Abstract
To cope with harsh circumstances, bacterial cells must initiate cellular stress response programs, which demands the de novo synthesis of many stress defense proteins. Reactive oxygen species (ROS) is a universal environmental stressor for both prokaryotic cells and eukaryotic cells. However, the physiological burden that limits the survival of bacterial cells during oxidative stress remains elusive. Here we quantitatively characterize the cell growth and translational elongation rate of Escherichia coli cells treated with different doses of hydrogen peroxide. Cell growth is immediately arrested by low to moderate levels of hydrogen peroxide, but completely recovers after a certain lag time. The lag time depends positively on the dose of hydrogen peroxide. During the lag time, translational elongation rate drops by as much as ∼90% at initial stage and recovers to its normal state later, a phenomenon resulting from the dramatic alteration in cellular tRNA pools during oxidative stress. However, translational elongation is completely stalled at a certain threshold-level of hydrogen peroxide, at which cells ultimately fail to resume growth. Although the mRNA transcription of oxidative defense genes in oxyR regulon is dramatically induced upon hydrogen peroxide treatment, the extreme slow-down of translational elongation during high levels of hydrogen peroxide has severely compromised the timely synthesis of those oxidative defense proteins. Our study demonstrates that the tRNA-limited translational elongation is a key physiological bottleneck that the bacteria must overcome to counteract ROS, and the maintenance of translational elongation rate for timely synthesis of stress defense proteins is crucial for cells to smoothly get over the oxidative stress.
INTRODUCTION
In nature, bacterial cells frequently undergo many harsh environmental conditions such as nutrient deprivation, oxidants, heat, low pH and high osmolarity, which inhibit the growth and survival of bacteria cells. To maintain viability and ultimately overcome stress conditions, bacterial cells must initiate certain stress response programs, triggering the de novo synthesis of many stress-defense proteins (1). One of best-characterized example is the rpoS-regulon (2,3), which contains a list of over 100 genes encoding a diverse set of proteins functioning in stress management, DNA repair, central metabolism and cell morphology control (1,2,4). During nutrient starvation and other stress conditions, the expression of genes in rpoS-regulon is strongly induced, protecting bacteria against the potential damage of external stress (4–6). Failure in timely synthesis of those stress-defense proteins could cause significant loss in cell viability (4,7).
Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide anions (O2−) and hydroxyl radical (•OH), is a universal environmental stressor for almost all types of cells including bacterial cells, yeast cells and mammalian cells (8–10). For human beings, it has long been proposed that ROS-induced oxidative stress is strongly implicated in the emergence of many important diseases and disorders such as aging/senescence, cancer, cardiovascular diseases, neurodegenerative disorder, rheumatoid arthritis and inflammation (8,9,11). Severe oxidative stress causes damage of intracellular macromolecules including proteins, nucleic acids and lipids (8,9,12). When confronting oxidative stress, bacterial cells, yeast cells as well as mammalian cells undergo immediate growth arrest due to inhibition of the activities of certain key metabolic enzymes (13–16). Cells then must initiate specific ROS-defense signaling pathways to remove intracellular ROS and resume growth (8,14,16,17). The cellular response to oxidative stress in the model bacteria species has been largely elucidated. For Escherichia coli, the oxyR regulon (primarily responds to H2O2) and soxRS regulon (primarily responds to O2−) defend cells against the damage of ROS (16–19). When E. coli cells are treated with a low dose of H2O2, growth arrest occurs immediately and the expression of ∼30 genes is maximally induced within 10–30 min (18,20–22). Among them, the oxidized form of the transcriptional regulator, OxyR, induces a dozen of genes such as katG (encoding catalase G), ahpCF (encoding alkyl hydroperoxide reductase), trxC (encoding reduced thioredoxin 2),to remove intracellular H2O2, maintain redox homeostasis and ultimately enable cells to resume growth (16,17,22). The oxyR mutant strain, which fails to induce related defense proteins, becomes hypersensitive to H2O2 shock (18,23,24). However, even for wild type cells, it cannot survive and resume growth when the external H2O2 level becomes too high. Therefore, some fundamental questions remain open: what's the major physiological burden that limits the survival of bacteria during oxidative stress? What's the major factor that determines whether bacteria could smoothly survive and ultimately get over the oxidative stress?
Under stress conditions, cell growth and the overall protein synthesis are severely inhibited (5,6,25,26). The overall protein synthesis rate is limited by the number of actively translating ribosomes and the translational elongation rate (alternatively, polypeptide chain elongation rate) (25–27). When E. coli cells grow in rich nutrient conditions, the ribosome translates proteins at a high elongation rate (16–17 amino acids per sec, aa/s) (25,27–29). When growth is arrested during nutrient deprivation (e.g. carbon, nitrogen and amino acid), the overall protein synthesis rate is severely inhibited. However, E. coli cells still maintain a moderate translational elongation rate (8-9 aa/s) so that it can timely synthesize stress-related proteins to survive in those extreme poor conditions (25,27,30,31). In this study, we quantitatively characterize the cell growth and translational elongation rate of E. coli being subjected to hydrogen peroxide (H2O2) treatment. We find that oxidative stress causes unusually dramatic slow-down or even complete stalling of the translational elongation in E. coli through substantially down-regulating cellular tRNA pools. The tRNA-limited translational elongation process, being crucial for the timely synthesis of stress defense proteins, becomes a key physiological bottleneck that limits the survival of E. coli cells during oxidative stress.
MATERIALS AND METHODS
Strain and medium
Bacterial strains used in this study were wild type K-12 E. coli NCM3722 strain (32,33) and its derivatives NQ1468 (25), FL174, FL175, FL189, FL190, FL191 and FL192 strains. The NQ1468 strain was used in LacZα induction assay for calibration of initiation time cost in the translational elongation rate measurement. FL174 and FL175 strain were used for measuring the translational elongation rate of ManA-GFP and FusA-GFP protein, respectively. The FL189 strain was used for Rnase D overexpression experiment. The FL190, FL191 and FL192 strains are strains harboring OxyR-regulated translational-fused GFP proteins.
The FL174 strain and FL175 strain harbored pFL-manA-gfp vector and pFL-fusA-gfp vector, respectively. To make these two vectors, a Placq-lacI cassette together with its downstream Ptac promoter (without RBS) were first inserted into the XhoI/EcoRI sites of the pZE11 vector; a pair of NdeI/BamHI sites was introduced downstream of Ptac promoter through point mutation. The manA and fusA gene of E. coli were then PCR amplified and inserted into the NdeI/BamHI sites. Finally, the coding sequence of egfp, together with a N-terminal 30 bp sequence encoding (GGGGS)2 linker was placed downstream of the manA and fusA, respectively, yielding pFL-manA-gfp and pFL-fusA-gfp vector, respectively. The pFL-manA-gfp and pFL-fusA-gfp were then transformed into E. coli, generating FL174 and FL175 strain for measuring translational elongation rates of GFP fusion proteins.
To construct three strains (FL190, FL191 and FL192) harboring OxyR-regulated translational-fused GFP proteins, the ORFs of dps, trxC and grxA genes together with their upstream ∼200 bp transcriptional regulator regions were PCR amplified and inserted into the XhoI/BamHI site of the pFL-manA-gfp vector to replace the whole Placq-lacI-Ptac-manA cassette. In the case, the three translational-fused GFP proteins were controlled by the native OxyR-regulated promoters. The three vectors were then transformed into NCM3722 strain to obtain FL190, Fl191 and FL192 strain, respectively. These three strains were used for the experiments of Figure 5H–J.
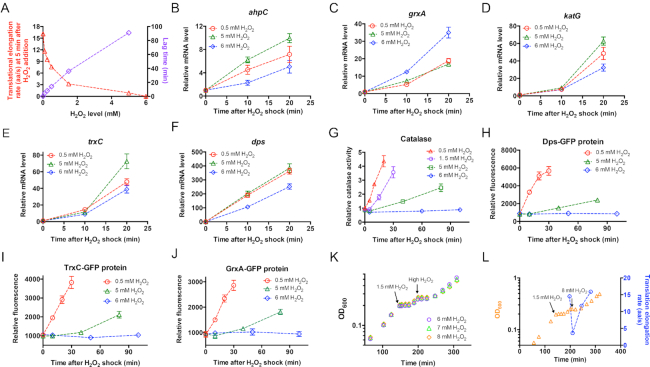
Figure 5.
Inhibition of translational elongation by oxidative stress limits the de novo synthesis of catalase. (A) The correlation of translational elongation rate (at 5 min after the addition with H2O2) and lag time with the dose of H2O2. (B–F) The mRNA levels of five genes in oxyR-regulon at various time points after the addition of 0.5, 5 and 6 mM H2O2. (G) The total catalase activity of E. coli at various time points after the addition of 0.5, 1.5, 5 and 6 mM H2O2. The data at time zero of 0.5 mM H2O2 is set as 1. (H–J) The levels of three GFP-fused proteins at various time points after the addition of 0.5 mM, 5 mM and 6 mM H2O2. (K) Pre-adaption of a low H2O2 dose allows cell to counteract a higher lethal H2O2 dose than no pre-adapted cells. E. coli cells were first subject to the treatment of 1.5 mM H2O2; after the growth recovery, high doses of H2O2 (6, 7 and 8 mM) were respectively added to the cultures to initiate a second round of oxidative stress. (L) The translational elongation rate and growth curve of E. coli cells (pre-adapted to 1.5 mM H2O2 treatment) upon subjecting to 8 mM H2O2 treatment.
To construct an Rnase D-overexpression strain, FL189, the coding sequence of rnd gene was PCR amplified using T5 direct PCR kit (Tsingke Biotech) and first inserted into pClone007 Blunt vector (Tsingke Biotech) for sequence verification. The sequence-verified rnd fragment was then inserted into the NdeI/SpeI of pFL-manA-gfp vector to replace the manA-gfp gene so that rnd expression was controlled by Ptac promoter, yielding pFL-rnd vector. The pFL-rnd vector was further transformed into NCM3722 strain for Rnase D overexpression experiments in Figure 6.
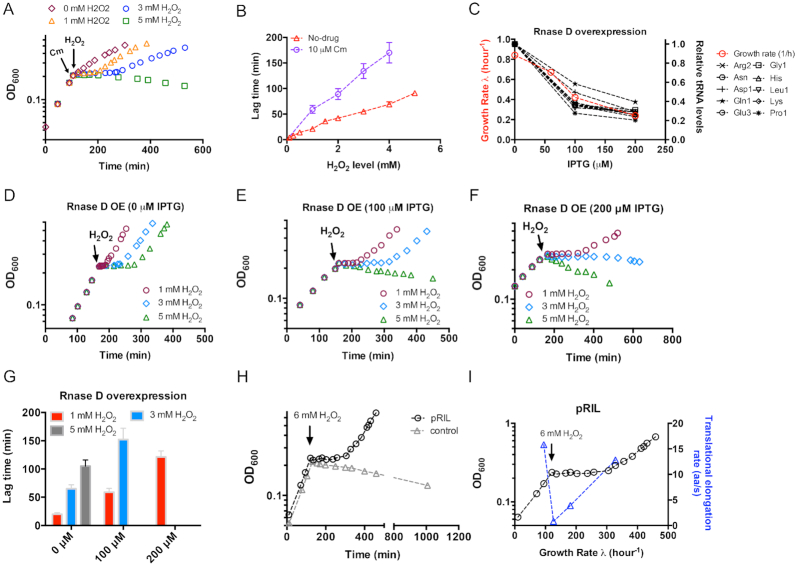
Figure 6.
The tRNA-limited translational elongation process significantly affects cell fitness during oxidative stress. (A) Growth curve of chloramphenicol (Cm)-treated E. coli cells being subjected to H2O2 treatment. Cells were first exponentially growing in glucose medium to OD600 ∼0.2, 10 μM Cm was then added to inhibit the translation process. After 5 min, different doses of H2O2 were added to the cultures. (B) The correlation between the lag time and the H2O2 doses for cells treated with or without Cm. (C) The growth rate and individual tRNA levels of E. coli upon different degrees of Rnase D overexpression. The expression of Rnase D was controlled by the IPTG-inducible Ptac promoter. (D–F) Growth curve of H2O2-treated E. coli cells upon different degrees of Rnase D overexpression. (G) Lag time of H2O2-treated E. coli cells upon different degrees of Rnase D overexpression. (H) Effect of tRNA upregulation by pRIL plasmid on the growth of E. coli cells subjecting to 6 mM H2O2 treatment. The control group corresponds to E. coli cells harboring pACYC184 plasmid (with the same chloramphenicol resistance marker and p15A origin as pRIL). (I) Translational elongation rate of E. coli cells harboring pRIL plasmid during 6 mM H2O2 treatment. The growth curve of panel D is shown here.
MOPS-buffered minimal medium was the same as used in Cayley et al. (34) using 0.2% glucose as the carbon source and 10 mM NH4Cl as the nitrogen source. The MOPS reagent was ordered from Coolaber Biotech in Beijing.
Cell growth
Cell growth experiments were performed in a water bath shaker (220 rpm under 37°C). A standard procedure of cell growth experiments included three steps: seed culture, pre-culture and experimental culture. Cells from a fresh colony were inoculated into LB medium (Coolaber Biotech) and grew for several hours as seed culture. Seed culture was inoculated into MOPS glucose minimal medium for growing overnight as pre-culture. At the next day, the pre-culture was inoculated into the same medium at an initial OD600∼0.01 as the final experimental culture. The final experimental culture was first exponentially growing to OD600 ∼0.2; different doses of H2O2 were then added to induce oxidative stress. The OD600 of the culture was measured throughout the whole process to obtain a complete growth curve. Related parameters such as translational elongation rate, cellular tRNA abundance and total catalase activity were measured at specific time points. For cells harboring pFL-series vector, the cultures were always supplemented with 50-μg/ml ampicillin (Coolaber Biotech).
Translational elongation rate measurement
Measurement of translational elongation rate was based on LacZ induction assay as well as GFP fusion protein induction assay (ManA-GFP and FusA-GFP). The LacZ induction assay with a 10-s initiation time calibration was performed the same as described in Dai et al. (25), as described in Supplementary Figure S3 and S4. The procedure of GFP induction assay was slight different from that of LacZ induction assay. In brief, 5 mM IPTG was added to the FL174 and FL175 culture to induce the expression of ManA-GFP or FusA-GFP. Immediately after addition of IPTG, at 10- to 30-s intervals (depends on the translational elongation rate), 20–30 aliquots of 300-μl cultures were transferred into pre-chilled microfuge tube containing 10-μl chloramphenicol (34 mg/ml) (Coolaber Biotech). The samples were kept on ice for over 6 h before measuring the GFP fluorescence. The GFP fluorescence intensity was measured by a micro-plate reader (485 nm excitation filter, 528 emission filters). The GFP induction curve was made by plotting the fluorescence intensity against the induction time and analyzed using the square root plot (Schleif plot). From the Schleif plot, we could obtain the time needed for the appearance of first round of GFP fusion protein, Tfirst. The translational elongation rate was obtained using the GFP protein length to divide (Tfirst = 10 s), where the 10-s was the time cost of initiation steps. The length of ManA-GFP and FusA-GFP protein are 639 and 952 aa, respectively.
Measurement of the GFP fluorescence units
A 300-μl GFP cell sample was directly pipetted into a pre-cooled 1.5 mL eppendorf tube containing 10-μl chloramphenicol (34 mg/ml) and further put on ice for 6 h before measuring fluorescence units. GFP fluorescence was measured with a synergy-2 micro-plate reader (Biotek) at 485-nm/528 nm Ex/Em mode. The GFP value of each sample was subtracted by the background fluorescence of wild type NCM3722 strain at the same OD600 point.
Measurement of cellular individual tRNA and mRNA by qRT-PCR
The cellular tRNA abundance was measured by qRT-PCR. In brief, 0.8 ml of cell culture was transferred to a plastic tube containing 0.8 ml pre-cooled stop solution (60% ethanol, 2% phenol and 10 mM EDTA). The total cellular RNA was then extracted with a bacterial total RNA extraction kit (TianGen, China). The final concentration of RNA was then measured with a NanoDrop-1000 micro-spectrophotometer. 1-μg total cellular RNA sample was first pre-treated in 80°C for 15 min to remove the secondary structure of tRNA and immediately put into ice. The first-strand cDNA synthesis was performed using a first-strand cDNA synthesis reverse transcriptase kit (TianGen Biotech, China) using random primers. The qRT-PCR reaction was performed based on the Super-premix SYBR green Plus kit (Yeasen Biotech, Shanghai, China) using Bio-rad CFX96 Touch real-time PCR system. Detailed qRT-PCR reaction protocol was as follows: 95°C for 15 min, followed by 40 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 30 s. The 5S rRNA was used as the internal references since rRNA concentration or total RNA concentration remains constant upon oxidative stress (35). The related qRT-PCR primers were based on ref. (35). The Ct value (for both tRNA and 5S rRNA) of the sample at each time point after H2O2 addition was obtained from the machine. The relative tRNA level of time 0 sample was set as ‘1’. Relative expression of tRNA at different time points was calculated according to the 2−ΔΔCT method. The mRNA quantification of oxyR-regulon genes was similar with tRNA quantification except that the total cellular RNA did not require pre-treatment in 80°C before cDNA synthesis.
For measurement of the transcriptional kinetics of the full-length lacZ mRNA and manA-gfp mRNA shown in Supplementary Figure S6, E. coli cells were exponentially growing to OD600 ∼0.4 followed by the induction of lac operon or Ptac-manA-gfp expression through addition of 5 mM isopropyl-β-D-thiogalactoside (IPTG). Immediately after the IPTG induction, 0.8 ml of cell culture was taken at a 15-s interval and transferred into a pre-cooled plastic tube containing 0.8 mL stop solution containing 60% ethanol, 2% phenol and 10 mM EDTA. The RNA extraction and reverse transcription process was then performed the same as described above. Two pairs of qRT-PCR primers were used to detect the 3′-end region of lacZ mRNA and manA-gfp mRNA respectively. For lacZ mRNA, forward primer: GCACATGGCTGAATATCGACG; Reverse primer: P3105-R: GACACCAGACCAACTGGTAATGG. For manA-gfp mRNA, forward primer: TCCACACAATCTGCCCTTTCG; reverse primer: TGTGTAATCCCAGCAGCTGTTAC. The relative mRNA abundance in each time point equals to 2Ct(0)-Ct(t), where Ct(0) means the Ct value of the sample taken immediately before IPTG addition and Ct(t) means the Ct value at each time point. The mRNA abundance was plotted with the time to obtain the transcriptional kinetics curve.
Measurement of total catalase activity
The total catalase activity was performed similarly as described in (36). Catalase activity determination was based on measuring the rate of decomposition of hydrogen peroxide, which was proportional to the reduction of the absorbance at λ = 240 nm. Briefly, 10 mL E. coli cells were collected by centrifuge and suspended in 1.5 ml 50 mM phosphate buffer (pH 7.0). Cells were further lysed by sonication and centrifuged at 13 000 rpm at 4°C for 10 min, after which the supernatant was taken for catalase activity measurement. 0.1 ml crude extract was added to 0.9 ml phosphate buffer (pH 7.0); hydrogen peroxide was then added to a final concentration of 5 mM. The absorbance of the samples at 240 nm was measured every 30 s for 10 min by a micro-plate reader (Biotek). Catalase activities were further normalized by the total protein concentration of the crude extracts.
RESULTS
Growth arrest of E. coli cells upon H2O2 shock
We focus on the effect of H2O2 treatment on the wild type E. coli K-12 strain (NCM3722) exponentially growing in glucose medium (Figure 1A). Escherichia coli cells were first growing exponentially in glucose minimal medium at a growth rate of λ1: 0.96/h. When the optical density of the culture at 600 nm (OD600) reached ∼0.2, 1.5 mM H2O2 was added to impose oxidative stress on the cell culture (red arrow of Figure 1A); cell growth was immediately arrested into zero. After a lag time of 38 min (gray region of Figure 1A), cell growth completely recovered to its normal state, λ2: 0.94/h, indicating that the excess intracellular H2O2 had been successfully removed during the lag time. This phenomenon of transient growth arrest of E. coli cells upon H2O2 shock is consistent with previous literatures focusing on E. coli cells, yeast cells and animal cells (14). Earlier studies had found that E. coli K-12 cells could smoothly survive and resume normal growth upon 2 mM H2O2 shock, which is consistent with our finding (37,38).
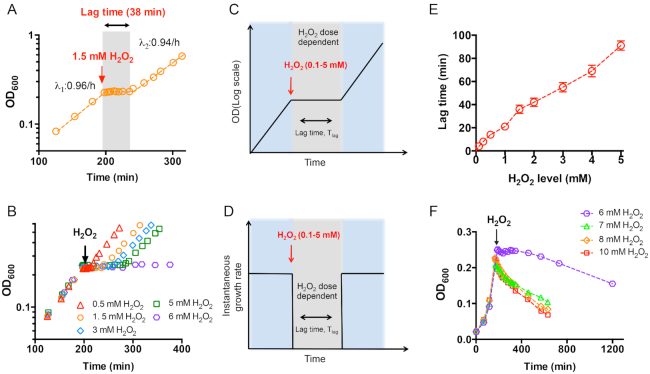
Figure 1.
Growth of E. coli cells subjecting to the treatment of different doses of hydrogen peroxide (H2O2). (A) Growth curve of E. coli cells in glucose minimal medium treated with 1.5 mM H2O2. Cells were first exponentially growing to OD600∼0.2; 1.5 mM H2O2 was then added (red arrow) and cell growth immediately stopped. After a lag time of 38 min (gray part), cell growth recovered to normal speed. (B) Growth curve of E. coli cells in glucose minimal medium treated with various levels of H2O2 (from 0.5 to 6 mM). The full range of 6 mM data is shown in panel F. (C, D) Growth pattern of E. coli cells upon treatment of 0.1 to 5 mM H2O2. Cells were first exponentially growing at a constant growth rate (panel D), H2O2 (red arrow) was then added and cell growth was immediately arrested (panel C). At this stage, instantaneous growth rate dropped into zero (panel D). After a certain lag time, cell growth completely recovered (panel C). The growth rate after growth recovery was the same as that before H2O2 treatment. The lag time is positively dependent on H2O2 dose. (E) The correlation between the lag time and the H2O2 dose. (F) Growth curve of E. coli cells in glucose minimal medium treated with ≥6 mM H2O2.
We next repeated the same growth experiments at different doses of H2O2 (the different symbols in Figure 1B and Supplementary Figure S1). At the range of 0.1–5 mM H2O2, the general growth patterns of E. coli cells were similar at different H2O2 levels. Growth was immediately arrested but completely recovered after a certain lag time (Figure 1B–D). However, the lag time depends positively on the external H2O2 levels (Figure 1E). The lag time was only 5–15 minutes at low H2O2 range (0.1–0.5 mM, Figure 1E and Supplementary Figure S1), but dramatically increased to ∼90 min at 5 mM H2O2 (green square in Figure 1B and E). During the lag time, the cell viability was not affected at all (Supplementary Figure S2), indicating that cells could tolerate potential oxidative damage for a period of time. At 6 mM H2O2, cell growth was also immediately arrested (purple hexagon in Figure 1B and F). However, cell mass kept constant for ∼3 h and then dropped gradually (purple hexagon in Figure 1F), indicating that 6 mM is the threshold H2O2 level at which E. coli cells ultimately fail to tolerate. When the external H2O2 levels were further increased, the cell mass could not be sustained and decreased quickly (Figure 1F). In summary, as schematically illustrated in Figure 1C and D, cell growth is immediately arrested at a certain range of H2O2 levels (0.1–5 mM). After a certain lag time (depending positively on the H2O2 dose), cell growth completely recovers. However, when the external H2O2 level is higher than the threshold level (6 mM), cells ultimately fail to survive and resume growth.
Time-course analysis of translational elongation rate and cellular tRNA pools upon H2O2 shock
There are two important observations in the above section: (i) At the range of 0.1-5 mM H2O2 level, the lag time depends positively on the H2O2 levels, and (ii) E. coli fails to tolerate a certain high level of H2O2 (≥6 mM). These findings pose a fundamental question: what's the physiological bottleneck that limits the survival of bacterial cells during oxidative stress? If it is simply an issue that higher levels of H2O2 require more time to be removed by catalases, it is difficult to understand why E. coli cells could completely resume normal growth at 5 mM H2O2 but fail to tolerate a slightly higher H2O2 level of 6 mM.
H2O2 shock activates the oxyR regulon of E. coli cells, inducing the expression of related genes such as ahpCF, katG and trxC, which enable bacterial cells to scavenge the intracellular H2O2, maintain redox homeostasis and resume cell growth. The de novo synthesis of stress defense proteins can be strongly affected by the overall protein translational elongation status of E. coli. Recent indirect evidence such as polysome profiling, has indicated that oxidative stress could inhibit the translational elongation of E. coli (35). However, it remains unclear to what extent the translational elongation is suppressed in vivo, and, moreover, the effect of translation elongation status on the oxidative defense response. We next quantitatively characterized the ribosome translational elongation rate (ER) of E. coli during H2O2 treatment. The ER was measured using the classical β-galactosidase (LacZ) induction assay (Supplementary Figure S3) (25) with correction of a 10-s time cost for initiation steps (Supplementary Figure S4). The LacZ induction assay was performed at various time points before and after the addition of 1.5 mM H2O2 (Figure 2A). Under the normal growth condition, the ER value was high, ∼16 aa/s (blue diamond in Figure 2B). Strikingly, at 5 min after the addition of 1.5 mM H2O2, the synthesis of full-length LacZ protein took a much longer time (5-fold) than it did under the normal condition, ∼350 s (red triangle of Figure 2B), corresponding to an ER of only ∼3 aa/s (Figure 2C, red bar). This result directly supports that the oxidative stress strongly inhibits the translational elongation process. ER then gradually recovered and reached its normal state at 60 min after the onset of H2O2 treatment (Figure 2C). To rule out the possibility that our result is specific to LacZ translation, we also measured the ER of two GFP fusion proteins, ManA-GFP and FusA-GFP, upon H2O2 treatment (Supplementary Figure S5). The time-course behavior of ER of these two proteins is quantitatively consistent with that of LacZ protein (Figure 2C). By plotting the growth curve and ER in the same plot (Figure 2D), it can be clearly seen that ER dramatically decreased at the initial stage (∼5 min) of H2O2 shock, but gradually recovered during the lag time and reached a normal value shortly after growth recovery. To investigate whether the slow-down of translational elongation was related to a slow-down of mRNA transcription, we measured the induction kinetics of lacZ mRNA and manA-gfp mRNA of E. coli cells at 5 min after addition of 1.5 mM H2O2 using qRT-PCR. As shown in Supplementary Figure S6 (blue circles), the transcription of full-length lacZ mRNA and manA-gfp mRNA was strongly induced by IPTG addition and required a much shorter time (∼170 s for lacZ mRNA) to synthesize than the full-length proteins. Therefore, the translational ER under oxidative stress was not limited by the mRNA transcriptional elongation.
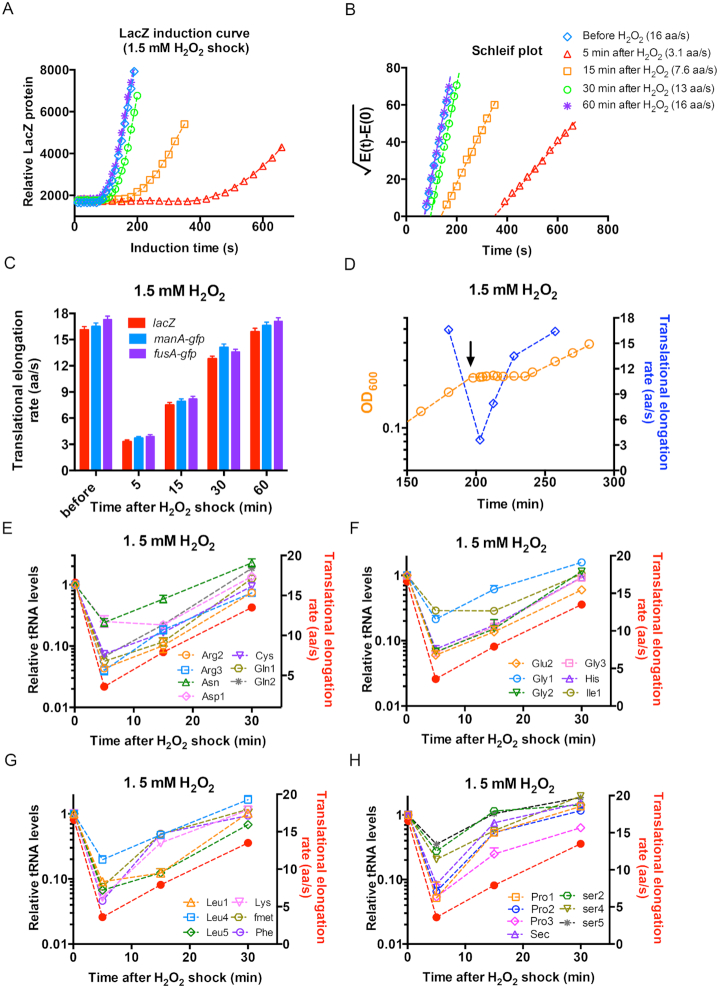
Figure 2.
The translational elongation rate and cellular tRNA pools of E. coli subjecting to 1.5 mM H2O2 treatment. (A) The induction curves of LacZ protein for E. coli NCM3722 strain growing in glucose minimal medium at various time points (5, 15, 30, 60 min) after the addition of 1.5 mM H2O2. The same experiment was also conducted for culture before the addition of H2O2. (B) The Schleif plot of the LacZ induction curve shown in panel A. (C) The translational elongation rate of three genes of E. coli cells before H2O2 treatment as well as at various time points (5 min, 15 min, 30 min, 60 min) after the addition of 1.5 mM H2O2. (D) The translational elongation rate is shown together with the growth curve of E. coli cells subjecting to 1.5 mM H2O2 treatment. The data of translational elongation rate is the average of the values of three genes shown in panel C. (E–H) The cellular tRNA pools of E. coli cells at various time points (0, 5, 15, 30 min) after the addition of 1.5 mM H2O2. The translational elongation rates at those time points were shown as red solid symbols. The data at time 0 for each individual tRNA species is set as 1. The standard deviations of triplicates are shown but are very small in the log-scale plot.
A fast ER requires highly abundant intracellular tRNA pools (25,39). It is known that substantial degradation of full-length tRNA occurs during oxidative stress and other stress conditions for many types of cells (35,40–43). Therefore, the dramatic slow-down of translational elongation upon H2O2 shock might be due to lower cellular tRNA pools. We next used qRT-PCR to quantitatively characterize the time-course behavior of 26 individual tRNA levels of E. coli cells during H2O2 shock. Substantial alteration in cellular tRNA pools was observed shortly after the onset of H2O2 shock. At 5 min, the abundance of most investigated tRNA species was reduced by ∼90% (Figure 2E–H). Moreover, similar to the trend of ER (solid red circles in Figure 2E–H), tRNA pools also gradually recovered later during the lag time (Figure 2E–H). In summary, the alterations in tRNA pools correlate well with the dramatic change in ER during H2O2 shock.
H2O2-dose dependent translational elongation rate and cellular tRNA pools
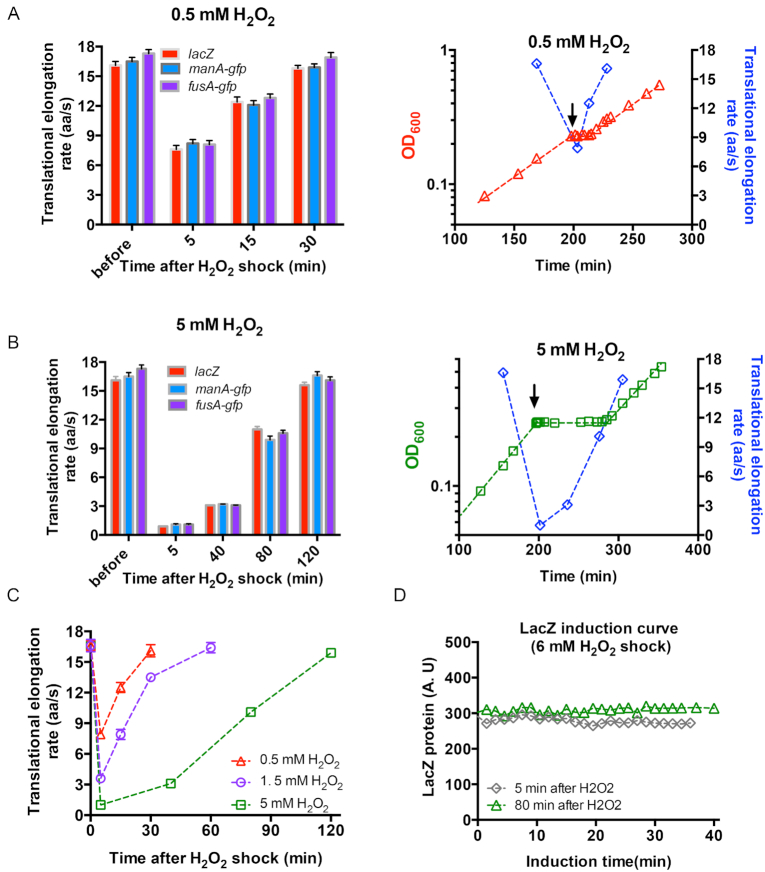
To gain a more systematic insight into the effect of H2O2 shock on ER and cellular tRNA pools, we repeated the above study with a lower H2O2 level (0.5 mM) and a higher H2O2 level (5 mM) (Figure 1B and E). In the case of a 0.5 mM H2O2 shock, we measured the ER at 5, 15 and 30 min after the addition of H2O2 (Figure 3A, left panel). At 5 min after a 0.5 mM H2O2 shock, ER dropped by 50%, to ∼8 aa/s, which was significantly higher than the value in the case of a 1.5 mM H2O2 shock (∼3 aa/s). Furthermore, ER recovered to its normal value at 30 min (Figure 3A, right panel), which was shorter than the time required for ER recovery in the case of 1.5 mM H2O2 treatment. We also measured the ER at 5, 40, 80 and 120 min after the addition of 5 mM H2O2. At 5 min, the synthesis of full-length LacZ protein took nearly 20 min (Supplementary Figure S7A), corresponding to an extremely slow ER of 1 aa/s, which was also found for ManA-GFP and FusA-GFP protein (Figure 3B, left panel; Supplementary Figure S7B and C). Although ER also gradually recovered latter, the recovery rate was much slower than that in the case of 1.5 and 0.5 mM H2O2 treatment (Figure 3C). As shown in Figure 3B, the ER increased to only ∼3 aa/s at 40 min and remained sub-optimal, at 10 aa/s, at the end of lag time (80 min) (Figure 3B, left panel). Those results show that the stress on translational elongation dramatically aggravates with increasing H2O2 levels. From this perspective, we expected that translational elongation might stall completely at an even higher H2O2 dose. We thus performed the LacZ induction assay at 6 mM H2O2, in which cells fail to survive and resume growth (Figure 1F). At both 5 and 80 min after the onset of 6 mM H2O2 shock, no synthesis of new LacZ protein was observed after an induction time of 40 min (Figure 3D). The same result was observed in the case of ManA-GFP and FusA-GFP proteins (Supplementary Figure S8), supporting that translational elongation process is completely arrested at 6 mM H2O2 (ER: 0 aa/s).
Figure 3.
The translational elongation rate of E. coli subjecting to 0.5, 5 and 6 mM H2O2 treatment. (A) The translational elongation rate of three genes of E. coli cells before treatment as well as at various time points (5, 15, 30 min) after the addition of 0.5 mM H2O2. At the right panel, the translational elongation rate is shown together with the growth curve of E. coli cells subjecting to 0.5 mM H2O2 treatment. The data of translational elongation rate is the average of the values of three genes. (B) The translational elongation rate of three genes of E. coli cells before H2O2 treatment as well as at various time points (5, 40, 80 and 120 min) after the addition of 5 mM H2O2. At the right panel, the translational elongation rate is shown together with the growth curve of E. coli cells subjecting to 5 mM H2O2 treatment. The data of translational elongation rate is the average of the values of three genes. (C) The translational elongation rate of E. coli at various times points after the addition of 0.5, 1.5 and 5 mM H2O2. The data at time 0 corresponds to the normal value before H2O2 addition. (D) The induction curve of LacZ protein for E. coli cells growing in glucose minimal medium at two time points (5 min and 80 min) after the addition of 6 mM H2O2.
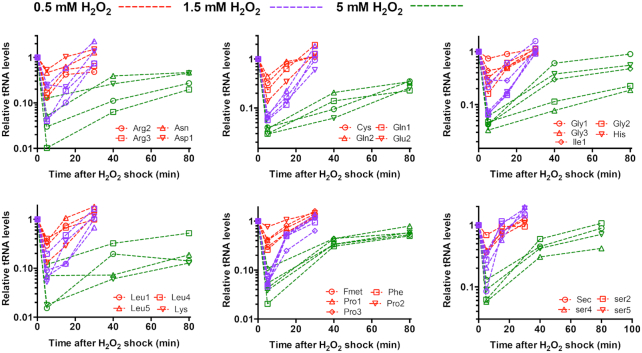
The time-course behavior of cellular tRNA pools of E. coli cells (Figure 4) exhibited a similar time-course pattern as ER (Figure 3C). However, the extent of the alteration of tRNA pools exhibited a strong H2O2-dose dependence. The drop in cellular tRNA pools at 5 min after addition of 0.5 mM H2O2 was remarkable but much milder than the drop after addition of 1.5 mM H2O2. In contrast, the abundances of most tRNA species dropped by 95% at 5 min after addition of 5 mM H2O2 (green symbol in Figure 4), which was a more severe drop than that of addition of 1.5 mM H2O2. Therefore, the severity of the stress on translational elongation at different H2O2 levels was largely determined by the cellular tRNA pools.
Figure 4.
The relative cellular tRNA abundances of E. coli at various times points after the addition of 0.5, 1.5 and 5 mM H2O2. The data at time 0 for each individual tRNA species is set as 1. The standard deviations of triplicates are shown but are very small in the log-scale plot.
Severely delayed synthesis of oxidative stress-defense proteins at high H2O2
The above result demonstrates that E. coli cells face severe problem in maintaining the ER during oxidative stress, which may significantly compromise the process of cellular stress response to oxidative stress. When confronting H2O2 shock, bacterial cells need to immediately initiate stress defense programs such as the oxyR regulon to defend them against H2O2 stress. The oxyR regulon lies at the core of bacterial defense against oxidative stress. The oxyR-null strain, which fails to induce the expression of related oxidative defense proteins, becomes hypersensitive to H2O2 treatment (20,22,23,44). It is known that oxidative defense genes such as ahpCF, katG are maximally induced within as short as ∼10 min at a low dose of H2O2 (18,22). However, the stress on translational elongation at high H2O2 levels might significantly delay the timely synthesis of those oxidative defense proteins. For example, ER is only ∼1 aa/s at 5 min after the addition of 5 mM H2O2, in which case it would take ∼12 min to translate a full-length KatG protein (726 amino acids). As shown in Figure 5A, the increase in lag time required for growth recovery coincided strongly with the decrease of ER at 5 min after H2O2 addition. Specially, 6 mM was the threshold H2O2 level at which ER drops to zero and cells fail to resume growth.
We next directly investigated whether the stress on translational elongation upon H2O2 treatment could also inhibit the synthesis of oxidative defense proteins. We first measured the mRNA levels of five genes in oxyR-regulon, ahpC, grxA, katG, trxC and dps, at three H2O2 levels. As shown in Figure 5B-F, the mRNA levels of all five genes strongly increased regardless of the level of H2O2, indicating that the oxyR regulon was strongly activated in the transcriptional level upon H2O2 shock. We then investigated the synthesis of catalase upon H2O2 treatment. The E. coli cells have two major catalases, KatG (HPI) and KatE (HPII). Although katE is not induced by H2O2 shock, katG is strongly induced by OxyR upon H2O2 shock (17,18). Therefore, low doses of H2O2 should remarkably elevate the total catalase activities of E. coli cells. As shown in Figure 5G, the total catalase activity of E. coli indeed strongly increased by several-fold within 30 min at two low levels of H2O2 (red triangle and purple circles). However, the process of catalase accumulation became much slower in E. coli cells treated by 5 mM H2O2 (green squares at Figure 5G). When the H2O2 level reached 6 mM, no catalase accumulation was observed within 100 min (blue diamond, Figure 5G), which would be as expected if translational elongation of oxidative defense proteins also gets completely stalled as observed in the cases of LacZ and GFP proteins. To see whether other oxidative defense proteins exhibit similar behaviors as catalases, we focused on three additional OxyR-regulated proteins, Dps, TrxC and GrxA. We directly fused the three proteins with GFP protein and placed the three translational-fusion GFP proteins downstream of their native OxyR-regulated promoters. The synthesis of all the three proteins were also strongly induced at low H2O2 level (red circles in Figure 5H–J) but severely inhibited or completely abolished at 5 or 6 mM H2O2, respectively. Overall, the stress on translational elongation upon H2O2 shock could indeed limit the timely synthesis of oxidative defense proteins.
At low levels of H2O2, E. coli cells could smoothly induce the oxyR-defense program and accumulate related defense proteins. If it is indeed the inhibition of the timely synthesis of oxidative defense proteins that underlies the failure of E. coli in tolerating high levels of H2O2, we expect cells pre-adapted in a low dose of H2O2 could acquire increased H2O2 tolerance. To test this scenario, we first had the exponentially growing E. coli treated with 1.5 mM H2O2; after the recovery of growth, we added 6 mM, 7 mM and 8 mM H2O2 to the cell culture (Figure 5K). Strikingly, E. coli cells could resume growth in all the three high H2O2 levels after a ∼30 min lag time (Figure 5K). Moreover, ER of pre-adapted cells was still significant (3.6 aa/s) at 5 min after 8 mM H2O2 shock and again recovered into the normal value later (blue circles in Figure 5L). Therefore, if cells have pre-accumulated stress-related proteins, translation stress would be substantially alleviated and the cells could overcome a higher H2O2 dose than cells without pre-adaptation.
tRNA-limited translational elongation strongly affects the growth fitness during oxidative stress
The stress on translational elongation, which limits the timely synthesis of stress defense proteins, is likely to impose a severe physiological burden on E. coli during counteracting oxidative stress. To further test the above notion, we performed three experiments. In the first experiment, we added a sublethal dose (10 μM) of chloramphenicol (Cm) to the exponentially growing culture to inhibit protein translation. Cm is a bacteriostatic antibiotic that blocks the elongation process of bacterial translation without affecting cell viability (45,46). At 5 min after the addition of Cm, we treated the cells with different doses of H2O2 (Figure 6A). The growth rate of E. coli cells at 10 μM Cm (doubling time: 170 min) was only one fourth of that at no-drug condition (doubling time 43 min) so that the overall translational rate dropped by 75% (Figure 6A, magenta diamonds). We found that the Cm-treated cells could not tolerate 5 mM H2O2 (Figure 6A, green squares). Moreover, the lag times of Cm-treated cells at lower H2O2 doses (1–4 mM) were much longer than that of drug-free cells (Figure 6B). These results indicate that decreased translational rate indeed affect cell fitness upon oxidative stress.
In the second experiment, we attempted to artificially enlarge the tRNA translational stress of E. coli cells and test their fitness during oxidative stress. The Rnase D protein (47,48), which has high specificity to tRNA, was placed downstream of the IPTG-inducible Ptac promoter. It is known that overexpression of Rnase D proteins could effectively down-regulate cellular tRNA pools and reduce cell growth rate (48–50), as confirmed by our measurements of individual tRNA pools and growth rate (Figure 6C). We next characterized cell growth upon H2O2 treatment upon different degrees of Rnase D overexpression. At 0 μM IPTG level, cells could smoothly survive and resume growth at all three H2O2 levels (Figure 6D), which was similar with the behavior of wild type cells. However, at 100 μM IPTG level, cells could not tolerate 5 mM H2O2 (green triangles in Figure 6E), and had a much longer lag time at 1 and 3 mM H2O2 than they did at no IPTG condition (red and blue bars in Figure 6G). The situation became even more severe for the condition of 200 μM IPTG, at which cells failed to tolerate 3 mM H2O2 (Figure 6F). In addition, the lag time of cells treated by 1 mM H2O2 at 200 μM IPTG level increased to as long as ∼2 h, which was 6-fold of no-IPTG condition (red bars in Figure 6G). In contrast, cells overexpressing a control protein, ManA-GFP, could still smoothly resume growth at all three H2O2 levels (Supplementary Figure S9). Those observations, being qualitatively consistent with the result of Cm treatment, reinforce the notion that translation stress severely limit cell survival during oxidative stress.
Finally, we transformed a tRNA up-regulated plasmid, pRIL (51), into E. coli cells. pRIL plasmid harbors extra copies of related rare tRNA species including argU encoding tRNA4Arg (AGA, AGG), ileY encoding tRNA2Ile (AUA) and leuW encodes tRNA3Leu (CUA) (51). The E. coli cells harboring pRIL could indeed overcome 6 mM H2O2 after a certain lag time (∼ 2h) as shown in Figure 6H (black circle). In contrast, E. coli cells harboring the control plasmid still could not survive over 6 mM H2O2 treatment as found for wild type cells (Figure 1F). Throughout the whole process of H2O2 shock, translational elongation rate of pRIL-harboring cells was 0.8 aa/s at 5-min time point and again recovered to normal status during the recovery of cell growth (Figure 6I), being similar with the pattern of wild type cells during sublethal H2O2 treatment (0.5 to 5 mM). Overall, those various pieces of evidences support the scenario that tRNA-limited translational elongation process, being crucial for timely synthesis of stress defense proteins, becomes a key physiological bottleneck that limits the survival of E. coli cells during oxidative stress.
DISCUSSION
Timely synthesis of stress defense proteins is important for E. coli to counteract stress conditions. In this work, we quantitatively characterize the growth of E. coli cell during H2O2-induced oxidative stress. We show that cells undergo temporary growth arrest at 0.1-5 mM H2O2 but completely resume normal growth after a certain lag time. The lag time is positively correlated with the dose of external H2O2. However, when the H2O2 dose reaches a threshold concentration of 6 mM, cells ultimately fail to survive and resume growth. This phenomenon poses an important question regarding the physiological bottleneck that limits bacterial fitness during oxidative stress. It has been known before that extensive tRNA degradation by Rnase occurs under oxidative stress in both bacterial cells and eukaryotic cells (42), however, its physiological consequence is not well understood. Here, we systematically combine the quantitative characterizations of tRNA and translational elongation rate of E. coli and establish their time-course behavior under various H2O2 levels. Surprisingly, we found that the decrease in tRNA pools is most substantial during the initial stage (∼5 min) of oxidative stress. At 5 min after the onset of 5 mM H2O2 shock, the abundances of most cellular tRNA species drop by ∼95%, leading to an unusually dramatic slow-down of translational elongation process (∼1 aa/s). The extent of the drop in ER during oxidative stress (1 aa/s at ∼ 5 min after the onset of 5 mM H2O2 shock) is much more remarkable than that found during any other kinds of stress conditions (e.g. nutrient deprivation, osmotic stress) (25–27). The severity of translational elongation stress positively depends on the level of H2O2. At the threshold H2O2 level of 6 mM, translation is even completely stalled.
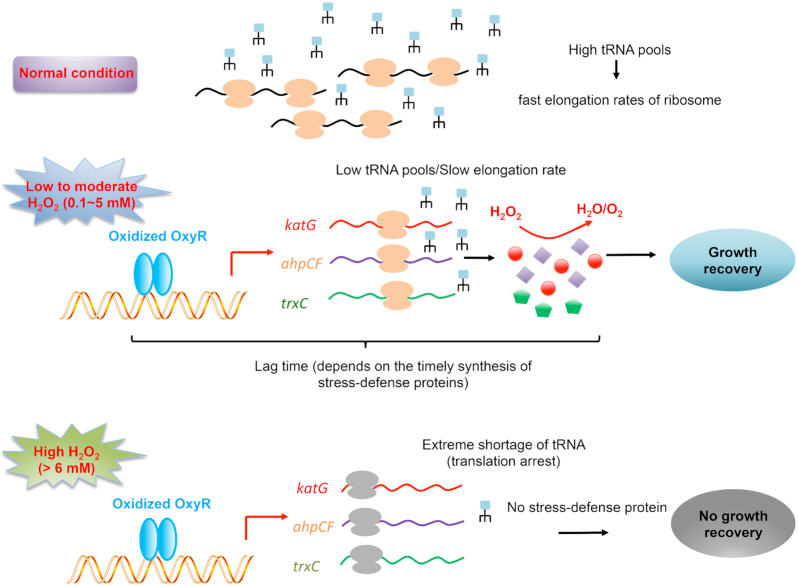
Those findings support a scenario in which the tRNA-limited translational elongation process is a key physiological bottleneck that the cells need to overcome in order to smoothly survive and counteract H2O2 shock. As depicted in Figure 7, at normal growth condition, the highly abundant cellular tRNA pools support a high translational elongation rate; at 0.1–5 mM H2O2, the ribosome translational elongation slows down due to lower cellular tRNA pools; a higher H2O2 dose causes slower translational elongation rate so that cell needs a longer time to synthesize stress-defense proteins such as KatG, AhpCF and TrxC to get rid of H2O2 and maintain cellular redox homeostasis. Therefore, cells treated by a high level of H2O2 have a longer lag time for growth recovery than cells treated with a low level of H2O2. However, when external H2O2 level is higher than 6 mM, cells fail to synthesize related stress defense proteins due to complete arrest of translational elongation and thus cannot tolerate the oxidative damage (e.g. damage on nucleic acids, lipids and protein) from excess H2O2. The time-course behavior of oxidative stress-defensive proteins under different H2O2 provides direct evidence for the above picture (Figure 5G–J). In addition, artificial aggravating the situation of translation stress by either Cm treatment or Rnase D overexpression could further compromise the fitness of cells upon oxidative stress (Figure 6A–G). On the contrary, artificial overexpression of tRNA could alleviate the translational elongation stress and enable cells to better tolerate H2O2 treatment (Figure 6H and I).
Figure 7.
Schematic illustration depicting the maintenance of translational elongation rate as the physiological bottleneck limiting the survival of E. coli cells during oxidative stress. At normal condition, the cellular tRNA pools are highly abundant, supporting fast translational elongation rate of ribosomes. Upon the treatment of 0.1–5 mM H2O2, translational elongation significantly slows down due to lower tRNA pools. Therefore, cells need a certain lag time (depends on the H2O2 dosage) to synthesize stress defense proteins (e.g. KatG, AhpCF and TrxC) to remove H2O2 and maintain redox homeostasis before the growth can be resumed. When the external H2O2 level is higher than a threshold level (6 mM), translational elongation gets completely stalled so that cells lose the capacity to synthesize stress-defense proteins and ultimately fail to survive from the oxidative damage and resume growth.
The H2O2-mediated oxidative stress presents a striking example in which bacterial cells face severe problem in maintaining the elongation rate of ribosomes and further timely synthesis of stress defense proteins. Although stress defense programs such as oxyR-regulon have been strongly activated in the transcription level by H2O2 shock, it was significantly inhibited or completely abolished by high H2O2 level due to the severely compromised translational capacity. Upon stress conditions, timely synthesis of stress defense proteins depends on two crucial parameters, number of actively translating ribosomes (determined by translational initiation) and ER (the speed of ribosomes) (25,26). Since stress defense proteins are preferentially expressed under stress conditions, the number of actively ribosomes translating stress defense proteins is guaranteed. However, for ER, our study shows that the case of oxidative stress is quite different from that of nutrient starvation conditions (carbon starvation and amino acid starvation) since at the later case, E. coli still maintains a significant ER (8–9 aa/s) so that timely synthesis of related stress defense protein is guaranteed (25,27,30). Although cellular tRNA levels also drop significantly during amino acid starvation due to degradation (43,52), the drop seems to be much milder than the case of H2O2 shock. This may account for the moderate ER under nutrient starvation. Overall, our study has shown that the slow ER becomes a major bottleneck for synthesizing stress-defensive protein in E. coli during oxidative stress, which has not observed in other stress conditions. Given such a severe physiological burden of translational elongation stress under oxidative stress, an intriguing question is why E. coli cells evolve such tRNA-degradation response. A possible explanation may lie in the concern of translational accuracy (52,53). It has been known that oxidative stress could cause mild protein mistranslation (54). In this case, high tRNA pools and the resultant high translational elongation rate may further exacerbate the translation error problem and compromise cell viability under oxidative stress (52).
At 0.1–5 mM H2O2, E. coli cells could resume growth after a certain lag time. It should be noted that although the cellular tRNA pools and translation capacity gradually recover during the lag time, cell growth does not gradually recover. Instead, the growth recovery occurs rapidly at the end of lag time. The origin is likely to be that: bacterial cells are very sensitive to the treatment of H2O2. H2O2 causes cell growth arrest through inhibiting the activity of related metabolic enzymes in TCA cycle and some key biosynthesis pathways (16,17). As little as micromoles (∼μM) levels of H2O2 are enough to inhibit cell growth (16,55). Therefore, although translation capacity is recovering with the removal of H2O2 during the lag time, cell might not be able to resume growth until most H2O2 has been removed. On the other hand, it is intriguing that the E. coli cells can completely resume its normal growth rate after the lag time. This observation indicates that the concentrations of essential components such as ribosome, RNAP and tRNA charging enzymes are maintained during H2O2 shock and are ready for use immediately after the lag time. For example, the cellular ribosome content (indicated by RNA/protein ratio) of E. coli cells does not change during H2O2 shock (blue asterisk in Supplementary Figure S10).
In summary, our finding elucidate that the tRNA-limited translational elongation process is the key physiological bottleneck that limits the bacterial survival during oxidative stress. The oxidative-induced growth arrest and cellular defense response is also a conserved phenomenon in yeast cells and mammalian cells (11,14,15). Furthermore, substantial tRNA degradation under oxidative stress has also been reported in yeast cells and mammalian cells (40–42,56). Therefore, the notion of our study might be applicable to eukaryotic cells as well, which deserves to be explored in the future for better understanding of the ROS physiology of eukaryotes.
Supplementary Material
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Natural Science Fund of China [31700089, 31700039, 31870028]; self-determined research funds of CCNU from the colleges’ basic research and operation of MOE. Funding for open access charge: National Natural Science Fund of China [31700089, 31700039, 31870028]; self-determined research funds of CCNU from the colleges' basic research and operation of MOE.
Conflict of interest statement. None declared.
REFERENCES
- 1. Ron E.Z. Dworkin M, Fallow S, Rosenberg E, Schleifer K-H, Stackebrandt E. Bacterial stress response. The prokaryotes. 2006; NY: Springer; 1012–1027. [Google Scholar]
- 2. Battesti A., Majdalani N., Gottesman S.. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 2011; 65:189–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiang S.M., Schellhorn H.E.. Evolution of the RpoS regulon: origin of RpoS and the conservation of RpoS-dependent regulation in bacteria. J. Mol. Evol. 2010; 70:557–571. [DOI] [PubMed] [Google Scholar]
- 4. Henggearonis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993; 72:165–168. [DOI] [PubMed] [Google Scholar]
- 5. Kolter R., Siegele D.A., Tormo A.. THE stationary phase of the bacterial life cycle. Annu. Rev. Microbiol. 1993; 47:855–874. [DOI] [PubMed] [Google Scholar]
- 6. Nyström T. Stationary-phase physiology. Annu. Rev. Microbiol. 2004; 58:161–181. [DOI] [PubMed] [Google Scholar]
- 7. Reeve C.A., Amy P.S., Matin A.. Role of protein synthesis in the survival of carbon-starved Escherichia coli K-12. J. Bacteriol. 1984; 160:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schieber M., Chandel N.S.. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014; 24:R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sies H., Berndt C., Jones D.P.. Oxidative stress. Annu. Rev. Biochem. 2017; 86:715–748. [DOI] [PubMed] [Google Scholar]
- 10. Reichmann D., Voth W., Jakob U.. Maintaining a healthy proteome during oxidative stress. Mol. Cell. 2018; 69:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finkel T., Holbrook N.J.. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408:239–47. [DOI] [PubMed] [Google Scholar]
- 12. Ezraty B., Gennaris A., Barras F., Collet J.F.. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017; 15:385. [DOI] [PubMed] [Google Scholar]
- 13. Dukan S., Nystrom T.. Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J. Biol. Chem. 1999; 274:26027–26032. [DOI] [PubMed] [Google Scholar]
- 14. Davies K.J.A. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2010; 50:279–289. [DOI] [PubMed] [Google Scholar]
- 15. Wu M.J., O’Doherty P.J., Fernandez H.R., Lyons V., Rogers P.J., Dawes I.W., Higgins V.J.. An antioxidant screening assay based on oxidant-induced growth arrest in Saccharomyces cerevisiae. FEMS Yeast Res. 2011; 11:379–387. [DOI] [PubMed] [Google Scholar]
- 16. Imlay J.A. Transcription factors that defend bacteria against reactive oxygen species. Annu. Rev. Microbiol. 2015; 69:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imlay J.A. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 2013; 11:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farr S.B., Kogoma T.. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 1991; 55:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Storz G., Tartaglia L.A., Ames B.N.. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990; 248:189–194. [DOI] [PubMed] [Google Scholar]
- 20. Christman M.F., Morgan R.W., Jacobson F.S., Ames B.N.. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985; 41:753–762. [DOI] [PubMed] [Google Scholar]
- 21. Walkup L.K., Kogoma T.. Escherichia coli proteins inducible by oxidative stress mediated by the superoxide radical. J. Bacteriol. 1989; 171:1476–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng M., Wang X., Templeton L.J., Smulski D.R., LaRossa R.A., Storz G.. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 2001; 183:4562–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Storz G., Tartaglia L.A.. OxyR: a regulator of antioxidant genes. J. Nutr. 1992; 122:627–630. [DOI] [PubMed] [Google Scholar]
- 24. Christman M.F., Storz G., Ames B.N.. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. U.S.A. 1989; 86:3484–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dai X., Zhu M., Warren M., Balakrishnan R., Patsalo V., Okano H., Williamson J.R., Fredrick K., Wang Y.-P., Hwa T.. Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat. Microbiol. 2016; 2:16231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai X., Zhu M., Warren M., Balakrishnan R., Okano H., Williamson J.R., Fredrick K., Hwa T.. Slowdown of translational elongation in escherichia coli under hyperosmotic stress. mBio. 2018; 9:e02375-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu M., Dai X., Wang Y.P.. Real time determination of bacterial in vivo ribosome translation elongation speed based on LacZalpha complementation system. Nucleic Acids Res. 2016; 44:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bremer H., Dennis P.P.. Neidhardt FC. Modulation of chemical composition and other parameters of the cell at different exponential growth rates. Escherichia coli and Salmonella. 1996; 2nd ednWashington, DC: Am Soc Microbiol; 1553–1569. [DOI] [PubMed] [Google Scholar]
- 29. Pedersen S. Escherichia coli ribosomes translate in vivo with variable rate. EMBO J. 1984; 3:2895–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vogel U., Sorensen M., Pedersen S., Jensen K.F., Kilstrup M.. Decreasing transcription elongation rate in Escherichia coli exposed to amino acid starvation. Mol. Microbiol. 1992; 6:2191–2200. [DOI] [PubMed] [Google Scholar]
- 31. Iyer S., Le D., Park B.R., Kim M.. Distinct mechanisms coordinate transcription and translation under carbon and nitrogen starvation in Escherichia coli. Nat. Microbiol. 2018; 3:741. [DOI] [PubMed] [Google Scholar]
- 32. Lyons E., Freeling M., Kustu S., Inwood W.. Using genomic sequencing for classical genetics in E. coli K12. PLoS One. 2011; 6:e16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soupene E., van Heeswijk W.C., Plumbridge J., Stewart V., Bertenthal D., Lee H., Prasad G., Paliy O., Charernnoppakul P., Kustu S.. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J. Bacteriol. 2003; 185:5611–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cayley S., Lewis B.A., Guttman H.J., Record M.T. Jr. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J. Mol. Biol. 1991; 222:281–300. [DOI] [PubMed] [Google Scholar]
- 35. Zhong J., Xiao C., Gu W., Du G., Sun X., He Q.-Y., Zhang G.. Transfer RNAs mediate the rapid adaptation of escherichia coli to oxidative stress. PLos Genet. 2015; 11:e1005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Visick J.E., Clarke S.. RpoS- and OxyR-independent induction of HPI catalase at stationary phase in Escherichia coli and identification of rpoS mutations in common laboratory strains. J. Bacteriol. 1997; 179:4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenberg J.T., Demple B.. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J. Bacteriol. 1989; 171:3933–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tamarit J., Cabiscol E., Ros J.. Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J. Biol. Chem. 1998; 273:3027–3032. [DOI] [PubMed] [Google Scholar]
- 39. Klumpp S., Scott M., Pedersen S., Hwa T.. Molecular crowding limits translation and cell growth. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:16754–16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thompson D.M., Cheng L., Green P.J., Roy P.. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008; 14:2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson D.M., Parker R.. Stressing out over tRNA cleavage. Cell. 2009; 138:215–219. [DOI] [PubMed] [Google Scholar]
- 42. Nawrot B., Sochacka E., Düchler M.. tRNA structural and functional changes induced by oxidative stress. Cell Mol. Life Sci. 2011; 68:4023–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Svenningsen S.L., Kongstad M., Stenum T.S., Muñoz-Gómez A.J., Sørensen M.A.. Transfer RNA is highly unstable during early amino acid starvation in Escherichia coli. Nucleic Acids Res. 2017; 45:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Storz G., Altuvia S.. OxyR regulon. Methods Enzymol. 1994; 234:217–223. [DOI] [PubMed] [Google Scholar]
- 45. Deris J.B., Kim M., Zhang Z., Okano H., Hermsen R., Groisman A., Hwa T.. The innate growth bistability and fitness landscapes of antibiotic-resistant bacteria. Science. 2013; 342:1237435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greulich P., Scott M., Evans M.R., Allen R.J.. Growth-dependent bacterial susceptibility to ribosome-targeting antibiotics. Mol. Syst. Biol. 2015; 11:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ghosh R.K., Deutscher M.P.. Identification of an Escherichia coli nuclease acting on structurally altered transfer RNA molecules. J. Biol. Chem. 1978; 253:997–1000. [PubMed] [Google Scholar]
- 48. Deutscher M.P. How bacterial cells keep ribonucleases under control. FEMS Microbiol. Rev. 2015; 39:350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang J.R., Deutscher M.P.. Cloning, characterization, and effects of overexpression of the Escherichia coli rnd gene encoding RNase D. J. Bacteriol. 1988; 170:522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang J.R., Deutscher M.P.. Transfer RNA is a substrate for RNase D in vivo. J. Biol. Chem. 1988; 263:17909–17912. [PubMed] [Google Scholar]
- 51. Fedyunin I., Lehnhardt L., Böhmer N., Kaufmann P., Zhang G., Ignatova Z.. tRNA concentration fine tunes protein solubility. FEBS Lett. 2012; 586:3336–3340. [DOI] [PubMed] [Google Scholar]
- 52. Sørensen M.A., Fehler A.O., Svenningsen S.L.. Transfer RNA instability as a stress response in Escherichia coli: Rapid dynamics of the tRNA pool as a function of demand. Rna Biology. 2017; 15:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ehrenberg M., Kurland C.G.. Costs of accuracy determined by a maximal growth rate constraint. Q. Rev. Biophys. 1984; 17:45–82. [DOI] [PubMed] [Google Scholar]
- 54. Jiqiang L., Dieter S.L.. Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proc. Natl. Acad. Sci. U.S.A. 2010; 107:4028–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Seaver L.C., Imlay J.A.. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 2001; 183:7173–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andreas C., Sandra W., Mario M.R., Tao P., Zoya I.. Reversible and rapid transfer-RNA deactivation as a mechanism of translational repression in stress. PLos Genet. 2013; 9:535–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.