Abstract
Introduction
Causes of pain due to spinal metastases have been insufficiently investigated. Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were the focus of this study. Both are known as proinflammatory cytokines associated with the pathophysiology of pain syndromes1). It is well known that cancer cells produce these cytokines, but whether osteoclasts produce them as well remains unclear. We hypothesize that osteoclasts produce these cytokines; in other words, pain from spinal metastasis is stronger than pain from the primary tumor.
Methods
We made a rat spinal metastasis model of breast cancer (metastasis group) and models with a hole in the vertebrae (puncture group) and resected the vertebrae. Tartrate-resistant acid phosphatase (TRAP) staining was performed to reconfirm that osteoclasts increase in vertebrae with spinal metastasis. We then evaluated TNF-α and IL-6 expression using immunohistochemistry and real-time polymerase chain reaction (PCR).
Results
The results of TRAP staining showed that osteoclasts increase in metastatic vertebrae. The osteoclasts in the puncture models were TNF-α negative but were TNF-α positive in the metastasis model. The osteoclasts in the puncture models and metastasis model were both IL-6 positive. According to the real-time PCR results, TNF-α in vertebrae increased in the metastasis model, but IL-6 did not increase in the metastasis model compared with in the puncture model.
Conclusions
The number of osteoclasts is higher in the metastasis model. While TNF in the osteoclasts increased in the spinal metastasis model, IL-6 did not. This probably means that breast cancer affects TNF production in osteoclasts. This increase of TNF-α may lead to pain from spinal metastasis.
Keywords: osteoclast, TNF-α, IL-6, proinflammatory cytokines, spinal metastasis, breast cancer, pain
Introduction
Medical advances have improved the life expectancy of cancer patients, with an increase in metastatic patients. A bone is one of the common sites of metastasis, causing refractory bone pain2). In fact, 83% of patients, not all patients, who have bone metastasis experience pain3). In particular, breast cancer is responsible for bone metastasis and 65%-75% patients who have advanced breast cancer suffer from bone metastasis4). The spine is the most common site of metastases in breast cancer, and it can bring severe pain which impairs patients' quality of life and is difficult to cure5). However, pain from spinal metastasis also occurs when there are neither fractures nor nerve compression. It has recently been reported that osteoclasts cause pain through proton secretion6). Tumor necrosis factor-α (TNF-α) is a known activator of osteoclasts7) and a mediator of neuropathic pain8,9). In this study, we focused on TNF-α and interleukin-6 (IL-6), which are known as proinflammatory cytokines related to pain. We hypothesized that osteoclasts produce these cytokines so that pain from spinal metastasis is stronger than pain from the primary tumor.
Materials and Methods
Rat model with spinal metastasis of breast cancer
All animal procedures and protocols were approved by the Ethics Committee of our University and were conducted according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996 revision).
We selected a rat model of spinal metastasis10), which was reported in the study by Mantha. We used 42 eight-week-old female Sprague-Dawley rats weighing 200-250 g. First, we cultured rat breast adenocarcinoma cell line CRL-1666 in 10 cm dishes. We then injected the CRL-1666 subcutaneously to make subcutaneous tumors in six donor rats. Secondly, the remaining 36 rats were anesthetized with a mixture of 0.15 mg/kg body weight (b.w.) medetomidine, 2.0 mg/kg b.w. midazolam, and 2.5 mg/kg b.w. butorphanol and were treated aseptically throughout the experiments. A midline dorsal longitudinal incision was made over the lumbar spine. The L6 vertebra was exposed, and a hole was made with an 18-gage needle 1 mm left of the midline in this L6 vertebra. When the needle point reached a depth of 1.5 mm, the needle was rotated to create a 1 mm-wide hole (puncture model, n = 18) as Mantha reported10,11). A tumor section measuring 1 × 1 × 1 mm was excised from the donor rats and then implanted into the hole (metastasis model, n = 18). After the puncture and implantation, the hole was sealed with LUKENS Bone Wax (Lukens Corp., Richmond, VA) and both the fascia and skin were closed. We assessed the rats attentively after surgery every day, detecting signs of discomfort such as decreased activity, lack of mobility, hunched posture, and paresis.
Decalcification and making sections of vertebrae
As Mantha reported that the rats showed paraparesis by day 15 and it had started from day 10, most spinal metastasis rats showed paraparesis from day 9 to day 11. To decide the day of sacrifice, we sacrificed rats at 5, 7, 9, and 11 days and dissected vertebrae for macroscopic and microscopic observation. We intended to exclude the impact by the tumor of spinal compression, but we wanted the tumor to grow as big as possible. After the observation of the vertebrae, we hypothesized that sacrificing the rats on day 7 would be ideal for our study. Thus, we decided to make sections with rats on day 7. Seven days after the placement of the tumor sections, the rats were anesthetized with a mixture of 0.15 mg/kg body weight (b.w.) medetomidine, 2.0 mg/kg b.w. midazolam, and 2.5 mg/kg b.w. butorphanol. After anesthesia, the L6 vertebrae were resected and soaked in 10% neutral formalin at 4℃ for 48 h. After the fixation, the specimens were decalcified with weekly solution changes with 10% ethylenediaminetetraacetic acid (EDTA) for three weeks. We then made paraffin blocks and divided them into sections using a sliding microtome (LS113, Yamato-Kohki Industrial Co. Ltd, Saitama, Japan). We then cut 10 sections from one block and made slides.
TRAP staining
TRAP staining was performed to identify osteoclasts. We chose four sections per rat and performed TRAP staining using a TRAP/ALP stain kit (#294-67001, Wako Pure Chemical Co. Ltd, Osaka, Japan). We placed the TRAP Staining Solution Mix in a staining dish and used a water bath to pre-warm it to 37℃. Then we deparaffinized the slides and rehydrated them through grade ethanol to distilled water. The slides were placed in the pre-warmed TRAP Staining Solution and incubated at 37℃ for 30 min. Finally, the slides were rinsed in distilled water.
TRAP-positive multinucleated cells existing close to the trabecular bone in the L6 vertebrae were regarded as osteoclasts. They were counted by our group members, who were blind to the experiments, and the average number of osteoclasts in 1 mm2 was used for statistical analysis.
Immunohistochemistry
We chose four sections per rat and performed immunohistochemistry. The primary antibodies used for anti-TNF-α were a mouse monoclonal antibody (ab199013, 1/25, Abcam, Cambridge, UK) and an anti-IL6 mouse monoclonal antibody (ab9324, 1/300, Abcam, Cambridge, UK). The slides were incubated at 4℃ overnight. Then, the specimens were incubated with a horseradish-peroxidase polymer-labeled goat anti-mouse immunoglobulin antibody (Histofine Simple Stain MAX-PO (M), #424134; Nichirei Corporation, Tokyo, Japan) for 30 min at room temperature. After that, the reaction was visualized by treatment with 0.003% hydrogen peroxide and 0.2 mg/ml 3,3'-diaminobenzidine (DAB) in 50 mm Tris-HCl, pH 7.6, for 2 min at room temperature. The counter-staining was performed with Mayer's hematoxylin (#30142, Muto Pure Chemicals Co. Ltd., Tokyo, Japan).
Real-time PCR
Seven days after the placement of the tumor sections, the rats were anesthetized, and the L6 vertebrae were resected. We put the vertebrae into liquid nitrogen to make frozen specimens. The frozen specimens were crushed with Cell DestroyerⓇ (Biomedical Science, Tokyo, Japan). Then the RNA of the crushed specimens was extracted using IsogenⓇ (Nippon Gene, Tokyo, Japan). We performed real-time polymerase chain reaction (PCR) for TNF-α and IL-6.
Statistical analysis
A statistical analysis of the number of TRAP-positive cells was performed using the Student t-test, and the statistical analysis of the relative expression of TNF-α and IL-6 was performed using the Mann-Whitney U-test. P-values less than 0.05 were considered statistically significant. All of the results were expressed as the mean ± standard error unless otherwise indicated.
Results
TRAP staining and immunohistochemistry
There were more osteoclasts in the L6 vertebrae of the metastasis model as compared to the puncture model (P < 0.05) (Fig. 1, 2). This result indicates that CRL-1666 may induce osteoclast precursors to osteoclasts.
Figure 1.
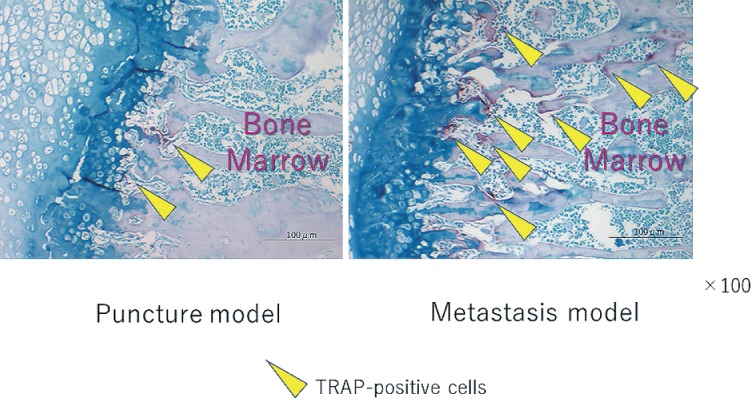
Tartrate-resistant acid phosphatase (TRAP)-staining of the L6 vertebrae. The arrowheads show TRAP-positive multinucleated cells. TRAP-positive multinucleated cells existing close to the trabecular bone in a L6 vertebra were regarded as osteoclasts. The scale bars are 100μm.
Figure 2.
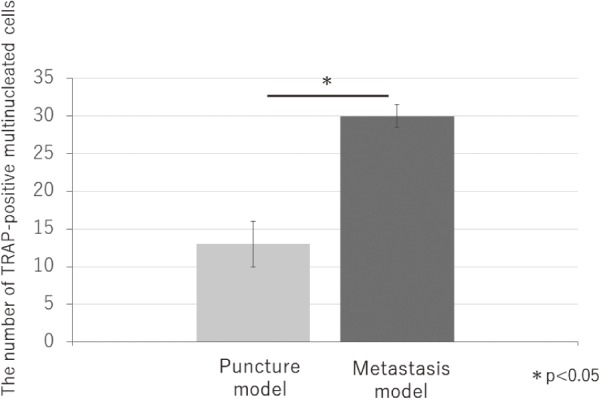
The number of TRAP-positive multinucleated cells in 1mm2. There were more osteoclasts in metastasis model compared with puncture model. (p<0.05)
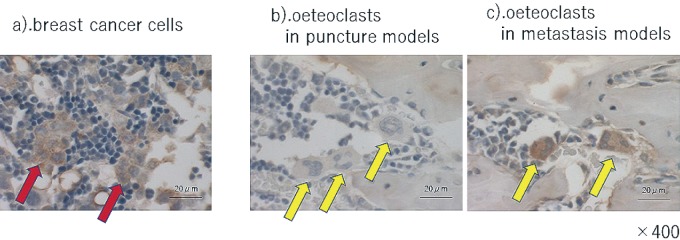
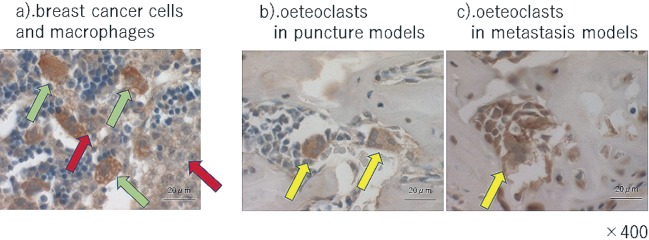
The osteoclasts in the puncture models were TNF-α negative, but TNF-α positive in the metastasis model (Fig. 3). The osteoclasts in the puncture models and metastasis model were both IL-6 positive (Fig. 4). This result implies that CRL-1666 increases TNF-α in the osteoclasts, but does not increase IL-6.
Figure 3.
Immunohistochemistry of tumor necrosis factor-α (TNF-α) a). breast cancer cells. Arrowheads indicate the breast cancer cells. They were TNF-α-positive. b).and c). osteoclasts in puncture models and metastasis model. Arrowheads indicate the osteoclasts. The osteoclasts in the puncture models are -negative, while TNF-α-positive in the metastasis model. The scale bars are 20μm.
Figure 4.
Immunohistochemistry of IL-6 a) breast cancer cells. Red arrow heads indicate the breast cancer cells and green arrowheads shows macrophages surrounding the breast cancer cells. They were IL-6-positive. b).and c). osteoclasts in puncture models and metastasis models. Arrow heads indicate the osteoclasts. The osteoclasts in the puncture models and metastasis models are both IL- 6 positive. The scale bars are 20μm.
Real-time PCR
According to the results of real-time PCR, TNF-α in vertebrae increased in the metastasis model, but IL-6 did not as compared to TNF-α and IL-6 in the puncture model (P < 0.05) (Fig. 5). The results from immunohistochemistry and real-time PCR are consistent.
Figure 5.
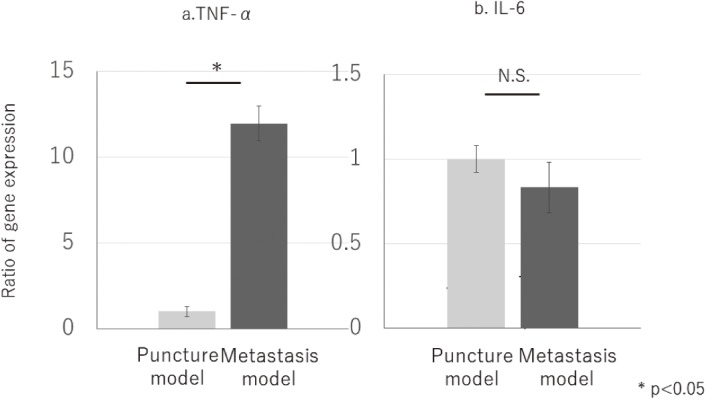
Ratio of gene expression. TNF-α in vertebrae increased significantly in metastasis model (a.), but IL-6 has no significant differences between puncture model and metastasis model (b.) (p<0.05).
Discussion
Pain due to spinal metastases occurs even when there are not vertebral fractures, and several mechanisms have been reported. It is well known that bone resorption causes pain and that TNF-α induces osteoclast differentiation by stimulating osteoclast progenitors7). Osteoclasts create an acidic microenvironment and cause bone pain12,13) via transient receptor potential vanilloid type 1 (TRPV1), an acid-sensing nociceptor14).
The proinflammatory cytokines not only induce the differentiation of osteoclasts but also cause pain by themselves. Xu et al. in their study reported that the upregulation of TNF-α and tumor necrosis factor receptor (TNFR) 1 is essential for the initiation of neuropathic pain15). Several studies provide that the epineural or intrathecal application of TNF-α causes a hyperalgesia, allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli and enhanced dorsal horn neuronal responses, including the acute responses to C-fiber stimulation, wind-up, and post-discharge in rats16-18). It has also been documented that TTX-resistant sodium currents in acute TNF-α-mediated increase in nociceptors' excitability depending on p38 MAPK19). TNF-α also causes hyperalgesia through the sensitization of TRPV1 channels, related to acidity20). As can be seen from these studies, TNF-α itself causes pain including bone cancer pain.
Previous studies have reported that the vertebral body is innervated by pain-related intravertebral sensory nerve fibers21,22), and mechanical, thermal, and chemical stimuli are transmitted to the nerves. Assuming the microenvironment of spinal metastases, TNF-α, which increased in the vertebrae stimulates the nerves, and they may activate nociceptors and lower the threshold23,24).
Moreover, the study by Geis reported that systemic antagonism of TNF significantly alleviated tactile hypersensitivity and spontaneous bone cancer-related pain behavior in the mouse model. This supports our theory that the production of TNF may lead to bone cancer pain.
Limitations
Several studies show that von Frey test, Gait analysis (Catwalk XT, Noldus) test, and other pain behavior tests are useful for evaluation of pain due to spinal metastasis25,26). We tried to evaluate pain by means of pain behavior with CAT WALK. However, the evaluation could not be completed because the rats of the metastasis model showed a significant decrease in activity due to possible pain and/or motor dysfunction. Considering that the primary purpose of the current study was to investigate the pain mechanism of spinal metastasis, our discussion was limited to the possible relationship between TNF-α and pain.
To conclude, the increase of TNF-α observed in our study may be one of the mechanisms of pain in the rat spinal metastasis model.
Disclaimer: Sumihisa Orita is one of the Editors of Spine Surgery and Related Research and on the journal's Editorial Committee. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Author Contributions: Ai Mazaki wrote and prepared the manuscript, Ai Mazaki and Kazuyo Yamauchi developed the study design and conducted the study, and all of the authors participated in the study design. All authors have read, reviewed, and approved the article.
Acknowledgement
The authors would like to thank Ikuko Tajiri (Department of Orthopaedic Surgery, Graduate School of Medicine, Chiba University, Chiba, Japan) for technical assistance with the experiments.
References
- 1.de Oliveira CM, Sakata RK, Issy AM, et al. Cytokines and pain. Rev Bras Anestesiol. 2011;61(2):255-9,60-5,137-42. [Article in English, Portuguese, Spanish] [DOI] [PubMed] [Google Scholar]
- 2.Smith HS, Mohsin I. Painful boney metastases. Korean J Pain. 2013;26(3):223-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laird BJ, Walley J, Murray GD, et al. Characterization of cancer-induced bone pain: an exploratory study. Supportive Care Cancer. 2011;19(9):1393-401. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(S8):1588-94. [DOI] [PubMed] [Google Scholar]
- 5.Briasoulis E, Karavasilis V, Kostadima L, et al. Metastatic breast carcinoma confined to bone. Cancer. 2004;101(7):1524-8. [DOI] [PubMed] [Google Scholar]
- 6.Nagae M, Hiraga T, Wakabayashi H, et al. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39(5):1107-15. [DOI] [PubMed] [Google Scholar]
- 7.Azuma Y, Kaji K, Katogi R, et al. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275(7):4858-64. [DOI] [PubMed] [Google Scholar]
- 8.Schafers M, Lee DH, Brors D, et al. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23(7):3028-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson GM, Nakada MT, DeWitte M. Tumor necrosis factor-alpha in the pathogenesis and treatment of cancer. Curr Opin Pharmacol. 2004;4(4):314-20. [DOI] [PubMed] [Google Scholar]
- 10.Mantha A, Legnani FG, Bagley CA, et al. A novel rat model for the study of intraosseous metastatic spine cancer. J. Neurosurg Spine. 2005;2(3):303-7. [DOI] [PubMed] [Google Scholar]
- 11.Funayama T, Sakane M, Abe T, et al. Photodynamic therapy with indocyanine green injection and near-infrared light irradiation has phototoxic effects and delays paralysis in spinal metastasis. Photomed Laser Surg. 2012;30(1):47-53. [DOI] [PubMed] [Google Scholar]
- 12.Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. 2007;25(2):99-104. [DOI] [PubMed] [Google Scholar]
- 13.Yoneda T, Hata K, Nakanishi M, et al. Involvement of acidic microenvironment in the pathophysiology of cancer-associated bone pain. Bone. 2011;48(1):100-5. [DOI] [PubMed] [Google Scholar]
- 14.Kanaya K, Iba K, Abe Y, et al. Acid-sensing ion channel 3 or P2X2/3 is involved in the pain-like behavior under a high bone turnover state in ovariectomized mice. J Orthop Res. 2016;34(4):566-73. [DOI] [PubMed] [Google Scholar]
- 15.Xu JT, Xin WJ, Zang Y, et al. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123(3):306-21. [DOI] [PubMed] [Google Scholar]
- 16.Reeve AJ, Patel S, Fox A, et al. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain. 2000;4(3):247-57. [DOI] [PubMed] [Google Scholar]
- 17.Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst. 2000;5(2):96-100. [DOI] [PubMed] [Google Scholar]
- 18.Liu YL, Zhou LJ, Hu NW, et al. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology. 2007;52(3):708-15. [DOI] [PubMed] [Google Scholar]
- 19.Jin X, Gereau RW. Acute p38-Mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-α. J Neurosci. 2006;26(1):246-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantin CE, Mair N, Sailer CA, et al. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28(19):5072-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orita S, Ohtori S, Koshi T, et al. The effects of risedronate and exercise on osteoporotic lumbar rat vertebrae and their sensory innervation. Spine. 2010;35(22):1974-82. [DOI] [PubMed] [Google Scholar]
- 22.Bailey JF, Liebenberg E, Degmetich S, et al. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat. 2011;218(3):263-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haegerstam GA. Pathophysiology of bone pain: a review. Acta Orthop Scand. 2001;72(3):308-17. [DOI] [PubMed] [Google Scholar]
- 24.Payne R. Mechanisms and management of bone pain. Cancer. 1997;80(8 Suppl):1608-13. [DOI] [PubMed] [Google Scholar]
- 25.Aielli F, Ponzetti M, Rucci N. Bone metastasis pain, from the bench to the bedside. Int J Mol Sci. 2019 Jan;20(2):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luger NM, Sabino MA, Schwei MJ, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain. 2002;99(3):397-406. [DOI] [PubMed] [Google Scholar]