Abstract
Introduction
Many patients suffer from discogenic low back pain. However, the mechanisms, diagnosistic strategy, and treatment of discogenic low back pain all remain controversial. The purpose of this paper was to review the pathological mechanisms of discogenic low back pain.
Methods
Many authors have investigated the pathological mechanisms of discogenic low back pain using animal models and examining human patients. Central to most investigations is understanding the innervation and instabilities of diseased intervertebral discs and the role of inflammatory mediators. We discuss three pathological mechanisms of discogenic low back pain: innervation, inflammation, and mechanical hypermobility of the intervertebral disc.
Results
Sensory nerve fibers include C-fibers and A delta-fibers, which relay pain signals from the innervated outer layers of the intervertebral disc under normal conditions. However, ingrowth of these sensory nerve fibers into the inner layers of intervertebral disc occurs under disease conditions. Levels of neurotrophic factors and some cytokines are significantly higher in diseased discs than in normal discs. Stablization of the segmental hypermobility, which can be induced by intervertebral disc degeneration, suppresses inflammation and prevents sensitization of sensory nerve fibers innervating the disc.
Conclusions
Pathological mechanisms of discogenic low back pain include sensory nerve ingrowth into inner layers of the intervertebral disc, upregulation of neurotrophic factors and cytokines, and instability. Inhibition of these mechanisms is important in the treatment of discogenic low back pain.
Keywords: low back pain, mechanisms, intervertebral disc
Introduction
Low back pain affects much of the world's population, and has significant adverse socioeconomic implications. The one-time occurrence of low back pain is about 15%-30% of the population; the one-month prevalence is 19%-43% of the population; and the lifetime prevalence is up to 60%-80% of the population1).
Low back pain may arise from different sites, such as an intervertebral disc, facet joint, or the sacroiliac joint(s). Several authors have reported on the prevalence of different origins of chronic low back pain. By injecting lidocaine into different structures in patients with chronic low back pain, the intervertebral disc was reported as the source in 39%-41%, facet joint 15%-32%, and sacroiliac joint 13%-18.5%2-4). Age distribution of discogenic low back pain was thought to be 36-47 years old, which is significantly younger than that in patients suffering low back pain from a facet joint or sacroiliac joint origin2-4).
We have previously reported that animal models and specimens from humans have revealed sensory innervation of lumbar intervertebral discs and sensory nerve ingrowth into the inner layer of intervertebral discs, causing painful conditions5). Cytokines such as tumor necrosis factor-α and interleukins induce this ingrowth. Nerve growth factor has also been recently identified as an inducer of ingrowth6). Finally, disc degeneration induces several collagenases; their action results in hypermobility and pain7).
In this paper, we review innervation, inflammation, and mechanical hypermobility of discogenic low back pain from studies of animal and humans (Fig. 1).
Figure 1.
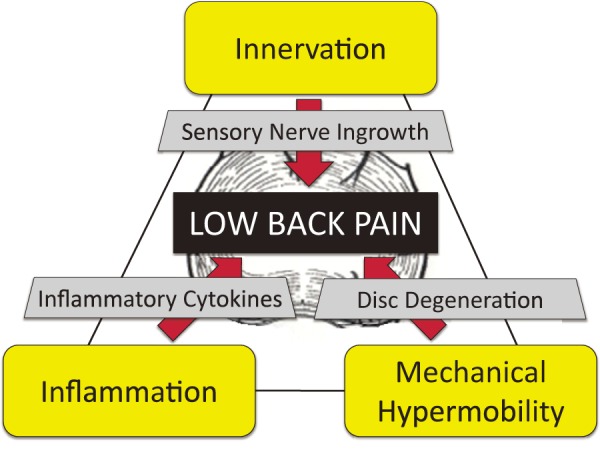
Pathomechanisms of discogenic low back pain. Innervation: animal model and specimens from humans revealed sensory nerve innervation of lumbar intervertebral discs (IVDs) and sensory nerve ingrowth into the inner layer (deep nerve ingrowth) of the degenerated IVD. Inflammation: many researchers have identified various proinflammatory molecules. Hypermobility: hypermobility of motion segment is usually induced in degenerated IVD. These factors are thought to be the major factors that induce discogenic low back pain.
Innervation
The vertebra, disc, facet joint, posterior longitudinal ligament (PLL), and dura mater are innervated segmentally by the dorsal ramus and the sinu-vertebral nerves branching from the spinal nerve of the corresponding levels8-12). Many studies have described the existence of sensory nerve endings in the annulus fibrosus13,14). It is believed that such nerve endings originate from the sinu-vertebral nerves branching from the ventral ramus of the spinal nerve and the ramus communicans of the corresponding level9,10). However, the level of the spinal cord or dorsal root ganglia (DRG) innervating the intervertebral disc has not been elucidated.
Takahashi et al. have reported direct evidence for groin pain corresponding to the L2 dermatome referred from the L4/5 intervertebral disc using an animal model15). Using another animal study, sensory nerve fibers from the lower intervertebral disc are thought to be innervated by DRGs at the corresponding level and in multiple segments by DRGs at upper levels. In nonsegmental innervation, sensory nerve fibers are thought to enter the paravertebral sympathetic trunks and reach the L2 DRGs16-19).
Human sensory innervation to intervertebral disc.
Patients who have degenerated lumbar discs in lower segments (L4-L5 or L5-S1) occasionally report groin pain20,21). Yukawa et al. reported that 21 of a total of 512 patients (4.1%) with groin pain were diagnosed with singular lower lumbar disc herniation (L4-L5 and L5-S1), and concluded that the sinu-vertebral nerve that innervates the posterior annulus fibrosus, the posterior longitudinal ligament, and the dura was the afferent nerve of the groin pain21). We have reported the efficacy of an L2 spinal nerve block for discogenic pain patients22,23). Finally, we have reported efficacy of lumbar interbody fusion surgery for groin pain without low back pain24). The patients suffered from groin pain and showed disc degeneration only at one level on magnetic resonance imaging (MRI). Patients did not show any hip joint abnormality on radiography or MRI. Anterior lumbar interbody fusion surgery resulted in a significant decrease in groin pain24). These results suggest L2 DRG innervation to lower intervertebral discs in human.
Pathogenesis of sensory nerve ingrowth into intervertebral discs causing painful conditions.
Some investigators disagree with the notion that nerve endings are present in the intervertebral disc and thereby deny the possibility of pain originating from discs themselves25,26). There is evidence to support the idea that sensory fibers are present in the outer layers of the annulus fibrosus under normal conditions7). Some reports have suggested that the presence of sensory nerve fibers in the deeper layer of the annulus fibrosus and the production of inflammatory mediators in the degenerated nucleus pulposus lead to discogenic low back pain in patients with degenerated intervetebral discs5,27). Burke et al. reported that intervertebral discs from patients with discogenic low back pain contained more inflammatory mediators than did intervetebral discs from patients with intervertebral disc herniation6). These reports strongly suggest an association between sensory nerve ingrowth, inflammatory mediators, and discogenic low back pain.
In animal models, the inflammatory mediators in the discs may promote CGRP-IR axonal ingrowth and may, at least in part, explain the mechanism of nerve ingrowth into the inner annulus28). It is also possible that nerve ingrowth is induced as a consequence of reduction of the barrier provided by proteoglycan and the human cartilage large aggregating proteoglycan, aggrecan, to axonal growth after degeneration of the lumbar intervertebral discs29,30). In fact, animal models of disc degeneration showed the induction of nerve ingrowth in association with a depletion of proteoglycan30). Considering our present and these previous reports, ingrowth of sensory nerve fibers might be closely associated with the pathogenesis of discogenic low back pain.
Inflammation
Human samples
Multiple authors have reported pain-related molecules, including tumor necrosis factor (TNF) alpha, Interleukin (IL)-1 beta, IL-4, IL-6, IL-8, IL-12, prostaglandin E2 (PGE2), interferon-gamma, and nitric oxide (NO) are up-regulated in herniated intervertebral discs resected during surgery6,30-33). Kepler et al. has reported pain-related molecules including chemokine regulated upon activation in normal T cells, expressed, probably secreted (RANTES) and its promoter, IL-1 beta, were significantly elevated in painful discs compared to painless discs34). In addition, Burke et al. documented that discs from patients with low back pain produced significantly more pain-related molecules than discs from patients with sciatica6). These findings suggested that there is persistent inflammation in painful discs, and that the production of these molecules may be a major factor in discogenic low back pain.
In vivo studies
Several animal models of intervertebral disc degeneration have been used as animal models of discogenic low back pain. Disc injury models including disc puncture by needles and disc stab by blade have been reported, and several pain-related molecules have been detected in the injured discs. In their study of disc puncture models in rabbits, using a 16-gauge needle, Sobajima et al. reported that disc injury induced the upregulation of IL-1 and nitric oxide synthase (iNOS) transiently (within 3 weeks)35). In our study using a disc puncture model, nerve growth factor and TNF-alpha levels (over 1 week) and IL-6 levels (over 4 days) were significantly increased in the injured disc, but the upregulation of these molecules resolved within 2 weeks after disc injury36). This type of disc injury induces transient inflammation, but degenerated discs in humans show persistent inflammation. This discrepancy might be one of the limitations of animal models of disc injury. Several authors have attempted to solve this discrepancy between animal models and human samples. Ulrich et al. reported repeated disc injury induced inflammatory response with elevated levels of TNF-alpha, IL-1 beta, and IL-8 up to 28 days after injury37). Lotz et al. developed a different animal model of disc degeneration, and reported disc static compression induced disc cell death depending on the magnitude and duration of spinal loading38). We modified this disc compression model and reported that the combination of disc dynamic compression and disc injury induced long-lasting upregulation of inflammatory mediators, including TNF-alpha, IL-1 beta, IL-6, and NGF39). This indicates that not only disc injury but also repetitive trauma or mechanical stress are important for representative animal models of disc degeneration in humans.
In vitro studies
Several authors have evaluated the role of pain-related molecules found in human samples or in vivo. Goupille et al. reported TNF-alpha is known to promote irreversible degradation of aggrecan; disc catabolism; and expression of inflammatory mediators and NGF40). In their in vitro study, Hoyland et al. reported that IL-1 beta was up-regulated in discs clinically associated with chronic low back pain and that IL-1 beta antagonists inhibited matrix degradation. They concluded that IL-1 is a key cytokine mediator in degenerated discs and therefore a therapeutic target41).
The role of immune cells
Despite controversy surrounding which cells produce pain-related molecules, it has been reported there were several immune cells including macrophages, T-cells, B-cells, and natural killer cells in degenerated discs42,43). In addition, inflammatory cytokines studies have reported that pain-related molecules are expressed by immune cells, including macrophages33,44,45). Takata et al. demonstrated that interaction between disc tissue and macrophages is necessary for upregulation of IL-6 production44). However, whether macrophages exist in normal healthey disc was still unclear. Nerlich et al. reported that the intact nucleus pulposus contains a high number of resident macrophages46). On the other hand, it has been reported that the healthy normal intervertebral disc was an immunologically privileged environment47). One hypothesis for the possible entry pathway of immune cells is that the injury of annulus fibrosus, the leakage of nucleus pulposus, and deep nerve and vessels ingrowth into the disc might be a trigger of immune cell supply in discs48).
Therapeutic targets for discogenic low back pain
These inflammatory mediators are potential targets for discogenic low back pain. In a rat disc injury model, intradiscal injection of etanercept (TNF-alpha inhibitor) suppressed pain-related neuropeptide expression in DRGs innervating injured discs49). Clinical studies have revealed the efficacy of these inhibitors in discogenic low back pain. Tobinick et al. reported that TNF-alpha inhibition by etanercept delivered by perispinal administration may offer clinical benefit to patients with chronic, treatment-resistant discogenic pain50). Sainoh et al. reported the efficacy of etanercept and anti-IL6 antibody for disc pain patients compared to placebo51,52). Tanezumab is a humanized monoclonal antibody that specifically inhibits nerve growth factor as a treatment for chronic pain. In a study where patients (n = 1,347) received intravenous tanezumab, naproxen, or placebo, tanezumab provided significantly greater improvement in pain, function, and global scores vs. placebo and naproxen in patients with chronic low back pain53).
Mechanical Hypermobility
Segmental hypermobility is considered another major factor associated with discogenic low back pain54-56). Hypermobility is induced through disc degeneration because the lumbar intervertebral discs have a major load-bearing role in humans56), but disc degeneration itself is reported not to be associated with hypermobility57). Of course, not all degenerated discs are symptomatic, and symptomatic and asymptomatic degenerated intervertebral discs show similar structural and biochemical features58,59). Disc degeneration is markedly common, but a definitive and widely accepted definition remains unclear. In clinical studies using MRI, disc degeneration has been suggested to be one of the most remarkable risk factors for discogenic low back pain60-63). Many papers identified genetic influences and unidentified factors, which include complex and unpredictable interactions for the presence of disc degeneration, besides, disc degeneration might not be induced by most environmental factors64-68). Even more, several mechanisms have sought to explain how degenerative changes in the disc cause pain. In 1989, Nachemson suggested in a presentation at the AAOS, that environmentally or genetically induced premature aging changes may render the disc mechanically incompetent, creating abnormal motion patterns that subject various spinal structures to undue stress, causing pain64).
Some studies have reported instability for mild degeneration65,66,69), while other studies have showed increasing spinal stiffness with progressing degeneration70,71). Some papers indicated that hypermobility between flexion and extension is associated with degenerative disc disease72,73). Fujiwara et al. evaluated 110 lumbar motion segments from 44 human cadavers, and reported that segmental motion increased with increasing severity of disc degeneration to grade IV and decreased in grade V, as classified by MRI. Also, such segmental motion changes were greater in axial rotation compared with the other motion as lateral bending, flexion and extension65). Tanaka et al. evaluated 114 lumbar spine segments taken from 47 fresh cadavers and suggested that lumbar spine angular mobility is related to disc degeneration, and angulation was greater in grades III and IV degeneration, in which radial tears of the annulus fibrosus are found in Thompson's grading system69). Several animal studies advocated that torsional loads are important factors for the degeneration of the motion segments74-76). In cadaveric study, a relationship between the severity of disc degeneration and increases of the torsional movement was reported77). Although, it is consensus in previous papers that severe loss of height in the intervertebral disc, sclerosis in the endplate, and osteophyte formation around bone structure were induced in the final stage of disc degeneration, resulting in stabilization of the motion segments, as first reported in 1980s by Kirkaldy-Willis55,69,78-80).
Histologically, based on the loss of differentiation between the annulus fibrosus and nucleus pulposus, as well as changes in collagen content from Type II to Type I and decreased proteoglycan, hypermobility at the specific lumbar segment is induced by the loss of structural integrity, insufficient hydration, and the lack of tolerance against motion of lumbar spine67,81-86). At the next stage, decreased hydration within the nucleus pulposus results in decreased disc pressure and reduced disc height, and at that time, degeneration is characterized by a fibrotic nucleus pulposus and an annulus fibrosis with many clefts or fissures86). As the inner part of the annulus fibrosus increases in size and the interface between the nucleus pulposus and annulus fibrosus becomes unclear, segmental hypermobility is gradually stabilized with a disc height narrowing55).
To create a model of discogenic low back pain, puncture incision of discs has been widely used in various animals37,87,88). Using this puncture-induced discogenic low back pain model, stabilization established by lumbar posterolateral fusion, inhibited sensory nerve ingrowth into punctured intervertebral discs and upregulation of CGRP expression in DRG neurons innervating intervertebral discs in rats, suggests that stabilization itself can reduce discogenic low back pain89). It is still unclear how hypermobility of the segment is related to the development of discogenic low back pain, but at least, pain induced by the puncture of the intervertebral disc was suppressed by stabilization of the affected segment. This may suggest that transient or persistent inflammation in the disc, which is induced by hypermobility of the lumbar spine, is suppressed, resulting in pain relief. However, there is evidence in human studies that suggests that histomorphological features, instability of the lumbar spine, and low back pain bear no relationship to one another90), and that the clinical outcome of lumbar fusion surgery is highly variable91). This suggests that many factors, including patient selection or background, may affect the surgical outcome. Definite evidence of stabilization efficacy remains elusive. Further studies should be undertaken to shed light on the difference between symptomatic and asymptomatic degenerated discs, and the pathological mechanisms of discogenic low back pain.
Conclusions
Animal models and samples from humans have revealed that sensory nerve innervation of lumbar intervertebral discs and increases in levels of cytokines such as TNF-alpha, interleukins, and NGF, may be accelerated by disc degeneration and hypermobility. In this regard, it is important to prevent sensitization of sensory nerve fibers innervating the disc, suppress increases of cytokines, and possibly decrease disc hypermobility for the treatment of discogenic low back pain.
Conflicts of Interest: The authors declare no conflicts of interest with respect to the authorship and publication of this article.
References
- 1.Nachemson A. Epidemiology and the economics of low back pain. In: Herkowits HN, Dvorak J, Bell G, Nordin M, Grob D, eds. The Lumbar Spine, 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 3-10. [Google Scholar]
- 2.Schwarzer AC, Aprill CN, Derby R, et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20(17):1878-83. [DOI] [PubMed] [Google Scholar]
- 3.Manchikanti L, Manchikanti KN, Cash KA, et al. Age-related prevalence of facet-joint involvement in chronic neck and low back pain. Pain Physician. 2008;11(1):67-75. [PubMed] [Google Scholar]
- 4.Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21(16):1889-92. [DOI] [PubMed] [Google Scholar]
- 5.Shinohara H. A study on lumbar disc lesion. Significance of histology of free nerve endings in lumbar disc. J Jap Orthop Ass. 1970;44:553-70. Japanese. [PubMed] [Google Scholar]
- 6.Burke JG, Watson RW, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84(2):196-201. [DOI] [PubMed] [Google Scholar]
- 7.Ohtori S, Inoue G, Miyagi M, et al. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15(6):1347-55. [DOI] [PubMed] [Google Scholar]
- 8.Bogduk N, Long DM. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low back pain. J Neurosurg. 1979;51(2):172-7. [DOI] [PubMed] [Google Scholar]
- 9.Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132(1):39-56. [PMC free article] [PubMed] [Google Scholar]
- 10.Bogduk N. The innervation of the lumbar spine. Spine 1983;8(3):286-93. [DOI] [PubMed] [Google Scholar]
- 11.Edgar MA, Nundy S. Innervation of spinal dura mater. J Neurol Neurosurg Psychiatry. 1966;29:530-4. [Google Scholar]
- 12.Edgar MA, Ghadially JA. Innervation of the lumbar spine. Clin Orthop Relat Res. 1976;(115):35-41. [PubMed] [Google Scholar]
- 13.Hirsch C, Ingelmark B, Miller M. The anatomical basis for low back pain: Studies on the presence of sensory nerve endings in ligamentous, capsular, and intervertebral disc structures in the human lumbar spine. Acta Orthop Scand. 1963;33:1-17. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizawa H, O'Brien JP, Smith WT, et al. The neuropathology of intervertebral discs removed for low back pain. J Pathol. 1980;132(2):95-104. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Nakajima Y, Sakamoto T, et al. Capsaicin applied to rat lumbar intervertebral disc causes extravasation in the groin skin: A possible mechanism of referred pain of the intervertebral disc. Neurosci Lett. 1993;161(1):1-3. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura S, Takahashi K, Takahashi Y, et al. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine. 1996;21(8):917-24. [DOI] [PubMed] [Google Scholar]
- 17.Ohtori S, Takahashi Y, Takahashi K, et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24(22):2295-9. [DOI] [PubMed] [Google Scholar]
- 18.Ohtori S, Takahashi K, Chiba T, et al. Sensory innervation of the dorsal portion of the lumbar intervertebral discs in rats. Spine. 2001;26(8):946-50. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Hou S, Peng B, et al. Effect of the L2 ramus communicans on the nociceptive pathway in lumbar intervertebral discs in rats. Eur J Pain. 2008;12(6):798-803. [DOI] [PubMed] [Google Scholar]
- 20.Murphey F. Sources and patterns of pain in disc disease. Clin Neurosurg. 1968;15:343-51. [DOI] [PubMed] [Google Scholar]
- 21.Yukawa Y, Kato F, Kajino G, et al. Groin pain associated with lower lumbar disc herniation. Spine. 1997;22(15):1736-9. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura S, Takahashi K, Takahashi Y, et al. The afferent pathways of discogenic low back pain. J Bone Joint Surg Br. 1996;78(4):606-12. [PubMed] [Google Scholar]
- 23.Ohtori S, Nakamura S, Koshi T, et al. Effectiveness of L2 spinal nerve infiltration for selective discogenic low back pain patients. J Orthop Sci. 2010;15(6):731-6. [DOI] [PubMed] [Google Scholar]
- 24.Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-8. [DOI] [PubMed] [Google Scholar]
- 25.Roofe PG. Innervation of annulus fibrosus and posterior longitudinal ligament. Arch Neurol Psychiatry. 1940;44(1):100-3. [Google Scholar]
- 26.Wiberg G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand. 1949;19(2):211-21. [DOI] [PubMed] [Google Scholar]
- 27.Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350(9072):178-81. [DOI] [PubMed] [Google Scholar]
- 28.Aoki Y, Akeda K, An H, et al. Nerve fiber ingrowth into scar tissue formed following nucleus pulposus extrusion in the rabbit annular-puncture disc degeneration model: Effects of depth of puncture. Spine. 2006;31(21):E774-80. [DOI] [PubMed] [Google Scholar]
- 29.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136(1):32-43. [DOI] [PubMed] [Google Scholar]
- 30.Davies SJ, Fitch MT, Memberg SP, et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390(6661):680-3. [DOI] [PubMed] [Google Scholar]
- 31.Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21(3):271-7. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi H, Suguro T, Okazime Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21(2):218-24. [DOI] [PubMed] [Google Scholar]
- 33.Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kepler CK, Markova DZ, Dibra F, et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1beta in painful human intervertebral discs. Spine. 2013;38(11):873-80. [DOI] [PubMed] [Google Scholar]
- 35.Sobajima S, Shimer AL, Chadderdon RC, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14-23. [DOI] [PubMed] [Google Scholar]
- 36.Miyagi M, Ishikawa T, Orita S, et al. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: Pathomechanism of chronic diskogenic low back pain. Spine. 2011;36(26):2260-6. [DOI] [PubMed] [Google Scholar]
- 37.Ulrich JA, Liebenberg EC, Thuillier DU, et al. ISSLS prize winner: Repeated disc injury causes persistent inflammation. Spine. 2007;32(25):2812-9. [DOI] [PubMed] [Google Scholar]
- 38.Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25(12):1477-83. [DOI] [PubMed] [Google Scholar]
- 39.Miyagi M, Ishikawa T, Kamoda H, et al. Disk dynamic compression in rats produces long-lasting increases in inflammatory mediators in disks and induces long-lasting nerve injury and regeneration of the afferent fibers innervating disks: A pathomechanism for chronic diskogenic low back pain. Spine. 2012;37(21):1810-8. [DOI] [PubMed] [Google Scholar]
- 40.Goupille P, Mulleman D, Paintaud G, et al. Can sciatica induced by disc herniation be treated with tumor necrosis factor alpha blockade? Arthritis Rheum. 2007;56(12):3887-95. [DOI] [PubMed] [Google Scholar]
- 41.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology. 2008;47(6):809-14. [DOI] [PubMed] [Google Scholar]
- 42.Geiss A, Larsson K, Rydevil B, et al. Autoimmune properties of nucleus pulposus: An experimental study in pigs. Spine. 2007;32(2):168-73. [DOI] [PubMed] [Google Scholar]
- 43.Mukai K, Sakai D, Nakamura Y, et al. Primary immune system responders to nucleus pulposus cells: Evidence for immune response in disc herniation. Eur Cell Mater. 2010;19:13-21. [DOI] [PubMed] [Google Scholar]
- 44.Takada T, Nishida K, Doita M et al. Interleukin-6 production is upregulated by interaction between disc tissue and macrophages. Spine. 2004;29(10):1089-92. [DOI] [PubMed] [Google Scholar]
- 45.Punt IM, Cleutjens JP, de Bruin T, et al. Periprosthetic tissue reactions observed at revision of total intervertebral disc arthroplasty. Biomaterials. 2009;30(11):2079-84. [DOI] [PubMed] [Google Scholar]
- 46.Nerlich AG, Weiler C, Zipperer J, et al. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine. 2002;27(22):2484-90. [DOI] [PubMed] [Google Scholar]
- 47.Takada T, Nishida K, Doita M, et al. Fas ligand exists on intervertebral disc cells: A potential molecular mechanism for immune privilege of the disc. Spine. 2002;27(14):1526-30. [DOI] [PubMed] [Google Scholar]
- 48.Miyagi M, Millecamps M, Danco AT, et al. Increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine. 2014;39(17):1345-54. [DOI] [PubMed] [Google Scholar]
- 49.Horii M, Orita S, Nagata M, et al. Direct application of the tumor necrosis factor-α inhibitor, etanercept, into a punctured intervertebral disc decreases calcitonin gene-related peptide expression in rat dorsal root ganglion neurons. Spine. 2011;36(2):E80-5. [DOI] [PubMed] [Google Scholar]
- 50.Tobinick EL, Britschgi-Davoodifar S. Perispinal TNF-alpha inhibition for discogenic pain. Swiss Med Wkly. 2003;133(11-12):170-7. [DOI] [PubMed] [Google Scholar]
- 51.Sainoh T, Orita S, Miyagi M, et al. Single intradiscal injection of the interleukin-6 receptor antibody tocilizumab provides short-term relief of discogenic low back pain; prospective comparative cohort study. J Orthop Sci. 2016;21(1):2-6. [DOI] [PubMed] [Google Scholar]
- 52.Sainoh T, Orita S, Miyagi M, et al. Single intradiscal administration of the tumor necrosis factor-alpha inhibitor, Etanercept, for patients with discogenic low back pain. Pain Med. 2016;17(1):40-5. [DOI] [PubMed] [Google Scholar]
- 53.Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013;154(7):1009-21. [DOI] [PubMed] [Google Scholar]
- 54.Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: Review of animal model data. J Bone Joint Surg Am. 2006;88(Suppl 2):76-82. [DOI] [PubMed] [Google Scholar]
- 55.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;(165):110-23 [PubMed] [Google Scholar]
- 56.Nachemson AL, Schultz AB, Berkson MH. Mechanical properties of human lumbar spine motion segments: Influences of age, sex, disc level, and degeneration. Spine. 1979;4(1):1-8. [DOI] [PubMed] [Google Scholar]
- 57.Burton AK, Battié MC, Gibbons L, et al. Lumbar disc degeneration and sagittal flexibility. J Spinal Disord. 1996;9(5):418-24. [PubMed] [Google Scholar]
- 58.Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69-73. [DOI] [PubMed] [Google Scholar]
- 59.Kanayama M, Togawa D, Takahashi C, et al. Cross-sectional magnetic resonance imaging study of lumbar disc degeneration in 200 healthy individuals. J Neurosurg Spine. 2009;11(4):501-7. [DOI] [PubMed] [Google Scholar]
- 60.Erkintalo MO, Salminen JJ, Alanen AM, et al. Development of degenerative changes in the lumbar intervertebral disk: Results of a prospective MR imaging study in adolescents with and without low-back pain. Radiology. 1995;196:529-33. [DOI] [PubMed] [Google Scholar]
- 61.Luoma K, Riihimäki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487-92. [DOI] [PubMed] [Google Scholar]
- 62.Takatalo J, Karppinen J, Niinimäki J, et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults? Spine. 2011;36:2180-9. [DOI] [PubMed] [Google Scholar]
- 63.Samartzis D, Borthakur A, Belfer I, et al. Novel diagnostic and prognostic methods for disc degeneration and low back pain. Spine J. 2015;15:1919-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Battié MC, Videman T, Gibbons LE, et al. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration: A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine. 1995;20:2601-12. [PubMed] [Google Scholar]
- 65.Fujiwara A, Tamai K, An HS, et al. The relationship between disc degeneration, facet joint osteoarthritis, and stability of the degenerative lumbar spine. J Spinal Disord. 2000;13:444-50. [DOI] [PubMed] [Google Scholar]
- 66.Krismer M, Haid C, Behensky H, et al. Motion in lumbar functional spine units during side bending and axial rotation moments depending on the degree of degeneration. Spine. 2000;25:2020-7. [DOI] [PubMed] [Google Scholar]
- 67.Seki S, Kawaguchi Y, Chiba K, et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet. 2005;37:607-12. [DOI] [PubMed] [Google Scholar]
- 68.Battié MC, Videman T. Lumbar disc degeneration: Epidemiology and genetics. J Bone Joint Surg Am. 2006;88(Suppl 2):3-9. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka N, An HS, Lim TH, et al. The relationship between disc degeneration and flexibility of the lumbar spine. Spine J. 2001;1:47-56. [DOI] [PubMed] [Google Scholar]
- 70.Kettler A, Rohlmann F, Ring C, et al. Do early stages of lumbar intervertebral disc degeneration really cause instability? Evaluation of an in vitro database. Eur Spine J. 2011;20:578-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oxland TR, Lund T, Jost B, et al. The relative importance of vertebral bone density and disc degeneration in spinal flexibility and interbody implant performance. An in vitro study. Spine. 1996;21:2558-69. [DOI] [PubMed] [Google Scholar]
- 72.Hayes MA, Howard TC, Gruel CR, et al. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine. 1989;14:327-31. [DOI] [PubMed] [Google Scholar]
- 73.Stokes IA, Frymoyer JW. Segmental motion and instability. Spine. 1987;12:688-91. [DOI] [PubMed] [Google Scholar]
- 74.Barbir A, Godburn KE, Michalek AJ, et al. Effects of torsion on intervertebral disc gene expression and biomechanics, using a rat tail model. Spine. 2011;36:607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veres SP, Robertson PA, Broom ND. The influence of torsion on disc herniation when combined with flexion. Eur Spine J. 2010;19:1468-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farfan HF, Cossette JW, Robertson GH, et al. The effects of torsion on the lumbar intervertebral joints: The role of torsion in the production of disc degeneration. Bone Joint Surg Am. 1970;52:468-97. [PubMed] [Google Scholar]
- 77.Lim TH, Eck JC, An HS, et al. A noninvasive, three-dimensional spinal motion analysis method. Spine. 1997;22:1996-2000. [DOI] [PubMed] [Google Scholar]
- 78.Jang SY, Kong MH, Hymanson HJ, et al. Radiographic parameters of segmental instability in lumbar spine using kinetic MRI. J Korean Neurosurg Soc. 2009;45:24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kong MH, Morishita Y, He W, et al. Lumbar segmental mobility according to the grade of the disc, the facet joint, the muscle, and the ligament pathology by using kinetic magnetic resonance imaging. Spine. 2009;34:2537-44. [DOI] [PubMed] [Google Scholar]
- 80.Wilke HJ, Rohlmann F, Neidlinger-Wilke C, et al. Validity and interobserver agreement of a new radiographic grading system for intervertebral disc degeneration: Part I. Lumbar spine. Eur Spine J. 2006;15:720-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869-74. [DOI] [PubMed] [Google Scholar]
- 82.Roughley PJ. Biology of intervertebral disc aging and degeneration: Involvement of the extracellular matrix. Spine. 2004;29:2691-9. [DOI] [PubMed] [Google Scholar]
- 83.Niosi CA, Oxland TR. Degenerative mechanics of the lumbar spine. Spine J. 2004;4:202S-8S. [DOI] [PubMed] [Google Scholar]
- 84.Zhao F, Pollintine P, Hole BD, et al. Discogenic origins of spinal instability. Spine. 2005;30:2621-30. [DOI] [PubMed] [Google Scholar]
- 85.Mimura M, Panjabi MM, Oxland TR, et al. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371-80. [DOI] [PubMed] [Google Scholar]
- 86.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307-14. [DOI] [PubMed] [Google Scholar]
- 87.Inoue G, Ohtori S, Aoki Y, et al. Exposure of the nucleus pulposus to the outside of the annulus fibrosus induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine. 2006;31:1433-8. [DOI] [PubMed] [Google Scholar]
- 88.Rousseau MA, Ulrich JA, Bass EC. Stab incision for inducing intervertebral disc degeneration in the rat. Spine. 2007;32:17-24. [DOI] [PubMed] [Google Scholar]
- 89.Koshi T, Ohtori S, Inoue G, et al. Lumbar posterolateral fusion inhibits sensory nerve ingrowth into punctured lumbar intervertebral discs and upregulation of CGRP immunoreactive DRG neuron innervating punctured discs in rats. Eur Spine J. 2010;19:593-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyauchi A, Baba I, Sumida T, et al. Relationship between the histological findings of spondylolytic tissue, instability of the loose lamina, and low back pain. Spine 2008;33:687-93. [DOI] [PubMed] [Google Scholar]
- 91.Carreon LY, Glassman SD, Howard J. Fusion and nonsurgical treatment for symptomatic lumbar degenerative disease: A systematic review of Oswestry Disability Index and MOS Short Form-36 outcomes. Spine J. 2008;8:747-55. [DOI] [PubMed] [Google Scholar]


