Abstract
Introduction
A 91-year-old sedentary man presenting exhaustion, lower-limb weakness, hypertension, and history of multiple falls was diagnosed with sarcopenia – appendicular skeletal muscle mass index (ASM) of 7.10 kg/m2.
Purpose
To investigate the effects of strength training performed with low intensity in isolation (LI) or with blood flow restriction (LI-BFR) on strength, muscle mass, IGF-1, endothelial function, microcirculation, inflammatory biomarkers, and oxidative stress.
Methods
In the first 3 months, LI was performed with intensity corresponding to 30% of 1 repetition maximum, followed by 1 month of inactivity, and another 3 months of LI-BFR (similar load than LI concomitant to BFR equivalent to 50% of resting systolic blood pressure).
Results
LI-BFR, but not LI improved muscle mass, ASM, handgrip strength, isokinetic peak torque, IL-6, and IGF-1. Endothelial function, red blood cell velocity, and concentrations of C-reactive protein, and soluble intercellular adhesion molecules-1 improved after both LI and LI-BFR. Endothelin-1 and oxidative stress increased after LI-BFR, and lowered after LI.
Conclusion
LI-BFR, but not LI improved strength, muscle mass, IGF-1, endothelial function, and selected inflammatory markers in a nonagenarian sarcopenic patient. These results are promising and suggest that LI-BFR should be considered as an alternative to prevent muscle loss and improve functional fitness in frail older populations.
Keywords: aging, muscle mass, resistance exercise, vascular occlusion, endothelial function, microcirculation
Introduction
In 1987, Irwin Rosenberg defined sarcopenia as a decline in skeletal muscle mass (SMM) and muscle strength, related to aging, being highly predictive of adverse events, such as falls, hospitalization, morbidity, and mortality.1 Strength training is acknowledged as a strategy to preserve the muscle function throughout the aging process.2,3 For these purposes, intensities >60–70% of 1 repetition maximum (RM) are recommended.4 However, in clinical settings it is not always feasible to implement high-intensity (HI) routines, particularly in the case of frail older patients.5
As a possible alternative, previous studies have shown that low intensity (LI) routines associated with blood flow restriction (BFR) would be capable to promote neural and hypertrophic adaptations through a variety of endocrine, neural and metabolic mechanisms when compared to traditional protocols with higher intensity. Therefore, LI performed with BFR may be an alternative for individuals who are intolerant to vigorous strength training.6–8
Although relevant as a clinical intervention with respect to muscle and strength adaptations for older populations, few studies have evaluated the effects of BFR training on vascular health.7,8 This is an important aspect to consider, since retrograde flow has been associated with endothelial dysfunction.9,10 Jenkins et al (2013) demonstrated that retrograde flow would relate to acute cellular apoptosis, producing an atherogenic endothelial phenotype.9 From a clinical perspective, these data indicate that alterations in blood flow promoted by BFR might impair endothelial function, being an adverse effect of this training method.
To date, the concomitant effects of BFR on muscle, strength, and vascular function have not been investigated, especially in very older sarcopenic patients. The comprehension of the effects of BFR in this group may expand the current knowledge about the benefits and safety of this modality of intervention in individual incapable to perform strength training with HI.
In 2014, a 91-year-old frail man was attended on an outpatient care unit, with report excessive exhaustion, accentuated reduction of lean mass and muscle strength of upper and lower limbs, hypertension and history of falls. Thus, the present study describes the effects of strength training performed with LI, isolate or concomitant to BFR on the strength, muscle mass (hypertrophy), endothelial function, inflammatory biomarkers and oxidative stress in a nonagenarian sarcopenic patient, sedentary, unable to perform moderate do HI strength training.
Materials and methods
Patient
A 91-year-old physically inactive man (Minimental State Examination 26 pts, height 165.5 cm, body mass 58 kg, BMI 21.2 kg/m2) was recruited from the Frailty in Brazilian Older People Study (FIBRA-RJ).11 In his admission, the patient reported excessive exhaustion, muscle atrophy in upper and lower limbs, muscle weakness in lower limbs, and history of multiple falls. Family history did not reveal any disease related to these symptoms. The patient had been diagnosed with systemic arterial hypertension and, in 2011, underwent angioplasty for placement of two coronary stents. He had preserved ventricular systolic function and left ventricular diastolic function with relaxation alteration pattern (E/E’ ratio: 8, reflecting normal filling pressures in the left atrium). No clinical abnormalities were found in carotid and vertebral ecocolor Doppler.
In 2014, he was classified as sarcopenic, with appendicular skeletal muscle mass (ASM) index of 7.10 kg/m2 measured by dual-energy X-ray absorptiometry (DXA).12 In the pre-participation clinical evaluation, the patient performed a pharmacological myocardial perfusion scintigraphy and did not exhibit abnormalities suggestive of ischemia. During the strength training intervention, the patient was under daily prescription of Losartan Potassium 50 mg, Carvedilol 6.25 mg, Warfarin 5 mg, Finasteride 5 mg, Spirolactone 25 mg, and Simvastatin 20 mg.
Study design
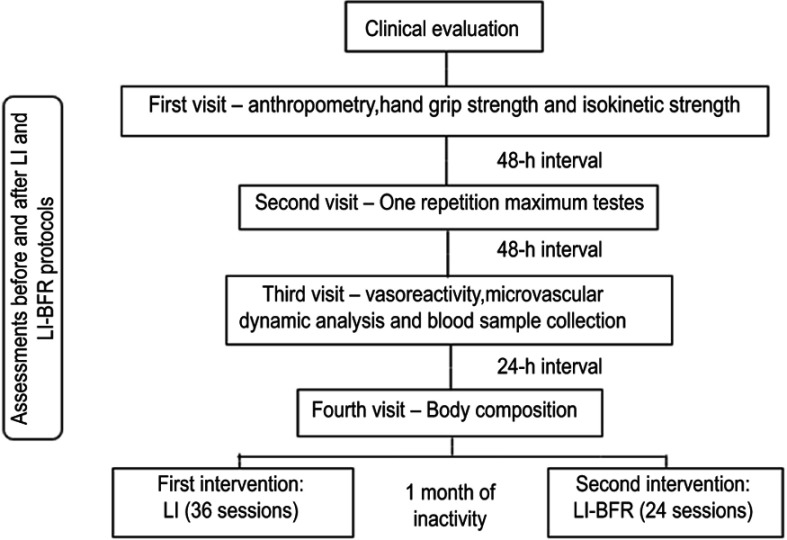
All assessments were performed before and after the intervention period, as shown in the flow chart of the experimental design (Figure 1). The study design was approved by the Ethics Committee of the University of Rio de Janeiro State, Rio de Janeiro, Brazil (CAAE: 17782513.0.0000.5282, opinion number 512.917, in January 2014) and has been registered in the Thai Clinical Trials Registry office (identification number TCTR20170131001, approved in 31-01-2017). The subject was informed about benefits and risks of the research prior to signing the written informed consent. In addition, a written informed consent was obtained from the subject for the publication of this case report.
Figure 1.
Experimental design.
Abbreviations: LI, low intensity; BFR, blood flow restriction.
Strength training
The strength training program included a 10-min warm-up walking on treadmill with intensity corresponding to 30% of heart rate reserve, followed by exercises for the upper and lower limbs (elbow flexion, elbow extension, leg press, and leg extension). All exercises were performed with three sets of 10 RM (1-min intervals between sets and exercises). The blood pressure was monitored before and at the end of each training session, using an automated Omron 705IT device (Omron Healthcare Co., Kyoto, Japan). Once a week, the heart rate was continuously measured during treadmill exercise by means of a cardiotachometer (RS800cx; Polar, Kempele, Finland) and the speed was adjusted to maintain the target intensity of 30% of heart rate reserve. The training sessions took place three times a week interspersed with 24–48 hrs intervals. In the first 12 weeks, the training intervention was performed with loads corresponding to 30% of 1 RM without BFR (LI), followed by 1 month of inactivity, ie, the patient was asked to maintain the instrumental activities of daily living, avoiding any changes in his routine. Then, the patient underwent another 8 weeks of training, with loads corresponding to 30% of 1 RM applied alongside with BFR (LI-BFR). The maximal dynamic strength in each exercise was established by 1 RM tests.
All exercise sessions were applied by certified exercise instructors (physical education professional or physical therapist) and supervised by physicians (geriatric and cardiologist). After each exercise session (LI and LI-BFR), nutritional management protein supplement was provided by a certified nutritionist (15 g dissolved in 150 mL water, Isofort Whey Protein Isolate, Vitafor Nutritional Supplements, Sao Paulo, SP, Brazil).
Blood flow restriction
BFR was applied by placing a nylon cuff size (11×85 cm) connected to a pneumatic cuff inflator (TD312 HokansonTM, Bellevue, WA, USA) to the proximal third of upper of lower limbs, inflated at 50% of resting systolic blood pressure (SBP).13 Pressure was maintained during the whole duration of the three sets of all exercises, including the rest intervals between sets. The total duration per session of occlusion cuff was of 12 mins on average.
Body composition
Body composition was evaluated by DXA (DPX-IQ Lunar Radiation CorporationTM, Madison, WI, USA) using standard protocols. The equipment was calibrated according to manufacturer instructions and the scans were performed in high resolution and analyzed by the same trained technician.14
Sarcopenia cutoff points
Lean mass of upper and lower limbs was used to calculate the ASM index. Cutoff point to diagnose sarcopenia proposed by Baumgartner et al (1998) was of 7.26 kg/m2 for men. This index has been associated to functional impairment and disability.12 Janssen et al (2004) estimated total SMM from ASM measured by DXA and normalized the SMM for height15 and termed it as SMM index. Cutoff points from 8.51 to 10.75 and ≤8.50 kg/m2 were reported to denote SMM index related to moderate to the elevated risk of physical disability in sarcopenic men.15
Handgrip and isokinetic strength
Handgrip strength was assessed by hand dynamometer (PC 5030J1 Fred SammonsTM, Burr Ridge, IL, USA), according to the American Association of Hand Therapists protocol.16 Three attempts were performed by each hand with 1 min of recovery between measurements. The highest value for each hand was recorded. Maximal isokinetic force of knee extensors was measured by means of an isokinetic dynamometer (Biodex System 4 ProTM, Biodex Medical Systems, NY, USA). Protocol and procedures were administered according to previous studies for isokinetic evaluation in the elderly.17 The patient had no previous experience with supervised resistance training programs. Therefore, he underwent familiarization sessions before baseline physical tests (ie, handgrip, 1 RM, and isokinetic strength tests).
Venous occlusion plethysmography and nailfold videocapillaroscopy
Forearm blood flow (FBF) was evaluated by venous occlusion plethysmography (HokansonTM AI6, Bellevue, WA, USA), as previously described.18 There were four stages as follows: 1) baseline FBF 1, 2) FBF during reactive hyperemia (after 5-min forearm arterial occlusion with pressure 50 mmHg above SBP), 3) baseline FBF 2, and 4) FBF after 5 mins of 0.4 mg sublingual nitroglycerin (Nitrolingual BurnsAdler PharmaceuticalsTM, Charlotte, NC, USA). FBF was measured for 2 mins at each stage with 3 mins interval for each phase were performed, except between reactive hyperemia and baseline FBF 2, which was 15 mins for washout of vasodilating substances created during ischemia. Microvascular function was evaluated by nailfold videocapillaroscopy, as described elsewhere.18 Baseline red blood cell velocity (RBCV) and maximal RBCV (after 1-min ischemia) were measured. The ratio between RBCVmax and RBCVbas was calculated.
Blood biomarkers analysis
Venous blood samples were harvested for biomarkers analysis, after a fasting period of 8 hrs. Plasma levels of soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), IL-6, and endothelin-1 (ET-1) were assessed by Human QuantikineTM ELISA kits (R&D Systems, MN, USA). Insulin-like growth factor-1 (IGF-1) plasma concentrations were evaluated by Human IGF-I and II magnetic bead panel (EMD Millipore CorporationTM, MA, USA). Serum levels of oxidated low-density lipoprotein (oxLDL), tumor necrosis factor α (TNF-α), and high sensitivity C-reactive protein analysis (hs-CRP) were determined by Mercodia oxidized LDL ELISA (MercodiaTM, Uppsala, Sweden), Human QuantikineTM HS ELISA kit (R&D Systems, MN, USA), and latex turbidimetric kit (BiosystemsTM S.A., Barcelona, Spain), respectively. Sample collection, storage, and all assays were performed according to protocols provided by manufacturers. All the samples were analyzed via the same assay. Intra-assay precision (CV%) for different biomarkers were: oxLDL=3.15%, sICAM-1=2.93%, sVCAM-1=2.38%, ET-1=1.93%, IL-6=7.65%, TNF-α=9.40% and IGF-1=5.35%.
Results
No clinical intercurrence during exercise protocols was recorded. The patient did not report any discomfort or pain during LI-BFR, having tolerated very well the occlusion level. All planned exercise sessions were accomplished by the patient with 100% adherence.
Body composition, muscle mass indices, and muscle strength
Table 1 depicts the results of body composition, muscle mass indices and muscle strength, as well as percent change (Δ%) before and after LI and LI-BFR. Body mass, BMI, fat mass, ASM, and SMM decreased after LI, while BMD remained stable. After LI-BFR, there was an increase in fat mass, ASM, and SMM. Body mass, BMI, and BMD were not different after strength training performed with BFR. Muscle strength of upper and lower limbs, including handgrip strength, improved only after LI-BFR. Peak torque and total work of knee extension increased in LI-BFR and reduced in LI. However, work fatigue of knee extension lowered after LI-BFR.
Table 1.
Body composition, handgrip, and isokinetic strength before and after strength training performed without (LI) and with blood flow restriction (LI-BFR) in a 91-year-old sarcopenic patient
| Variable | LI | LI-BFR | ||||
|---|---|---|---|---|---|---|
| Before | After | ∆ % | Before | After | ∆ % | |
| Total composition | ||||||
| Height (cm) | 165.5 | 165.5 | 0.0 | 165.0 | 165.0 | 0.0 |
| Body mass (kg) | 59.1 | 55.8 | −5.6 | 56.8 | 55.3 | −2.7 |
| Body mass index (kg/m2) | 21.6 | 20.4 | −5.6 | 20.9 | 20.3 | −2.7 |
| Fat mass (kg) | 13.0 | 10.8 | −16.9 | 11.0 | 11.1 | 1.3 |
| Fat free mass (kg) | 46.1 | 45.0 | −2.4 | 45.8 | 44.2 | −3.6 |
| Bone mineral density (kg) | 2.61 | 2.59 | −0.7 | 2.58 | 2.60 | 0.9 |
| ASM (kg) | 19.4 | 18.9 | −2.7 | 18.5 | 18.9 | 2.3 |
| SMM (kg) | 21.8 | 21.2 | −2.7 | 22.4 | 22.9 | 2.1 |
| Sarcopenia cut points | ||||||
| ASM index (kg/m2) | 7.1 | 6.9 | −2.7 | 6.8 | 6.9 | 2.4 |
| SMM index (kg/m2) | 7.9 | 7.7 | −2.8 | 8.2 | 8.4 | 2.1 |
| Handgrip strength (kgf) | 29.0 | 28.0 | −3.4 | 28.0 | 33.0 | 17.9 |
| Isokinetic strength | ||||||
| Peak torque ext (nm) | 78.7 | 65.4 | −16.9 | 78.8 | 82.4 | 4.6 |
| Total work ext (I) | 651.1 | 593.8 | −8.8 | 657.9 | 668.0 | 1.5 |
| Work fatigue ext (%) | 32.3 | 25.7 | −20.4 | 28.0 | 35.7 | 27.5 |
Abbreviations: LI, low intensity; BFR, blood flow restriction; ASM, appendicular skeletal muscle mass; SMM, total skeletal muscle mass; Ext, extension; Δ%, percent variation from baseline.
Hemodynamic outcomes, endothelial function, and blood biomarkers
Table 2 shows the results of hemodynamic outcomes, vasoreactivity and microvascular function, as well as inflammatory biomarkers, endothelial injury, oxidative stress, and muscle hypertrophy before and after LI-BFR and Δ% in LI and LI-BFR. All hemodynamic outcomes improved after LI. After LI-BFR, only heart rate decreased. The SBP remained unaltered, while diastolic and mean blood pressure increased.
Table 2.
Hemodynamic outcomes, vasoreactivity, microvascular function, and blood biomarkers before and after strength training performed without (LI) and with blood flow restriction (LI-BFR) in a 91-year-old sarcopenic patient
| Variable | LI | LI-BFR | ||||
|---|---|---|---|---|---|---|
| Before | After | ∆ % | Before | After | ∆ % | |
| Hemodynamic parameters | ||||||
| HR (bpm) | 69 | 57 | −17.39 | 83 | 78 | −6.02 |
| SBP (mmHg) | 149 | 141 | −5.37 | 130 | 130 | 0 |
| DBP (mmHg) | 77 | 65 | −15.58 | 68 | 75 | 10.29 |
| MBP (mmHg) | 101 | 90 | −10.89 | 88 | 93 | 5.68 |
| Vasoreactivity | ||||||
| FBF bas 1 (mL/min/100 mL) | 0.56 | 0.78 | 39.29 | 1.63 | 1.95 | 19.63 |
| FBF hyper (ml/min/100 mL) | 5.23 | 10.72 | 104.97 | 8.26 | 10.80 | 30.75 |
| FBF bas 2 (mL/min/100 mL) | 0.42 | 0.93 | 121.43 | 1.12 | 1.27 | 13.39 |
| FBF nitro (mL/min/100 mL) | 1.44 | 1.48 | 2.78 | 2.75 | 2.78 | 1.09 |
| Microvascular function | ||||||
| RBCVmax/RBCVbas (mm/s) | 1.27 | 1.40 | 10.24 | 1.40 | 1.75 | 25.27 |
| Blood biomarkers | ||||||
| hs-CRP (mg/di) | 0.12 | 0.02 | −83.33 | 0.13 | 0.06 | −53.85 |
| TNF-a (pg/mL) | 1.36 | 1.37 | 0.56 | 1.21 | 1.44 | 19.30 |
| IL-6 (pg/mL) | 2.73 | 2.76 | 0.88 | 6.75 | 3.20 | −52.55 |
| sVCAM-1 (ng/mL) | 1272.33 | 1337.76 | 5.14 | 1134.40 | 1123.98 | −0.92 |
| sICAM-1 (ng/mL) | 243.20 | 223.72 | −8.01 | 264.86 | 222.39 | −16.03 |
| ET-1 (pg/m1) | 2.16 | 1.80 | −16.87 | 2.00 | 2.22 | 10.91 |
| oxLDL (μ/L) | 30.46 | 26.60 | −12.86 | 37.25 | 40.21 | 7.96 |
| IGF-1 (na/m1) | 24.00 | 18.66 | −22.25 | 10.68 | 12.66 | 18.54 |
Abbreviations: LI, low intensity; BFR, blood flow restriction; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; FBF, forearm blood flow; Bas, baseline; Hyper, hyperemia; Nitro, nitroglycerin; RBCVmax/RBCVbas, increment RBCVmax of baseline; hs-CRP, high sensitivity c-reactive protein; TNF-α, tumor necrosis factor-alpha; IL-6, interleukin-6; sVCAM-1, soluble vascular cell adhesion molecule-1; sICAM-1, soluble intercellular adhesion molecule-1; ET-1, endothelin-1; oxLDL, oxidized low density lipoprotein; IGF-1, insulin-like growth factor-1; Δ%, percent variation from baseline.
The endothelium-dependent vasodilation during reactive hyperemia improved after both LI and LI-BFR. No difference concerning endothelium-independent vasodilation (after sublingual nitroglycerine) was detected after LI or LI-BFR. The RBCVmax/RBCVbas ratio also increased after both interventions. Plasma concentrations of hs-CRP and sICAM-1 improved after strength training performed with or without BFR. After LI-BFR, concentrations of TNF-α increased and IL-6 decreased, being stable after LI. Increased levels of sVCAM-1 were found after LI, while no change was detected after LI-BFR. ET-1 and oxLDL concentrations increased in LI-BFR and reduced in LI. Finally, IGF-1 reduced after LI and increased after LI-BFR.
Discussion
This case report observed that strength training performed with low BFR (~65 mmHg) was capable to induce positive health adaptations in a very old and frail nonagenarian individual. In comparison with isolate LI training (30% of 1 RM), greater improvements were observed in muscle mass and strength (with consequent increase in ASM). Moreover, positive effects in several inflammatory markers (hs-CRP, IL-6, and sICAM-1) and IGF1 were found. On the other hand, increased levels of TNF-α, ET-1, and oxLDL were detected after LI-BFR compared to LI. Lastly, endothelial and microvascular function seemed to be preserved after strength training performed with or without BFR. At the end of the 8th week of BFR, the volunteer decided to interrupt his participation, due to personal reasons not related to the intervention or clinical aspects. Despite the relatively short training time with BFR, the results have been consistently positive over microcirculation, muscle mass, and strength.
These results suggest that exercise with BFR may attenuate sarcopenia in frail older individuals,12 reducing the risk of disability associated with aging15 without impairment of endothelial function and microcirculation. The fact that the muscle mass of our patient reduced after 36 sessions of isolate LI (eg, without BFR) supports the clinical potential of BFR exercise (LI-BFR) as an alternative approach to optimize strength training routines designed for sarcopenic elderly. Some recent studies reinforce this premise. Kambic et al19 compared two groups of older patients with coronary artery disease, which performed resistance exercises along with LI-BFR (1 RM of unilateral knee extension with 30–40% occlusion) and continuous aerobic exercise. The strength increased in both groups, while brachial artery flow-mediated vasodilation and insulin resistance remained unchanged. In a recent meta-analysis including data from 238 older patients, Centner et al20 have shown that LI-BFR seemed to induce similar changes in muscle mass, but lower increase in muscular strength in comparison with high-load resistance training.
Several prior studies involving older people have demonstrated the efficacy of high levels of BFR associated with LI to magnify muscular responses.7,8 However, our results demonstrated that it is possible to increase both muscle mass and strength in a frail 91-year-old individual using light workloads combined to moderate BFR levels. Prior studies have also shown an inverse dose–response relationship between the magnitude of vascular occlusion and endothelial function,10 which is another important aspect to consider when prescribing strength training with BFR. Our findings indicate that, at least when implemented with LI (eg, loads corresponding to 30% 1RM) the strength training performed in isolation or combined with BFR did not compromise the endothelial function of a very old individual. In frail older patients, it is difficult to apply high workloads within strength training routines. Workloads of LI, on the other hand, are unlikely to produce substantial favorable effects on muscle mass.21 The present case report suggests that the effects of LI strength training can be optimized by moderate and perfectly tolerable levels of BFR, which seem to be compatible with the preservation of endothelial function. In practical terms, this is a very promising result warranting further studies.
Some potential confounding factors should be considered when LI-BFR resistance exercise is applied in the elderly, as the nutritional status, polypharmacy, and level of occlusion-pressure. Our patient used whey protein supplementation after evaluation by a nutritionist. Older patients are often under treatment with an expressive number of medications that cannot be withdrawn, particularly those for cardiometabolic diseases. Finally, there is an inherent concern with the risk of venous thromboembolism due to the occlusion pressure during BFR exercise. It is acknowledged that this risk increases with the level of occlusion. However, the literature shows minimal adverse events related to venous thromboembolism when the occlusion pressure is low.22
Several studies demonstrated that the underlying mechanisms responsible for potential beneficial effects in muscle mass and strength were associated with mechanical tension and metabolic stress which act synergistically to numerous other processes,23 including increased heat shock proteins,24 upregulation of mTOR signaling pathway,8 higher motor unit recruitment of fast twitch muscle fibers,25 and decreased myostatin expression26 and increased growth hormone and IGF-127,28 production. In the present study, plasma concentrations of IGF-1 decreased in LI and increased after LI-BFR (Table 2). It is therefore feasible to speculate that the combination of strength training with BFR provoked an increase in anabolism, which did not occur in response to isolate exercise, being an important find for maintaining the integrity of musculoskeletal system, considering that IGF-1 is acknowledged to decline in older age, being a determinant of losses in strength and muscle mass.29
The vascular reactivity during post occlusive reactive hyperemia increased in both training protocols, indicating an improvement of endothelial function in our patient. Endothelium-independent vasodilation remained unchanged, which concurs with prior research showing that vascular reactivity in response to nitrate seems not to be influenced by neither aging or physical exercise.30 The present data are indicative that even when performed with LI, strength training might help preventing age-related decline in endothelial function.18
Shear stress is an important physiological stimulus that modulates endothelial function in response to exercise.31,32 Increased expression of endothelial nitric oxide synthase (eNOS) and consequent release of nitric oxide (NO) production and reduction of vasoconstrictors, such as ET-131 have been reported. Recent studies showed that strength training performed with BFR would promote greater expression of vascular endothelial growth factor (VEGF) mRNA than isolate chronic strength exercise.33,34 In short, this means that BFR training would be able to increase the number of capillaries. Studies in vitro showed that VEGF enhances eNOS expression, resulting in NO production, an important effect in angiogenesis regulation.35 The enhanced response of angiogenic factors seems to be mediated through greater metabolic activity, low oxygen pressure, and shear stress.25 These mechanisms may explain the improvement in vascular health of our patient, even with a potential reduction of shear stress during exercise performed with BFR.
Even though the beneficial effects of physical exercise on vascular health are well documented in young people, as reflected by improvements in FMD of brachial artery,36 some studies also indicate that regular exercise can restore endothelium-dependent vasodilation in sedentary older individuals.30,35 Increases of approximately 45% in brachial artery reactivity37 and 30% in acetylcholine-induced FBF have been reported.30 The restriction of blood flow during chronic exercise seems to induce vascular adaptations counteracting the possible deleterious effects promoted by retrograde shear rate during acute exercise.38,39
The ratio between RBCVmax and RBCVbas increased in both protocols (Table 2), suggesting improvement of endothelium-dependent vasodilation (with increased bioavailability of NO) and preservation of smooth muscle cells. Previous studies assessing the skin microcirculation showed a relationship between microvascular dysfunction, aging,18,40 and cardiovascular risk,41 emphasizing the microvascular benefits of therapeutic approaches with ST, proposed in the present study, in nonagenarian subjects.
The interventions without and with BFR promoted different responses in inflammatory, oxidative stress, and vascular biomarkers. Reductions in hs-CRP, IL-6 and sICAM-1, no change in sVCAM-1, and increases in TNF-α, ET-1, and oxLDL were observed after LI-BFR. On the other hand, after LI there was a decline in hs-CRP, sICAM-1, ET-1, and oxLDL, while TNF-α and IL-6 remained unaltered, and sVCAM-1 increased. It is possible to speculate that the magnitude of variations in vascular reactivity observed after LI and LI-BFR (Δ% FBF hyper=104.97 vs 30.75 mL/min/100 mL tissue, respectively) could relate to increased levels of inflammatory and vasoconstrictor factors, as reflected by greater TNF-α and ET-1 following LI-BFR, but not LI (Δ% TNF-α=19.30 vs 0.56 pg/mL; Δ% ET-1=10.91 vs -16.87 pg/mL, respectively). A potential role in this context could be attributed to oxidative stress as well, which also increased after LI-BFR, but not LI (Δ% oxLDL =7.96 vs -12.86 u/L, respectively). Increased oxidative stress and systemic inflammation are acknowledged to predispose to impaired endothelial function.42 This might be a concern for individuals with high risk of cardiovascular events; in this case, interventions with BFR would be problematic. However, previous studies did not report significant changes in oxidative stress following strength exercise performed with BFR.6 It is possible that, in our patient, 2 months of LI-BFR have not been enough to improve antioxidant defenses, which could mitigate or prevent oxidative stress associated with aging.43 Further experimental studies are certainly warranted to elucidate this point.
Conclusion
In conclusion, strength training performed with LI and BFR (LI-BFR) improved the strength, ASM, IGF-1, and endothelial function in a 91-year-old sarcopenic patient. These effects were not detected after isolate strength training performed with similar intensity (LI). Increased circulating levels of TNF-α, ET-1, and oxidative stress were observed following strength training with BFR, but inflammatory profile measured by hs-CRP and IL-6 was improved. Moreover, there was a reduction in sICAM-1 and no change in sVCAM-1. These results are promising and suggest that LI-BFR should be considered as an alternative clinical intervention to prevent muscle loss and improve functional fitness in very old sarcopenic populations, which are often refractory to HI exercise routines. Additional research is needed to ratify the present findings and to elucidate the mechanisms underlying the potential effects of BFR training upon muscle function and vascular health of frail elderly.
Acknowledgment
This study was supported by Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.Rosenberg IH. Sarcopenia: origins and clinical relevance. Am Soc Nutr Sci. 1997;127:990–991. [DOI] [PubMed] [Google Scholar]
- 2.Chodzko-Zajko WJ, Proctor DN, Singh MA, et al; American College of Sports Medicine. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 3.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3181453546 [DOI] [PubMed] [Google Scholar]
- 4.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34(2):364–380. doi: 10.1097/00005768-200202000-00027 [DOI] [PubMed] [Google Scholar]
- 5.Ferraz RB, Gualano B, Rodrigues R, et al. Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med Sci Sports Exerc. 2018;50(5):897–905. doi: 10.1249/MSS.0000000000001530 [DOI] [PubMed] [Google Scholar]
- 6.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88(6):2097–2106. doi: 10.1152/jappl.2000.88.6.2097 [DOI] [PubMed] [Google Scholar]
- 7.Yasuda T, Fukumura K, Fukuda T, et al. Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand J Med Sci Sport. 2014;24(5):799–806. doi: 10.1111/sms.12087 [DOI] [PubMed] [Google Scholar]
- 8.Fry CS, Glynn EL, Drummond MJ, et al. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108:1199–1209. doi: 10.1152/japplphysiol.01266.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins NT, Padilla J, Boyle LJ, Credeur DP, Harold Laughlin M, Fadel PJ. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension. 2013;61(3):615–621. doi: 10.1161/HYPERTENSIONAHA.111.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thijssen DHJ, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53(6):986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508 [DOI] [PubMed] [Google Scholar]
- 11.Lourenço RA, Sanchez MA, Moreira VG, et al. Frailty in older Brazilians - Fibra-RJ: research methodology on frailty, cognitive disorders and sarcopenia. Rev Hosp Univ Pedro Ernesto. 2015;14(4):13–23. [Google Scholar]
- 12.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520 [DOI] [PubMed] [Google Scholar]
- 13.Gualano B, Neves M, Lima FR, et al. Resistance training with vascular occlusion in inclusion body myositis: a case study. Med Sci Sports Exerc. 2010;42(2):250–254. doi: 10.1249/MSS.0b013e3181b18fb8 [DOI] [PubMed] [Google Scholar]
- 14.Kelly TL, Berger N, Richardson TL. DXA body composition: theory and practice. Appl Radiat Isot. 1998;49(5–6):511–513. doi: 10.1016/S0969-8043(97)00226-1 [DOI] [PubMed] [Google Scholar]
- 15.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413–421. doi: 10.1093/aje/kwh058 [DOI] [PubMed] [Google Scholar]
- 16.Richards LG, Olson B, Palmiter-Thomas P. How forearm position affects grip strength. Am J Occup Ther. 1996;50(2):133–138. doi: 10.5014/ajot.50.2.133 [DOI] [PubMed] [Google Scholar]
- 17.Bottaro M, Ernesto C, Celes R, Farinatti PTV, Brown LE, Oliveira RJ. Effects of age and rest interval on strength recovery. Int J Sports Med. 2010;31(1):22–25. doi: 10.1055/s-0030-1249083 [DOI] [PubMed] [Google Scholar]
- 18.Bottino DA, Lopes FG, de Oliveira FJ, de Souza Mecenas A, Clapauch R, Bouskela E. Relationship between biomarkers of inflammation, oxidative stress and endothelial/microcirculatory function in successful aging versus healthy youth: a transversal study. BMC Geriatr. 2015;15(1):41. doi: 10.1186/s12877-015-0044-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kambic T, Novakovic M, Tomazin K, Stronjnik V, Jug B. Blood flow restriction resistance exercise inproves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: a pilot randomized controlled trial. Front Physiol. 2019; eCollection 2019. doi: 10.3389/fphys.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centner C, Wiegel P, Gollhofer A, König D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: a systematic review and meta-analysis. Sports Med. 2019;49:95–108. doi: 10.1007/s40279-018-0994-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loenneke JP, Wilson JM, Marín PJ, Zourdos MC, Bemben MG. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(5):1849–1859. doi: 10.1007/s00421-011-1978-0 [DOI] [PubMed] [Google Scholar]
- 22.Patterson SD, Hughes L, Warmington S, et al. Blood flow restriction exercise position stand: considerations of methodology, application and safety. Front Physiol. 2019;10. doi: 10.3389/fphys.2019.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sport Med. 2015;45(2):187–200. doi: 10.1007/s40279-014-0264-9 [DOI] [PubMed] [Google Scholar]
- 24.Kawada S, Ishii N. Skeletal muscle hypertrophy after chronic restriction of venous blood flow in rats. Med Sci Sports Exerc. 2005;37(7):1144–1150. doi: 10.1249/01.mss.0000170097.59514.bb [DOI] [PubMed] [Google Scholar]
- 25.Suga T, Okita K, Takada S, et al. Effect of multiple set on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. Eur J Appl Physiol. 2012;112(11):3915–3920. doi: 10.1007/s00421-011-1978-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurentino GC, Ugrinowitsch C, Roschel H, et al. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc. 2012;44(3):406–412. doi: 10.1249/MSS.0b013e318233b4bc [DOI] [PubMed] [Google Scholar]
- 27.Takano H, Morita T, Iida H, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol. 2005;95(1):65–73. doi: 10.1007/s00421-005-1389-1 [DOI] [PubMed] [Google Scholar]
- 28.Abe T, Yasuda T, Midorikawa T, et al. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int J KAATSU Train Res. 2005;1(1):6–12. doi: 10.3806/ijktr.1.6 [DOI] [Google Scholar]
- 29.Manini TM, Yarrow JF, Buford TW, Clark BC, Conover CF, Borst SE. Growth hormone responses to acute resistance exercise with vascular restriction in young and old men. Growth Horm IGF Res. 2012;22(5):167–172. doi: 10.1016/j.ghir.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–1357. doi: 10.1161/01.CIR.102.12.1351 [DOI] [PubMed] [Google Scholar]
- 31.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling. molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49(25):2379–2393. doi: 10.1016/j.jacc.2007.02.059 [DOI] [PubMed] [Google Scholar]
- 32.Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55(2):312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282 [DOI] [PubMed] [Google Scholar]
- 33.Ferguson RA, Hunt JEA, Lewis MP, et al. The acute angiogenic signalling response to low-load resistance exercise with blood flow restriction. Eur J Sport Sci. 2018;18(3):397–406. doi: 10.1080/17461391.2017.1422281 [DOI] [PubMed] [Google Scholar]
- 34.Larkin KA, MacNeil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc. 2012;44(11):2077–2083. doi: 10.1249/MSS.0b013e3182625928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999;41(3):773–780. doi: 10.1016/S0008-6363(98)00228-4 [DOI] [PubMed] [Google Scholar]
- 36.Allen JD, Geaghan JP, Greenway F, Welsch MA. Time course of improved flow-mediated dilation after short-term exercise training. Med Sci Sports Exerc. 2003;35(5):847–853. doi: 10.1249/01.MSS.0000064931.62916.8A [DOI] [PubMed] [Google Scholar]
- 37.Dobrosielski DA, Greenway FL, Welsh DA, Jazwinski SM. Modification of vascular function after handgrip exercise training in 73- to 90-yr-old men. Med Sci Sport Exerc. 2010;41(7):1429–1435. doi: 10.1249/MSS.0b013e318199bef4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renzi CP, Tanaka H, Sugawara J. Effects of leg blood flow restriction during walking on cardiovascular function. Med Sci Sports Exerc. 2010;42(4):726–732. doi: 10.1249/MSS.0b013e3181bdb454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreuder THA, Green DJ, Hopman MTE, Thijssen DHJ. Impact of retrograde shear rate on brachial and super fi cial femoral artery flow-mediated dilation in older subjects. Atherosclerosis 2015;241(1):199-204. [DOI] [PubMed] [Google Scholar]
- 40.Rossi M, Cupisti A, Mariani S, Santoro G, Pentimone F. Endothelium-dependent and endothelium-independent skin vasoreactivity in the elderly. Aging Clin Exp Res. 2002;14(5):343–346. [DOI] [PubMed] [Google Scholar]
- 41.Serné EH, Gans ROB, Ter Maaten JC, Tangelder GJ, Donker AJM, Stehouwer CDA. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension. 2001;38(2):238–242. [DOI] [PubMed] [Google Scholar]
- 42.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485 [DOI] [PubMed] [Google Scholar]
- 43.Franzoni F, Ghiadoni L, Galetta F, et al. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens. 2005;18(4):510–516. doi: 10.1016/j.amjhyper.2004.11.006 [DOI] [PubMed] [Google Scholar]