Abstract
Introduction
This study aimed to evaluate morphological changes in the L5 nerve roots in control and symptomatic patients using magnetic resonance myelography [MRM]. Moreover, the utility of MRM for the diagnosis of intraforaminal or extraforaminal lesions in patients with L5 radiculopathy was evaluated using healthy subjects as controls.
Methods
Of 270 subjects who underwent MRM of the lumbar spine at our institution between April 2007 and December 2010, 135 patients (78 men and 57 women; average age: 61.3 years) with no history of spinal surgeries and nerve roots without infections, tumors, or malformations were selected for this study.
The end-point measurements included the bifurcation angle of inclination (proximal tilting angle [PTA]) of the L5 nerve root as observed via MRM, lateral angle of inclination (lateral tilting angle [LTA]), bifurcation diameter (proximal nerve root width [PW]), and dorsal root ganglion (DRG) diameter (DRG width [DW]). DW ratio was then calculated for healthy controls and symptomatic subjects. We measured each parameter using the image information unification system ShadeQuest (Yokogawa, Tokyo, Japan). Two spinal surgeons conducted the magnetic resonance imaging evaluation.
Results
Swelling of the L5 DRG was detected in cases with intraforaminal or extraforaminal stenosis. With regard to the cutoff value of 6.5 mm for L5 DW, foraminal stenosis can be confirmed if DW is ≥6.5 mm or more via MRM. In cases where L5 DRG was swollen to ≥1.2 times the size in healthy subjects, L5 radiculopathy with foraminal lesions can be diagnosed.
Conclusions
Our findings indicated that 3D MRM is a noninvasive technique and a useful tool for the diagnosis of intraforaminal or extraforaminal lesions in the lumbar spine. Therefore, it can be combined with other diagnostic methods used for the identification of intraforaminal or extraforaminal L5 nerve root lesion.
Keywords: magnetic resonance myelography, lumbar foraminal stenosis, radiological assessments
Introduction
Diagnosis of intraforaminal and extraforaminal lesions has undergone exceptional progress over the last 10 years due to the development of novel imaging devices. However, the majority of these new devices have yet to be made commonly available for routine diagnosis. One such new technology, three-dimensional magnetic resonance myelography (MRM) is already in wide usage, but only a small number of quantitative studies involving this technology have been performed. Despite the fact that L5 radiculopathy can now be clearly visualized by various types of imaging studies, both false negatives and positives are still common when diagnosing intraforaminal and extraforaminal lesions. It has been reported that the frequency of foraminal stenosis is between 3.7% and 13.4%1-3). Preoperative assessment is the key to reduce the number of overlooked foraminal lesions, which represent a cause of failed back surgery syndrome4). Previously, it was believed that foraminal lesions occurred in what was known as the “hidden zone”1). This was because conventional diagnostic imaging technologies, such as myelography and post-myelography computed tomography (CT), were limited. Recently, there have been reports of a definitive diagnosis being performed with electrophysiological diagnostic tests using superficial peroneal nerve sensory nerve action potential on sites identified as possible culprits on imaging tests and stimulus-induced lumbar nerve distal motor latency. Moreover, there have been recent reports on the use of MRM in the field of diagnostic imaging, which has become relatively easy and effective due to advances in MR imaging technology3,6,7) as well as MR neurography7) and diffusion tensor tractography8) using diffusion-weighted images. The reported diagnostic criteria for foraminal stenosis when using MRM are the lateral course of the nerve root or spinal nerves, unclear morphology of the dorsal root ganglion (DRG), and spinal nerve entrapment3). Although it became possible to identify foraminal stenosis based on these findings, there are asymptomatic cases that satisfy the diagnostic imaging criteria. Therefore, there are asymptomatic cases with intraforaminal defects (false positives). However, it has been known that, in some cases of foraminal stenosis, clinical symptoms can be improved through decompression of the site where intraoperative findings have indicated the compression of the nerve root and DRG enlargement. This type of pathology appears on MRM as a secondary DRG enlargement accompanying radiculopathy. Nerve root enlargement is attributable to infiltration of inflammatory cells, interstitial edema, and onion-bulb formation due to repeated demyelination and remyelination and frequently produces conduction block9-11). If such DRG enlargement can be quantitatively assessed and a high correlation with the clinical symptoms can be established, this can lead to the reduction of failed back surgery syndrome. This study was a single-center retrospective study on the usefulness of imaging findings of the L5 nerve root in the foramen that compared the L5 nerve root morphology between asymptomatic cases (control) and symptomatic cases to quantitatively determine whether MRM-measured values suggest the presence of pathology.
Materials and Methods
Subjects consisted of 135 patients (270 nerve roots) selected from among those that underwent lumbar MRM as a follow-up examination for leg pain between April 2007 and December 2010. Cases with history of infection, tumor, nerve root malformations, or lumbar surgery were excluded. In total, there were 78 males and 57 females with the mean age of 61.3 years. All subjects were Japanese. The magnetic resonance imaging (MRI) device used was Phillips Acheva 1.5T. The balanced TSE method was used under the following conditions: TR/TE of 4.4/2.2 ms, flip angle of 45°, averaging three 2-mm thick slices (1 mm overlapping), field-of-view of 230 mm, and a matrix of 224×168. Nerve compression findings via MRM were defined as the “compression of the dural tube or nerve root.” Diagnosis of L5 radiculopathy, which was used as a supplementary diagnostic procedure to identify the culprit nerve root, was made based on the reproducibility of pain or the effectiveness of selective radiculography or block (transforaminal epidural injection). We categorized MRM findings as either an intraspinal lesion (IL group) or foraminal stenosis (FS group). The control (CO) group consisted of healthy individuals imaged using MRM due to lumbar pain without leg pain or lumbar discopathy. The unaffected sides in cases of unilateral foraminal stenosis were also included in the CO group. One goal of this series was to determine how to avoid overlooking foraminal stenosis on MRM examinations, and as a result, we created an FS group consisting of patients with stenosis in both the intraspinal and foraminal sites (i.e., “double-crush lesion”). In each case, we followed Aota et al.6) and diagnosed foraminal stenosis when there was a compression of the periradicular fat tissue on the parasagittal lumbar MRI images. Outcome measures were assessed by measuring all parameters using the ShadeQuest image data integration system manufactured by the Yokogawa Electric Corporation. MRM parameters used to measure L5 were as follows: proximal tilting angle (PTA), lateral tilting angle (LTA), proximal nerve root width (PW), and DRG width (DW). We also calculated the ratio of DW in the symptomatic side to that in the healthy side (DW ratio; Fig. 1). All nerve roots subjected to MRM were included in the three groups: FS, IL, or CO group. Assessments of each MRM parameters were performed by two spinal surgeons (AK and SM), and mean values were used as measured values. Interobserver reliability in each parameter was calculated using the interclass correlation coefficient (ICC). All statistical analyses of recorded data were performed using the Excel statistical software package (Ekuseru-Toukei 2012; Social Survey Research Information Co., Ltd., Tokyo, Japan). Statistical analyses were performed using Scheffe's F test, and a difference was considered significant when p was <0.05.
Figure 1.
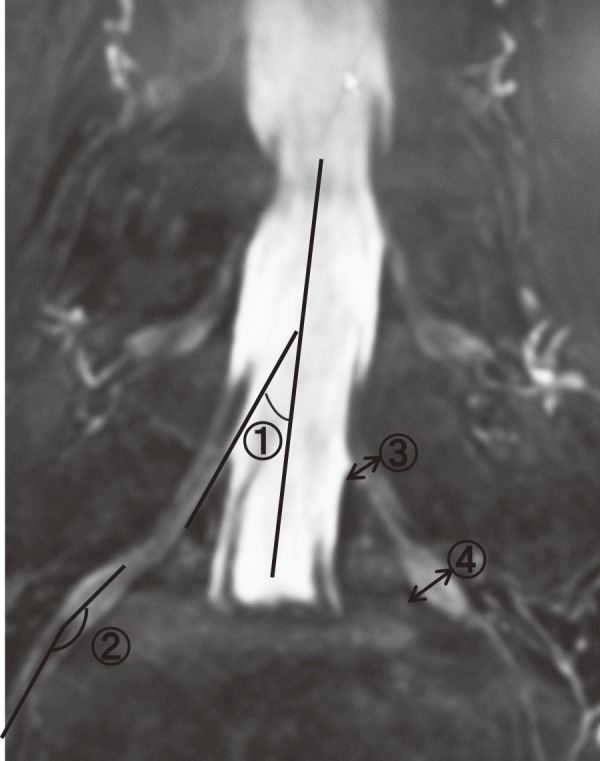
Measurement of ① the proximal tilting angle of L5 nerve root: PTA, ② the lateral tilting angle of L5 nerve root: LTA, ③ the proximal nerve root width of L5 nerve root: PW, ④ the dorsal root ganglion width of L5 nerve root: DW. The PTA of the nerve root was measured using a midline to the dural sac and another line passing through the medial line of L5 nerve root. The LTA of the nerve root was measured using a midline to the DRG and another line passing through the center of the spinal nerve.
Results
Of the total 270 roots, 112 were assigned to the IL group, 122 were assigned to the CO group, and 36 were assigned to the FS group. As shown in Fig. 2, 3, PTA was 37.5°±0.9° (ICC 0.94) in the IL group, 45.8°±2.8° (ICC 0.91) in the FS group, and 34.8°±0.9° (ICC 0.93) in the CO group, whereas LTA was 153.1°±0.9° (ICC 0.88) in the IL group, 139.2°±2.1° (ICC 0.87) in the FS group, and 153.4°±0.9° (ICC 0.90) in the CO group, indicating significant differences for the FS group compared with those for the other two groups (p<0.05). As shown in Fig. 4, PW was 4.48±0.1 mm (ICC 0.94) in the IL group, 4.59±0.2 mm (ICC 0.92) in the FS group, and 4.27±0.1 (ICC 0.94) mm in the CO group, showing no significant intergroup differences. As shown in Fig. 5, DW was 6.28±0.1 mm (ICC 0.91) in the IL group, 7.60±0.2 mm (ICC 0.93) in the FS group, and 5.89±0.1 mm (ICC 0.89) in the CO group, indicating that DW was significantly thicker in the FS group compared with those in the other two groups and that it was significantly thicker in the IL group compared with that in the CO group (p<0.05). DW ratio was 1.15±0.0 in the IL group, 1.29±0.0 in the FS group, and 1.01±0.0 in the CO group, indicating that it was significantly higher in the FS group than that in the other two groups and that it was significantly higher in the IL group than that in the CO group (p<0.05). We created ROC curves for the purpose of assessing the association of DW and DW ratio obtained in this study with foraminal stenosis (Fig. 6, 7). In cases of foraminal stenosis in the FS group, DW cutoff value was 6.5 mm, sensitivity was 87.5%, and specificity was 78.4%. For DW ratio, the cutoff value was 1.2 times the size of the unaffected side, sensitivity was 69.4%, and specificity was 79.8%. As shown in Fig. 8, a typical case of foraminal stenosis has large DW and DW ratio.
Figure 2.
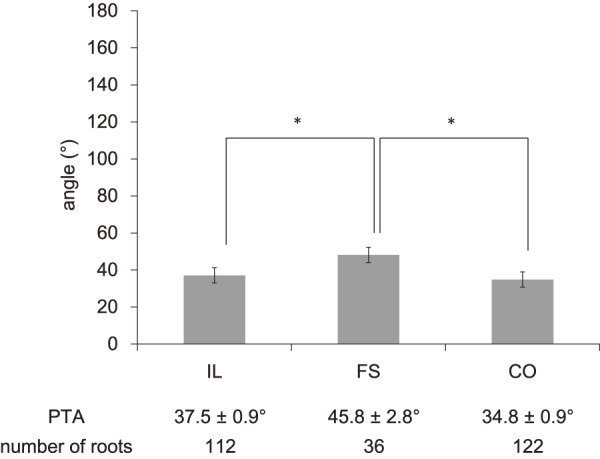
The values of PTA in each group. *p<0.01.
Figure 3.
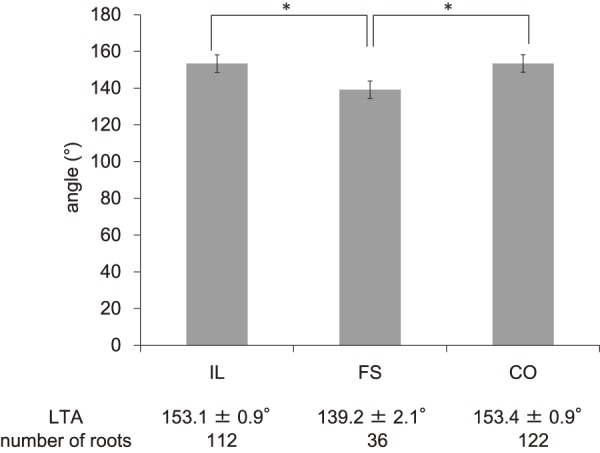
The values of L5LA in each group. *p<0.01.
Figure 4.
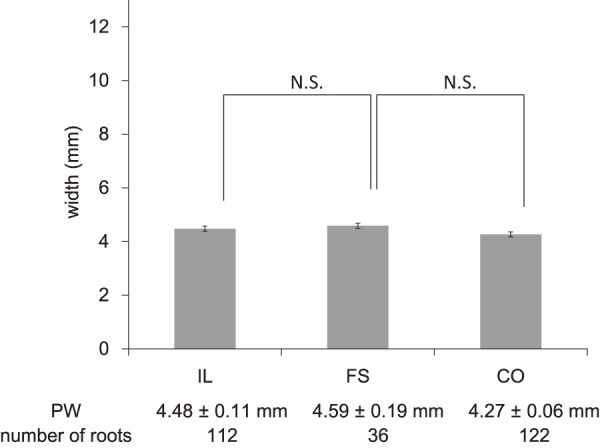
The values of PW in each group.
Figure 5.
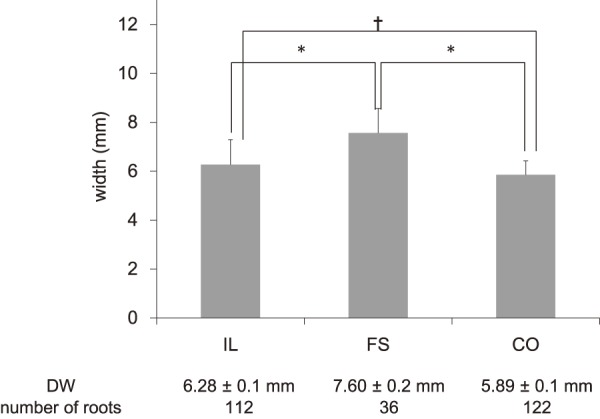
The values of DW in each group. *p<0.01, †p<0.05.
Figure 6.
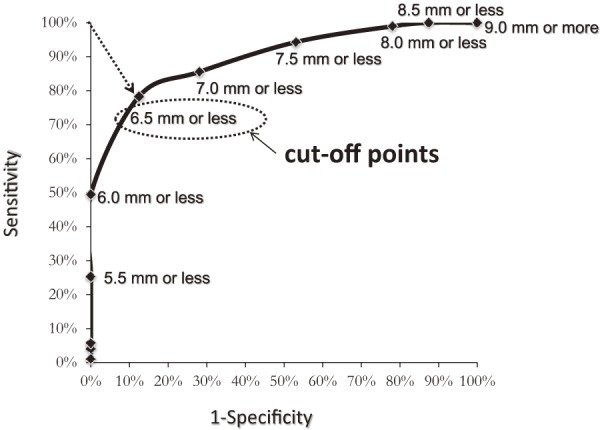
The values of sensitivity and false positive by several cutoff points of DW were plotted.
Figure 7.
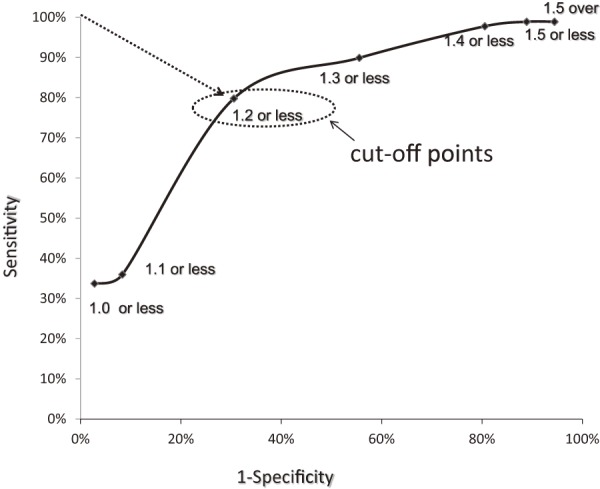
The values of sensitivity and false positive by several cutoff points of DW ratio were plotted.
Figure 8.
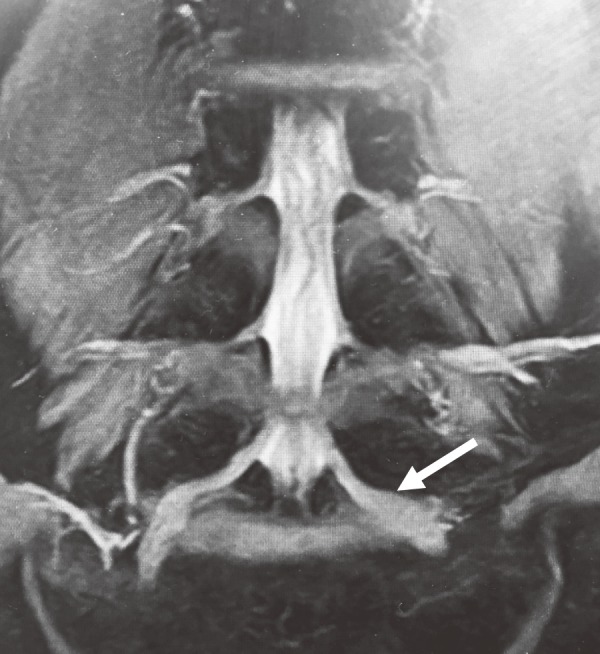
A typical case of foraminal stenosis with large DW and DW ratio (white arrow). Left L5 DW is 9.04 mm and DW ratio is 1.50.
Discussion
Foraminal lesions are considered to be in the “hidden zone”1). Therefore, they have been difficult to assess using conventional myelography and post-myelography CT. Hasegawa et al.12) assessed the lumbar nerve roots of healthy individuals using MRI frontal plane images. Their results indicated that in young adult male subjects, the angle of the L5 nerve root branch was 27.7°, which differed from that of the CO group (34.8°) in this study. In the present study, although we measured all nerve root parameters based on the methods used by Hasegawa et al.12), the reasons for the differences between the branch angles in this study compared with those in other studies include the fact that the mean age of the subjects in this study was 61 years. Because of this, age-associated intervertebral narrowing and wedging were more pronounced in our study, and differences in MRI imaging techniques used may have had an additional impact. DW in the CO group was nearly a perfect match to the findings reported by Hasegawa et al.12). However, there were some differences between our findings and those reported by Shen et al.13), who used the same imaging technique but reported a DW of 6.4±0.9 mm in subjects with a mean age of 40.3 years. In their comparison of 14 foraminal stenosis patients and 14 healthy volunteers, Eguchi et al.14) reported a DW of 6.3 and 5.3 mm, respectively, indicating a significant difference. Based on the findings of both the present study and previous reports, the maximum DRG for the L5 nerve root on MRI images of healthy individuals is approximately 6.0 mm.
In general, radiculopathy due to lumbar spinal canal stenosis is attributed to nerve root entrapment (nerve entrapment syndrome), whereas radiculopathy due to a herniated lumbar disc is attributed to localized compression and traction on the nerve root (nerve root displacement syndrome). Regardless of the cause, physical or chemical stimulation of the nerve root causes reflexive swelling of the root. Considering the both the present results and previous reports, nerve root enlargement observed on MRM images in the present study depicts secondary inflammation of the nerve root and especially the DRG due to either nerve root compression, entrapment, or both9-11).
MRM-based diagnostic criteria reported for foraminal stenosis consist of the lateral course of the nerve root or spinal nerves, unclear morphology of DRG, and spinal nerve entrapment3). In addition to these diagnostic criteria, the results of the present study indicate that the DRG swelling observed on MRM images is a finding unique to symptomatic patients that is caused by foraminal stenosis. Kim et al.7) have reported that the definition of DRG swelling on MRM images is a larger size compared with the healthy side. Moreover, using this method they found that 60% of their cases had foraminal stenosis with a specificity of 99.1%7). Aota et al.6) reported a sensitivity of 67% and specificity of 96% when MRM images were used to identify DRG swelling in cases of foraminal stenosis. The three diagnostic criteria proposed by Yamada et al.3) for MRM examinations of foraminal stenosis are a qualitative assessment. The novel finding of the present study is that as a result of performing a quantitative assessment, we found that in addition to the three diagnostic criteria mentioned above, DRG swelling in the FS group was found in 87.5% of the cases, and the thickness was at least 6.5 mm. Furthermore, DRG ratio on MRM images compared with the healthy side indicates the presence of foraminal stenosis, and the cutoff value is 1.2 times the size of the unaffected side. It has been reported that a lateral course of the nerve root suggests foraminal stenosis15) and that while the nerve root running course abnormality (RCA) is found in 93% of L5 foraminal stenosis cases, it is also found in 22% of healthy volunteers6). In the FS group, the mean PTA value was 45.8°, which was a significantly larger value. This result is a numerical expression of the lateral course of the nerve root, and as such, supports the findings of previous studies that the RCA of the nerve root suggests foraminal stenosis.
The limitations and issues with this study include the fact that there are some cases in which it is difficult to identify swelling on MRM slices even in cases of direct compression of the DRG. In the present study, there were some cases with low L5DW values even in symptomatic cases that did not exhibit compression within the lateral recess or intervertebral foramen. In their study of cadavers, Sato et al.16) reported that several L5 nerve root DRGs were within the intervertebral foramen and that this was closely associated with localized clinical symptoms and the therapeutic outcome16). Moreover, as we did not perform a quantitative investigation of the morphological changes within the intervertebral foramen, the actual degree of foraminal stenosis, bilateral differences in this degree, and the relationship with kinetic instability remain to be issues for future study.
Conclusions
1. In this study, we investigated the morphological changes in the L5 nerve roots of healthy and symptomatic individuals using 3D MRM. We also assessed the usefulness of 3D MRM in evaluating foraminal L5 radiculopathy with focus on comparison with the unaffected side.
2. In cases of foraminal stenosis, we found swelling of the L5 DRG. The cutoff value for 3D MRM-based L5 DRG width for cases of foraminal stenosis was 6.5 mm, strongly suggesting the presence of foraminal stenosis.
3. Swelling of the L5 DRG at least 1.2 times the size of the unaffected side strongly suggests foraminal L5 radiculopathy.
4. 3D MRM is noninvasive and can be used as a supplementary diagnostic technique for foraminal L5 radiculopathy.
Conflicts of Interest: The authors declare that there are no conflicts of interest.
References
- 1.Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53(5):891-903. [PubMed] [Google Scholar]
- 2.Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine. 1991;16(11):1312-20. [DOI] [PubMed] [Google Scholar]
- 3.Yamada H, Yoshida M, Kido M, et al. 3D MRI of the spinal nerve root. Spine & Spinal Cord. 2008;21(2):115-121. In Japanese. [Google Scholar]
- 4.Nakao S, Yoshida M, Yamada H, et al. A new 3-dimensional computed tomography imaging method to diagnose extraforaminal stenosis at the lumbosacral junction. J Spinal Disord Tech. 2010;23(8):e47-52. [DOI] [PubMed] [Google Scholar]
- 5.Burton CV, Kirkaldy-Willis WH, Yong-Hing K, et al. Causes of failure of surgery on the lumbar spine. Clin Orthop Related Res. 1981;(157):191-9. [PubMed] [Google Scholar]
- 6.Aota Y, Niwa T, Yoshikawa K, et al. Magnetic resonance imaging and magnetic resonance myelography in the presurgical diagnosis of lumbar foraminal stenosis. Spine. 2007;32(8):896-903. [DOI] [PubMed] [Google Scholar]
- 7.SB Kim, JS Jang, SH Lee. Morphologic changes of L5 root at coronal source images of MR myelography in cases of foraminal or extraforaminal compression. J Korean Neurosurg Soc. 2009;46(1):11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eguchi Y, Ohtori S, Yamashita M, et al. Clinical applications of diffusion magnetic resonance imaging of the lumbar foraminal nerve root entrapment. Eur Spine J. 2010;19(11):1874-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaji R, Oka N, Tsuji T, et al. Pathological findings at the site of conduction block in multifocal motor neuropathy. Ann Neurol. 1993;33(2):152-8. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, Ikeda S, Sakurai S, et al. Hypertrophic neuritis due to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): a postmortem pathological study. Muscle Nerve. 1996;19(2):163-9. [DOI] [PubMed] [Google Scholar]
- 11.Kuwabara S, Nakajima M, Matsuda S. Magnetic resonance imaging at the demyelinating foci in chronic inflammatory demyelinating polyneuropathy. Neurology. 1997;48(4):874-7. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa T, Mikawa Y, Watanabe R, et al. Morphometric analysis of the lumbosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine. 1996;21(9):1005-9. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi Y, Ohtori S, Orita S, et al. Quantitative evaluation and visualization of lumbar foraminal nerve root entrapment by using diffusion tensor imaging: preliminary results. AJNR Am J Neuroradiol. 2011;32(10):1824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen J, Wang HY, Chen JY, et al. Morphologic analysis of normal human lumbar dorsal root ganglion by 3D MR imaging. Am J Neuroradiol. 2006;27(10):2098-103. [PMC free article] [PubMed] [Google Scholar]
- 15.Hasue M, Kunogi J, Konno S, et al. Classification by position of dorsal root ganglia in the lumbosacral region. Spine. 1989;14(11):1261-4. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Kikuchi S. An anatomic study of foraminal nerve root lesions in the lumbar spine. Spine. 1993;18(15):2246-51. [DOI] [PubMed] [Google Scholar]


