Abstract
Objective
Glucocorticoid-induced osteoporosis (GIOP) is the most common form of secondary osteoporosis. In May 2018, denosumab was approved for the treatment of GIOP in men and women at high risk of fracture. We undertook a systematic review and meta-analysis to summarize the efficacy and safety of denosumab in the prevention and treatment of GIOP.
Methods
We searched PubMed, CINAHL, American College of Rheumatology and American Society for Bone and Mineral Research meeting abstracts for relevant studies. We included studies in which subjects were taking systemic glucocorticoid therapy and were assigned to take denosumab or control therapy, and assessed the effect of treatment on areal bone mineral density (BMD), fractures and/or safety.
Results
Three eligible studies were included in the primary meta-analysis. Denosumab significantly increased lumbar spine BMD (2.32%, 95% CI 1.73%, 2.91%, P<0.0001) and hip BMD (1.52%, 95% CI 1.1%,1.94%, P<0.0001) compared to bisphosphonates. Adverse events, serious adverse events and fractures were similar between denosumab and bisphosphonate arms.
Conclusion
Results suggest that denosumab is superior to bisphosphonates in its effects on lumbar spine and total hip BMD in patients with GIOP. There was no difference in the incidence of infections, adverse events or serious adverse events. Studies were underpowered to detect differences in the risk of fracture. Denosumab is a reasonable option for treatment of GIOP. However, further studies are needed to guide transitions off denosumab.
Keywords: denosumab, glucocorticoid-induced osteoporosis, bone mineral density, fractures, safety
Plain language summary
The collective data from three clinical trials shows that one year of denosumab therapy increased spine and hip bone mineral density more than bisphosphonate therapy. The collective data from the completed trials showed no difference in the risk of infections, mild or serious side effects between people who took denosumab, compared to people who took bisphosphonate therapy.
Introduction
Clinicians frequently prescribe glucocorticoids to treat common medical conditions including asthma, chronic obstructive pulmonary disease, rheumatoid arthritis, polymyalgia rheumatica, giant cell arteritis and inflammatory bowel disease. Over 20 years, the use of long-term oral glucocorticoid therapy increased in the UK by 34%, with nearly 1% of the population taking long-term (≥3 months) glucocorticoid therapy in 2008.1 In some geographic regions, use is even more common. For example, 17% of the population in France used oral glucocorticoids at least once in 2014.2 While most use was short-term, nearly 2% of the population filled ≥6 prescriptions per year.2
Glucocorticoid use is the most common cause of secondary osteoporosis, and the most common form of drug-induced osteoporosis. The cellular mechanisms by which glucocorticoids harm the skeleton are complex. Initially, glucocorticoids increase expression of receptor-activator nuclear kappa B ligand (RANKL), a cytokine that increases osteoclast differentiation and activation. Simultaneously, glucocorticoids reduce expression of the RANKL decoy receptor, osteoprotegerin. In concert, these actions increase osteoclastic bone resorption, primarily in the initial phase of glucocorticoid therapy.3 With long-term use, the main effect of glucocorticoid therapy is reduced bone formation, via increased osteoblast and osteocyte apoptosis.4 Glucocorticoid therapy can also contribute to osteoporosis pathogenesis by causing hypogonadotropic hypogonadism, reduced intestinal calcium absorption and hypercalciuria.5
Glucocorticoid therapy causes a rapid decline in areal bone mineral density (BMD). In one study,6 rheumatoid arthritis patients who took prednisone 10 mg daily for 12 weeks and then tapered off by the 20th week experienced an 8% decline in spine BMD, with partial recovery of BMD by week 44. Not surprisingly, up to 25% of patients taking systemic glucocorticoid therapy will develop fractures.7–10
Until recently, only four medications were Food and Drug Administration (FDA)-approved to treat glucocorticoid-induced osteoporosis (GIOP): alendronate, risedronate, zoledronate and teriparatide. In clinical trials, all medications increased spine BMD. Placebo-controlled trials documented fewer fractures with risedronate11,12 and alendronate.13,14 Zoledronate was compared to risedronate in a double-blind-controlled trial15 1 year of either medication was associated with low fracture rates in both arms, and no significant difference between the two drugs. Teriparatide was compared to alendronate in a clinical trial lasting 36 months16 teriparatide was associated with greater increments in BMD and fewer vertebral fractures.
Denosumab is a human monoclonal antibody against RANKL that is FDA approved to reduce osteoporotic fractures in postmenopausal women at high risk of fracture, as placebo-controlled clinical trials documented its ability to reduce major osteoporotic fractures. Denosumab is also FDA approved to increase BMD in men with osteoporosis or men at high risk of fracture. In May 2018, the FDA approved the use of denosumab to treat GIOP in adults.
In RANKL knock-in mice exposed to glucocorticoids, denosumab-preserved spine and hip BMD while concurrently reducing osteoclastic bone resorption, compared to control mice.17 The efficacy of denosumab in the murine model of GIOP, coupled with its efficacy in treating postmenopausal osteoporosis, prompted human studies using denosumab to treat GIOP. The purpose of this systematic review and meta-analysis is to summarize published data describing the efficacy and safety of denosumab in the treatment of GIOP in adults. We registered our study with PROSPERO (registration number CRD42019129233); no other systematic reviews focusing on denosumab use for GIOP were found in the PROSPERO database.
Materials and methods
We searched PubMed and CINAHL from January 1, 2000 to September 1, 2017 using the terms “denosumab,” “glucocorticoid,” “osteoporosis,” “glucocorticoid induced osteoporosis” and “safety.” Studies in any language were included. Two authors independently reviewed the abstracts of all publications to determine eligibility. We also searched the titles of abstracts presented at the American College of Rheumatology and American Society for Bone and Mineral Research in 2013, 2014, 2015 and 2016 using search terms “denosumab” and “glucocorticoid.” We searched Pubmed to determine whether relevant abstracts presented at these meetings were subsequently published. If not published, we next contacted authors via email to inquire on the date of anticipated publication. We updated our literature search in February 2019.
Studies were included if they recruited subjects taking systemic glucocorticoid therapy, used denosumab and a control or placebo arm, and assessed the effect of treatment on BMD, fractures and/or safety. We excluded review articles, case reports and case series. Figure 1 summarizes the total number of articles identified and reasons for exclusion.
Figure 1.
Flow diagram of literature search and study inclusion.
Both authors independently extracted data from included publications including the year of publication, number of subjects assigned to control and denosumab therapy, and study outcomes including changes in spine and hip areal BMD, clinical and radiographic fractures and side effects. Of note, two clinical trials supported by Amgen Pharmaceuticals20,23 did not report data in the format needed to perform a meta-analysis (mean and SD for the change in BMD). Thus, the authors formally requested, and received, the needed data for these two clinical trials from the company.
Both authors independently rated the quality of each included publication, using the Downs and Black quality scale for intervention studies.18 One author prepared a table summarizing details of the included studies.
Statistical analysis
All data were summarized using the mean and SD, then entered in duplicate into an Excel file and analyzed using R software version 3.1.2 and the package “meta.” We compared the effect of denosumab versus control therapy on BMD, using Forest plots and a random effect model. We also compared the odds of fractures, infections, adverse events and serious adverse events between denosumab and control therapy, using a random effect model. We viewed Funnel plots to evaluate publication bias and used the I2 statistic to assess study heterogeneity, with 25%, 50%, and 75% indicating low, moderate, and high heterogeneity.19 Our primary analyses focused on the three trials comparing denosumab to bisphosphonate therapy. We performed additional analyses by including a fourth trial in which placebo was the control arm.
Results
From our literature search, we identified 95 articles of interest. After screening for eligibility based on the aforementioned inclusion criteria, 88 articles were excluded (Figure 1). We assessed the remaining 7 full-length articles for eligibility. Of these, 3 publications were excluded due to lack of a control or placebo arm, leaving 4 publications for inclusion in the meta-analysis. Table 1 and the paragraphs below summarize the main findings of these studies.
Table 1.
Summary of studies included in the meta-analysis of denosumab to treat glucocorticoid-induced osteoporosis
| Study, year | Study design | Sample size | Study intervention and duration | Primary endpoint | Study outcome |
|---|---|---|---|---|---|
|
Dore, 201020 |
Multicenter, randomized, double-blind, placebo-controlled trial in patients with rheumatoid arthritis | Placebo or denosumab 60 mg or denosumab 120 mg at 0 and 6 months x 12 months | Change in structural damage in patients with rheumatoid arthritis | Denosumab inhibited structural damage. Denosumab increased BMD in patients taking glucocorticoids or bisphosphonates. | |
| Mok, 201521 | Single center, randomized, open-label study in patients taking bisphosphonates and prednisolone ≥2.5 mg daily | 42 entered, 40 completed | Continue bisphosphonate or switch to denosumab 60 mg at 0 and 6 months x 12 months | Change in lumbar spine BMD between treatment arms | Subjects who switched to denosumab had greater increases in spine BMD than those continuing bisphosphonates |
| Iseri, 201822 | Single center, randomized, open-label study in patients with glomerular disease who had glucocorticoid induced osteoporosis | 32 entered, 28 completed | Denosumab 60 mg at 0 and 6 months or alendronate 35 mg once a week x 12 months | Change in lumbar spine BMD between treatment arms | Denosumab increased spine BMD greater than alendronate at 12 months |
| Saag, 201823 | Multicenter, randomized, double-blind trial in patients taking ≥7.5 mg prednisone daily | 795 entered, 691 completed 12- month visit | Denosumab 60 mg at 0 and 6 months or risedronate 5 mg once daily x 24 months (12 month data reported) | Change in lumbar spine BMD between treatment arms | Denosumab increased spine BMD greater than risedronate in patients starting or continuing glucocorticoids. |
Abbreviation: BMD, bone mineral density.
Dore et al20 reported a subgroup analysis of a multicenter, double-blind, placebo-controlled, randomized trial comparing the effects of denosumab and placebo on structural damage in rheumatoid arthritis patients.20 The trial was sponsored by Amgen Incorporated (Thousand Oaks, CA, USA). The subgroup analysis focused on changes in BMD among 90 participants taking a median prednisone dose of 5 mg daily at baseline and 4 mg daily at the end of the study. Participants’ mean age was 56 years, 62% were women and their mean lumbar T score was −0.6. Participants were randomized to denosumab 60 mg, 180 mg or matching placebo every 6 months for 12 months. Herein, we report the results for the placebo and 60 mg denosumab arms, since the 60 mg dose is FDA approved for GIOP and for osteoporosis in postmenopausal women and men. We also restricted our analysis to the subset of subjects who were not taking concurrent denosumab with bisphosphonate therapy. At 12 months, subjects randomized to denosumab (n=21) experienced numerically greater increases in lumbar spine BMD compared to participants randomized to placebo (n=15) therapy (3.5±2.9% vs 0.4±3.7%). Likewise, the denosumab arm experienced numerically greater gains in total hip BMD (1.6±1.9% vs −1.2±2.6%). Authors did not provide P-values for this subset of subjects.
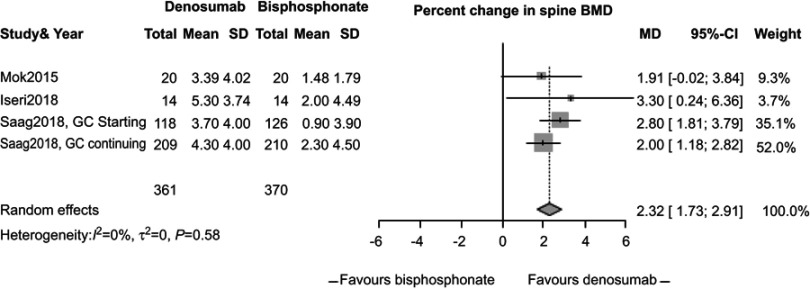
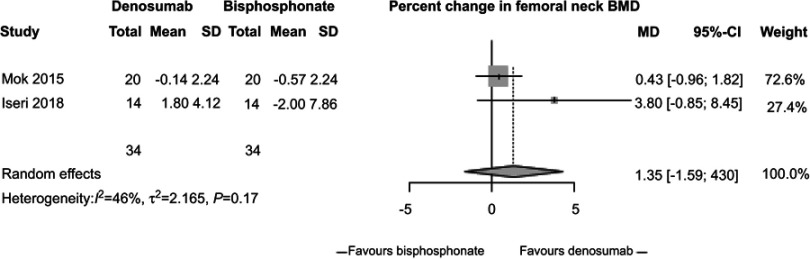
Mok et al21 conducted a 12-month, parallel-group, open-label, randomized-controlled trial in a single center in Hong Kong. Researchers recruited patients with GIOP and compared the change in BMD between patients who continued bisphosphonates and those who switched to denosumab. Participants must have taken bisphosphonate therapy for >2 years to be included in the study. Participants were randomized to continue bisphosphonate therapy or switch to denosumab subcutaneously every 6 months for 12 months. Researchers enrolled 42 women with a mean age of 55±13 years, of whom 71% were postmenopausal. All participants had rheumatic diseases; systemic lupus erythematosus (76%) was the most common condition. The mean prednisolone dose was 4±2 mg daily. Alendronate was used in 79%, risedronate in 12% and ibandronate in 10% of the group at baseline. At 12 months, subjects randomized to denosumab experienced a greater gain in lumbar spine BMD compared to subjects who continued bisphosphonates, after adjustment for multiple co-variates affecting BMD (3.39±4.02% vs 1.48±1.79%, P=0.01). By contrast, the between-arm changes in total hip and femoral neck BMD were not statistically significant.
Iseri et al22 conducted a 12-month prospective, open-label, randomized, controlled study in a single center in Japan, comparing denosumab to alendronate in 32 patients with GIOP and concomitant glomerular disease. Participants’ mean age was 66 years; 43% were female including 9 women past menopause, and 18% had a prior fracture. 71% of participants were continuing prednisolone at a median dose of 5 mg daily for at least 3 months, while the remainder were initiating prednisolone. Participants were randomized to denosumab 60 mg subcutaneous every 6 months or alendronate 35 mg by mouth weekly for 12 months. Participants randomized to denosumab experienced a greater increase in lumbar spine BMD compared to participants randomized to alendronate (+5.3±3.7% vs +2.0±4.5%, P<0.05). By contrast, there were no significant between-arm differences in femoral neck or ultra-distal radius BMD.
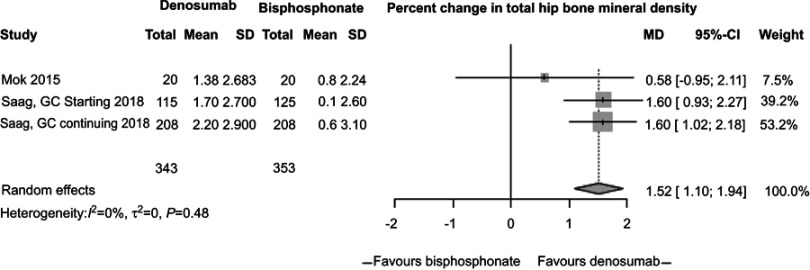
Saag et al23 conducted a 24-month, double-blind clinical trial comparing denosumab to risedronate in adults starting or continuing glucocorticoid therapy; 795 participants were enrolled across Europe, Latin America, Asia and North America, including 505 individuals in the “glucocorticoid continuing” and 290 individuals in the “glucocorticoid initiating” group. Subjects must be taking >7.5 mg prednisone daily to be eligible. Subjects’ mean age was 64 years, the majority were female (70%) and White (88%) while just under half of subjects (47%) reported a prior osteoporotic fracture. The primary reason for glucocorticoid treatment was a rheumatologic disorder (77%). The mean prednisone dose was ~14 mg daily and 45% of subjects took concomitant immunosuppression, although only 4% were taking biologic medications. Patients were randomly assigned to 24 months of denosumab 60 mg subcutaneously every 6 months and daily oral placebo, or risedronate 5 mg daily and subcutaneous placebo every 6 months. Denosumab treatment was associated with a significantly greater 12 months increase in spine BMD compared to risedronate in both the glucocorticoid continuing (4.3±4.0% vs 2.3±4.5%; P<0.0001) and glucocorticoid initiating groups (3.7±4.0% vs 0.9±3.9%; P<0.0001). Likewise, treatment with denosumab was associated with a greater increase in total hip BMD compared to risedronate in both the glucocorticoid continuing (2.2±2.9% vs 0.6±3.1%; P<0.0001) and glucocorticoid initiating (1.7±2.7% vs 0.1±2.6%; P<0.0001) groups.
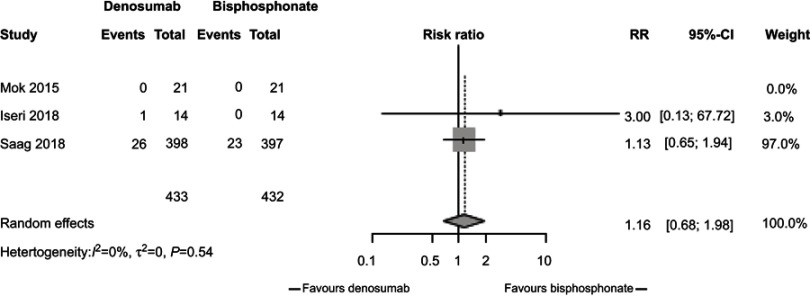
In our meta-analysis, we focused on three studies21–23 comparing changes in BMD between participants randomized to denosumab or bisphosphonates. In these studies, participants receiving denosumab had a greater increase in lumbar spine BMD compared to those receiving bisphosphonates (2.32%, 95% CI 1.73%, 2.91%, P<0.0001, Figure 2) with low study heterogeneity (I2=0%). Likewise, participants assigned to denosumab had greater increase in hip BMD compared to those assigned to bisphosphonates (1.52%, 95% CI 1.1%, 1.94%, P<0.0001, Figure 3) with low study heterogeneity (I2 0%). Finally, in the two studies21,22 that measured changes in femoral neck BMD, there was no difference between subjects assigned to denosumab versus those assigned to bisphosphonates (1.35%, 95% CI −1.59%, 4.30%, P=0.37, Figure 4) with moderate heterogeneity between studies (I2 46%). We found no difference in fracture incidence between participants randomized to denosumab or bisphosphonates (1.16%, 95% CI 0.68%, 1.98%, P=0.59, Figure 5) with low heterogeneity among the 3 studies (I2 0%). Funnel plots of these studies are included in the Supplementary materials (Figures S1–S4).
Figure 2.
Percent change in spine bone mineral density (BMD) between subjects randomized to denosumab or bisphosphonate therapy.
Figure 3.
Percent change in total hip bone mineral density (BMD) between subjects randomized to denosumab versus bisphosphonate.
Figure 4.
Percent change in femoral bone mineral density (BMD) between subjects randomized to denosumab versus bisphosphonate.
Figure 5.
Relative risk of fractures by randomization to denosumab or bisphosphonate therapy.
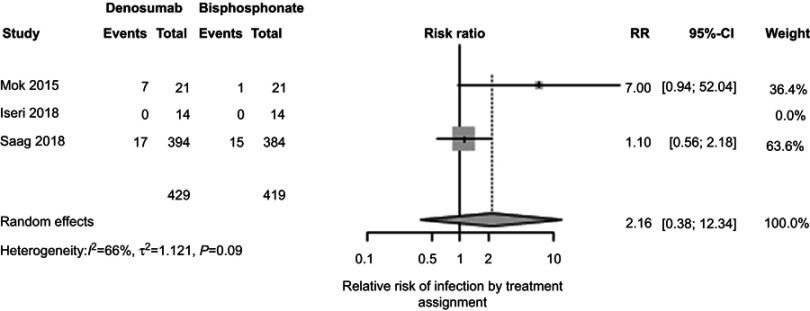
In clinical trials among postmenopausal women, denosumab therapy was associated with a higher risk of infection.24 Therefore, patients prescribed prednisone with denosumab might experience substantially more infections, compared to patients treated with prednisone and bisphosphonates. Reassuringly, we found no significant difference in the rate of infections between participants treated with denosumab or bisphosphonate therapy (2.16%, 95% CI 0.38%, 12.34%, P=0.39, Figure 6). However, we observed moderate heterogeneity among the 3 studies (I2 66%, Figure S5).
Figure 6.
Relative risk of infection by treatment assignment.
Adverse events were similar among subjects assigned to denosumab or bisphosphonate therapy (2.23%, 95% CI 0.70%, 7.08%, P=0.17, Figures S6 and S7) but study heterogeneity was high (I2 83%). Finally, rates of serious adverse events were similar among subjects assigned to denosumab and those assigned to bisphosphonate therapy (1.11%, 95% CI 0.42%, 2.93%, P=0.83, Figures S8 and S9), with low study heterogeneity (I2 18%).
We performed additional meta-analyses, including a fourth trial20 in which placebo was the control arm. Changes in lumbar spine and total hip BMD were significantly higher among subjects assigned to denosumab compared to subjects assigned to bisphosphonate or placebo, with no significant difference in the rates of fracture, infection, adverse events or serious adverse events between treatment arms (Figures S10–S21).
Downs and Black scores indicated that two studies were of good quality, and two were of excellent quality (Table 2).
Table 2.
Downs and black quality score for randomized and non-randomized studies of health care interventions
| Dore 2010 | Mok 2015 | Iseri 2018 | Saag 2018 | ||
|---|---|---|---|---|---|
| Yes=1, no or unable to determine=0 | |||||
| Reporting | |||||
| 1 | Hypothesis or aims clearly described | 1 | 1 | 1 | 1 |
| 2 | Main outcomes in introduction or methods | 1 | 1 | 1 | 1 |
| 3 | Patient characteristics clearly described | 1 | 1 | 1 | 1 |
| 4 | Interventions clearly described | 1 | 1 | 1 | 1 |
| 5 | Are the distribution of confounders clearly described in both groups (0, 1, 2) | 1 | 1 | 2 | 2 |
| 6 | Main findings clearly reported | 1 | 1 | 1 | 1 |
| 7 | Estimates of random variability given for main outcome(s) | 0 | 1 | 1 | 1 |
| 8 | All adverse events of intervention reported | 1 | 1 | 1 | 1 |
| 9 | Characteristics of subjects lost to follow up reported | 0 | 1 | 1 | 1 |
| 10 | Actual probability (P-values) reported for main outcomes (not <0.05 but P=0.035) | 1 | 1 | 0 | 1 |
| External validity | |||||
| 11 | Participants were representative of the source population | 1 | 1 | 1 | 1 |
| 12 | Subjects prepared to participate represented source population (% declined described) | 1 | 1 | 0 | 1 |
| 13 | Location and delivery of study intervention represented that of source population | 1 | 1 | 1 | 1 |
| Internal validity – bias & confounding | |||||
| 14 | Participants blinded to treatment | 1 | 0 | 0 | 1 |
| 15 | Blinded outcome assessment | 0 | 1 | 1 | 1 |
| 16 | Any data dreding clearly described | 0 | 1 | 1 | 1 |
| 17 | Analyses adjustedfor differing length of follow up | 1 | 1 | 1 | 1 |
| 18 | Appropriate statistical analysis used | 1 | 1 | 1 | 1 |
| 19 | Compliance with study intervention was reliable | 0 | 0 | 0 | 1 |
| 20 | Outcome measures were valid and reliable | 1 | 1 | 1 | 1 |
| 21 | All participants were recruited from same source population | 1 | 1 | 1 | 1 |
| 22 | All participants were recruited over same time frame | 1 | 1 | 0 | 1 |
| 23 | Participants were randomized | 1 | 1 | 1 | 1 |
| 24 | Treatment assignment concealed from subjects and investigators | 1 | 0 | 0 | 1 |
| 25 | Adequate adjustment for confounding | 1 | 1 | 1 | 1 |
| 26 | Losses to follow up accounted for | 1 | 1 | 1 | 1 |
| Power | |||||
| 27 | Adequate power to detect a treatment effect at α level of 0.05 | 0 | 1 | 1 | 1 |
| 21 | 24 | 22 | 28 | ||
Discussion
We performed a systematic review and meta-analysis of 4 randomized-controlled trials evaluating the efficacy and safety of denosumab for the prevention and/or treatment of GIOP. Treatment with denosumab provided significantly greater increments in lumbar spine and total hip BMD, compared to bisphosphonate therapy or placebo. In a recent meta-analysis excluding GIOP studies,24 denosumab likewise increased spine and hip BMD greater than that observed with bisphosphonate therapy. In our meta-analysis, there was no difference in fracture incidence; however, the total number of reported fractures across trials was low, and the studies were not powered to detect fracture differences between treatment groups. By contrast, in the recent meta-analysis excluding GIOP studies,24 denosumab was associated with significantly fewer fractures at 24 months, when compared to alendronate (risk ratio 0.51, 95% CI 0.27–0.97).
A previous meta-analysis of 11 studies using denosumab to treat postmenopausal women with osteoporosis indicated an increased risk of serious adverse events related to infections.25 However, we did not detect a difference in the frequency of infections between denosumab and control groups. Rates of adverse events and serious adverse events were also similar between denosumab and control groups. In summary, denosumab represents a reasonable therapeutic choice for patients with GIOP.
We acknowledge several limitations of our study. First, there were few randomized-controlled trials that met our criteria for inclusion in the meta-analysis, leading us to include open-label study designs. Second, none of the studies were powered to detect a difference in fracture between denosumab and comparator arms. Third, all studies were short in duration (12 months); thus the long-term efficacy and safety of denosumab for GIOP cannot be addressed at this time.
Discontinuation of denosumab warrants caution. Researchers have recently recognized that discontinuation of denosumab can markedly increase bone resorption, leading to sizeable declines in spine and hip BMD.25 Moreover, some individuals have sustained one or more painful compression fractures after stopping denosumab.26,27 There is an especially high risk of new compression fractures among individuals with prior vertebral fractures.27 At this time, it remains unclear whether to recommend long-term denosumab or switch to an alternative agent. Ongoing trials in postmenopausal osteoporosis will clarify the best “exit strategy” from denosumab, and might inform transitions off denosumab for patients with GIOP.
In conclusion, data from this systematic review and meta-analysis indicate that denosumab is a reasonable drug to prescribe, in the prevention and treatment of GIOP. Its use is particularly relevant in patients who have contraindications or side effects from bisphosphonates or anabolic therapy, or when patient compliance must be ensured. The American College of Rheumatology guidelines to prevent and treat GIOP,29 suggest use of denosumab as 4th line therapy, after oral bisphosphonates, intravenous bisphosphonates and teriparatide. Based on our literature review and meta-analysis, and concerns about skeletal health after discontinuation of denosumab, its place as 4th line therapy for GIOP seems reasonable.
Abbreviations
BMD, bone mineral density; FDA, Food and Drug Administration; GIOP, glucocorticoid-induced osteoporosis; RANKL, receptor-activator nuclear kappa B ligand.
Author contributions
YZ and KEH contributed equally to the design of the study. YZ performed the primary literature review, which was replicated by KEH. Both authors performed data extraction and entry. KEH performed statistical analysis. YZ and KEH contributed equally to drafting of the manuscript and approval of the final version. Both authors agree to be accountable for all aspects of the work including data accuracy.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford). 2011;50(11):1982–1990. doi: 10.1093/rheumatology/ker017 [DOI] [PubMed] [Google Scholar]
- 2.Benard-Laribiere A, Pariente A, Pambrun E, Begaud B, Fardet L, Noize P. Prevalence and prescription patterns of oral glucocorticoids in adults: a retrospective cross-sectional and cohort analysis in France. BMJ Open. 2017;7(7):e015905. doi: 10.1136/bmjopen-2017-015905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein RS, Chen JR, Powers CC, et al. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109(8):1041–1048. doi: 10.1172/JCI14538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2):274–282. doi: 10.1172/JCI2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirza F, Canalis E. Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol. 2015;173(3):R131–R151. doi: 10.1530/EJE-15-0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laan RF, van Riel PL, van de Putte LB, van Erning LJ, Van’t Hof MA, Lemmens JA. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. A randomized, controlled study. Ann Intern Med. 1993;119(10):963–968. doi: 10.7326/0003-4819-119-10-199311150-00001 [DOI] [PubMed] [Google Scholar]
- 7.Rossini M, Viapiana O, Vitiello M, et al. Prevalence and incidence of osteoporotic fractures in patients on long-term glucocorticoid treatment for rheumatic diseases: the Glucocorticoid Induced OsTeoporosis Tool (GIOTTO) study. Reumatismo. 2017;69(1):30–39. doi: 10.4081/reumatismo.2017.922 [DOI] [PubMed] [Google Scholar]
- 8.Rentero ML, Amigo E, Chozas N, et al. Prevalence of fractures in women with rheumatoid arthritis and/or systemic lupus erythematosus on chronic glucocorticoid therapy. BMC Musculoskelet Disord. 2015;16:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodd C, Lang B, Ramsay T, et al. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: a national observational study. Arthritis Care Res (Hoboken). 2012;64(1):122–131. doi: 10.1002/acr.20589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeBlanc CM, Ma J, Taljaard M, et al. Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. J Bone Miner Res. 2015;30(9):1667–1675. doi: 10.1002/jbmr.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European corticosteroid-induced osteoporosis treatment study. J Bone Miner Res. 2000;15(6):1006–1013. doi: 10.1359/jbmr.2000.15.6.1006 [DOI] [PubMed] [Google Scholar]
- 12.Wallach S, Cohen S, Reid DM, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67(4):277–285. [DOI] [PubMed] [Google Scholar]
- 13.Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44(1):202–211. doi: [DOI] [PubMed] [Google Scholar]
- 14.Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-induced osteoporosis intervention study group. N Engl J Med. 1998;339(5):292–299. doi: 10.1056/NEJM199807303390502 [DOI] [PubMed] [Google Scholar]
- 15.Reid DM, Devogelaer JP, Saag K, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2009;373(9671):1253–1263. doi: 10.1016/S0140-6736(09)60250-6 [DOI] [PubMed] [Google Scholar]
- 16.Saag KG, Zanchetta JR, Devogelaer JP, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60(11):3346–3355. doi: 10.1002/art.24879 [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer LC, Zeitz U, Schoppet M, et al. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum. 2009;60(5):1427–1437. doi: 10.1002/art.24445 [DOI] [PubMed] [Google Scholar]
- 18.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7420.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dore RK, Cohen SB, Lane NE, Palmer W, Shergy W, Zhou L. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis. 2010;69:872–875. doi: 10.1136/ard.2009.112920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok CC, Ho LY, Ma KM. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone. 2015;75:222–228. doi: 10.1016/j.bone.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 22.Iseri K, Iyoda M, Watanabe M, et al. The effects of denosumab and alendronate on glucocorticoid-induced osteoporosis in patients with glomerular disease: A randomized, controlled trial. PLoS One. 2018;13(3):e0193846. doi: 10.1371/journal.pone.0193846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018;6(6):445–454. doi: 10.1016/S2213-8587(18)30075-5 [DOI] [PubMed] [Google Scholar]
- 24.Lyu H, Jundi B, Xu C, et al. Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2019;104(5):1753–1765. doi: 10.1210/jc.2018-02236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol. 2014;7(5):2113–2122. [PMC free article] [PubMed] [Google Scholar]
- 26.Zanchetta MB, Boailchuck J, Massari F, Silveira F, Bogado C, Zanchetta JR. Significant bone loss after stopping long-term denosumab treatment: apost FREEDOM study. Osteoporos Int. 2018;29(1):41–47. doi: 10.1007/s00198-017-4242-6 [DOI] [PubMed] [Google Scholar]
- 27.Tsourdi E, Langdahl B, Cohen‐Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 28.Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33:190–198. doi: 10.1002/jbmr.3337 [DOI] [PubMed] [Google Scholar]
- 29.Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017;69(8):1521–1537. doi: 10.1002/art.40137 [DOI] [PubMed] [Google Scholar]