Abstract
Background
Skeletal muscle mass to visceral fat area ratio (SVR) were shown to be related to some chronic diseases, such as non-alcoholic fatty liver diseases. The aim of this study is to determine whether the SVR is associated with metabolic syndrome (MS) and type 2 diabetes (T2DM).
Methods
A total of 798 subjects were included in this cross-sectional study. Lipid profiles, plasma glucose, blood pressure, waist circumference (WC) and body mass index (BMI) were grouped by the SVR. The associations between the SVR and T2DM and MS were examined using logistic regression to determine whether the SVR was associated with T2DM and MS.
Results
Lipid profiles, glucose levels, blood pressure, WC and BMI showed significant differences when stratified based on the extent of SVR. The SVR levels were also significantly higher in subjects without MS or T2DM than in those with MS or T2DM. The SVR was inversely correlated with lipid profiles and WC and was especially correlated with BMI, with an r>0.5. The SVR was identified as a risk factor for T2DM and MS after adjusting age and sex. SVR can predict T2DM [area under the curve =0.726, 95% CI (0.669–0.782), p<0.001] and MS [area under the curve =0.730, 95% CI (0.694–0.766), p<0.001]. The suitable cut-off value is 0.230 for T2DM (sensitivity 0.696, specificity 0.694) and 0.278 for the onset of MS (sensitivity 0.518, specificity 0.862).
Conclusion
The SVR is closely associated with an increased risk for exacerbating T2DM and MS and can be used as a diagnostic indicator for T2DM and MS.
Keywords: metabolic syndrome, diabetes mellitus, skeletal muscle mass, visceral fat area
Background
The prevalence and incidence of type 2 diabetes mellitus (T2DM) are increasing rapidly worldwide. Approximately 400 million people have been diagnosed with diabetes mellitus1 worldwide, while the International Diabetes Federation predicted that the number of diabetic individuals would increase to 650 million in the next 30 years. Metabolic syndrome (MS) is closely related to DM with a prevalence of 34.7% in the United States in 2012.2 DM and MS have now become global health problems that increase the risk of cardiovascular disease (CVD),3,4 chronic liver disease and hepatocellular carcinoma.5,6 DM collectively killed an estimated 12.9 million people globally in 2010 as a result of CVD related diseases.7
In recent years, with the emphasis on exercise therapy and the prevention of T2DM and MS, an increasing number of people are aware of the importance of skeletal muscle mass and abdominal obesity for metabolism. The loss of skeletal muscle mass has become a worldwide problem with the global aging population, and reduced skeletal mass was identified as a risk factor for MS and CVD.8 Since skeletal muscle is one of the main sites of glucose uptake and utilization,9 decreased muscle mass increases insulin resistance, thereby increasing the risk of T2DM and MS.10 Abdominal obesity has been reported to be a high risk factor not only for T2DM11 and MS,12 but also for CVD13 and fatty liver disease.14 Though widely used, both BMI and WC are not precise parameters to represent abdominal obesity.15,16 However, visceral fat area (VFA) has been reported to be more accurate measuring abdominal obesity and has a stronger association with the risk for MS than WC or BMI.16,17 Some recent studies have found that the increased VFA seemed to be more strongly associated with the prevalence of MS and is the only best predictor of MS among females.18 In the context of the previous studies, reduced skeletal muscle mass and VFA were reported to increase the risk of metabolic impairment more than any other single factor alone.19
Considering the interaction of reduced skeletal muscle mass with elevated VFA, we used the index skeletal muscle mass to visceral fat area ratio (SVR) to investigate its relationship with chronic diseases such as T2DM and MS. Only a few studies have been carried out on the SVR. Much uncertainty still exists regarding the relationship between SVR and chronic diseases. Although the SVR has been associated with MS in nondiabetic participants,20 its predictive potential for MS and T2DM in a general population remains unknown.
This study was attempted to evaluate the relationship between T2DM, MS and the SVR, and to investigate whether the SVR could be a good predictor of T2DM and MS.
Methods
Study population
All eligible participants in each community or village were over 18 years old and had been living in their current residence for at least 5 years. All of them signed informed consent before the examination. Exclusion criteria were as follows: (1) with missing vital basic data, such as age, gender, results of OGTT, lipid profiles, WC or blood pressure; (2) individuals without taking the body composition analysis; (3) individuals with malignant tumors or serious liver (either alanine aminotransferase or aspartate aminotransferase higher than 100 U/L) or renal dysfunction (creatinine higher than 105 μmol/L and a glomerular filtration rate below 60 mL/min).
Data collection
Data were collected at local health stations by trained medical staff. Blood samples were collected from all participants after an overnight fast of at least 10 h. The serum lipid profiles and plasma glucose and insulin levels were measured using the ARCHITECT ci16200 Integrated System (Abbott, Illinois, USA).
Weight was measured in kilograms, while height was measured in centimeters. WC was measured at the umbilicus level with the participants in the standing position. BMI was equal to weight (kg) divided by squared height (m2). Blood pressure was measured on the nondominant arm three times in succession with a 3-min interval between the measurements with the subjects in a sitting position. The three readings were averaged for data analysis. Each participant with no history of T2DM underwent an oral glucose tolerance test. Skeletal muscle mass and VFA were measured using an InBody720 (Biospace Co., Ltd., Seoul, Korea). VFA defined as a cross sectional area of abdominal visceral fat at the umbilical level (L4–L5), was measured with multifrequency bioimpedance analysis, which has been considered a more precise technique for measuring the VFA than computed tomography (CT).21,22 Skeletal muscle mass was calculated from the appendicular muscle mass, which is mainly composed of skeletal muscle and accounts for approximately 70% of the total body skeletal muscle.
Definitions of T2DM and MS
According to the Chinese guidelines for DM,23 a person was diagnosed with DM if he or she met one or more of the following criteria: (1) Typical symptoms of T2DM and casual plasma glucose level of 11.1 mmol/L or higher (2) fasting plasma glucose (FPG)level of 7.0 mmol/L or higher,24 and (3) 2 h plasma glucose (2h-PG) level of 11.1 mmol/L or higher. Patients with type 1 diabetes were excluded. MS was defined as three or more of the following criteria being met:23 (1) abdominal obesity: WC male ≥90 cm, female ≥85 cm; (2) hyperglycemia: FPG level of 6.1 mmol/L or higher, 2-h PG level of 7.8 mmol/L or higher, or a diagnosis of T2DM;24 (3) hypertension: blood pressure of 135/80 or higher or a diagnosis of hypertension; (4) high triglyceride (TG): fasting plasma TG of 1.7 mmol/L or higher; and (5) low high-density lipoprotein(HDL-C): fasting plasma HDL-C lower than 1.04 mmol/L. Overweight was defined as a BMI of 24.0–27.9, and obesity was defined as a BMI of 28.0 or higher.23
Data analysis
Quartile division was used on SVR to group the participants into Q1-Q4 since the SVR does not yet have a partition criterion. Sex, age, BMI, WC, HOMA-IR, total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), FPG, 2h-PG, WC, and diastolic blood pressure (DBP) were analyzed in Q1–Q4. Differences between mean values were tested using variance analysis. A chi-squared test was used to analyze whether if there were any statistically significant differences in the prevalence of T2DM and MS between different SVR quartiles. The Spearman correlation coefficients were calculated to compare clinical and laboratory measurements and the SVR, which was logarithmically transformed. Logistic regression models were used to analyze the association between SVR quartiles and T2DM and MS. Odds ratios (ORs) were obtained from logistic regression analysis, and the results were presented as ORs with a 95% confidence intervals.25 A p-value of <0.05 was defined as statistically significant. The ability of the SVR to predict T2DM and MS was evaluated using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. All statistical analyses were performed using Statistical Product and Service Solutions (SPSS) version 22.0.
Result
The basic characteristics of the eligible participants according to SVR quartiles (n=798, mean 40.12±10.23 age years) are shown in Table 1. Men accounted for 35.0% of the total participants. Among the total subject population, the prevalence rates of T2DM and MS were 25.92% and 41.19% respectively.
Table 1.
Clinical, anthropometric and metabolic characteristics of all study subjects with quartiles stratification according to the SVR
| Total | Q1 (n=200) | Q2 (n=200) | Q3 (n=199) | Q4 (n=199) | p-value | |
|---|---|---|---|---|---|---|
| Male (n) | 35.01% | 39.0% | 36.0% | 35.7% | 30.2% | 0.005 |
| Age | 40.12±10.22 | 49.27±11.19 | 40.35±8.07 | 37.07±6.88 | 33.75±6.89 | <0.001 |
| FPG | 5.67±1.61 | 6.32±2.15 | 5.71±1.50 | 5.46±1.28 | 5.20±1.07 | <0.001 |
| 2h-PG | 7.30±3.32 | 8.29±4.69 | 7.36±3.11 | 6.95±2.61 | 6.58±2.05 | <0.001 |
| TC | 4.99±1.08 | 5.46±1.08 | 5.12±1.07 | 4.91±0.94 | 4.45±0.95 | <0.001 |
| TG | 1.54±1.76 | 1.98±2.16 | 1.80±2.27 | 1.32±0.96 | 1.04±1.04 | <0.001 |
| LDL-C | 2.66±0.78 | 3.02±0.78 | 2.79±0.75 | 2.60±0.69 | 2.21±0.66 | <0.001 |
| HDL-C | 1.23±0.31 | 1.20±0.32 | 1.18±0.30 | 1.24±0.32 | 1.29±0.27 | 0.001 |
| HOMA-IR | 3.10±2.30 | 3.92±2.6 | 3.64±2.72 | 2.88±1.89 | 1.95±1.05 | <0.001 |
| BMI | 25.46±4.00 | 27.81±3.96 | 26.96±3.50 | 25.17±2.96 | 21.86±2.57 | <0.001 |
| WC | 86.02±11.55 | 92.50±10.05 | 88.97±10.24 | 85.66±10.03 | 76.88±8.55 | <0.001 |
| SBP | 126.16±18.88 | 136.04±20.52 | 127.86±20.38 | 131.91±18.38 | 131.03±19.21 | <0.001 |
| DBP | 76.27±12.01 | 80.68±12.36 | 78.37±11.85 | 75.98±10.88 | 70.03±10.22 | <0.001 |
Abbreviations: BMI, body mass index; WC, waist circumference; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose, 2h-PG, 2 hr plasma glucose; SVR, Skeletal muscle mass to visceral fat area ratio.
The SVR values were divided into four groups by quartile, increasing from Q1 to Q4. The prevalence of T2DM and MS decreased with an increasing SVR quartiles in Table 2. The prevalence of MS with different numbers of MS components also decreased with an increasing SVR quartiles.
Table 2.
The prevalence rates of T2DM, MS and the rates of numbers of MS components with quartile stratification according to the SVR
| Q1 (n=200) | Q2 (n=200) | Q3 (n=199) | Q4 (n=199) | p-value | |
|---|---|---|---|---|---|
| T2DM (%) | 23.5 | 9.0 | 7.0 | 3.0 | <0.001 |
| MS | 47.5 | 36.5 | 22.1 | 6.5 | <0.001 |
| Number of MS components | |||||
| 3 | 24.5 | 18.5 | 12.7 | 4.0 | <0.001 |
| 4 | 15.0 | 14.0 | 7.5 | 2.0 | <0.001 |
| 5 | 8.0 | 4.0 | 2.0 | 0.5 | <0.001 |
Abbreviations: T2DM, Type 2 Diabetes Mellitus; MS, metabolic syndrome; SVR, Skeletal muscle mass to visceral fat area ratio.
Table 3 shows the correlation between SVR and metabolic parameters in the overall population: FPG (r=−0.227, p=0.000), 2h-PG (r=−0.114, p=0.000), SBP (r=−0.353, p=0.000), DBP (r=−0.303, p=0.000), LDL (r=−0.384, p=0.000), TC (r=−0.341, p=0.000), TG (r=−0.312, p=0.000), HOMA-IR(r=−0.386, p=0.000), WC (r=−0.493, p=0.000), BMI (r=−0.541, p=0.000), and HDL-C (r=0.100, p=0.005). There was a difference in the correlation between SVR and metabolic indicators between males and females. In addition to the strong correlation of BMI and WC with SVR in both men and women, the correlation between female SVR and blood lipids was stronger among females (TC: r=−0.391, p=0.000; LDL-C: r=−0.445, p=0.000), while in men, the SVR showed a stronger correlation with HOMA-IR (r=−0.462, p=0.000).
Table 3.
Correlation of SVR with clinical, anthropometric and metabolic characteristics*
| Total | Female | Male | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| SBP | −0.353 | 0.000 | −0.386 | 0.000 | −0.325 | 0.000 |
| DBP | −0.303 | 0.000 | −0.281 | 0.000 | −0.354 | 0.000 |
| TC | −0.341 | 0.000 | −0.391 | 0.000 | −0.271 | 0.000 |
| TG | −0.312 | 0.000 | −0.347 | 0.000 | −0.282 | 0.000 |
| LDL-C | −0.384 | 0.000 | −0.445 | 0.000 | −0.303 | 0.000 |
| HDL-C | 0.100 | 0.005 | 0.089 | 0.043 | 0.102 | 0.078 |
| WC | −0.493 | 0.000 | −0.570 | 0.000 | −0.502 | 0.000 |
| BMI | −0.541 | 0.000 | −0.566 | 0.000 | −0.528 | 0.000 |
| FPG | −0.227 | 0.000 | −0.262 | 0.000 | −0.207 | 0.000 |
| 2h-PG | −0.114 | 0.000 | −0.133 | 0.000 | −0.160 | 0.013 |
| HOMA-IR | −0.386 | 0.000 | −0.324 | 0.000 | −0.462 | 0.000 |
Notes: *SVR, TG, HOMA-IR was log-linearized in this analysis.
Abbreviations: BMI, body mass index; WC, waist circumference; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose, 2h-PG, 2 hr plasma glucose; SVR, Skeletal muscle mass to visceral fat area ratio.
The SVR values were divided into four groups by quartile, increasing from Q1 to Q4. The mean age decreased from Q1 to Q4 and there was a significant difference according to sex (p=0.005). BMI, HOMA-IR, TC, TG, LDL-C, FBG, 2h-PG, WC and DBP decreased from Q1 to Q4, while a positive trend was observed in HDL-C. There were significant differences of the parameters above. No trend was observed in systolic blood pressure (SBP). The prevalence rates of T2DM and MS were also showed decreasing trends with increasing SVR from Q1 to Q4. Considering that MS was diagnosed by the number of components, we analyzed the prevalence of MS comprised of different numbers of components. The prevalence rate of MS with different numbers of components decreased from Q1 to Q4. Significant differences were shown in the abovementioned prevalence rates when grouped by SVR quartiles.
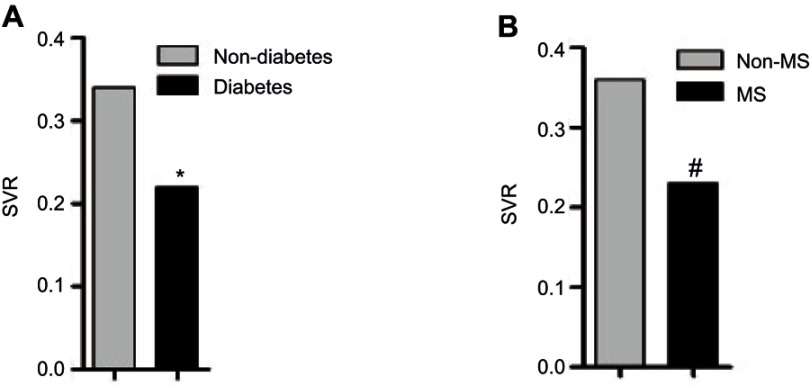
Consistently, the SVR levels were significantly higher in subjects without MS or T2DM than in those with MS or T2DM. As shown in Figure 1, when comparing SVR levels in different groups according to the diagnosis of T2DM and MS, a significant difference was found in the comparisons. Furthermore, as shown in Table 3, SVR was strongly inversely correlated with SBP, DBP, LDL, TC, TG, HOMA-IR and WC, and especially BMI, with an r >0.5 (all p<0.001). A rather weak correlation was found with HDL-C(r=0.100), FPG(r=−0.227) and 2h-PG(r=−0.114).
Figure 1.
The comparisons of SVR in study subjects grouped according to the diagnosis of T2DM and MS.
Notes: *Significant different showed (p=0.014) between diabetes participant and non-diabetes participants. #p<0.001 between MS participants and participants with no MS.
Abbreviations: T2DM, diabetes; MS, metabolic syndrome; SVR, Skeletal muscle mass to visceral fat area ratio.
Logistic regression analysis was performed to evaluate the relationship between SVR quartiles and T2DM and MS, as shown in Table 4. As a result, using Q4 as the reference, the relative risks for T2DM were 2.43 (95% CI: 0.92–6.47) in Q3, and 3.18 (95% CI: 1.24–8.19) in Q2, and 9.88 (95% CI: 4.11–23.72) in Q1 (model 1). Moreover, the relative risks for MS using Q4 as the reference were 4.06 (95% CI: 2.11–7.81) in Q3, 8.22 (95% CI: 4.37–15.47) in Q2, and 12.95 (95% CI: 6.92–24.23) in Q1 (model 1). Univariate logistic regression was performed in Model 1. The results of the analyses adjusted for age and sex (model 2) were similar. These results indicated that a decreased SVR was a risk factor for MS and T2DM. We analyzed the relationship between the SVR and T2DM or MS in males and females (Table 5). In addition to the overall trend that was consistent with that observed in the overall population, a lower SVR in males showed a stronger correlation with MS. Compared with males, lower SVR was a stronger risk factor for MS in females.
Table 4.
Logistic regression analysis to identify the association between SVR stratifications and MS, T2DM
| T2DM | MS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MODEL1 | MODEL2* | MODEL1 | MODEL2* | |||||||||
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| 4th quartile | 1 | 1 | 1 | 1 | ||||||||
| 3rd quartile | 2.43 | 0.92–6.47 | 0.074 | 2.14 | 0.80–5.73 | 0.128 | 4.06 | 2.11–7.81 | 0.000 | 4.01 | 2.03–7.80 | 0.000 |
| 2nd quartile | 3.18 | 1.24–8.19 | 0.016 | 2.46 | 0.94–6.44 | 0.067 | 8.22 | 4.37–15.47 | 0.000 | 8.48 | 4.36–16.51 | 0.000 |
| 1st quartile | 9.88 | 4.11–23.72 | 0.000 | 5.51 | 2.11–14.35 | 0.000 | 12.95 | 6.92–24.23 | 0.000 | 12.13 | 5.96–24.69 | 0.000 |
Notes: *Model 2 were adjusted for sex and age.
Table 5.
Logistic regression analysis to identify the association between SVR stratifications and type 2 diabetes, metabolic syndrome in different genders
| Female | Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T2DM* | MS* | T2DM* | MS* | |||||||||
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| 4th quartile | 1 | 1 | 1 | 1 | ||||||||
| 3rd quartile | 2.34 | 0.60–9.09 | 0.22 | 2.82 | 0.88–9.03 | 0.081 | 2.04 | 0.49–8.54 | 0.33 | 5.48 | 2.51–11.97 | 0.000 |
| 2nd quartile | 1.98 | 0.50–7.79 | 0.33 | 5.03 | 1.66–15.28 | 0.004 | 3.42 | 0.88–13.20 | 0.075 | 10.29 | 4.56–23.19 | 0.000 |
| 1st quartile | 4.79 | 1.23–18.62 | 0.024 | 11.00 | 3.51–34.48 | 0.000 | 6.78 | 1.75–26.25 | 0.006 | 7.09 | 2.99–16.81 | 0.000 |
Notes: *Model 2 were adjusted for age.
Abbreviations: T2DM, type 2 diabetes; MS, metabolic syndrome; SVR, Skeletal muscle mass to visceral fat area ratio.
To evaluate the predictive performance of the SVR for T2DM and MS, the AUC of the ROC curve was calculated and was 0.726 [95% CI (0.669–0.782), p<0.001] for T2DM, and 0.730[95% CI (0.694–0.766), p<0.001] for MS (Figure 2). The suitable cut-off value was 0.230 for type 2 diabetes (sensitivity 0.696, specificity 0.694) and 0.278 for the onset of MS (sensitivity 0.518, specificity 0.862) (Table 6).
Figure 2.
ROC curves of the SVR to predict T2DM and MS.
Notes: (A) ROC curves for T2DM; (B) ROC curves for MS.
Abbreviations: T2DM, type 2 diabetes; MS, metabolic syndrome; SVR, Skeletal muscle mass to visceral fat area ratio.
Table 6.
Receiver operator curve characteristics for SVR in predicting type 2 diabetes and metabolic syndrome and cut-off points for SVR
| ROC | Cut-off | Sensitivity | Specificity | Youden index | ||
|---|---|---|---|---|---|---|
| A (95% CI) | p-value | |||||
| T2DM | 0.726 (0.669–0.782) | 0.000 | 0.230 | 0.696 | 0.694 | 0.390 |
| MS | 0.730 (0.694–0.766) | 0.000 | 0.278 | 0.518 | 0.862 | 0.381 |
Abbreviations: ROC, receiver operating characteristic; AUC, area under ROC curve; T2DM, type 2 diabetes; MS, metabolic syndrome; SVR, skeletal muscle mass to visceral fat area ratio.
Discussion
There were five main findings in our study. First, the lipid profiles, FPG, 2h-PG, HOMA-IR, BMI, WC, SBP and DBP decreased significantly from Q1 to Q4. Second, the SVR was lower in participants with T2DM or MS. Third, a strong negative correlation was found between the SVR and parameters such as SBP, DBP, LDL, TC, TG, HOMA-IR and WC. Fourth, a lower SVR was a risk factor for MS and T2DM. Fifth, the SVR can predict T2DM and MS.
Although the most common method to measure VFA is CT, limitations were exposed related to for its high cost and harmful radiation which makes it difficult to popularize in clinical practice. In contrast the measurement by Inbody 720 is nonradioactive and repeatable, and is considered a more precise technique for measuring VFA than CT.21,22
Only a few studies have considered both the decreased muscle mass and increased VFA and focused on the SVR and its relationships with chronic diseases. The SVR were shown to be a risk factor for NAFLD in a Japanese population.26 The SVR was inversely related to BMI and HOMA-IR, which was also verified in our research. One study focused on the relationship between the SVR and MS development without T2DM or hypertension.20 This study did not demonstrate the relationship between the SVR and glucose levels. Considering the limitations of the population included in that study, we conducted our study in a general population to investigate the potential association between the SVR and diseases.
Table 3 indicates that the SVR had a better correlation with HOMA-IR (r=−0.386) than FPG (r=−0.227) or 2h-PG (r=−0.114). A possible explanation for this might be that skeletal muscle is one of the main organs for glucose uptake and is related to insulin resistance. Additionally, visceral obesity could directly affect inflammation and insulin resistance.27 A possible explanation for the SVR’s poor correlation with FBG or 2h-PG may be the lack of a confirmed connection between skeletal muscle, visceral fat and islet function. More research should be conducted to test the relationships between the SVR and islet function. These results suggest that a low SVR may be partly related to T2DM by increasing insulin resistance but not insulin secretion. When we analyzed the correlation between the SVR and metabolic indicators, we found differences between the sexes. In men, the SVR showed a better correlation with MS and insulin resistance, while in women the SVR was more strongly correlated with blood lipids. Previous studies have also found sex differences in fat distribution.28 In previous studies, women were more likely to deposit fat in their limbs than in the viscera. A study by Heymsfield29 also showed that BMI is more closely related to male muscle development. This may explain why men are more likely to exhibit greater changes in muscle mass for the same change in BMI. This suggests that men may be more likely than women to experience a decrease in muscle mass as a result of the same reduction in the SVR, which also explains why the correlation between male SVR and the insulin resistance index was stronger in our study. Moreover, as shown in Table 4, only Q1 was found to be significantly associated with T2DM. No significant associations of Q2 or Q3 were observed with T2DM, which indicated that the SVR is not a rather strong risk factor for T2DM.
SVR showed a good correlation with serum lipid parameters and other components of MS (Table 3). Q1–Q3 were shown to be strong risk factors for MS when Q4 were used as the reference which indicated that SVR is a good index for predicting MS. The result was in line with those of the previous study which included a nondiabetic population.20 First, a recent investigation had suggested that reduced skeletal muscle mass and increased visceral fat were correlated with inflammatory cytokines.30,31 The results of this study indicated that a low SVR may reflect a proinflammatory state. Second, the two factors of SVR, skeletal muscle and visceral fat, are both closely related to energy metabolism and insulin resistance. These results might explain the close relationship between the SVR and MS. These findings in our research could help clinical physicians identify a group with high risk of MS. The relationship indicates that the SVR is a good way to assess healthy status without the use of an invasive technique.
The relationship between T2DM, MS and the SVR may be explained by the fact that skeletal muscle mass and physical activity are closely related.32,33 Studies have shown that the prevalence of T2DM or MS is lower in people with high exercise capacity. It is well established that the risk of T2DM and MS can be decreased by several lifestyle interventions, especially physical activity. However, physical activity is less feasible to quantify because it is difficult to measure exercise intensity and forms, and the data obtained are subjective to some extent. More research could be performed to investigate the relationship between physical activity and the SVR.
The main strength of the study is that it is the first study to assess the relationship between the SVR and T2DM and MS in a general survey-based population. The diagnosis of T2DM and MS in our study was strictly based on the criteria of the T2DM and MS guidelines in China.
A few limitations still exist in our study. First, it was based on a cross-sectional analysis; thus, it is not possible to obtain a prediction model. More prospective studies should be conducted in the future. Second, no data on physical activity or inflammatory factors were included in our study, which resulted in no further discussion of the mechanism. Third, the sample size of the population was relatively small, and all the participants were from one region, which might cause some bias. Forth, the most precise way to measure skeletal muscle mass is CT or DXA. Considering of the portability of the machines, the compliance of the local individuals and the cost in the whole study, we used impedance method instead of CT as a tool for the measurement of skeletal muscle mass. Last, we discussed about the difference correlation between SVR and blood profiles were stronger in females. Sex hormones and the menstrual and obstetrical histories were crucial factors to affect lipid profiles. However, these data were not included in our study. More research should be done to analyze the difference correlation between SVR and blood profiles in the future.
Conclusion
Significant differences were observed from Q1–Q4 when compared anthropometric parameters were compared according to the degree of SVR. The SVR is inversely correlated with metabolic profiles, including BMI, WC, SBP, DBP and HOMA-IR. The SVR was also associated with the diagnosis of T2DM and MS, independent of sex and age.
Acknowledgment
The authors express their gratitude to the TIDE study group, which was hold by the department of Endocrinology and Metabolism of the first affiliated hospital of China Medical University. This work was supported by grants from National Key Research and Development Program of China (2017YFC1309800 and 2017YFC0909600), The Clinical Research Fund of Chinese Medical Association (15010010589), The Research Fund for Public Welfare, National Health and Family Planning (201402005), Key Research and Department of Shandong Province (2016GSF201013), Key Research and Department of Shandong Province (2016GSF201015), Key Research and Department of Shandong Province (2017GSF18184, 2017GSF18129), National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2015ZX0910019), Jinan Science and Technology Program (201704111), Natural science foundation of shandong province (ZR2019MH061).
Abbreviations
MS, metabolic syndrome; DM, diabetes; T2DM, Type 2 diabetes; SVR, skeletal muscle mass to visceral fat area ratio; CVD, cardiovascular disease; BMI, body mass index; WC, waist circumference; FPG, fasting plasm glucose; 2h-PG, 2h-plasm glucose; VFA, visceral fat area; CT, computed tomography; SBP, systolic blood pressure; DBP, diastolic blood pressure; OR, odds ratios; CI, confidence interval; AUC, area under the curve; ROC, receiver operating characteristic.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of China Medical University. Written informed consent was obtained from all participants following a detailed description of the purpose of the study. This study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
All authors have read and approved the submission of the manuscript; the manuscript has not been published and is not being considered for publication elsewhere.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Orwoll ES, Lapidus J, Wang PY, et al. The limited clinical utility of testosterone, estradiol, and sex hormone binding globulin measurements in the prediction of fracture risk and bone loss in older men. J Bone Miner Res. 2017;32(3):633–640. doi: 10.1002/jbmr.3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Mooyaart EA, Dannemann N, et al. Relation of the metabolic syndrome to quantity of coronary atherosclerotic plaque. Am J Cardiol. 2008;101(8):1127–1130. doi: 10.1016/j.amjcard.2008.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371(9626):1800–1809. doi: 10.1016/S0140-6736(08)60768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–468. doi: 10.1053/j.gastro.2003.10.065 [DOI] [PubMed] [Google Scholar]
- 6.Halmos T, Suba I. [Non-alcoholic fatty liver disease, as a component of the metabolic syndrome, and its causal correlations with other extrahepatic diseases]. Orv Hetil. 2017;158(52):2051–2061. doi: 10.1556/650.2017.30936 [DOI] [PubMed] [Google Scholar]
- 7.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)61137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klip A, Paquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990;13(3):228–243. doi: 10.2337/diacare.13.3.228 [DOI] [PubMed] [Google Scholar]
- 10.Reaven GM. Banting Lecture 1988. Role of insulin resistance in human disease. 1988. Nutrition. 1997;13(1):65; discussion 64, 66. [DOI] [PubMed] [Google Scholar]
- 11.Standl E. Dysglycemia and abdominal obesity. Curr Vasc Pharmacol. 2012;10(6):678–679. doi: 10.2174/157016112803520936 [DOI] [PubMed] [Google Scholar]
- 12.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05376 [DOI] [PubMed] [Google Scholar]
- 13.Anand SS, Yusuf S, Jacobs R, et al. Risk factors, atherosclerosis, and cardiovascular disease among Aboriginal people in Canada: the Study of Health Assessment and Risk Evaluation in Aboriginal Peoples (SHARE-AP). Lancet. 2001;358(9288):1147–1153. doi: 10.1016/S0140-6736(01)06255-9 [DOI] [PubMed] [Google Scholar]
- 14.Jakobsen MU, Berentzen T, Sorensen TI, Overvad K. Abdominal obesity and fatty liver. Epidemiol Rev. 2007;29:77–87. doi: 10.1093/epirev/mxm002 [DOI] [PubMed] [Google Scholar]
- 15.Doyle SL, Bennett AM, Donohoe CL, et al. Establishing computed tomography-defined visceral fat area thresholds for use in obesity-related cancer research. Nutr Res. 2013;33(3):171–179. [DOI] [PubMed] [Google Scholar]
- 16.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng AC, Wai DC, Tai ES, Ng KM, Chan LL. Visceral adipose tissue, but not waist circumference is a better measure of metabolic risk in Singaporean Chinese and Indian men. Nutr Diabetes. 2012;2:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HI, Kim JT, Yu SH, et al. Gender differences in diagnostic values of visceral fat area and waist circumference for predicting metabolic syndrome in Koreans. J Korean Med Sci. 2011;26(7):906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TN, Yang SJ, Yoo HJ, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes. 2009;33(8):885–892. [DOI] [PubMed] [Google Scholar]
- 20.Kim TN, Park MS, Lim KI, et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Res Clin Pract. 2011;93(2):285–291. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa H, Fujitani K, Tsujinaka T, et al. InBody 720 as a new method of evaluating visceral obesity. Hepato-Gastroenterology. 2011;58(105):42–44. [PubMed] [Google Scholar]
- 22.Park KS, Lee DH, Lee J, et al. Comparison between two methods of bioelectrical impedance analyses for accuracy in measuring abdominal visceral fat area. J Diabetes Complications. 2016;30(2):343–349. [DOI] [PubMed] [Google Scholar]
- 23.China Guideline for Type 2 Diabetes. 2010. Available from: http://www.diab.net.cn/UploadFile/Ueditor/file/20160811/6360650768334000005174021.pdf. Accessed 9 August, 2019.
- 24.Kohl HW 3rd, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: global action for public health. Lancet. 2012;380(9838):294–305. [DOI] [PubMed] [Google Scholar]
- 25.Chinese Diabetes Society. China guideline for type 2 diabetes [in Chinese]. [M]. Beijing University Medical Press; 2014. [Google Scholar]
- 26.Shida T, Akiyama K, Oh S, et al. Skeletal muscle mass to visceral fat area ratio is an important determinant affecting hepatic conditions of non-alcoholic fatty liver disease. J Gastroenterol. 2018;53(4):535–547. [DOI] [PubMed] [Google Scholar]
- 27.Lemieux S. Contribution of visceral obesity to the insulin resistance syndrome. Can J Appl Physiol. 2001;26(3):273–290. [DOI] [PubMed] [Google Scholar]
- 28.Schautz B, Later W, Heller M, Muller MJ, Bosy-Westphal A. Total and regional relationship between lean and fat mass with increasing adiposity–impact for the diagnosis of sarcopenic obesity. Eur J Clin Nutr. 2012;66(12):1356–1361. [DOI] [PubMed] [Google Scholar]
- 29.Heymsfield SB, Scherzer R, Pietrobelli A, Lewis CE, Grunfeld C. Body mass index as a phenotypic expression of adiposity: quantitative contribution of muscularity in a population-based sample. Int J Obes. 2009;33(12):1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito T, Murata M, Otani T, Tamemoto H, Kawakami M, Ishikawa SE. Association of subcutaneous and visceral fat mass with serum concentrations of adipokines in subjects with type 2 diabetes mellitus. Endocr J. 2012;59(1):39–45. [DOI] [PubMed] [Google Scholar]
- 31.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–332. [DOI] [PubMed] [Google Scholar]
- 32.Piastra G, Perasso L, Lucarini S, et al. Effects of two types of 9-month adapted physical activity program on muscle mass, muscle strength, and balance in moderate sarcopenic older women. Biomed Res Int. 2018;2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francaux M, Deldicque L. Exercise and the control of muscle mass in human. Pflugers Archiv. 2019;471(3):397–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- China Guideline for Type 2 Diabetes. 2010. Available from: http://www.diab.net.cn/UploadFile/Ueditor/file/20160811/6360650768334000005174021.pdf. Accessed 9 August, 2019.