Blakebrough IS, Gilles HM. The effect of rifampicin on meningococcal carriage in family contacts in northern Nigeria. Journal of Infection 1980;2(2):137‐43. [DOI] [PubMed] [Google Scholar]
Borgono 1981 {published data only}
- Borgono JM, Rodriguez H, Garcia J, Canepa I. Efficacy of rifampicin in the treatment of Meningococcus carriers [Eficacia de la rifampicina en el tratamiento de los portadores de meningococo]. Revista Chilena de Pediatria 1981;52(2):146‐8. [PubMed] [Google Scholar]
Cuevas 1995 {published data only}
- Cuevas LE, Kazembe P, Mughogho GK, Tillotson GS, Hart CA. Eradication of nasopharyngeal carriage of Neisseria meningitidis in children and adults in rural Africa: a comparison of ciprofloxacin and rifampicin. Journal of Infectious Disease 1995;171(3):728‐31. [DOI] [PubMed] [Google Scholar]
- Hart CA, Cuevas LE, Kazembe P, Mughogho GK, Tillotson GS. The use of ciprofloxacin to eradicate oropharyngeal carriage of Neisseria meningitidis: a preliminary report. Advances in Antimicrobial and Antineoplastic Chemotherapy 1992;11:167‐71. [Google Scholar]
Deal 1969a {published data only}
- Deal WB, Sanders E. Efficacy of rifampin in treatment of meningococcal carriers. New England Journal of Medicine 1969;281(12):641‐5. [DOI] [PubMed] [Google Scholar]
Deal 1969b {published data only}
- Deal WB, Sanders E. Therapeutic trial of cephalexin in meningococcal carriers. Antimicrobial Agents and Chemotherapy 1969;9:441‐4. [PubMed] [Google Scholar]
Deviatkina 1978 {published data only}
- Deviatkina NP, Demina AA, Orlova EV, Timina VP, Petrova IS. Evaluation of the sanative action of rifampicin on the meningococcal carrier state [Otsenka saniruiushchego deistviia rifampitsina na nositel'stvo meningokokkov]. Antibiotiki 1978;23(9):794‐7. [PubMed] [Google Scholar]
Devine 1970a {published data only}
- Devine LF, Johnson DP, Hagerman CR, Pierce WE, Rhode SL 3rd, Peckinpaugh RO. The effect of coumermycin A on the meningococcal carrier state. American Journal of the Medical Sciences 1970;260(3):165‐70. [DOI] [PubMed] [Google Scholar]
Devine 1970b {published data only}
- Devine LF, Johnson DP, Hagerman CR, Pierce WE, Rhode SL, Peckinpaugh RO. Rifampin levels in serum and saliva and effect on the meningococcal carrier state. JAMA 1970;214(6):1055‐9. [DOI] [PubMed] [Google Scholar]
Devine 1971a {published data only}
- Devine LF, Johnson DP, Hagerman CR, Pierce WE, Rhode SL 3rd, Peckinpaugh RO. The effect of minocycline on meningococcal nasopharyngeal carrier state in naval personnel. American Journal of Epidemiology 1971;93(5):337‐45. [DOI] [PubMed] [Google Scholar]
Devine 1971b {published data only}
- Devine LF, Johnson DP, Hagerman CR, Pierce WE, Rhode SL 3rd, Peckinpaugh RO. The effect of minocycline on meningococcal nasopharyngeal carrier state in naval personnel. American Journal of Epidemiology 1971;93(5):337‐45. [DOI] [PubMed] [Google Scholar]
Dowd 1966 {published data only}
- Dowd JM, Blink D, Miller CH, Frank PF, Pierce WE. Antibiotic prophylaxis of carriers of sulfadiazine resistant meningococci. Journal of Infectious Diseases 1966;116(4):473‐80. [DOI] [PubMed] [Google Scholar]
Dworzack 1988 {published data only}
- Dworzack DL, Sanders CC, Horowitz EA, Allais JM, Sookpranee M, Sanders WE Jr, et al. Evaluation of single dose ciprofloxacin in the eradication of Neisseria meningitidis from nasopharyngeal carriers. Antimicrobial Agents and Chemotherapy 1988;32(11):1740‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 1984 {published data only}
- Edwards LD, Gartner T. Comparison between bacampicillin and amoxycillin in treating genital and extragenital infection with Neisseria gonorrhoeae and pharyngeal infection with Neisseria meningitidis. British Journal of Venereal Diseases 1984;60(6):380‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Girgis 1998 {published data only}
- Girgis N, Sultan Y, Frenck RW Jr, Gendy A, Farid Z, Mateczun A. Azithromycin compared with rifampin for eradication of nasopharyngeal colonization by Neisseria meningitidis. Pediatric Infectious Disease Journal 1998;17(9):816‐9. [DOI] [PubMed] [Google Scholar]
Guttler 1971 {published data only}
- Guttler RB, Counts GW, Avent CK, Beaty HN. Effect of rifampin and minocycline on meningococcal carrier rates. Journal of Infectious Diseases 1971;124(2):199‐205. [DOI] [PubMed] [Google Scholar]
Judson 1984 {published data only}
- Judson FN, Ehret JM. Single‐dose ceftriaxone to eradicate pharyngeal Neisseria meningitidis. Lancet 1984;2(8417‐8):1462‐3. [DOI] [PubMed] [Google Scholar]
Kaiser 1974 {published data only}
- Kaiser AB, Hennekens CH, Saslaw MS, Hayes PS, Bennett JV. Seroepidemiology and chemoprophylaxis of disease due to sulfonamide‐resistant Neisseria meningitidis in a civilian population. Journal of Infectious Diseases 1974;130(3):217‐24. [DOI] [PubMed] [Google Scholar]
Kaya 1997 {published data only}
- Kaya A, Tasyaran MA, Celebi S, Yilmaz S. Efficacy of a single dose of ciprofloxacin vs. rifampicin in eradicating the nasopharyngeal carriage of Neisseria meningitidis. Turkish Journal of Medical Sciences 1997;27(2):153‐5. [Google Scholar]
Munford 1974 {published data only}
- Munford RS, Vasconcelos ZJS, Philips CJ, Gelli DS, Gorman GW, Risi JB, et al. Eradication of carriage of Neisseria meningitidis in families: a study in Brazil. Journal of Infectious Diseases 1974;129(6):644‐9. [DOI] [PubMed] [Google Scholar]
Pugsley 1984 {published data only}
- Pugsley MP, Dworzack DL, Sanders CC, Sanders WE Jr. Evaluation of Sch 29,482 in the eradication of Neisseria meningitidis from nasopharyngeal carriers. Antimicrobial Agents and Chemotherapy 1984;25(4):494‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pugsley 1987 {published data only}
- Pugsley MP, Dworzack DL, Horowitz EA, Cuevas TA, Sanders WE Jr, Sanders CC. Efficacy of ciprofloxacin in the treatment of nasopharyngeal carriers of Neisseria meningitidis. Journal of Infectious Diseases 1987;156(1):211‐3. [DOI] [PubMed] [Google Scholar]
Renkonen 1987 {published data only}
- Renkonen OV, Sivonen A, Visakorpi R. Effect of ciprofloxacin on carrier rate of Neisseria meningitidis in army recruits in Finland. Antimicrobial Agents and Chemotherapy 1987;31(6):962‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 1988 {published data only}
- Schwartz B. Chemoprophylaxis for bacterial infections: principles of and application to meningococcal infections. Reviews of Infectious Diseases 1991;13(Suppl 2):170‐3. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Al Tobaiqi A, Al Ruwais A, Fontaine RE, A'ashi J, Hightower AW, et al. Comparative efficacy of ceftriaxone and rifampicin in eradicating pharyngeal carriage of group A Neisseria meningitidis. Lancet 1988;1(8597):1239‐42. [DOI] [PubMed] [Google Scholar]
Simmons 2000 {published data only}
- Simmons G, Jones N, Calder L. Comparison of ceftriaxone and rifampicin in eliminating nasopharyngeal carriage of serogroup B Neisseria meningitidis (abstract). Australian and New Zealand Journal of Medicine 1999;29:587. [DOI] [PubMed] [Google Scholar]
- Simmons G, Jones N, Calder L. Equivalence of ceftriaxone and rifampicin in eliminating nasopharyngeal carriage of serogroup B Neisseria meningitidis. Journal of Antimicrobial Chemotherapy 2000;45(6):909‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Artenstein 1967 {published data only}
- Artenstein MS, Lamson TH, Evans JR. Attempted prophylaxis against meningococcal infection using intramuscular penicillin. Military Medicine 1967;132(12):1009‐11. [PubMed] [Google Scholar]
Band 1984 {published data only}
- Band JD, Fraser DW. Adverse effects of two rifampicin dosage regimens for the prevention of meningococcal infection. Lancet 1984;1(8368):101‐2. [DOI] [PubMed] [Google Scholar]
Beam 1971 {published data only}
- Beam WE Jr, Newberg NR, Devine LF, Pierce WE, Davies JA. The effect of rifampin on the nasopharyngeal carriage of Neisseria meningitidis in a military population. Journal of Infectious Diseases 1971;124(1):39‐46. [DOI] [PubMed] [Google Scholar]
Beaty 1983 {published data only}
- Beaty HN. Rifampin and minocycline in meningococcal disease. Reviews of Infectious Diseases 1983;5(Suppl 3):451‐8. [DOI] [PubMed] [Google Scholar]
Cardenas 1995 {published data only}
- Cardenas AT, Contreras AG, Rojas CQ. Cefixime in meningococcal disease chemoprophylaxis [Cefixima para quimioprofilaxis antimeningococica]. Revista Chilena de Pediatria 1995;66(4):217‐9. [Google Scholar]
Chapalain 1992 {published data only}
- Chapalain JC, Guibourdenche M, Perrier Gros Claude JD, Bartoli M, Riou JY. The chemoprophylaxis of cerebrospinal meningitis using rifampin in a military population. Pathologie‐biologie 1992;40(3):230‐3. [PubMed] [Google Scholar]
Cheever 1943 {published data only}
- Cheever FS, Breeses BB, Upham HC. The treatment of meningococcus carriers with sulfadiazine. Annals of Internal Medicine 1943;19:602‐8. [Google Scholar]
Devine 1972 {published data only}
- Devine LF, Springer GL, Frazier WE, Rhode SL 3rd, Pierce WE, Johnson DP, et al. Selective minocycline and rifampin treatment of group C meningococcal carriers in a new naval recruit camp. American Journal of the Medical Sciences 1972;263(2):79‐93. [DOI] [PubMed] [Google Scholar]
Devine 1973 {published data only}
- Devine LF, Pollard RB, Krumpe PE, Hoy ES, Mammen RE, Miller CH, et al. Field trial of the efficacy of a previously proposed regimen using minocycline and rifampin sequentially for the elimination of meningococci from healthy carriers. American Journal of Epidemiology 1973;97(6):394‐401. [DOI] [PubMed] [Google Scholar]
Fairbrother 1940 {published data only}
- Fairbrother RW. Cerebrospinal meningitis: the use of sulphonamide derivatives in prophylaxis. British Medical Journal 1940;2:859‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaunt 1988 {published data only}
- Gaunt PN. Ciprofloxacin vs ceftriaxone for eradication of meningococcal carriage. Lancet 1988;2(8604):218‐9. [DOI] [PubMed] [Google Scholar]
Gilja 1993 {published data only}
- Gilja OH, Halstensen A, Digranes A, Mylvaganam H, Aksnes A, Hoiby EA. Use of single‐dose ofloxacin to eradicate tonsillopharyngeal carriage of Neisseria meningitidis. Antimicrobial Agents and Chemotherapy 1993;37(9):2024‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez 2000 {published data only}
- Gonzalez de Aledo Linos A, Garcia Merino J. Control of a school outbreak of serogroup B meningococcal disease by chemoprophylaxis with azithromycin and ciprofloxacin [Control de un brote escolar de enfermedad meningococica serogrupo B mediante quimioprofilaxis con azitromicina y ciprofloxacino]. Anales Espanoles de Pediatria 2000;53(5):412‐7. [PubMed] [Google Scholar]
Gray 1941 {published data only}
- Gray FC, Gear J. Sulphapyridine M & B 693 as a prophylactic against cerebrospinal meningitis. South African Medical Journal 1941;15:139‐40. [Google Scholar]
Halstensen 1995 {published data only}
- Halstensen A, Gilja OH, Digranes A, Mylvaganam H, Aksnes A, Hoiby EA, et al. Single dose ofloxacin in the eradication of pharyngeal carriage of Neisseria meningitidis. Drugs 1995;49(Suppl 2):399‐400. [DOI] [PubMed] [Google Scholar]
Kuhns 1943 {published data only}
- Kuhns DM, Nelson CT, Feldman HA, Kuhn LR. The prophylactic value of sulfadiazine. Journal of the American Medical Association 1943;123:335‐9. [Google Scholar]
Potter 1990 {published data only}
- Potter PC, Frasch CE, Sande WJ, Cooper RC, Patel Y, Orren A. Prophylaxis against Neisseria meningitidis infections and antibody responses in patients with deficiency of the sixth component of complement. Journal of Infectious Diseases 1990;161(5):932‐7. [DOI] [PubMed] [Google Scholar]
Sanders 1967 {published data only}
- Sanders E. Use of sulfonamide carbonic anhydrase inhibitors in treatment of meningococcal carriers: rationale and report of a clinical trial of ethoxzolamide. American Journal of the Medical Sciences 1967;254(5):709‐16. [DOI] [PubMed] [Google Scholar]
Sheehab 1991 {published data only}
- Shehab S, Leitner L, Bogokowsky B, Epstein I, Swartz TA, Shehab S, et al. Alternating rifampicin and ceftriaxone for Neisseria meningitidis eradication in contacts. Harefuah 1991;120(11):641‐3. [PubMed] [Google Scholar]
Sivonen 1978 {published data only}
- Sivonen A, Renkonen OV, Weckstrom P, Koskenvuo K, Raunio V, Makela PH. The effect of chemoprophylactic use of rifampin and minocycline on rates of carriage of Neisseria meningitidis in army recruits in Finland. Journal of Infectious Diseases 1978;137(3):238‐44. [DOI] [PubMed] [Google Scholar]
Wall 1982 {published data only}
- Wall AR, Grunberg RN. Rifampicin and erythromycin for the meningococcal carrier. Lancet 1982;2(8311):1346. [DOI] [PubMed] [Google Scholar]
Weidmer 1971 {published data only}
- Weidmer CE, Dunkel TB, Pettyjohn FS, Smith CD, Leibovitz A. Effectiveness of rifampin in eradicating the meningococcal carrier state in a relatively closed population: emergence of resistant strains. Journal of Infectious Diseases 1971;124(2):172‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Carter 1994
- Carter PE, Abadi FJ, Yakubu DE, Pennington TH. Molecular characterization of rifampin‐resistant Neisseria meningitidis. Antimicrobial Agents and Chemotherapy 1994;38(6):1256‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conterno 2006
- Conterno LO, Silva Filho CR, Ruggenberg JU, Heath PT. Conjugate vaccines for preventive meningococcal C meningitis and septicemia. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001834.pub2] [DOI] [PubMed] [Google Scholar]
Ferguson 2002
- Ferguson LE, Hormann MD. Neisseria meningitidis: presentation, treatment and prevention. Journal of Pediatric Health Care 2002;16:119‐24. [PubMed] [Google Scholar]
Hart 1993
- Hart CA, Rogers TRF. Meningococcal disease. Journal of Medical Microbiology 1993;39:3‐25. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kimmel 2005
- Kimmel SR. Prevention of meningococcal disease. American Family Physician 2005;72(10):2049‐56. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester, UK: John Wiley & Sons, 2011. [Google Scholar]
Manchanda 2006
- Manchanda V, Gupta S, Bhalla P. Meningococcal disease: history, epidemiology, pathogenesis, clinical manifestations, diagnosis, antimicrobial susceptibility and prevention. Indian Journal of Medical Microbiology 2006;24(1):7‐19. [DOI] [PubMed] [Google Scholar]
Mandell 2000
- Mandell GL, Douglas JE, Dolin R. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 5th Edition. Churchill Livingstone, 2000. [Google Scholar]
PHLS 2002
- Public Health Laboratory Service Meningococcus Forum. Guidelines for public health management of meningococcal disease in the UK. Communicable Disease and Public Health 2002;5(3):187‐204. [PubMed] [Google Scholar]
Purcell 2004
- Purcell B, Samuelsson S, Hahne SJM, Ehrars I, Heuberger S, Camaroni I, et al. Effectiveness of antibiotics in preventing meningococcal disease following a case: a systematic review. BMJ 2004;328(7452):1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenstein 2001
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. New England Journal of Medicine 2001;344(18):1378‐88. [DOI] [PubMed] [Google Scholar]
Samuelsson 2002
- Samuelsson S. Meningococcal disease ‐ still a major challenge. Communicable Disease and Public Health 2002;5(3):178‐80. [PubMed] [Google Scholar]
WHO 2003a
- WHO. Meningococcal disease. http://www.who.int/csr/disease/meningococcal/en/ (accessed December 2003).
WHO 2003b
- WHO. Meningococcal meningitis. http://www.who.int/mediacentre/factsheets/2003/fs141/en/ (accessed December 2003).
References to other published versions of this review
Fraser 2005
- Fraser A, Gafter‐Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004785.pub3] [DOI] [PubMed] [Google Scholar]
Fraser 2006
- Fraser A, Gafter‐Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004785.pub3] [DOI] [PubMed] [Google Scholar]
Fraser 2009
- Fraser A, Gafter‐Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004785.pub4] [DOI] [PubMed] [Google Scholar]
Zalmanovici Trestioreanu 2011
- Zalmanovici Trestioreanu A, Fraser A, Gafter‐Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD004785.pub4] [DOI] [PubMed] [Google Scholar]


