Introduction
Tuberculosis (TB) remains a global health threat. In 2013, 9 million new cases of TB disease were recorded, with 1.5 million deaths1. Modelling studies show that early diagnosis, optimal treatment and effective vaccines may significantly affect the pandemic2. Among these, an effective TB vaccine could be cost-effective in low- and middle-income countries3 and is the most sustainable intervention4. The only registered vaccine against TB, Bacillus Calmette-Guérin (BCG), has demonstrated highly variable efficacy in clinical trials5,6. In endemic settings such as South Africa, TB incidence in infants and young children is around 1% per annum with childhood TB making up about one fifth of all cases, despite good BCG coverage7, 8. There is an urgent need for a more effective TB vaccine for use in infants and children.
There are currently 16 candidate TB vaccines in clinical testing aimed at replacing BCG or at boosting its immune effects9. AERAS-402 is a replication-deficient human adenovirus 35-vectored vaccine expressing a fusion protein of the Mycobacterium tuberculosis (M.tb) antigens Ag85A, Ag85B and TB10.410. It was shown to be safe and immunogenic in adults and infants11,12, 13 and to reduce bacterial replication of M.tb in mice14, .
Here we present the results of a dose-finding, multicentre Phase 2 clinical trial, conducted over a period of three years, to test the safety and immunogenicity of selected doses of AERAS-402 in healthy, HIV-uninfected, BCG-vaccinated infants.
Methods
We conducted a Phase 2 multicentre, multinational, double-blind, randomised, placebo-controlled study of AERAS-402 at the South African Tuberculosis Vaccine Initiative (SATVI) site near Cape Town, South Africa; Kenya Medical Research Institute (KEMRI)/CDC, Kisumu site, Kenya; Centro de Investigação em Saúde de Manhiça (CISM), Manhiça, Mozambique; and the Perinatal HIV Research Unit (PHRU), Soweto, South Africa.
The study protocol was approved by the following regulatory agencies: Medicines Control Council of South Africa; Kenyan Pharmacy and Poisons Board; and the Ministry of Health of Mozambique. It was also reviewed and approved by the following Ethics Committees: the Human Research Ethics Committee of the Faculty of Health Sciences of the University of Cape Town; KEMRI National Ethics Committee; Comité Nacional de Bioética para a Saúde de Moçambique, Comité Ético de Investigación Clínica del Hospital Clinic I Provincial de Barcelona; and the University of the Witwatersrand Human Research Ethics Committee. The study was registered with the South African National Clinical Trial Register (DOH-27-0611-3044), the Pan African Clinical Trials Registry (PACTR201203000306280) and https://clinicaltrials.gov/ () .
The original trial design consisted of two phases and seven study groups: an initial dose-finding phase, which investigated 2 doses at three dose levels (Groups 1–3), followed by a safety and efficacy phase (Groups 4–7), which included immunogenicity cohorts. The trial design was initially modified to add a third dose of AERAS-402 at the selected dose in the safety and efficacy phase. Subsequently, when the predefined immunogenicity target was not met, the study was modified to remove the efficacy objective (Groups 6–7) and focus on the safety and immunogenicity of three doses of AERAS-402 (modified Group 5).
Infants were eligible for enrolment only if the parent or legal guardian provided written informed consent. We enrolled healthy infants, aged 16 -26 weeks, who had received BCG more than 3 months prior to randomisation, who had received all age-appropriate routine immunisations at least 14 days before randomisation, and whose mother’s HIV status was known. HIV-uninfected infants of HIV-infected mothers were eligible. Infants were excluded if they were acutely ill, or had clinically significant abnormalities of blood or urine tests, were HIV infected (PCR positive), or QuantiFERON®-TB Gold In-Tube test (QFT; Cellestis, Australia) positive, had history or evidence of active TB, or were exposed to a household contact with active TB.
Infants were randomised to receive two intramuscular doses of AERAS-402 or placebo on study days 0 and 28 using a paper-based system in Groups 1–3 and an interactive voice response system (IVRS) in Groups 4–5. The placebo was sterile vaccine buffer. Doses were prepared and labelled in syringes by an unblinded study pharmacist. Participants’ parents or legal guardians and all clinical study staff were blinded to the assigned intervention group. Randomisation in Groups 1–3 was in a 3:1 ratio (AERAS-402: placebo), Group 4 in a 1:1:1:1 ratio to receive one of three dose levels of AERAS-402 (1.5 × 1010, 3.0 × 1010, or 1 × 1011 viral particles (vp)) or placebo, and Group 5 in a 1:1 randomisation ratio (1×1011 vp: placebo). Group allocation was sequential, subsequent to Data Monitoring Committee (DMC) review of safety data. Dose selection for Group 5 was determined by sponsor review of cumulative safety and immunogenicity data for Groups 1–4 and all previous trials of AERAS-402 in both adults and infants12,13,11. The selection of the highest dose strength, 1×1011 vp, was based on these data and supported by the protocol which states that in case of ambiguous data the highest dose should be selected. During the enrolment period for Group 5, it was decided to add a third dose of AERAS-402 1×1011 vp at study day 280, to potentially enhance the immune responses observed following dose 2. To receive the third dose, the participant’s parent/guardian was required to consent separately.
All participants were followed up for adverse events (AEs) for 28 days after each dose, and for serious adverse events (SAEs) for the duration of their study participation. Solicited AEs were defined as tenderness, redness and swelling at the site of injection; upper respiratory tract infection, fatigue, conjunctivitis, fever, and diarrhea.
Peripheral blood for routine haematology and blood chemistry was taken from all participants at screening and seven days after each vaccination. Immunogenicity was assessed by intracellular cytokine staining (ICS), ELISpot, TB Antibody ELISA, and adenovirus 35 serum neutralisation assays at screening and day 56 for Groups 1–4; and at screening and days 56, 280, 308 and 448 / end of study for Group 5. QFT was measured at screening, upon suspicion of TB disease or history of household contact, and again at study completion. The required sample size was 442 for the final version of the protocol. Follow up was for 24 months.
Data on AEs were obtained from diary cards completed for 7 days post vaccination, and by direct questioning by study staff at each visit. Active surveillance of public health records allowed for timely SAE reporting. All AEs and SAEs were assessed by trial investigators. A blinded Local Medical Monitor (LMM) also assessed all SAEs. Despite removal of the TB efficacy objective participants with signs, symptoms or history of TB exposure were investigated for TB diagnosis, as previously described8.
The DMC met at pre-specified time points, and on an ad hoc basis to review safety data when a study pause rule was met, or if any AEs or SAEs were of concern to the sponsor, investigators, LMM or Global Medical Monitor.
Immunology
Immunogenicity was assessed with PBMC Intracellular Cytokine Staining (ICS) assay performed as previously described12, Enzyme-Linked ImmunoSpot (ELISpot) assay, ELISA for Anti-TB Antigen Antibodies, Anti-adenovirus 35 Neutralizing antibodies and Measles Antibodies. Details re above methods are contained in the Supplemental material Table 1
Statistical analysis
The primary outcome was safety, and included all solicited, unsolicited and serious adverse events in all participants who received at least one dose of study vaccine. The percentage of participants with AEs was presented by MedDRA Preferred Term. For categorical data, analysis was performed using the Chi-square test; and for AE grading analysis the Chi-square test for linear trend was used. Statistical significance was assigned to p ≤ 0.05.
Responder definition (ICS and ELISpot)
The determination of per-study day responder status for both ICS and ELISpot results was based upon principles outlined in Horton et al. 15 using Fisher’s Exact Test and Bonferroni Holm method.
Immunology results
Statistical tests were performed using SAS 9.4 (Cary, NC). For > 2 groups, Kruskal-Wallis tests were used to compare groups at a given time point for each T cell / antigen combination. If significant (p < 0.05), comparisons between pairs of groups were made using Mann-Whitney U tests, and p-values were adjusted for multiplicity by T cell and antigen using the Holm method.
Vaccine groups were compared to placebo at each time point by Mann-Whitney U Tests and were adjusted for multiplicity by T cell type (if applicable) and antigen using the Holm method.
Change in neutralising antibody response over time was determined initially by a Friedman test. Then changes from baseline by time point were analyzed using Wilcoxon signed-rank tests and adjusted for multiplicity by antigen using the Holm method.
Results
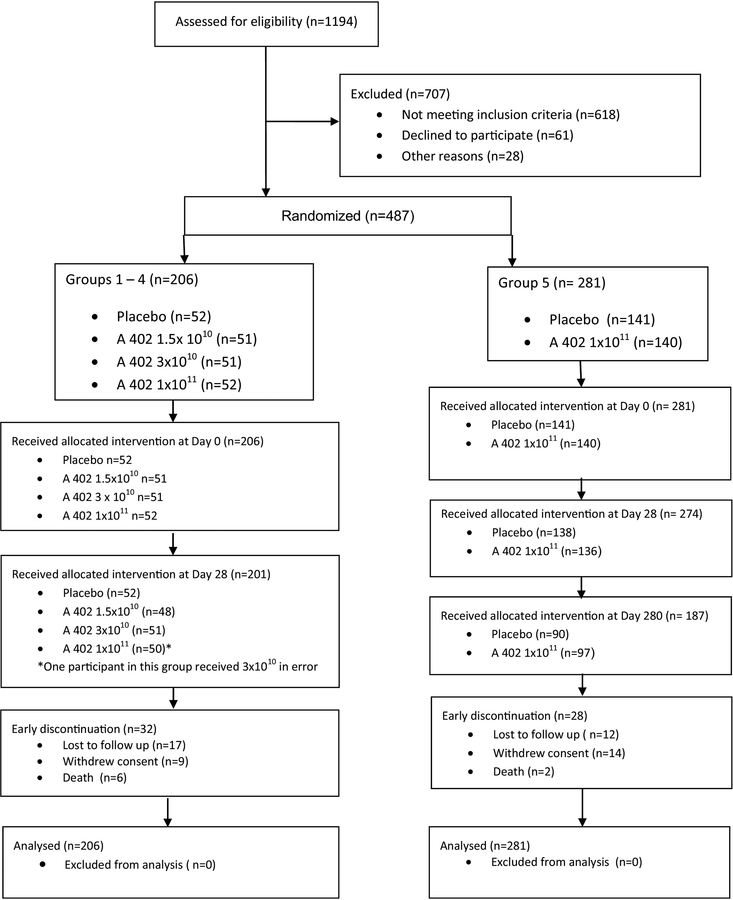
Between October 7, 2010 and April 27, 2012 we enrolled 487 of 1194 screened participants (Fig. 1). Reasons for screening failure included laboratory abnormalities, positive QFT, withdrawal of consent and age at study day 0 (Supplementary Table 2). In Groups 1–4, 206 infants were randomized to receive two doses of AERAS-402 or placebo. Five infants in Groups 1–4 either discontinued the study before study day 28, or did not meet criteria for receipt of the second dose. In Group 5, 281 infants were randomized to receive two doses of AERAS-402 or placebo, with a third dose added following trial design modification. Seven infants either discontinued the study before study day 28, or were not eligible to receive the second dose, and 94 infants either discontinued the study before study day 280, or were not eligible for the third dose. Demographic characteristics between the study groups were comparable (Table 1).
Figure 1.
Consort diagram
Table 1.
Demographics of enrolled population
| Groups 1–4 | Group 5 | All participants | |||||
|---|---|---|---|---|---|---|---|
| Placebo | 1.5×1010 | 3 × 1010 | 1 × 1011 | Placebo | 1 × 1011 | ||
| 52 | 51 | 51 | 52 | 141 | 140 | 487 | |
| Gender, n (%) male | 30 (57.7%) | 31 (60.8%) | 21 (41.2%) | 32 (61.5%) | 84 (59.6%) | 72 (51.4%) | 270 (55.4%) |
| Mean age (days) | 145.3 | 144.5 | 138.6 | 149.1 | 150.2 | 145.2 | 146.3 |
| Mean weight (kg) | 6.67 | 6.74 | 6.57 | 6.93 | 6.96 | 6.68 | 6.78 |
| Race, n (%) (black)1 | 42 (80.8%) | 44 (86.3%) | 41 (80.4%) | 43 (82.7%) | 98 (69.5%) | 102 (72.9%) | 370 (76.0%) |
| Mother HIV negative n (%) | Maternal HIV status not collected for groups 1–4 | 129 (91.5%) | 128 (91.4%) | 257 (91.5%) | |||
Race categories recorded Black, white, Asian and coloured / mixed. No white or Asian participants enrolled
Safety
At least one AE was reported in 292 (99.3%) of the 294 AERAS-402 recipients and 188 (97.4%) of the 193 placebo recipients (Table 2). At least one solicited AE was reported in 276 (93.9%) of the AERAS-402 and 170 (88.1%) of the placebo recipients. Chi-square test for trend showed significance for linear trend of AE severity in Group 5 AERAS-402 recipients compared with placebo recipients (Table 2). The proportions of participants with severe AEs were comparably distributed among the AERAS-402 and placebo groups. However, a higher proportion of participants receiving AERAS-402 at the highest dose level of 1 × 1011 vp had moderate events, compared to those receiving placebo, in Group 5. The frequency of local reactogenicity events, pyrexia and fatigue increased significantly with increasing dose level of AERAS-402 (Supplementary Table 3). There was no significant increase in AEs reported with number of doses of AERAS-402 received (Supplementary Table 4).
Table 2:
Adverse Events after Any Dose
| Groups 1–4 | Group 5 | |||||
|---|---|---|---|---|---|---|
| Placebo | 1.5 × l010vp | 3 × l010vp | 1 × l011vp | Placebo | 1 × l011vp | |
| N=52 | N=51 | N=51 | N=52 | N=141 | N=140 | |
| Participants with at least one AE, n (% of N) | 49 (94.2%) | 49 (96.1%) | 51 (100%) | 52 (100%) | 139 (98.6%) | 140 (100%) |
| Participants with at least one solicited AE, n (% of N) | 41 (78.8%) | 44 (86.3%) | 46 (90.2%) | 50 (96.2%) | 129 (91.5%) | 136 (97.1%) |
| Severity, n (% of N; participants counted once at highest severity level in each dose group) | ||||||
| Mild | 11 (21.2%) | 13 (25.5%) | 14 (27.5%) | 8 (15.4%) | 47 (33.3%) | 18(12.9%)1 |
| Moderate | 17 (32.7%) | 18 (35.3%) | 15 (29.4%) | 21 (40.4%) | 48 (34.0%) | 71 (50.7%)2 |
| Severe | 21 (40.4%) | 18 (35.3%) | 22 (43.1%) | 23 (44.2%) | 44 (31.2%) | 51 (36.4%) |
| *p=0.60 | p=0.86 | p=0.66 | p=0.005 | |||
| Relatedness, n (% of N; participants counted once at strongest relationship in each dose group ) | ||||||
| Not related | 4 (7.7%) | 3 (5.9%) | 2 (3.9%) | 2 (3.8%) | 6 (4.3%) | 3 (2.1%) |
| Related | 45 (86.5%) | 46 (90.2%) | 49 (96.1%) | 50 (96.2%) | 133 (94.3%) | 137 (97.9%) |
| Most common SAEs , n (% of N) | ||||||
| Participants with at least one SAE | 20 (38.5%) | 25 (49.0%) | 23 (45.1%) | 18 (34.6%) | 38 (27.0%) | 40 (28.6%) |
| p=0.289 | p=0.50 | p=0.69 | p=0.76 | |||
| Malaria | 12 (23.1%) | 18 (35.3%) | 11 (21.6%) | 8 (15.4%) | 12 (8.5%) | 14 (10.0%) |
| Pneumonia | 10 (19.2%) | 7 (13.7%) | 6 (11.8%) | 6 (11.5%) | 5 (3.5%) | 11 (7.9%) |
| Gastroenteritis | 2 (3.8%) | 2 (3.9%) | 7 (13.7%) | 4 (7.7%) | 11 (7.8%) | 8 (5.7%) |
| Deaths | ||||||
| All causes | 2 (3.8%) | 1 (2.0%) | 2 (3.9%) | 1 (1.9%) | 0 (0.0%) | 2 (1.4%) |
| p=0.63 | p=0.98 | p=0.62 | p=0.25 | |||
Distribution of adverse events across severity grading compared for each study group vs placebo (Chi-square test for trend)
Group 5 Placebo: AERAS 402 p = 0.000
Group 5 Placebo: AERAS 402 p = 0.04
Injection site erythema measuring >25mm was observed within 60 minutes post vaccination in seven infants at a single trial site, following the first (n=3), second (n=2) or third (n=2) dose of AERAS-402 1 × 1011 vp. Erythema was limited to the injection site, spontaneously decreased in size within a few hours of appearance and resolved 1–14 days later. No systemic signs or symptoms of allergic reaction were noted. Skin prick tests and mast cell tryptase were normal. Allergist assessment was of a local injection reaction, which was not IgE-mediated. These seven infants did not receive further doses of AERAS-402.
A total of 164 (33.7%) participants had at least one SAE, with a comparable incidence among AERAS-402 and placebo recipients. The most common SAEs were malaria (KEMRI and CISM sites), pneumonia, and gastroenteritis, as expected in this African infant population. One SAE of hospitalisation for tachypnea (respiratory rate in same range as at baseline) in a participant who received 1 × 1011 vp AERAS-402 was considered related to study vaccine based on timing of the event (Study Day 0). Malaria and pneumonia were excluded as causes and this event resolved without sequelae.
There were 8 deaths during the trial, none of which were considered vaccine related.
Tuberculosis
End of study QFT conversions occurred in 17 participants and were equally distributed between the placebo and AERAS-402 groups at 6 (3.6%) and 11 (4.3%), respectively. Of these, 3 participants(1 placebo, 2 AERAS-402) received anti-tubercular therapy during the study.
During the course of the study, 16 (8 Placebo, 8 AERAS-402) participants received anti-tubercular treatment based on the judgment of the treating physician. None of these participants were mycobacterial culture or GeneXpert MTB/RIF positive. Neither QFT status nor receipt of anti-tubercular therapy were criteria for exclusion from the safety and immunogenicity analysis, which was based on receipt of appropriate number and timing of doses of the study vaccine and (for immunogenicity) availability of samples.
Immunogenicity
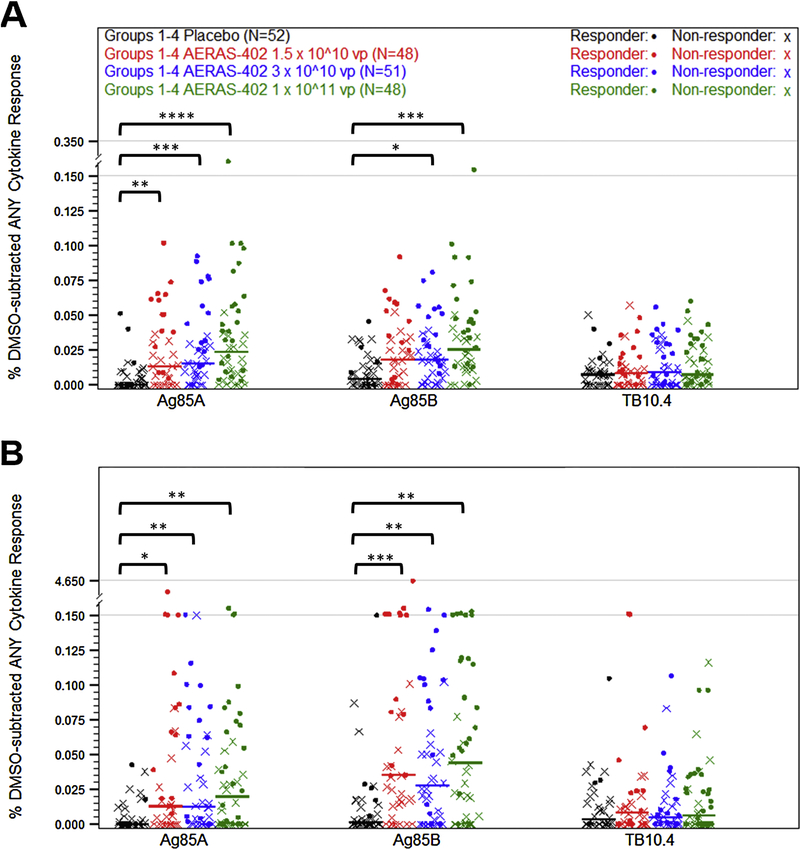
Participants in Groups 1–4 received placebo or increasing dose levels of AERAS-402 on study days 0 and 28 and blood for PBMCs was collected on study day 56. ICS was performed to evaluate expression of IFN-γ, TNF, and/or IL-2 in CD4+ and CD8+ T cells following ex vivo stimulation with Ag85A, Ag85B, or TB10.4 peptide pools. Median CD4+ T-cell responses to Ag85A and Ag85B stimulation following vaccination with high dose AERAS-402 were slightly higher than those observed with the two lower doses (Fig. 2A and Supplementary Table 4). CD8+ T-cell responses were higher than CD4+ T-cell responses and showed the same trend as the CD4+ T cells with higher responses in the high dose group (Fig. 2B and Supplementary Table 5). Responses to TB10.4 were very low in both CD4+ and CD8+ T cells, which is consistent with prior studies with AERAS-40211,12,13. Responder rates were calculated using Fisher’s Exact Test15. Using this definition, the ICS responder rates following two vaccinations with placebo, 1.5 × 1010, 3 × 1010, and 1 × 1011 vp were found to be 12.2%, 35.9%, 35.7%, and 50%, respectively.
Figure 2. PBMC ICS responses for dose selection.
Participants were vaccinated with placebo (black symbols) or AERAS-402 at doses of 1.5 × 10^10 (red symbols), 3 × 10^10 (blue symbols), or 1 × 10^11 (green symbols) vp on study day 0 and 28. Blood was collected 28 days following the second vaccination (study day 56). PBMC were isolate for the measurement of CD4+ (A) and CD8+ (B) DMSO-subtracted T-cell responses against Ag85A, Ag85B, and TB10.4 peptide pools using intracellular cytokine staining. Total responses (percentage of cells making any of the three IFN-γ, IL-2, or TNF alone or in combination) are shown as a percentage of the parent population. Fisher’s Exact Test was used to determine responder status. Closed circles (•) represent responders and (X) represents non-responders. Bars represent median responses by group. Statistical significance was determined initially by Kruskal-Wallis analysis, and vaccine groups were then compared to placebo using Mann-Whitney U Tests, which were adjusted for multiplicity by T cell and antigen using the Holm method. P-values are indicated by asterisks: <0.05 (*), <0.01 (**), <0.001 (***), ≤0.0001 (****).
ELISpot, an often more sensitive assay, was also performed to evaluate immunogenicity in the placebo and high dose groups. ELISpot results for study day 56 were consistent with the ICS results with medians of 0.4 and 127.1 SFU/106 PBMC, following stimulation with Ag85B in the placebo and high dose groups, respectively (Supplementary Fig. 1). As expected with a more sensitive assay, the responder rate was higher than for ICS with corresponding rates of 3.8% and 91.2% in the placebo and high dose groups, respectively.
Based on these data, as well as the safety data, the high dose (1 × 1011 vp) was selected for evaluation in Group 5.
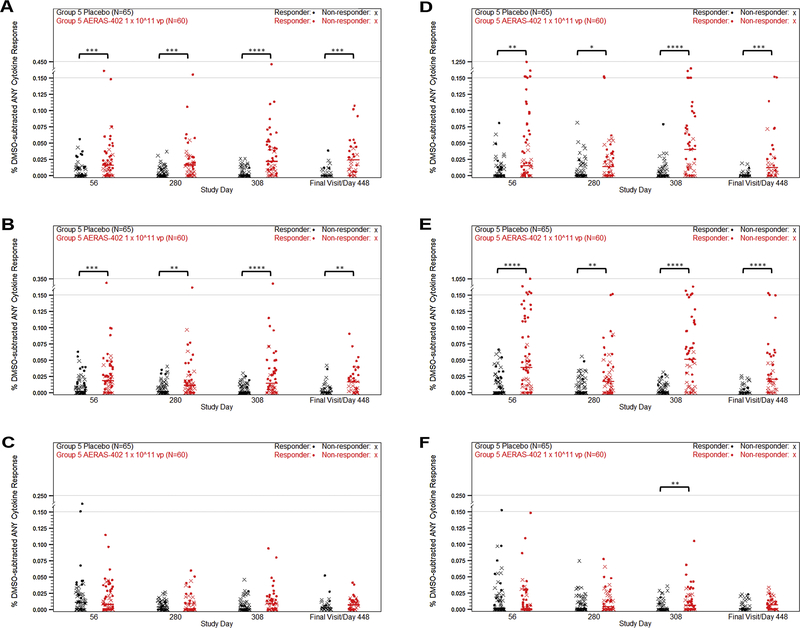
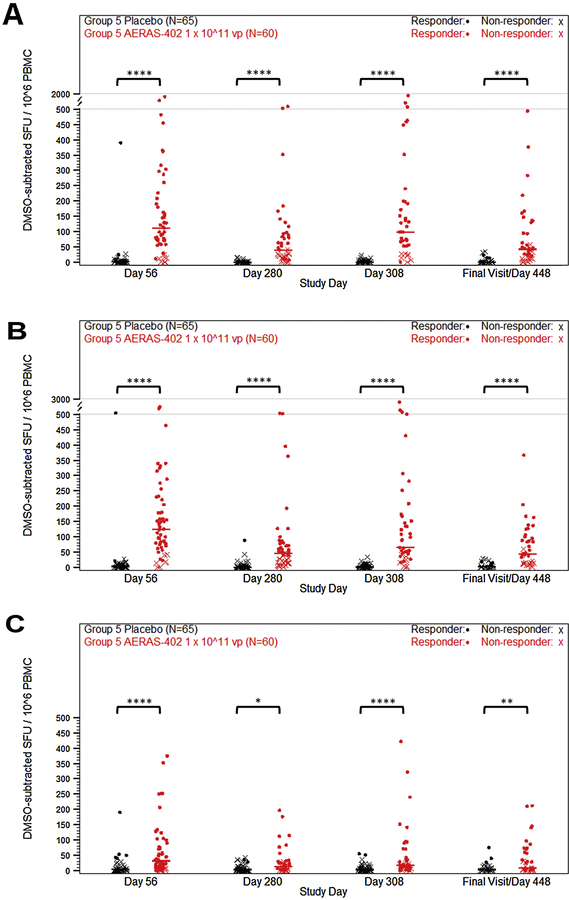
Group 5 evaluated responses to the high dose following 2 or 3 vaccinations of AERAS-402, with the third vaccination given to participants on study day 280. Responses were evaluated on study days 56, 280, 308, and at the end of the study (day 448 to 664, variable due to delays in approval of study protocol amendment). Consistent with the Group 1–4 data, lower responses were observed in CD4+ T cells compared to CD8+ cells (Fig. 3, A–C). The dominant response observed was a CD8+ T-cell response to Ag85B, with median responses of 0.04% and 0.05% for study days 56 and 308, respectively (Fig. 3, D–F). While some observed CD8+ responses were at or around 1%, the overall responses remained below 0.1% (Fig. 3). Responder rates were 26.7% for placebo and 65.6% for AERAS-402 at study day 56. For study day 308, the responder rates were 6.6% and 60.8%, respectively. Study day 56 Group 5 ELISpot data confirmed the prior dose finding results in infants who received AERAS-402 (124.17 SFU/106 PBMC for Ag85B, vs. 3.33 SFU/106 PBMC in the placebo group). Spot counts diminished by study day 280 (46.67 SFU/106 PBMC, vs. 0.42 SFU/106 PBMC in the placebo group) and were boosted with the third dose of AERAS-402 (65.00 SFU/106 PBMC vs. 0.83 SFU/106 PBMC in the placebo group, at study day 308). While responses were detectable at the end of study, they remained low with a median spot count of 44.17 SFU/106 PBMC (Fig. 4). ELISpot responder rates for placebo and AERAS-402 were 8.4% and 83.6%, respectively, at day 56 and 4.3% and 81.4%, respectively, at day 308.
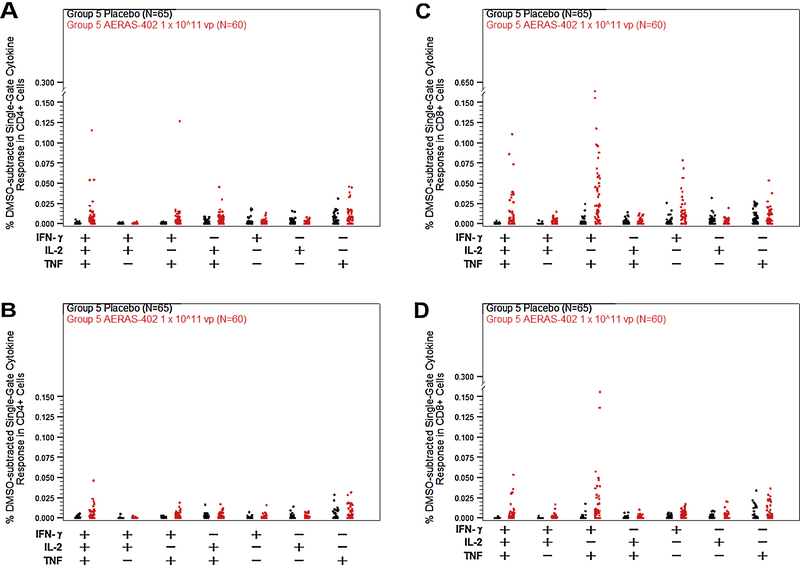
Figure 3. PBMC T cell ICS responses to high dose AERAS-402.
Participants were vaccinated with placebo (black symbols) or 1 × 10^11 vp AERAS-402 (red symbols) on study day 0, 28, and 280. Blood was collected on study days 56, 280, 308, and at the end of the study (study days 448–664). PBMC were isolate for the measurement of CD4+ (A–C) and CD8+ (D–F) DMSO-subtracted T-cell responses against Ag85A (A, D), Ag85B (B, E), and TB10.4 (C, F) peptide pools using intracellular cytokine staining. Total responses (percentage of cells making any of the three IFN-γ, IL-2, or TNF alone or in combination) are shown as a percentage of the parent population. Fisher’s Exact Test was used to determine responder status. Closed circles (•) represent responders and (X) represents non-responders. Bars represent group median responses. The high-dose AERAS-402 group was compared to placebo at each timepoint using Mann-Whitney U Tests, which were adjusted for multiplicity by T cell and antigen using the Holm method. P-values are indicated by asterisks: <0.05 (*), <0.01 (**), <0.001 (***), ≤0.0001 (****).
Figure 4. PBMC T cell ELISpot responses to high dose AERAS-402.
Participants were vaccinated with placebo (black symbols) or 1 × 10^11 vp AERAS-402 (red symbols) on study day 0, 28, and 280. Blood was collected on study days 56, 280, 308, and at the end of the study (study days 448–664). PBMC were isolate for the measurement of responses against Ag85A (A), Ag85B (B), and TB10.4 (C) peptide pools. Total spot-forming units (SFU) were background- (DMSO stimulation) subtracted and plotted. Fisher’s Exact Test was used to determine responder status. Closed circles (•) represent responders and (X) represents non-responders. Bars represent group median responses. High-dose AERAS-402 and placebo were compared at each timepoint using Mann-Whitney U Tests and adjusted for multiplicity by antigen using the Holm method. P-values are indicated by asterisks: <0.05 (*), <0.01 (**), <0.001 (***), ≤0.0001 (****).
Boolean gates were generated in order to examine the functionality of CD4+ and CD8+ T cell responses against Ag85B following vaccination with AERAS-402 in Group 5. Twenty-eight days following the third vaccination on study day 308, we observed low CD4 responses characterized by expression of polyfunctional (IFN-γ+, IL-2+, TNF+) and a lower level of bifunctional (IL-2+, TNF+) and monofunctional (TNF+) T cells. The dominant response was CD8+ T cells that were largely bifunctional (IFN-γ+, TNF+) but also included polyfunctional and monofunctional (IFN-γ+ or TNF+) T cells. By end of study only polyfunctional CD4+ T cells and polyfunctional and bifunctional CD8+ T cells remain (Fig. 5).
Figure 5. Polyfunctional analysis of PBMC T cell responses on days 308 and at the end of the study.
Participants were vaccinated with placebo (black symbols) or 1 × 10^11 vp AERAS-402 (red symbols) on study day 0, 28, and 280. Blood was collected on study day 308 (A, C) and at the end of the study (study days 448–664; B, D). PBMC were isolate for the measurement of CD4+ (A,B) and CD8+ (C,D) responses against the Ag85B peptide pool by intracellular cytokine staining. Boolean gating was performed to obtain the percentage of cells making IFN- γ, IL-2, or TNF alone or in combination. Symbols represent individual responses for each possible cytokine combination. Bars represent group median responses.
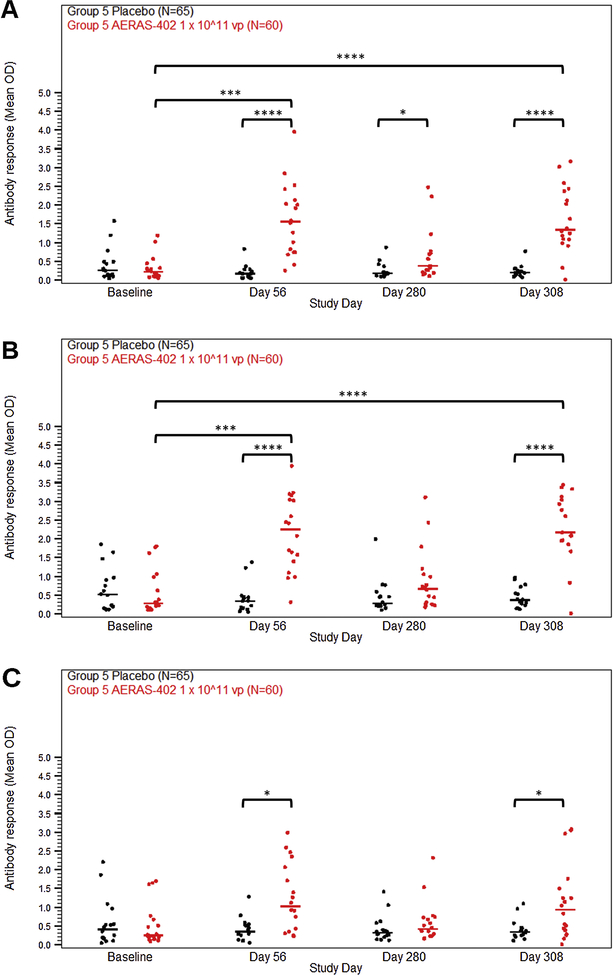
Humoral immune responses to TB antigens were measured by antigen-specific ELISA on plasma collected from all participants in Group 5. The majority of participants in the high dose group developed antibodies against Ag85A and Ag85B by study day 56 and to a lesser extent TB10.4. These responses diminished by study day 280 and were boosted to the day 56 level by the third dose of AERAS-402 (Fig. 6).
Figure 6. Antibody responses following vaccination with high dose AERAS-402.
Participants were vaccinated with placebo (black symbols) or 1 × 10^11 vp AERAS-402 (red symbols) on study day 0, 28, and 280. Blood was collected on study days 56, 280, 308, and at the end of the study (study days 448–664). Plasma was isolate for the measurement of antibody responses against Ag85A (A), Ag85B (B), and TB10.4 (C) by antigen-specific ELISA. The OD values, which correspond to the concentration of the antigen-specific antibodies in the plasma, are plotted. Symbols represent individual responses. Bars represent group median responses. Statistical significance of change in antibody response over time was determined initially by Friedman analysis. Then changes from baseline by timepoint were analyzed using Wilcoxon signed-rank tests and adjusted for multiplicity by antigen using the Holm method. High-dose AERAS-402 was then compared to placebo at each timepoint using Mann-Whitney U Tests, which were adjusted for multiplicity by antigen using the Holm method. P-values are indicated by asterisks: <0.05 (*), <0.01 (**), <0.001 (***), ≤0.0001 (****).
Only a limited number of participants had serum available for Ad35 neutralizing antibody testing. At day 280, prior to receiving the third dose of AERAS-402, 1 of 18 subjects had detectable anti-vector neutralizing antibodies. This increased to 17 of 18 subjects by day 308. Vaccination at 4–6 months of age and 13–15 months of age did not result in altered measles antibody levels in AERAS-402-vaccinated compared to placebo recipients (data not shown).
Discussion
We demonstrated that AERAS-402 has an acceptable safety profile and was well tolerated at all dose levels, across one, two and three doses. The AERAS-402 safety profile in young infants (aged 4–6 months) was similar to that shown in three previous adult trials and one trial in older infants (aged 6–9 months) of the same vaccine11–13,15. As previously shown, the rates of local reactogenicity events, pyrexia and fatigue were dose related. We observed injection site erythema that has previously been seen in an Ad5-vectored vaccine trial in adults16. In contrast to this previous study, we recorded seven participants with very rapid onset extensive injection site erythema that rapidly diminished in size within hours. This finding appears to be distinct from the slowly evolving, localized injection site erythema observed both in this study and in the previous studies with this vaccine11–13. This rapidly appearing and waning erythema was not associated with clinical or laboratory evidence of an allergic or IgE-mediated reaction (data not shown). One third of participants had SAEs, irrespective of study arm. The majority of the hospitalizations and causes of death reflect the region-specific morbidity of HIV-uninfected infants in sub-Saharan Africa, i.e. malaria, pneumonia, and gastroenteritis.
The response rate, as measured by flow cytometry, was between 50 and 65%; and 80 to 90% by the more sensitive ELISpot measure. In TB naïve adults primed with BCG, the response rate following boosting with a lower dose of AERAS-402 was 100% for both antigen-specific CD4+ T cells and antigen-specific CD8+ T cells13. The quantitative antigen specific CD8+ T cell responses in infants in this study had medians of between 0.04 and 0.05% of total CD8+ T cells. These levels contrast with medians of between 0.21 and 0.38 for studies of AERAS-402 at a lower dose level in BCG primed adults, reported by Hoft et al13. In order to determine if an increase in responder rate, and levels comparable to that seen in adults, could be achieved in infants, the protocol was modified to immunize with a third dose of AERAS-402. The additional dose did not increase the frequency and magnitude of the antigen 85A/B specific CD8+ T cell response to that seen previously in adults. Furthermore, CD4+ T cell responses, including polyfunctional CD4+ T cell responses, remain low even after the third dose of AERAS-402, which may have an impact on the durability of the immune response. These finding suggest that the infant immune system requires a stronger stimulus than BCG primed adults, in order to induce and maintain high levels of antigen specific CD8+ T cells. A recent study utilizing MVA encoding Ag85A also demonstrated lower responses in infants than had been seen previously in adults8. A study in BCG primed adults immunized with one or two doses of AERAS-402 and boosted with MVA is currently underway (H. McShane, Personal Communication). Results from such heterologous prime boost regimens may suggest a different approach to enhance the induction of antigen specific CD8+ T cell immunity in infants for protection against TB.
While a third dose of AERAS-402 was able to elicit Ad35 neutralizing antibodies, we failed to detect neutralizing activity at study day 280 (prior to the third dose), suggesting that the failure to provide a substantial boost over two doses was not due to the presence of neutralizing antibodies (data not shown). The dominant response, as seen before, was a CD8+ T cell response directed against Ag85B. which was largely polyfunctional or bifunctional. Notably, the monofunctional (IFN-γ+) T cells observed 28 days following the third dose with the highest dose (study day 308) were absent at the End of Study time point (which varied from study day 448 to 664). This is consistent with the loss of terminal effector T cells. In CD4+ T cells, we observed a population of bifunctional (IL-2+, TNF+) T cells that have been previously described to be central memory T cells, which may be important for control of M.Tb17. However, by the end of the study, the level of these cells was below the limit of detection.
One concern with infant vaccination is interference with the Expanded Program on Immunization scheduled vaccines, which includes measles vaccine at 9 and 18 months. Vaccination with study vaccine at 4–6 months of age and 13–15 months of age did not result in altered measles antibody levels in AERAS-402 vaccinated compared to placebo recipients. Antibodies directed against all three experimental vaccine antigens were detected, principally against Ag85A and Ag85B. While the role of antibodies in preventing and controlling TB disease is unknown, the presence of these antibodies provides additional evidence of immunogenicity.
The data confirm that AERAS-402 is safe and well tolerated in young infants, which suggests that the use of such adenovectors for applications other than TB might also be well tolerated in this age group. As a boost to newborn BCG vaccination, AERAS-402 was able to induce polyfunctional CD4+ and CD8+ T cell responses in more than 50% of the infants immunized, but at levels considerably lower than those previously achieved with this vaccine in adults. Utilization of heterologous prime boost regimens such as adenovector prime, followed by an MVA boost vaccination, may be able to solve the challenging problem of inducing higher levels of response in young infants.
Supplementary Material
Acknowledgements
We thank all study participants and their parents/ guardians; all members of the C 029–402 study teams – site, safety and laboratory; members of the Collaboration Oversight Group (Drs Tom Evans, Ann Ginsberg, Joseph Chiu, Jerry Sadoff, Macaya Douoguih, Sharon Nachman, James Kublin); Local Medical Monitors (Drs Tony Hawkridge, Zainab Waggie); Data Monitoring Committee members (Drs. Andreas Diacon, Benjamin (Gil) Price, James Balsley, Alfredo Mac-Arthur Junior, Christina Mwachari), statisticians Peggy Snowden and Katherine Rutkowski.
Funding: Aeras, EDCTP, NIAID/IMPAACT, Crucell
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. IMPAACT was involved in study design and in the decision to submit the article for publication
Footnotes
Aeras and Crucell were involved in study design, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
EDCTP had no role in the conduct of the study or preparation of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
None declared
References
- 1.World Health Organisation. Global Tuberculosis Report 2014. 2014. [Google Scholar]
- 2.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health 2013; 34: 271–86. [DOI] [PubMed] [Google Scholar]
- 3.Knight GM, Griffiths UK, Sumner T, et al. Impact and cost-effectiveness of new tuberculosis vaccines in low- and middle-income countries. Proc Natl Acad Sci U S A 2014; 111(43): 15520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dye C, Williams BG. Eliminating human tuberculosis in the twenty-first century. J R Soc Interface 2008; 5(23): 653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colditz GA, Berkey CS, Mosteller F, et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 1995; 96(1 Pt 1): 29–35. [PubMed] [Google Scholar]
- 6.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346(8986): 1339–45. [DOI] [PubMed] [Google Scholar]
- 7.Zar HJ, Eley B, Nicol MP, Figaji A, Hawkridge A. Advances in childhood tuberculosis - contributions from the University of Cape Town. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde 2012; 102(6): 518–21. [DOI] [PubMed] [Google Scholar]
- 8.Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polly Clayden SC, Colleen Daniels, Mike Frick, Mark Harrington, Tim Horn, Richard Jefferys,Karyn Kaplan, Erica Lessem, Lindsay McKenna, Tracy Swan Pipeline Report In: Benzacar A, editor. HIV, Hepatitis C (HCV) and Tuberculosis (TB) Drugs , diagnostics, vaccines, preventive technologies, research toward a cure, and immune- based and gene therapies in development : HIV i-Base; 2014. p. 260. [Google Scholar]
- 10.Vogels R, Zuijdgeest D, van Rijnsoever R, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 77(15): 8263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abel B, Tameris M, Mansoor N, et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am J Respir Crit Care Med 2010; 181(12): 1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagina BM, Tameris MD, Geldenhuys H, et al. The novel tuberculosis vaccine, AERAS-402, is safe in healthy infants previously vaccinated with BCG, and induces dose-dependent CD4 and CD8T cell responses. Vaccine 2014; 32(45): 5908–17. [DOI] [PubMed] [Google Scholar]
- 13.Hoft DF, Blazevic A, Stanley J, et al. A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity. Vaccine 2012; 30(12): 2098–108. [DOI] [PubMed] [Google Scholar]
- 14.Radosevic K, Wieland CW, Rodriguez A, et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun 2007; 75(8): 4105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horton H, Thomas EP, Stucky JA, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods 2007; 323(1): 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham BS, Enama ME, Nason MC, et al. DNA vaccine delivered by a needle-free injection device improves potency of priming for antibody and CD8+ T-cell responses after rAd5 boost in a randomized clinical trial. PLoS One 2013; 8(4): e59340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenstrom T, Knudsen NP, Agger EM, Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. Journal of immunology 2013; 190(12): 6311–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.