Abstract
Introduction: Multilevel total en bloc spondylectomy (TES) is required to secure oncologically adequate resection margins. However, no useful information has been reported for spinal reconstruction after multilevel TES. Therefore, this study set out to assess the clinical and radiological outcomes of spinal reconstruction after multilevel TES. Methods: Forty-eight patients treated with multilevel TES at our institute were included in the analysis. Reconstruction was achieved with posterior pedicle screw fixation and an anterior titanium mesh cage filled with iliac autograft in all cases. Spinal shortening was performed to increase spinal stability from the reconstruction. Instrumentation failure and radiological findings were evaluated with radiography and computerized tomography (CT). Results: After excluding one patient whose general condition was deteriorating, radiological evaluations of 47 patients were performed over a period of more than a year. The follow-up time was 17 to 120 months (mean: 70.2 months). Instrumentation failure occurred in one patient (5.9%) after thoracic multilevel TES, in 4 patients (25.0%) after thoracolumbar multilevel TES, and in 3 patients (42.9%) after lumbar multilevel TES. No instrumentation failure was observed in cervicothoracic cases. Cage subsidence (>2 mm) occurred in 30 patients (63.8%). In 22 of them, subsidence appeared on the CT one month after surgery. The risk factors of instrumentation failure included a multilevel TES below the thoracolumbar level and a long span of vertebral resection. There was no instrumentation failure in any of the 11 “disc-to-disc cutting” cases. Conclusions: This study identified the risk factors of instrumentation failure after multilevel TES. There is a high risk of instrumentation failure in cases of long vertebral resection below the thoracolumbar level. On the other hand, our reconstruction method can be successful for multilevel TES above the thoracic level.
Keywords: spinal tumor, multilevel total en bloc spondylectomy, instrumentation failure, cage subsidence
Introduction
Spondylectomy was first described as a treatment method for spinal tumors by Stener1) and Roy-Camille et al.2). Authors have since reported excellent clinical results from this operation3). In the 1990s, Tomita (from our institute) developed and popularized the procedure known as “total en bloc spondylectomy” (TES), which is designed to achieve the complete oncological resection of spinal tumors4,5). This procedure has proven to provide excellent local control6-10), and is now widely accepted by spinal and musculoskeletal tumor surgeons11-21). TES has been used in selected patients with solitary spinal metastasis, and long-term follow-up has also been reported22-24). Given the advances in surgical techniques25), TES indications have been expanded to include patients with extracompartmental or consecutive multilevel spinal tumor. We have already reported the results of a study in which 8 of 26 patients did not require blood transfusions after three-level TES26). TES is no longer regarded as a very high-risk procedure. On the other hand, there have been reports of instrumentation failure after TES26,27). From a biomechanical standpoint, TES causes a complete loss of spinal stability due to the need to detach soft tissue surrounding the vertebral body and to resect the vertebral body, including ligaments, to completely excise the spinal tumor. Spinal reconstruction after TES is particularly challenging when multiple spinal levels are resected. However, there is no consensus about the best reconstruction method for multilevel TES. Spondylectomy can be performed in any area from the upper thoracic spine to the lumbar spine. However, the results of spinal reconstruction may differ between the spinal levels. Therefore, this study set out to assess the clinical and radiological outcomes of our spinal reconstruction method after multilevel TES.
Materials and Methods
Forty-eight patients underwent multilevel TES at our institute between April 2006 and March 2010. Excluding one patient whose general condition was deteriorating, radiological evaluations of 47 patients were performed over a period of more than one year. In the present study, the indications for surgery included a primary spinal tumor in 15 patients, metastasis with neurological deficit in 15 patients, metastasis with recurrence after surgery and/or radiation therapy in 14 patients, and solitary metastasis without neurological deficit in 3 patients. There were 20 men and 27 women, with an average age of 53.3 years (range: 32-80 years). The primary tumors included the following: giant cell tumor (7 cases), chordoma (2), plasmacytoma (2), angiosarcoma (1), hemangiopericytoma (1), leiomyosarcoma (1), and aggressive hemangioma (1). The other 32 patients had metastatic tumors, which included renal cell carcinoma (14 patients), thyroid cancer (4), lung cancer (4), breast cancer (3), leiomyosarcoma (2), colon cancer (1), hepatocellular carcinoma (1), carcinoma of the maxilla (1), osteosarcoma (1), and unknown (1) as primary tumors. The resected vertebrae were cervicothoracic in 7 cases, thoracic in 17, thoracolumbar in 16, and lumbar in 7. One whole vertebra was resected with part of the adjacent vertebra (vertebral cutting) in 21 cases; two whole vertebral resections (disc-to-disc cutting) were performed in 4 cases; two whole vertebrae were resected with part of the adjacent vertebra in 10 cases; three whole vertebral resections were performed in 7 cases; and three whole vertebrae were resected with part of the adjacent vertebra in 5. In all cases, preoperative embolization of the bilateral segmental arteries on three levels was performed in the 72 hours before the surgery.
Surgical technique (Figure 1)
Figure 1.
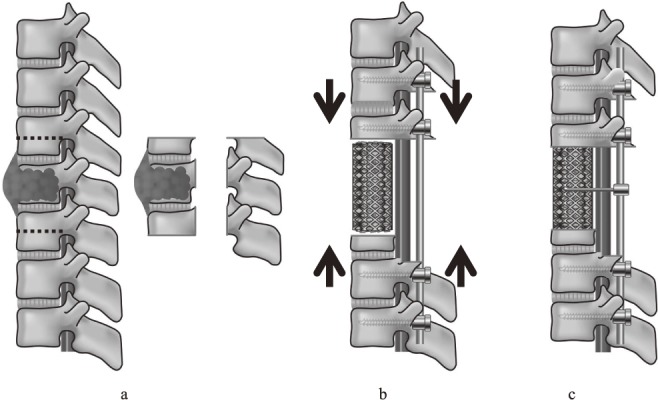
a: En bloc corpectomy was performed after pedicle screw fixation. b: Anterior reconstruction was performed with a titanium mesh cage (MOSS-Miami; DePuy Motech, Warsaw, Indiana) filled with autograft. To increase spinal stability, the posterior instrumentation was adjusted so as to slightly compress the inserted vertebral cage. c: The connector device was made up of threaded rod (diameter: 3 mm) and was attached bilaterally between the posterior rods and the anterior mesh cage to serve as artificial pedicles.
Total en bloc spondylectomy comprised two steps: en bloc laminectomy and en bloc corpectomy25). The surgical approach was based on the degree of tumor involvement and the affected spinal level. A single posterior approach was used in 33 patients. In 9 patients whose spinal tumor affected neighboring structures contiguously, an additional anterior approach was used to ensure safe surrounding tissue release from the tumor and to secure the subsequent posterior en bloc resection. A posterior-anterior-posterior approach was used in five cases, including three that involved the L4 and L5, one involving a recurrent tumor at the cervicothoracic level, and one involving a huge paravertebral extension at the thoracolumbar level. After en bloc laminectomy, two-above and two-below segmental fixation (using a total of eight screws) was performed in 43 patients. Three patients needed nine pedicle screws, as only one pedicle screw can be inserted into the vertebra next to the cage level. Three-above and two-below segmental fixation (using a total of 10 pedicle screws) was performed in one patient with osteoporosis. Iliac screws were used in 3 patients with an L5 resection. We did not use hooks or wires. The rods used in all patients were composed of titanium alloy (diameter: 5.5 mm). After en bloc corpectomy, anterior reconstruction was performed with a titanium mesh cage (MOSS-Miami; DePuy Motech, Warsaw, Indiana) filled with autograft in all 47 patients (Figure 1a). End caps were placed on both ends of the cage in 35 patients, while no end caps were used in the other 12 patients. To increase spinal stability from the reconstruction, the posterior instrumentation was adjusted to slightly compress the inserted vertebral cage (Figure 1b). After spinal shortening, a connector device made of threaded rod (diameter: 3 mm) was attached bilaterally between the posterior rods and the anterior mesh cage to serve as artificial pedicles for patients undergoing at least two whole vertebral resections (Figure 1c). Finally, at least two transverse connectors were applied to reinforce torsional rigidity.
In all cases, a rigid spinal brace was used for a post-operative period of three months, followed by a soft brace for another three months.
Evaluation
The cage subsidence (>2 mm), pedicle screw loosening, and radiolucent lines between the cage and the endplate were evaluated by radiography and CT with multiplanar reconstructions (MPRs). Each parameter was evaluated one month after surgery, and every year thereafter until the last follow-up point. The length of spinal shortening was also measured. The incidence of instrumentation failure was evaluated in relation to age, sex, use of radiation therapy, spinal level of the tumor, length of vertebral resection, use of cage end caps, cutting line of resection (vertebral body versus disc), cage subsidence one month after surgery, and cage subsidence progression.
Statistical analyses were performed with χ2 and Mann-Whitney U tests. A p-value of <0.05 was considered statistically significant throughout. All the statistical analyses were performed with SPSS v. 20 (IBM, Chicago, IL).
The study was approved by our institution's ethics committee, and informed consent was obtained from all patients.
Results
The patient characteristics are detailed in Table 1. The median operative time was 588 minutes (391-1618 min), and the median intraoperative blood loss was 1370 ml (360-5530 ml). In the final follow-up, 18 patients showed no sign of the disease, 14 were alive with the disease, and 15 had died from it. The follow-up period was 17 to 123 months (mean 71.3 months). Although 44 patients showed no evidence of local recurrence at the surgical site throughout the follow-up period, 2 patients developed metastasis on levels adjacent to the TES site, and one showed recurrence on the lateral side of the cage.
Table 1.
Patient Characteristics and Data.
| No. | Instrum entation failure | Age (yr.) | Sex | Radiation therapy | Resection level | Op.time (min) | Bleeding (ml) | Length of resected vertebrae (mm) | Cage subsidence (mm) | End cap use | Follow-up period | Onocological status | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One month | One year | ||||||||||||
| 1 | - | 77 | F | - | (C7), T1, (T2) | 558 | 1940 | 26 | 0 | 0 | - | 55 | AWD |
| 2 | - | 33 | F | - | C7, T1, T2, (T3) | 1618 | 4950 | 55 | 0 | 0 | - | 95 | NED |
| 3 | - | 55 | M | - | (T1), T2, (T3) | 631 | 750 | 45 | 2 | 2 | - | 31 | DOD |
| 4 | - | 48 | M | + | (T1), T2, (T3) | 765 | 1590 | 59 | 0 | 0 | - | 54 | NED |
| 5 | - | 42 | M | - | (C7), T1, T2, T3, (T4) | 1017 | 4000 | 52 | 8 | 8 | - | 97 | NED |
| 6 | - | 66 | F | + | T2, T3, T4 | 627 | 700 | 55 | 2 | 2 | + | 32 | DOD |
| 7 | - | 49 | M | - | T2, T3, T4 | 530 | 1930 | 65 | 2 | 2 | - | 72 | NED |
| 8 | - | 49 | M | - | (T3), T4, (T5) | 731 | 1710 | 38 | 6 | 6 | - | 52 | DOD |
| 9 | - | 50 | M | - | (T3), T4, (T5) | 630 | 2000 | 30 | 2 | 5 | + | 81 | AWD |
| 10 | - | 50 | M | - | (T4), T5 | 465 | 1980 | 33 | 0 | 0 | - | 70 | DOD |
| 11 | - | 50 | M | - | T4, T5, (T6) | 557 | 2460 | 52 | 4 | 4 | - | 24 | DOD |
| 12 | - | 62 | M | + | (T4), T5, (T6) | 503 | 730 | 45 | 1 | 1 | + | 94 | AWD |
| 13 | + | 58 | M | - | (T5), T6 | 445 | 1100 | 37 | 2 | 11 | - | 117 | NED |
| 14 | - | 39 | F | - | (T5), T6, (T7) | 625 | 1100 | 28 | 2 | 2 | - | 116 | AWD |
| 15 | - | 67 | M | - | T6, T7 | 397 | 1660 | 41 | 0 | 0 | + | 74 | AWD |
| 16 | - | 41 | F | - | T5, T6, T7, (T8) | 524 | 820 | 63 | 2 | 2 | - | 17 | DOD |
| 17 | - | 58 | F | - | (T6), T7, (T8) | 445 | 1100 | 34 | 0 | 0 | + | 123 | NED |
| 18 | - | 52 | F | - | (T6), T7, (T8) | 505 | 2875 | 38 | 1 | 3 | + | 67 | DOD |
| 19 | - | 50 | F | - | T6, T7, T8 | 565 | 550 | 66 | 6 | 6 | + | 83 | AWD |
| 20 | - | 59 | F | + | (T6), T7, T8 | 432 | 360 | 48 | 2 | 4 | + | 84 | AWD |
| 21 | - | 34 | F | + | (T6), T7, T8 | 685 | 1400 | 45 | 2 | 3 | + | 49 | DOD |
| 22 | - | 66 | M | - | (T7), T8, T9 | 510 | 1050 | 66 | 0 | 0 | + | 50 | DOD |
| 23 | - | 71 | F | - | (T8), T9 | 417 | 1200 | 30 | 0 | 4 | + | 75 | NED |
| 24 | - | 80 | M | + | (T8), T9, (T10) | 580 | 1200 | 48 | 0 | 3 | + | 90 | NED |
| 25 | - | 47 | F | + | T9, T10, T11 | 566 | 1340 | 85 | 1 | 1 | + | 71 | DOD |
| 26 | - | 62 | F | - | T10, T11 | 424 | 1070 | 52 | 0 | 0 | + | 72 | AWD |
| 27 | - | 66 | M | - | (T10), T11 | 655 | 1645 | 40 | 0 | 1 | + | 112 | NED |
| 28 | + | 40 | M | - | (T8), T9, T10, T11, (T12) | 748 | 780 | 111 | 2 | 2 | + | 94 | AWD |
| 29 | - | 57 | F | - | (T10), T11, (T12) | 391 | 1450 | 42 | 2 | 2 | + | 68 | DOD |
| 30 | - | 62 | M | - | (T9), T10, T11, T12, (L1) | 683 | 900 | 124 | 1 | 5 | + | 42 | DOD |
| 31 | - | 62 | F | + | (T11), T12, (L1) | 567 | 650 | 44 | 0 | 0 | + | 41 | AWD |
| 32 | - | 61 | M | - | (T11), T12, L1 | 592 | 650 | 86 | 0 | 3 | + | 89 | NED |
| 33 | - | 70 | F | + | T12, L1 | 515 | 870 | 57 | 0 | 0 | + | 48 | AWD |
| 34 | - | 32 | F | - | (T12), L1 | 488 | 1300 | 36 | 0 | 0 | + | 91 | NED |
| 35 | - | 58 | F | - | (T12), L1, (L2) | 500 | 650 | 47 | 0 | 3 | + | 92 | AWD |
| 36 | + | 60 | M | + | T12, L1, (L2) | 875 | 1410 | 73 | 0 | 7 | + | 70 | NED |
| 37 | + | 46 | F | - | T12, L1, (L2) | 655 | 1600 | 75 | 5 | 10 | + | 28 | NED |
| 38 | - | 59 | F | - | T12, L1, L2 | 753 | 2050 | 120 | 2 | 2 | + | 84 | AWD |
| 39 | - | 52 | M | + | T12, L1, L2 | 1185 | 5530 | 120 | 0 | 0 | + | 24 | DOD |
| 40 | + | 57 | M | + | (T12), L1, L2 | 760 | 2010 | 105 | 0 | 5 | + | 87 | DOD |
| 41 | - | 50 | F | - | (L1), L2, (L3) | 713 | 680 | 42 | 0 | 0 | + | 94 | DOD |
| 42 | - | 38 | F | - | (L1), L2, (L3) | 596 | 690 | 49 | 2 | 3 | + | 60 | NED |
| 43 | + | 63 | F | + | (L2), L3, L4 | 531 | 1800 | 111 | 2 | 10 | + | 95 | NED |
| 44 | - | 52 | F | + | L3, L4 | 583 | 980 | 74 | 0 | 0 | + | 106 | AWD |
| 45 | + | 37 | F | - | L3, L4, (L5) | 660 | 2540 | 82 | 3 | 3 | + | 39 | NED |
| 46 | + | 32 | F | - | (L3), L4, (L5) | 679 | 2000 | 58 | 2 | 2 | + | 79 | NED |
| 47 | - | 36 | F | - | L3, L4, L5 | 1516 | 5510 | 103 | 2 | 2 | + | 61 | NED |
NED: no evidence of disease, AWD: alive with disease, DOD: dead of disease
The mean length of the vertebral resection was 60.3 mm (median 52 mm, range 26-124 mm). We did not perform spinal shortening in 2 patients who presented vertebral collapse from pathological vertebral fractures. In these 2 patients, the posterior wall of the vertebral body had collapsed, and had therefore already shortened the spinal column. However, spinal shortening was performed in the other 45 patients. The mean length of spinal shortening was 6.9 mm (range 2-21 mm), and the mean shortening rate was 10.2% (range 3.5-29.7%).
There was no instrumentation failure in the seven cases involving the cervicothoracic spine. However, it was observed in 1 out of 17 (5.9%) thoracic cases, in 4 out of 16 (25.0%) thoracolumbar cases, and in 3 out of 7 (42.9%) lumbar cases (Table 2). The 11 patients who underwent disc-to-disc cutting showed stable reconstructions throughout the follow-up period, with no evidence of instrumentation failure. On the other hand, 8 of the 36 patients (22.2%) who had undergone vertebral cutting developed instrumentation failure. Instrumentation failure occurred within three years of the surgery in 6 patients, and more than seven years after the surgery in the other 2 patients. Four patients experienced rod breakage at the level of the inferior end of the cage. Among the cases of whole vertebra/e resections with part of the adjacent vertebra, the remaining vertebral body and endplate had collapsed (with consequent backing-out of the pedicle screws) in 2 patients (cases 40 and 43). The other 6 patients demonstrated nonunion with the appearance of a radiolucent line between the cage and the vertebral body on the CT (coronal or sagittal MPRs). Rod breakage also occurred at the L5/S1 disc level in 2 patients (cases 45 and 46), who developed iatrogenic spondylolisthesis at the L5, despite complete bony fusion between the cage and the L5 vertebral body. Revision surgery after instrumentation failure was performed in 7 patients. Posterior reinstrumentation with bone grafting was conducted in two cases, posterior and anterior reinstrumentation with bone grafting in three cases, posterior lumbar interbody fusion at the L5/S1 in one case, and multiple instrumented surgeries in one case. We carefully observed and deferred revision surgery for one patient who manifested slight low back pain from iatrogenic spondylolisthesis at the L5 (case 46).
Table 2.
Surgical Level and Number of Resected Vertebrae.
| No. of resected vertebrae | CT | T | TL | L | Total |
|---|---|---|---|---|---|
| 1" | 3 | 10 (1) | 5 | 3 (1) | 21 (2) |
| 2 | 0 | 1 | 2 | 1 | 4 |
| 2" | 0 | 4 | 4 (3) | 2 (2) | 10 (5) |
| 3 | 2 | 1 | 3 | 1 | 7 |
| 3" | 2 | 1 | 2 (1) | 0 | 5 (1) |
| Total | 7 | 17 (1) | 16 (4) | 7 (3) | 47 (8) |
| % of instrumentation failure | 0% | 5.9% | 25.0% | 42.9% | 17.0% |
": Resection coupled with part of the adjacent vertebra
( ): No. of instrumentation failure
CT: cervicothoracic, T: thoracic, TL: thoracolumbar, L: lumbar
The risk factors of instrumentation failure after multilevel TES were the resection level (p=0.004) and the length of the vertebral resection (p=0.017). On the other hand, preoperative irradiation, vertebral body cutting, cage subsidence, the progression of cage subsidence, and the use of end caps were not significant risk factors (Table 3).
Table 3.
Risk Factors of Instrumentation Failure.
| Parameter | p Value |
|---|---|
| Age | 0.308 |
| Sex | 0.707 |
| Resection level | 0.004* |
| Length of resected vertebrae | 0.017* |
| Radiation | 0.679 |
| Cutting vertebrae | 0.170 |
| Cage subsidence (>2mm) | 0.123 |
| Progression of cage subsidence | 0.101 |
| End cap use | 0.659 |
*Statistically significant
Radiolucent lines around the distal pedicle screws were observed in 3 patients, although the latter presented no clinical symptoms. Cage subsidence (>2 mm) was observed in 30 patients (63.8%), and was found one month after surgery in 22 of them. However, in 16 out of the 22 patients, there was no subsequent progression of the subsidence. Radiolucent lines between the cage and endplate were observed without any signs of instrumentation failure in 2 patients, both of whom remained without clinical symptoms for more than five years after the surgery. In 40 patients, bone fusion was achieved at the interface of the cage and endplate, with no radiolucent lines observed on the CT with MPRs taken more than one year after surgery.
Illustrative cases
Case 40
The patient was a 57-year-old man with a history of renal cell carcinoma who underwent TES of the inferior half of the T12 to L2 (Figure 2a and b). Cage subsidence developed over time, and the pedicle screws at the distal site backed out 15 months after surgery (Figure 2c and d). Moreover, tumor recurrence appeared on the lateral side of the cage. Revision surgery was performed with a posterior and anterior combined approach. Posterior instrumentation with three-above and three-below pedicle screw fixation was first performed, and a second-stage excision of the recurrent tumor beside the cage was then accomplished using a retroperitoneal approach. The remaining T12 and T11/12 disc and cage and L3 and L3/4 disc were totally removed, and subsequent anterior reconstruction was performed with a 150 mm-long cage, filled with iliac and fibula bone autografts. Posterior spinal shortening was performed, and four rods were used to reinforce the stability of the reconstruction (Figure 2e and f). As a result, solid bony fusion was obtained, with no signs of tumor recurrence at the surgical site. The patient was able to walk for more than six years after the second surgery. However, he died of the disease 87 months after the initial TES surgery.
Figure 2.
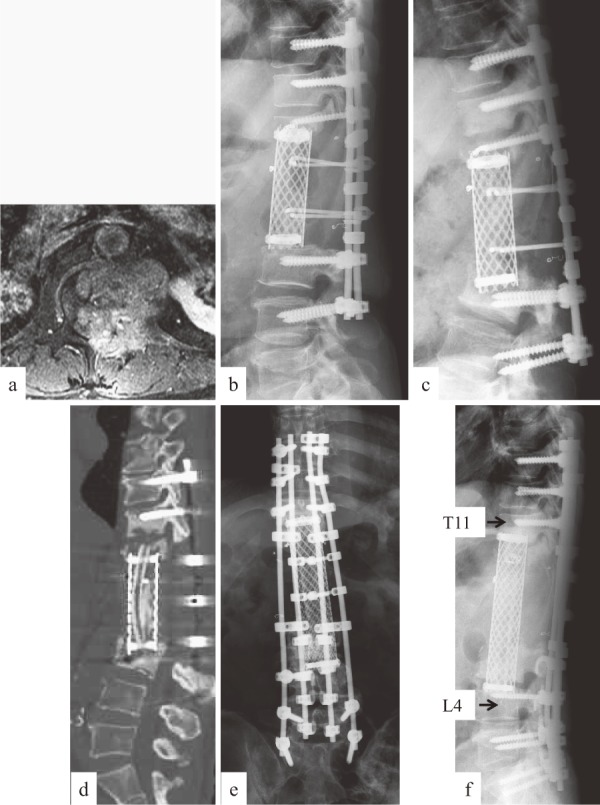
Case 40: RCC metastasis at T12-L2. a: Preoperative enhanced MRI showing extracompartmental tumor at L1 level. b: Lateral radiograph immediately after surgery showing no cage subsidence. c, d: Lateral radiograph (c) and sagittal CT reconstruction (d) at 15 months after surgery, demonstrating severe cage subsidence and screw back-out. e, f: Radiograph after revision surgery.
Case 36
The patient was a 52-year-old man with a history of renal cell carcinoma. His paralysis arising from a metastatic lesion at the L1 did not improve with 70 Gy radiation therapy. He underwent TES of the T12 to the superior 2/3rd of the L2 (Figure 3a and b). The remaining L2 eventually collapsed, with subsequent rod breakage documented at 28 months after the TES surgery (Figure 3c). Revision was performed using a combined posterior and anterior approach, and anterior reconstruction was conducted with iliac bone and rib grafts. Second-stage posterior instrumentation was performed with new pedicle screws and rods to replace the old ones. Solid bony fusion was achieved with no signs of tumor recurrence (Figure 3d, e and f). The patient was able to walk for more than 10 years after the TES surgery.
Figure 3.
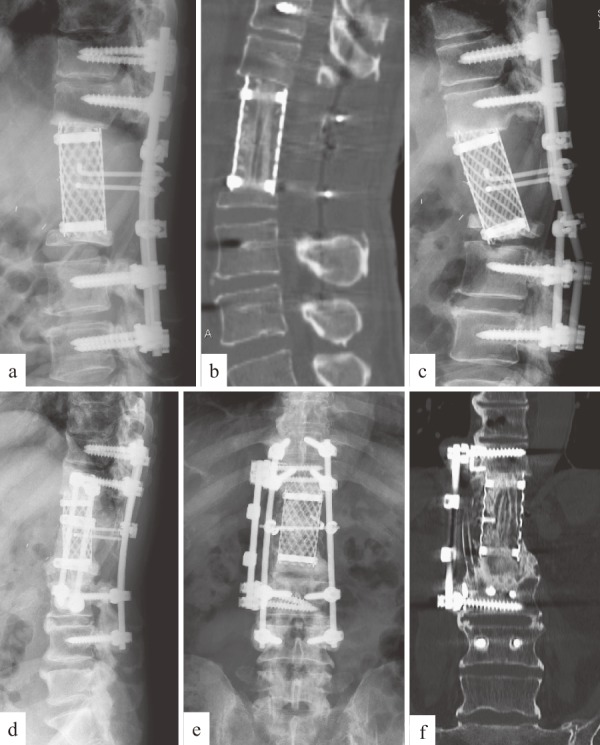
Case 36: RCC metastasis at T12-L2. a, b: Lateral radiograph (a) and sagittal CT reconstruction (b) immediately after surgery, showing no cage subsidence. c: Lateral radiograph at 28 months after surgery, showing rod breakage and collapse of remaining L2. d, e, f: Radiograph (d, e) and coronal CT reconstruction (f) after revision surgery, showing sufficient bony fusion.
Discussion
The treatment of patients with cancer has significantly evolved. As a result, patients are now surviving longer, and more of them are requiring treatment for spinal involvement. The surgical management of spinal tumors has witnessed tremendous advances in the past few decades. Indeed, we have developed our surgical techniques of both en bloc resection and instrumentation. Kato reported that most patients were satisfied and maintained a good ADL performance after undergoing TES for spinal tumors22,23). Therefore, it is now important to focus on spinal reconstruction after TES. The goal of spinal reconstruction after TES is to achieve bony fusion in patients with a long life expectancy. To achieve spinal fusion after multilevel TES, a number of adverse conditions have to be considered. These include the poor blood supply from the surrounding tissues caused by the wide dissection to remove the tumor vertebra, the need for a longer cage than a single vertebral resection, and the difficulty in obtaining robust stabilization. The number of segments removed influences spinal instability. Matsumoto identified spinal instrumentation failures in 40% of TES cases involving single-level resections27), and there have been some biomechanical reports of spinal reconstruction after single-level TES25,26,28-33). However, only one study has evaluated the biomechanical effects of spinal reconstruction after multilevel TES34). Therefore, the present article reports the largest case series of clinical outcomes from spinal reconstruction after multilevel TES performed in a single procedure.
This study took particular note of the spinal level. There was only one instrumentation failure (4.2%) above the T10 (cervicothoracic and thoracic spine), and it happened nearly 10 years after the TES surgery. In other words, despite the lack of bony fusion between the cage and vertebra, stability was sustained for a long time with our reconstruction technique. We consider that an upper spinal level promotes better stability than a lower spinal level due to the lower exposure of mechanical stresses. On the other hand, there were seven cases of instrumentation failure (30.4%) below the T10 (thoracolumbar and lumbar spine). We consider this to be related to two disadvantages from the low spinal level, namely, spinal mobility and a long resection length. After reconstruction, the mobility of the thoracolumbar and lumbar levels contributes to spinal instability. In addition, the vertebral size gets bigger in the lower spinal levels. The length of a three-whole vertebral resection was less than 70 mm in the thoracic spine, whereas it was more than 100 mm in the lumbar spine. It has been recommended to perform an additional anterior rod instrumentation and/or a longer posterior fixation after multilevel spondylectomy11,13,27). In our series, rod breakage without screw loosening occurred at the inferior end of the cage in four out of six cases. This result suggests that rod breakage was caused by instability from the delayed union between the distal end of the cage and the vertebral body. Although a longer posterior fixation may prevent screw loosening, it does not prevent rod breakage. We are in the process of considering CoCr rods, or additional rods (three or four rods) in the posterior instrumentation to reinforce the initial stabilization after a lower TES. However, we believe that a reconstruction method involving no additional anterior fixation should provide adequate stresses to allow the bone graft within the titanium mesh cages to undergo remodeling29).
In 2 patients, the remaining vertebral body had collapsed, with consequent backing-out of the pedicle screws (cases 40 and 43). Cage subsidence is believed to be one of the causes of instrumentation failure. The findings of this study suggested that cage subsidence is a common phenomenon after multilevel TES. In this study, it occurred within one month of the surgery in many cases. If the development of severe cage subsidence prevents bony fusion, the inherent stability of the fixed segments is damaged, thereby increasing the stress load on the rods. Yu reported that multilevel corpectomy was a unique risk factor of severe cage subsidence35). Therefore, we carefully evaluated the progression of cage subsidence as an important outcome of multilevel TES in the follow-up period. However, cage subsidence was not found to be a significant risk factor in this study. Most of the cases in our series showed no cage subsidence progression, and they eventually achieved bony fusion. We believe that, despite any observed cage subsidence, the eventual bony fusion prevented instrumentation failure. There was no progression of cage subsidence in any of the patients who underwent disc-to-disc cutting from one month after the multilevel TES. Although additional partial vertebral resection is understandably required to secure negative margins for extracompartmental spinal tumor, disc-to-disc cutting seems to be the ideal procedure. However, we have to balance the potential benefits and risks. The use of cage end caps is one possible way to increase the contact area and prevent cage subsidence. However, this study showed that 7of the 8 patients in whom cage end caps were used developed instrumentation failure. This finding may be associated with the spinal level of the resection, as end caps were mostly used at levels below the middle thoracic spine. We also suggest that the strength of the bony structure may be a factor, as we observed loss of bony contact when using an end cap. Further studies are required to evaluate the appropriateness of using cage end caps.
One component of our reconstruction method is spinal shortening. Spinal shortening presents three important advantages: 1) it reinforces the stability of the anterior and posterior spinal column; 2) it shortens the course of the bone remodeling, as compared with that involved in the use of an expandable cage; and 3) it increases spinal cord blood flow, which is beneficial to spinal cord function26). The safety limits and physiological effects of spinal shortening on the spinal cord have been investigated in dogs36). The results of that study suggested that spinal shortening within one-third of the vertebral segment was in the safe range, as characterized by the absence of deformity of the dural sac or the spinal cord36). The mean rate of spinal shortening in our study was 10.2% (maximum 29.7%), which was considered to be the allowed range. Spinal cord function was recovered or preserved in all 47 patients. Although expandable cages are one of the options for spinal reconstruction, they are inadequate for spinal shortening. This study demonstrated that two-above and two-below segmental fixation using pedicle screws is acceptable after a cervicothoracic or thoracic multilevel TES. We also analyzed the relationship between the shortening rate and the degree of cage subsidence. There was no significant correlation between these factors (Spearman correlation =-0.140, p=0.534). Therefore, we concluded that spinal shortening did not have a negative effect on cage subsidence.
Two patients developed rod breakage at the L5/S1 disc level, from iatrogenic spondylolisthesis at the L5 after complete bony fusion between the cage and the remaining L5. Only the caudal half of the L5 was left in both cases. As multilevel lumbar TES requires a longer construct for stability, L5/S1 interbody fusion may be considered a desirable component of spinal reconstruction. However, due to poor blood supply resulting from the wide dissection of surrounding tissues, the remaining L5 may not be in sufficiently good condition for an additional interbody fusion. A previous report showed high pseudarthrosis rates at the L5/S1 level in cases that required long spinal instrumentation with fusion to the sacrum37). As multilevel lumbar TES, including sacrum instrumentation, makes the planning of spinal reconstruction more difficult than TES at the other spinal levels, we have to weigh the improvement of spinal stability with the invasiveness of the technique. With this in mind, we have started to use a more robust cage and three or four rods for multilevel TES below the T10 level.
This study had some limitations. Throughout the follow-up period, the tumor histologies and adjuvant therapies were diverse. In addition, we did not consider the osteoporotic factors. Nonetheless, this report is valuable for its identification of the significant risk factors of instrumentation failure resulting from spinal reconstruction after multilevel TES.
Conclusion
Instrumentation failure occurred in one patient (5.9%) after thoracic multilevel TES, in 4 patients (25.0%) after thoracolumbar multilevel TES, and in 3 patients (42.9%) after lumbar multilevel TES. This study identified the significant risk factors of instrumentation failure after multilevel TES. There was a high risk of instrumentation failure at a lower spinal level of involvement, particularly below the thoracolumbar level. Moreover, a long vertebral resection length was another significant risk factor. After multilevel TES, cage subsidence was a common phenomenon (63.8%), occurring as early as within one month of the surgery in many cases. However, in most cases, the presence of cage subsidence did not have a negative effect on the spinal stability. Our spinal reconstruction method using pedicle screw fixation (two-above and two-below), a titanium mesh cage filled with autograft, and an artificial pedicle build-up can be a successful reconstruction technique for cases of multilevel TES above the T10 level, but may need to be reinforced in cases of TES below the T10 level.
Conflict of Interest: The authors declare that there are no conflicts of interest.
Acknowledgements
The authors thank Mamer S. Rosario for his kind criticism and advice.
References
- 1.Stener B. Total spondylectomy in chondrosarcoma arising from the seventh thoracic vertebra. J Bone Joint Surg [Br]. 1971; 53-B(2): 288-295. [PubMed] [Google Scholar]
- 2.Roy-Camille R, Saillant G, Bisserié M, et al. Total excision of thoracic vertebrae (author's transl). Rev Chir Orthop Reparatrice Appar Mot. 1981; 67(3): 421-430. (In French). [PubMed] [Google Scholar]
- 3.Sundaresan N, Rosen G, Huvos AG, Krol G. Combined treatment of osteosarcoma of the spine. Neurosurgery. 1988; 23(6): 714-719. [DOI] [PubMed] [Google Scholar]
- 4.Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine. 1997; 22(3): 324-333. [DOI] [PubMed] [Google Scholar]
- 5.Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy for solitary spinal metastases. Int Orthop. 1994; 18(5): 291-298. [DOI] [PubMed] [Google Scholar]
- 6.Tomita K, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006; 11(1): 3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami H, Kawahara N, Demura S, et al. Total en bloc spondylectomy for lung cancer metastasis to the spine. J Neurosurg Spine. 2010; 13(4): 414-417. [DOI] [PubMed] [Google Scholar]
- 8.Hart RA, Boriani S, Biagini R, et al. A System for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine. Spine. 1997; 22(15): 1773-1783. [DOI] [PubMed] [Google Scholar]
- 9.Boriani S, De Iure F, Bandiera S, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine. 2000; 25(7): 804-812. [DOI] [PubMed] [Google Scholar]
- 10.Demura S, Kawahara N, Murakami H, et al. Total en bloc spondylectomy for spinal metastases in thyroid carcinoma. J Neurosurg Spine. 2011; 14(2): 172-176. [DOI] [PubMed] [Google Scholar]
- 11.Disch AC, Schaser KD, Melcher I, et al. Oncosurgical results of multilevel thoracolumbar en-bloc spondylectomy and reconstruction with a carbon composite vertebral body replacement system. Spine. 2011; 36(10): E647-E655. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa K, Homma T, Hirano T, et al. Margin-free spondylectomy for extended malignant spine tumors: surgical technique and outcome of 13 cases. Spine. 2007; 32(1): 142-148. [DOI] [PubMed] [Google Scholar]
- 13.Liljenqvist U, Lerner T, Halm H, et al. En bloc spondylectomy in malignant tumors of the spine. Eur Spine J. 2008; 17(4): 600-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami H, Tomita K, Kawahara N, et al. Complete segmental resection of the spine, including the spinal cord, for telangiectatic osteosarcoma: a report of 2 cases. Spine. 2006; 31(4): E117-E122. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda Y, Sakayama K, Sugawara Y, et al. Mesenchymal chondrosarcoma treated with total en bloc spondylectomy for 2 consecutive lumbar vertebrae resulted in continuous disease-free survival for more than 5 years: case report. Spine. 2006; 31(8): E231-E236. [DOI] [PubMed] [Google Scholar]
- 16.Chanplakorn P, Chanplakorn N, Pongtippan A, et al. Recurrent epithelioid sarcoma in the thoracic spine successfully treated with multilevel total en bloc spondylectomy. Eur Spine J. 2011; 20(Suppl 2): S302-S308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Druschel C, Disch AC, Melcher I, et al. Surgical management of recurrent thoracolumbar spinal sarcoma with 4-level total en bloc spondylectomy: description of technique and report of two cases. Eur Spine J. 2012; 21(1): 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aryan HE, Acosta FL, Ames CP. Two-level total en bloc lumbar spondylectomy with dural resection for metastatic renal cell carcinoma. J Clin Neurosci. 2008; 15(1): 70-72. [DOI] [PubMed] [Google Scholar]
- 19.Boriani S, Biagini R, De Iure F, et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine. 1996; 21(16): 1927-1931. [DOI] [PubMed] [Google Scholar]
- 20.Kato S, Kawahara N, Murakami H, et al. Multi-level total en bloc spondylectomy for solitary lumbar metastasis of myxoid liposarcoma. Orthopedics. 2010; 33(6): 446. [DOI] [PubMed] [Google Scholar]
- 21.Cloyd JM, Acosta FL Jr, Polley MY, Ames CP. En bloc resection for primary and metastatic tumors of the spine: a systematic review of the literature. Neurosurgery. 2010; 67(2): 435-444 [DOI] [PubMed] [Google Scholar]
- 22.Kato S, Murakami H, Demura S, Yoshioka K, Kawahara N, Tomita K, Tsuchiya H. More than 10-year follow-up after total en bloc spondylectomy for spinal tumors. Ann Surg Oncol. 2014; 21(4): 1330-1336. [DOI] [PubMed] [Google Scholar]
- 23.Kato S, Murakami H, Demura S, Yoshioka K, Kawahara N, Tomita K, Tsuchiya H. Patient-reported outcome and quality of life after total en bloc spondylectomy for a primary spinal tumour. Bone Joint J. 2014; 96-B(12): 1693-1698. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Murakami H, Takeuchi A, Demura S, Yoshioka K, Kawahara N, Tomita K, Tsuchiya H. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013; 36(11): e1454-e1457. [DOI] [PubMed] [Google Scholar]
- 25.Kawahara N, Tomita K, Murakami H, et al. Total en bloc spondylectomy for spinal tumors: surgical techniques and related basic background. Orthop Clin North Am. 2009; 40(1): 47-63. [DOI] [PubMed] [Google Scholar]
- 26.Yoshioka K, Murakami H, Demura S, Kato S, Kawahara N, Tomita K, Tsuchiya H. Clinical outcome of spinal reconstruction after total en bloc spondylectomy at 3 or more levels. Spine. 2013; 38(24): E1511-E1516. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M, Watanabe K, Tsuji T, et al. Late instrumentation failure after total en bloc spondylectomy. J Neurosurg Spine. 2011; 15(3): 320-327. [DOI] [PubMed] [Google Scholar]
- 28.Disch AC, Schaser KD, Melcher I, et al. En bloc spondylectomy reconstructions in a biomechanical in-vitro study. Eur Spine J. 2008; 17(5): 715-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akamaru T, Kawahara N, Sakamoto J, et al. Transmission of the load sharing inside a titanium mesh cage used in anterior column reconstruction after total spondylectomy; a finite element analysis. Spine. 2005; 30(24): 2783-2787. [DOI] [PubMed] [Google Scholar]
- 30.Akamaru T, Kawahara N, Tsuchiya H, et al. Healing of autogenous bone in a titanium mesh cage used in anterior column reconstruction after total spondylectomy. Spine. 2002; 27(13): E329-E333. [DOI] [PubMed] [Google Scholar]
- 31.Kanayama M, Ng JTW, Cunningham BW, et al. Biomechanical analysis of anterior versus circumferential spinal reconstruction for various anatomic stages of tumor lesions. Spine. 1999; 24(5): 445-450. [DOI] [PubMed] [Google Scholar]
- 32.Oda I, Cunningham BW, Abumi K, et al. The stability of reconstruction methods after thoracolumbar total spondylectomy. Spine. 1999; 24(16): 1634-1638. [DOI] [PubMed] [Google Scholar]
- 33.Shannon FJ, DiResta GR, Ottaviano D, et al. Biomechanical analysis of anterior poly-methyl-methacrylate reconstruction following total spondylectomy for metastatic disease. Spine. 2004; 29(19): 2096-2102. [DOI] [PubMed] [Google Scholar]
- 34.Kato S, Murakami H, Higashino K, et al. The effect of spinal shortening after total en bloc spondylectomy: a biomechanical study in the thoracic spine. J Spinal Disord Tech. 2012; 25(6): E183-E190. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Chen D, Guo Y, et al. Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord Tech. 2008; 21(7): 489-492. [DOI] [PubMed] [Google Scholar]
- 36.Kawahara N, Tomita K, Kobayashi T, et al. Influence of acute shortening on the spinal cord. An experimental study. Spine. 2005; 30(6): 613-620. [DOI] [PubMed] [Google Scholar]
- 37.Kim YJ, Bridwell KH, Lenke LG, et al. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine. 2006; 31(20): 2329-2336. [DOI] [PubMed] [Google Scholar]


