Abstract
Intra-amniotic inflammation is strongly associated with spontaneous preterm labor and birth, the leading cause of perinatal mortality and morbidity worldwide. Previous studies have suggested a role for the NLRP3 (NLR family pyrin domain-containing protein 3) inflammasome in the mechanisms that lead to preterm labor and birth. However, a causal link between the NLRP3 inflammasome and preterm labor/birth induced by intra-amniotic inflammation has not been established. Herein, using an animal model of lipopolysaccharide-induced intra-amniotic inflammation (IAI), we demonstrated that there was priming of the NLRP3 inflammasome (1) at the transcriptional level, indicated by enhanced mRNA expression of inflammasome-related genes (Nlrp3, Casp1, Il1b); and (2) at the protein level, indicated by greater protein concentrations of NLRP3, in both the fetal membranes and decidua basalis prior to preterm birth. Additionally, we showed that there was canonical activation of the NLRP3 inflammasome in the fetal membranes, but not in the decidua basalis, prior to IAI-induced preterm birth as evidenced by increased protein levels of active caspase-1. Protein concentrations of released IL1β were also increased in both the fetal membranes and decidua basalis, as well as in the amniotic fluid, prior to IAI-induced preterm birth. Finally, using the specific NLRP3 inhibitor, MCC950, we showed that in vivo inhibition of the NLRP3 inflammasome reduced IAI-induced preterm birth and neonatal mortality. Collectively, these results provide a causal link between NLRP3 inflammasome activation and spontaneous preterm labor and birth in the context of intra-amniotic inflammation. We also showed that, by targeting the NLRP3 inflammasome, adverse pregnancy and neonatal outcomes can be significantly reduced.
Keywords: amniotic fluid, fetal inflammatory response syndrome, intra-amniotic infection, acute chorioamnionitis, funisitis, clinical chorioamnionitis, neutrophils, interleukin-1-beta, cytokines, caspase-1, NLRP3 inhibitor, fetal membranes, decidua, LPS
Intra-amniotic inflammation induces the activation of the NLRP3 inflammasome in the fetal membranes and decidua basalis prior to preterm birth, which is significantly reduced by inhibiting such a pathway.
Introduction
Intra-amniotic inflammation is a causal link to spontaneous preterm birth [1–8], the leading cause of perinatal mortality and morbidity worldwide [9–12]. Such inflammation can occur as the result of microbial invasion of the amniotic cavity (MIAC), referred to as intra-amniotic infection, or from danger signals or alarmins, known as sterile intra-amniotic inflammation [13–16]. Intra-amniotic infection is characterized by an increased white blood cell count [17–22] and elevated concentrations of cytokines [23–35] and lipid mediators (e.g. prostaglandins) [36–43] in the amniotic fluid. This intra-amniotic inflammatory response can result in deleterious effects on the offspring [27, 28, 44–49]. Therefore, the elucidation of the mechanisms involved in intra-amniotic inflammation leading to preterm birth is critical for the development of novel treatment strategies to reduce adverse neonatal outcomes [50, 51].
Recently, we provided evidence supporting a role for the inflammasome in the mechanisms leading to intra-amniotic inflammation associated with preterm labor [52–55]. Inflammasomes are cytoplasmic multi-protein complexes that are mainly expressed by innate immune cells [56–74]. Inflammasome activation includes two steps: the priming and the assembly of the multi-protein complex [75, 76]. In the first step, microbial products or alarmins are sensed by pattern recognition receptors, inducing the activation of the NF-κB pathway, which results in the upregulation (mRNA and protein) of the inflammasome sensor molecule (e.g. NLR family pyrin domain-containing protein or NLRP3) and other related proteins [75–77]. In the second step, the inflammasome complex is assembled [75, 76], inducing the activation of caspase-1 (CASP-1) [56, 61, 63, 71, 72, 74]. Active forms of CASP-1 can then lead to the maturation of pro-interleukin (IL)1β and pro-IL18 into their bioactive forms [78–88]. Both the priming and activation of the inflammasome have been described in the chorioamniotic membranes and amniotic cavity of women with term [89–92] or preterm [52, 55] labor. However, a causal link between inflammasome activation and spontaneous preterm labor in the context of intra-amniotic inflammation has not been established.
Herein, using a previously established model of intra-amniotic inflammation that resembles the subclinical syndrome of preterm labor associated with MIAC [93] (hereafter referred to as IAI-induced preterm birth model), we investigated whether a microbial product (lipopolysaccharide [LPS]) could induce the activation of the inflammasome at the maternal-fetal interface (fetal membranes and decidua basalis) prior to preterm birth. In addition, we investigated whether inhibition of such a pathway could prevent preterm birth and reduce adverse neonatal outcomes.
Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory in Bar Harbor, ME, USA, and bred in the animal care facility at the C.S. Mott Center for Human Growth and Development at Wayne State University, Detroit, MI, USA. All mice were housed under a circadian cycle (12 h light/12 h dark). Females 8–12 weeks old were mated with males of the same background and proven fertility. Female mice were checked daily between 8:00 a.m. and 9:00 a.m. for the appearance of a vaginal plug, which indicated 0.5 days post coitum (dpc). Females were then placed into new cages, and their weights were monitored daily. A gain of two or more grams by 12.5 dpc confirmed pregnancy. All procedures were approved by the Institutional Animal Care and Use Committee at Wayne State University (Protocol No. A-07–03-15).
Murine model of intra-amniotic inflammation-induced preterm birth
Intra-amniotic administration of LPS [93] (IAI-induced preterm birth): dams were anesthetized on 16.5 dpc by inhalation of 2–3% isoflurane (Aerrane, Baxter Healthcare Corporation, Deerfield, IL, USA) and 1–2 L/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.5–2% isoflurane and 1.5–2 L/min of oxygen. Dams were positioned on a heating pad and stabilized with adhesive tape. Fur removal from the abdomen was achieved by applying Nair cream (Church & Dwight Co., Inc., Ewing, NJ, USA) to this area. Body temperature was maintained in the range of 37 ± 1°C and detected with a rectal probe (VisualSonics, Toronto, ON, Canada), and respiratory and heart rates were monitored by electrodes embedded in the heating pad. An ultrasound probe was fixed and mobilized with a mechanical holder, and the transducer was slowly moved toward the abdomen. Ultrasound-guided intra-amniotic injection of LPS (Escherichia coli O111: B4; Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 100 ng dissolved in 25 μL of sterile 1X phosphate-buffered saline (PBS; Fisher Scientific Bioreagents, Fair Lawn, NJ, USA) was performed in each amniotic sac using a 30G needle (BD PrecisionGlide Needle, Becton Dickinson, Franklin Lakes, NJ, USA). Controls were injected with 25 μL of sterile 1X PBS. The syringe was stabilized by a mechanical holder (VisualSonics Inc., Toronto, ON, Canada). Following the ultrasound, mice were placed under a heat lamp for recovery (defined as when the mouse resumes normal activity, such as walking and responding), which typically occurred 10–20 min after removal from anesthesia.
RNA isolation, cDNA synthesis, and quantitative reverse transcription polymerase chain reaction analysis
Dams were intra-amniotically injected with either LPS or PBS on 16.5 dpc. Mice were euthanized on 17.5 dpc (15–17 h post-injection) and dissection to obtain the fetal membranes and decidua basalis was performed (Figure 1A and B, n = 12 per group). Tissues were placed in RNAlater™ Stabilization Solution (Invitrogen by Thermo Fisher Scientific, Carlsbad, CA, USA), according to the manufacturer's instructions. The samples were kept at 4°C overnight, after which the RNAlater™ solution was removed, and the tissues were stored at −80°C until analysis. Total RNA was isolated from the fetal membranes and decidua basalis using QIAshredders, RNase-Free DNase Sets, and RNeasy Mini Kits (all from Qiagen, Hilden, Germany), according to the manufacturer's instructions. RNA concentrations and purity were assessed with the NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and RNA integrity was evaluated with the Bioanalyzer 2100 (Agilent Technologies, Wilmington, DE, USA). Complementary (c)DNA was synthesized using SuperScript III First-Strand Synthesis SuperMix (Invitrogen by Thermo Fisher Scientific). Gene expression profiling was performed on the BioMark System (Fluidigm, San Francisco, CA, USA) for high-throughput qRT-PCR with the TaqMan gene expression assays (Applied Biosystems, Life Technologies Corporation, Foster City, CA, USA) listed in Supplemental Table S1.
Figure 1.
Inflammasome-related gene profiles are upregulated in the fetal membranes and decidua basalis prior to intra-amniotic inflammation (IAI)-induced preterm birth. A) Animal model of IAI-induced preterm labor and birth. B) Spatial localization of the fetal membranes and decidua basalis in the murine uterus. Heatmaps showing regulation of inflammasome-related genes in the C) fetal membranes and D) decidua basalis. LPS = lipopolysaccharide; PBS = phosphate-buffered saline (vehicle control). Green = downregulation, red = upregulation. N = 12 per group.
Western blots for inflammasome-related proteins
For analysis of the inflammasome-related proteins in the fetal membranes and decidua basalis, dams were intra-amniotically injected with either LPS or PBS on 16.5 dpc. Mice were euthanized on 17.5 dpc and dissection to obtain the fetal membranes and decidua basalis was performed (Figure 1A and B, n = 6 per group). Tissues were snap-frozen in liquid nitrogen and stored at −80°C until analysis. Tissue lysates were prepared by mechanically homogenizing snap-frozen fetal membranes and decidua basalis in 1X PBS containing a complete protease inhibitor cocktail (Roche Applied Sciences, Mannheim, Germany). Lysates were centrifuged at 15 700 x g for 5 min at 4°C and the supernatants were stored at −80°C until use.
For analysis of inflammasome-related proteins in murine macrophages, bone marrow was collected from C57BL/6 mice and the cells were differentiated in IMDM medium (Thermo Scientific) with 10% FBS (Invitrogen) and 10ng/mL of M-CSF (Cat#576402; BioLegend, San Diego, CA, USA) at 37°C and 5% CO2 for 7 days. Bone marrow-derived macrophages (BMDMs) were seeded into 6-well tissue culture plates (Fisher Scientific) at 5 × 105 cells/well and cultured at 37°C with 5% CO2 overnight. Following incubation, the culture medium was gently aspirated and replaced with fresh medium. For MCC950 pretreatment, 10 μM MCC950 (Cat#PZ0280; Sigma-Aldrich) was added to the culture media and the BMDMs were incubated at 37°C with 5% CO2 for 30 min. Following treatment with MCC950, the BMDMs were incubated with 0.5 μg/mL of LPS (Escherichia coli 0111: B4; Sigma-Aldrich) at 37°C with 5% CO2 for 4 h, followed by the addition of 10 μM of nigericin (Cat#N7143; Sigma-Aldrich) for an additional hour. Non-treated BMDMs were used as a negative control. The cell supernatants were collected and centrifuged at 1300 x g for 5 min to remove floating cells and debris. The cell-free supernatants were then concentrated to 10X with the Amicon Ultra Centrifuge filter (Cat#UFC800324, Ultracel 3K, EMD Millipore, Darmstadt, Germany) and stored at −20°C until use. Cultured BMDMs were then collected and lysed with RIPA buffer (Sigma-Aldrich) containing a complete protease inhibitor cocktail (Roche Applied Sciences). Lysates were centrifuged at 15 700 x g for 5 min at 4°C and the supernatants were collected and stored at −20°C until use.
Prior to immunoblotting, total protein concentration was determined using the Pierce BCA Protein Assay Kit (Cat#23225; Pierce Biotechnology, Thermo Fisher Scientific, Inc., Rockford, IL). Fetal membrane and decidua basalis tissue lysates (50 μg per well), cell lysates (10 μg per well), and concentrated cell supernatants (40 μl) were subjected to electrophoresis in 4%–12% sodium dodecyl sulphate-polyacrylamide gels (Cat#NP0336BOX, Invitrogen by Thermo Fisher Scientific, Carlsbad, CA). Separated proteins were then transferred onto nitrocellulose membranes (Cat#1620145, Bio-Rad, Hercules, CA). Next, the nitrocellulose membranes were submerged in blocking solution (StartingBlock T20 Blocking Buffer, Thermo Fisher Scientific, Inc.) for 30 min at room temperature and then probed overnight at 4°C with the following mouse antibodies: mouse anti-NLRP3 (Cat#AG-20B-0014-C100, 1μg/mL, Adipogen Life Sciences, San Diego, CA), rat anti-CASP-1 (Cat#14–9832-82, 5μg/mL, Invitrogen), and rat anti-IL1β (Cat#MAB4011, 1μg/mL, R&D Systems, Inc., Minneapolis, MN). Finally, nitrocellulose membranes were then stripped with Restore PLUS Western Blot Stripping Buffer (Pierce Biotechnology, Thermo Fisher Scientific, Inc.) for 15 min, washed with 1X PBS, blocked, and re-probed for 1 h at room temperature with a mouse anti-β-actin (ACTB) monoclonal antibody (Cat#A5441, Sigma-Aldrich). Chemiluminescence signals were detected with the ChemiGlow West Substrate Kit (ProteinSimple, San Jose, CA) and images were acquired using the Fujifilm ImageQuant LAS-4000 Imaging System (GE Life Sciences, Pittsburgh, PA). Quantification was performed using ImageJ.
ELISA determination of IL1β concentrations in the fetal membranes and decidua basalis
Dams were intra-amniotically injected with either LPS or PBS on 16.5 dpc. Mice were euthanized on 17.5 dpc and dissection to obtain the fetal membranes and decidua basalis was performed (Figure 1A and B, n = 10 per group). Tissues were snap-frozen in liquid nitrogen and stored at −80°C until analysis. Tissue lysates were prepared by mechanically homogenizing snap-frozen fetal membranes and decidua basalis in the Cell Lysis Buffer 2 (Cat#895347; R&D Systems, Inc.). Lysates were centrifuged at 15 700 x g for 5 min at 4°C and the supernatants were stored at −80°C until use. Prior to ELISA, total protein concentration was determined using the Pierce BCA Protein Assay Kit. Concentrations of IL1β in the fetal membranes and decidua basalis were determined by using a sensitive and specific ELISA assay kit (Cat#MLB00C; R&D Systems, Inc.). This ELISA kit was initially validated in our laboratory prior to the execution of this study. Tissue concentrations of IL1β were obtained by interpolation from the standard curve. The sensitivity of the assay was 2.31 pg/mL. The inter- and intra-assay coefficients of variation were less than 10%.
Amniotic fluid concentrations of IL1β were also determined, as previously reported [94].
MCC950 treatment
Dams were intra-peritoneally injected on 16.5 dpc with 50mg/kg of the NLRP3 inhibitor MCC950 dissolved in 200 μL of sterile 1X PBS (n = 8–10 per group). This dosage of MCC950 has been shown to inhibit the NLRP3 inflammasome in vivo [95]. Shortly after (1–2 h), dams received the intra-amniotic administration of LPS or PBS as described above. Controls were injected with LPS or PBS alone. The rates of preterm labor/birth and neonatal mortality were obtained for each group.
Video monitoring and definition of preterm labor/birth and neonatal mortality
Dams were monitored via video camera (Sony Corporation, Tokyo, Japan) until delivery to obtain the rates of preterm birth and neonatal mortality. Preterm birth was defined as delivery occurring before 18.5 dpc, and its rate was represented by the percentage of dams delivering preterm among the total number of mice injected. The rate of neonatal mortality for each litter was defined as the proportion of delivered pups found dead among the total litter size.
Immunofluorescence microscopy to detect ASC specks
BMDMs were obtained as described above and seeded into a four-well Lab-Tek chamber slide (Thermo Fisher Scientific, Rochester, NY, USA) at 1.25 × 105 cells/well and cultured at 37°C with 5% CO2 overnight. Following incubation, the BMDMs were treated with MCC950 and LPS + nigericin as described above. Next, the BMDMs were fixed using 4% paraformaldehyde (Electron Microscopy Sciences Hatfield, PA, USA) for 20 min at room temperature, rinsed with 1X PBS, and permeabilized using 0.25% Triton X-100 (Promega, Madison, WI, USA) for 5 min at room temperature. Prior to staining, non-specific antibody interactions were blocked using serum-free protein blocker (Cat#X09090; DAKO, Carpinteria, CA, USA) for 30 min at room temperature. BMDMs were then stained with a rabbit anti-ASC (Cat#AG-25B-0006-C100; Adipogen, San Diego, CA) antibody at room temperature for 1 h. Rabbit IgG was used as a negative control. Following staining, the BMDMs were washed with 1X PBS containing 0.1% Tween 20 (PBS-T) (Sigma-Aldrich). After blocking for 10 min with 10% goat serum (KPL, Gaithersburg, MD, USA), secondary goat anti-rabbit IgG–Alexa Fluor 594 (Life Technologies) was added and BMDMs were incubated for 1 h at room temperature in the dark. Finally, the BMDMs were washed with 1X PBS-T and mounted using ProLong Diamond Antifade Mountant with DAPI (Life Technologies). Immunofluorescence was visualized using an Olympus BX60 fluorescence microscope (Olympus, Tokyo, Japan) at 400X original magnification. The pictures were taken using an Olympus DP71 camera and DP Controller Software (Olympus).
Statistical analysis
Data were analyzed using IBM SPSS v19.0 (IBM Corporation, Armonk, NY) and the R statistical language and environment (www.r-project.org). Negative ΔCt values were determined using multiple reference genes (Gusb, Hsp90ab1, Gapdh, and Actb) averaged within each sample to determine gene expression levels. A heat map was created for the group mean expression matrix (gene x group mean), with individual gene expression level being standardized first. The heat map represents the Z-scores of the mean (−ΔCt) and the hierarchical clustering using correlation distance. Relative fold changes for mRNA expression of Nlrp3, Casp1, Il1b, and Il18 were calculated using the 2−ΔΔCT method [96]. For gene expression, protein expression, and protein concentrations, the statistical significance of group comparisons was assessed using the Mann–Whitney U-test. For rates of preterm birth and neonatal mortality, the statistical significance of group comparisons was assessed using the Fisher's exact test. A P-value < 0.05 was considered significant.
Results
Inflammasome-related genes are upregulated in the fetal membranes and decidua basalis prior to IAI-induced preterm birth
First, we determined whether the expression of inflammasome-related genes was altered in the fetal membranes and decidua basalis prior to IAI-induced preterm birth. Figure 1A shows the timeline of intra-amniotic injection of LPS and sampling of the fetal membranes and decidua basalis. Figure 1B is a representation of the spatial localization of the fetal membranes and decidua basalis in the murine uterus. The fetal membranes from dams injected with LPS showed upregulation of inflammasome-related genes compared to their PBS controls (Figure 1C). Likewise, the decidua basalis from dams injected with LPS had higher expression of inflammasome-related genes than PBS controls (Figure 1D). Yet, the inflammasome-related gene profiles between the fetal membranes and the decidua basalis were different prior to IAI-induced preterm birth (Figure 1C vs. 1D).
Given that the human chorioamniotic membranes from women with spontaneous preterm labor expressed high mRNA levels of NLRP3, CASP1, and IL1B [52], we investigated the expression of these molecules prior to IAI-induced preterm birth. In addition, we determined the expression of Il18 since its processing is also mediated by the inflammasome [57, 71, 82, 97]. The fetal membranes from dams injected with LPS had greater expression of Nlrp3, Casp1, and Il1b than those from PBS controls (Figure 2A). In the decidua basalis from dams injected with LPS, however, only Nlrp3 and Il1b were upregulated (Figure 2B). The expression of Il18 was not upregulated in either the fetal membranes or the decidua basalis prior to IAI-induced preterm birth (Figure 2A and B).
Figure 2.
NLRP3 inflammasome-related genes are upregulated in the fetal membranes and decidua basalis prior to intra-amniotic inflammation (IAI)-induced preterm birth. Expression of Nlrp3, Casp1, Il1b, and Il18 in the A) fetal membranes and B) decidua basalis prior to IAI-induced preterm birth. LPS = lipopolysaccharide; PBS = phosphate-buffered saline (vehicle control). Middle lines indicate medians. N = 12 per group.
Collectively, these findings indicate that, at the transcriptional level, there is priming of the NLRP3 inflammasome (i.e. upregulation of the inflammasome-related genes [75, 76]) in both the fetal membranes and decidua basalis prior to IAI-induced preterm birth.
The NLRP3 protein is increased in the fetal membranes and decidua basalis prior to IAI-induced preterm birth
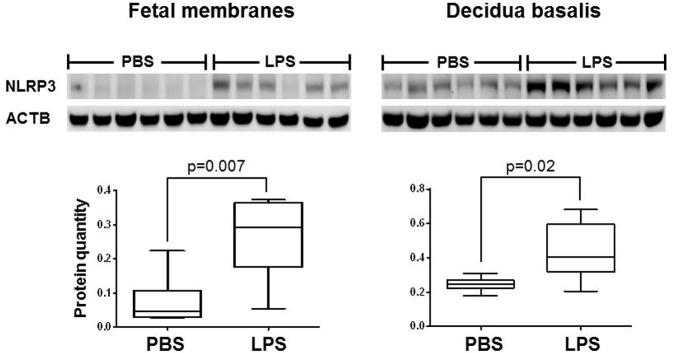
The chorioamniotic membranes from women with spontaneous preterm labor displayed increased amounts of the NLRP3 inflammasome sensor molecule [52]. Therefore, we next investigated whether this protein was overexpressed prior to IAI-induced preterm birth. Consistent with our human findings, the protein quantities of NLRP3 in the fetal membranes and decidua basalis were greater in dams injected with LPS than in PBS controls (Figure 3). This finding confirms that, at the protein level, there is priming of the NLRP3 inflammasome in both the fetal membranes and decidua basalis prior to IAI-induced preterm birth.
Figure 3.
NLRP3 protein concentrations are increased in the fetal membranes and decidua basalis prior to intra-amniotic inflammation (IAI)-induced preterm birth. LPS = lipopolysaccharide; PBS = phosphate-buffered saline (vehicle control). ACTB = β-actin. Midlines indicate medians, boxes show interquartile range, and whiskers indicate min-max range. N = 6 per group.
The activation of caspase-1 is increased in the fetal membranes, but not in the decidua basalis, prior to IAI-induced preterm birth
Following priming, the NLRP3 inflammasome is assembled, inducing the activation of CASP-1 [56, 61, 63, 71, 72, 74]. Thus, we next investigated whether there is activation of CASP-1 prior to IAI-induced preterm birth. Different forms of partially processed CASP-1 (e.g. p35) were detected by Western blot (Figure 4A and B); yet we focused on quantifying the p20 active form that has biological function [78]. The protein quantity of active CASP-1 (p20 form) in the fetal membranes was greater in dams injected with LPS than in PBS controls (Figure 4A). However, the active form of CASP-1 in the decidua basalis was not different between dams injected with LPS and PBS controls (Figure 4B). The protein quantity of pro-CASP-1 in the fetal membranes and decidua basalis was not different between dams injected with LPS and PBS controls (Figure 4A and B). These data show that in the fetal membranes, but not in the decidua basalis, there is canonical activation of the NLRP3 inflammasome prior to IAI-induced preterm birth.
Figure 4.
Active caspase-1 is increased in the fetal membranes prior to intra-amniotic inflammation (IAI)-induced preterm birth. Protein concentrations of pro-caspase-1 and active caspase-1 p20 in the A) fetal membranes and B) decidua basalis prior to IAI-induced preterm birth. LPS = lipopolysaccharide; PBS = phosphate-buffered saline (vehicle control). ACTB = β-actin. Midlines indicate medians, boxes show interquartile range, and whiskers indicate min-max range. N = 6 per group.
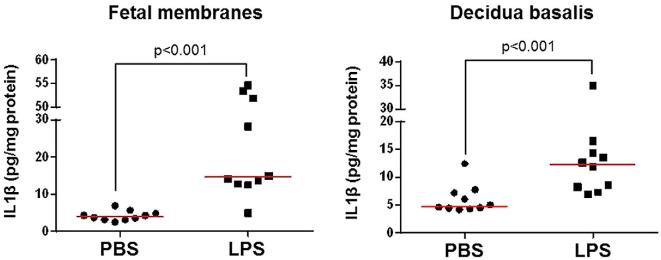
The concentration of IL1β is increased in the fetal membranes and decidua basalis prior to IAI-induced preterm birth
Following activation of the NLRP3 inflammasome, the active forms of CASP-1 can induce the release of mature IL1β into the extracellular space [78–88]. Hence, we determined the concentration of IL1β in the fetal membranes and decidua basalis prior to IAI-induced preterm birth. The concentration of released IL1β in the fetal membranes and decidua basalis from dams injected with LPS was greater than in those from PBS controls (Figure 5). In addition, we determined the concentration of released IL1β in the amniotic fluid. Similar to the protein extracts of the fetal membranes and decidua basalis, amniotic fluid concentrations of released IL1β were increased in dams injected with LPS (Supplementary Figure 1).
Figure 5.
Interleukin (IL)1β is increased in the fetal membranes and decidua basalis prior to intra-amniotic inflammation (IAI)-induced preterm birth. LPS = lipopolysaccharide; PBS = phosphate-buffered saline (vehicle control). Middle lines indicate medians. N = 10 per group.
It is worth mentioning that the mature form of IL18, another cytokine processed by the inflammasome [83], was neither detected in the fetal membranes nor in the decidua basalis of dams from any group (data not shown).
Inhibition of the NLRP3 inflammasome prevents IAI-induced preterm birth and reduces adverse neonatal outcomes
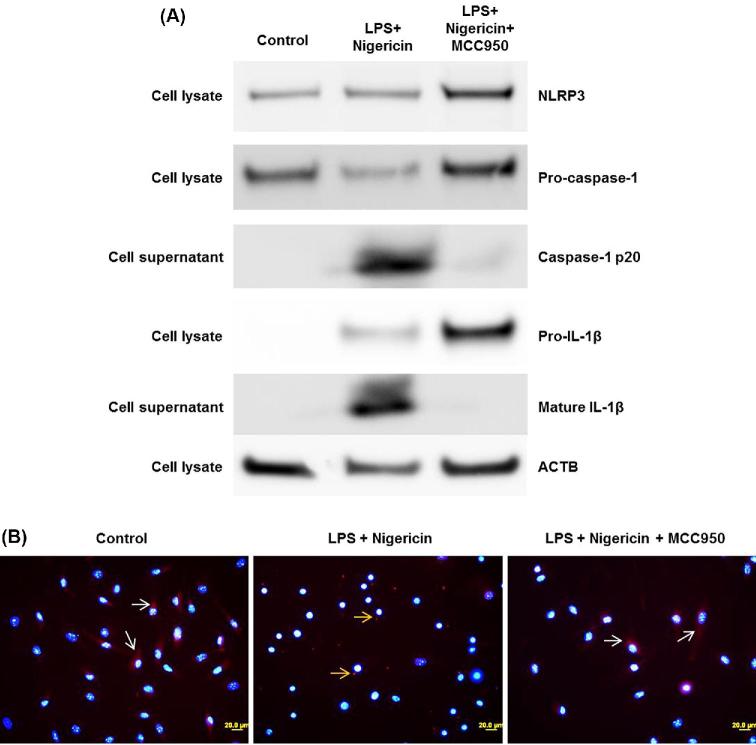
Up to this point, we have shown that the fetal membranes and decidua basalis released IL1β prior to IAI-induced preterm birth. Next, we tested whether inhibition of the NLRP3 inflammasome could prevent preterm birth and, more importantly, its adverse neonatal outcomes. In vivo inhibition of the NLRP3 inflammasome is achieved by systemic administration of the specific NLRP3 inhibitor, MCC950 [95]. Dams were randomized to be treated or untreated with MCC950 (Figure 6A). As expected [93, 94], most of the animals intra-amniotically injected with LPS had a shorter time interval from injection to delivery compared to PBS controls (Figure 6B) and delivered preterm (Figure 6C), whereas PBS controls delivered at term (Figure 6C). Treatment with MCC950 restored the time interval between LPS injection and delivery (Figure 6B) and reduced the rate of preterm birth by 30% (Figure 6C). Importantly, treating dams with MCC950 reduced the rate of neonatal mortality by approximately 30%, and such a reduction was statistically significant (Figure 6D). To test whether the activation of the NLRP3 inflammasome was inhibited in our model, murine BMDMs were incubated with LPS + nigericin with and without MCC950 treatment. BMDMs incubated with LPS + nigericin contained high amounts of active CASP-1 and mature IL1β, indicating inflammasome activation (Figure 7A). These effects were inhibited by treatment with MCC950 (Figure 7A). In addition, we proved that treatment with MCC950 inhibited ASC speck formation (red dots indicated by yellow arrows) induced by LPS + nigericin (Figure 7B). These results show that the NLRP3 inflammasome is implicated in the mechanisms that lead to spontaneous preterm labor in the context of intra-amniotic infection, and that by inhibiting this pathway adverse neonatal outcomes can be significantly reduced.
Figure 6.
Inhibition of the NLRP3 inflammasome via MCC950 reduces intra-amniotic inflammation (IAI)-induced preterm birth and neonatal mortality. A) Animal model of IAI-induced preterm labor and birth with MCC950 treatment. B) Time intervals (hours) from intra-amniotic injection to delivery Midlines indicate medians. C) Rates of preterm birth. D) Rates of neonatal mortality. Data are shown as percentages of the total group size. N = 8–10 per group.
Figure 7.
In vitro inhibition of NLRP3 inflammasome activation by MCC950. Bone marrow-derived macrophages were incubated with LPS and nigericin, with and without treatment with MCC950. A) Western blots showing the NLRP3 protein, pro-caspase-1, active caspase-1, pro-IL1β, and mature IL1β in the cell lysates or supernatants. Beta-actin (ACTB) was used as an internal control. B) ASC speck formation by bone marrow-derived macrophages incubated with LPS and nigericin, with and without treatment with MCC950. White arrows indicate cytoplasmic ASC, yellow arrows indicate ASC specks (red dots). Magnification 400X.
Discussion
Principle findings
In this study, we demonstrated that there was priming of the NLRP3 inflammasome (1) at the transcriptional level, indicated by enhanced mRNA expression of inflammasome-related genes (Nlrp3, Casp1, Il1b); and (2) at the protein level, indicated by greater protein concentrations of NLRP3, in both the fetal membranes and decidua basalis prior to IAI-induced preterm birth. Additionally, we showed that there was canonical activation of the NLRP3 inflammasome in the fetal membranes, but not in the decidua basalis, prior to IAI-induced preterm birth as evidenced by increased protein levels of active caspase-1. Protein concentrations of released IL1β were also increased in both the fetal membranes and decidua basalis, as well as in the amniotic fluid, prior to IAI-induced preterm birth. Finally, using the specific NLRP3 inhibitor, MCC950, we showed that in vivo inhibition of the NLRP3 inflammasome extended gestational length and significantly reduced IAI-induced preterm birth and neonatal mortality.
The NLRP3 inflammasome in the intra-amniotic space during spontaneous preterm labor
Inflammasome activation and assembly in the chorioamniotic membranes are associated with spontaneous preterm labor, either in the context of intra-amniotic infection [52, 55] or sterile intra-amniotic inflammation [55]. Expression of inflammasome-related genes (e.g. NLRP3, CASP1, CASP4, and IL1B) is upregulated in the chorioamniotic membranes of women who underwent spontaneous preterm labor with acute histologic chorioamnionitis (a placental lesion characterized by infiltration of neutrophils into the chorioamniotic membranes [98–104] and associated with both intra-amniotic infection and sterile intra-amniotic inflammation [13–16, 55]) compared to women who delivered preterm without this placental lesion [52]. The formation of ASC (apoptosis-associated speck-like protein containing a CARD; adaptor protein of the inflammasome)/CASP-1 complexes and the levels of active CASP-1, IL1β, and IL18 are also increased in the chorioamniotic membranes from these women [52]. Moreover, women with intra-amniotic infection or sterile intra-amniotic inflammation had increased protein expression of ASC, CASP-1, and IL1β in the chorioamniotic membranes compared to women who underwent spontaneous preterm labor without intra-amniotic inflammation/infection [55]. Such findings are in line with the current study in which we showed that the fetal membranes from mice intra-amniotically injected with LPS, a model of intra-amniotic inflammation [93], have greater mRNA and protein expression of NLRP3, active CASP-1, and IL1β, indicating that both priming and activation of the NLRP3 inflammasome [75, 76] occur in the chorioamniotic membranes during intra-amniotic inflammation-induced preterm labor and birth. Yet, dams intra-amniotically injected with LPS did not have higher mRNA expression or detectable mature IL18 in the fetal membranes, which contrasts with what is observed in the chorioamniotic membranes of women with acute histologic chorioamnionitis [52]. This discrepancy may be due to the fact that the murine fetal membranes are not attached to the decidua (i.e. decidua parietalis), which is the source of maternal neutrophils infiltrating the human chorioamniotic membranes [21, 103].
The amniotic fluid from women with spontaneous preterm labor and intra-amniotic inflammation/infection contains high concentrations of inflammasome-related proteins such as CASP-1 [105], IL1β [24, 106], IL18 [107, 108], and ASC [55]. A possible source for these proteins is the immune cells that invade the amniotic cavity in response to microbial invasion [17–22]. For example, neutrophils, monocytes/macrophages, T cells, NK cells, and B cells are abundant in the amniotic fluid of women with intra-amniotic inflammation/infection [17, 20–22], some of which can express inflammasome components [109–111]. Yet, further research is required to demonstrate that amniotic fluid immune cells express inflammasome components in the context of intra-amniotic infection and/or sterile intra-amniotic inflammation.
The NLRP3 inflammasome responds to both microbial [60, 112–119] and non-infectious or endogenous [120–128] signals. Thus, we and others have proposed that microbial products [94, 129–132] and alarmins [133] can activate the inflammasome pathway in the context of intra-amniotic inflammation-associated preterm labor. Recent in vitro studies showed that Ureaplasma spp [134], Gardnerella vaginalis [135], and group B streptococcus (GBS) [136], microorganisms commonly found in women with intra-amniotic infection [13, 35, 137–142], induce activation of the NLRP3 inflammasome in macrophages, further implicating this pathway in the mechanisms leading to preterm labor. Moreover, the herpes virus MHV-68 enhanced LPS-induced secretion of IL1β by chorioamniotic membranes, a process likely mediated through the NLRP3 inflammasome [131]. This finding provides evidence for the potential of viruses to prime and/or activate the NLRP3 inflammasome, especially in the context of an active polymicrobial infection [131].
The NLRP3 inflammasome in the decidua basalis during spontaneous preterm labor
Several studies showed that the decidua is a source of IL1β [143–146], suggesting that the inflammasome is implicated in the processing of this cytokine at the maternal-fetal interface. Recent studies have supported such a concept. For example, decidual stromal cells expressed high levels of inflammasome-related genes (NLRP3, CASP1, and IL1B) upon in vitro LPS stimulation [147]. Furthermore, choriodecidual leukocytes from women who underwent spontaneous labor at term had increased formation of ASC “specks” (intracellular aggregates of ASC protein), an indicator of inflammasome activation [148, 149], compared to those who delivered at term without labor [150]. In this study, we provided evidence demonstrating that, in the context of intra-amniotic inflammation induced by an endotoxin, the processing of IL1β in the decidua basalis could be mediated by an active caspase-1-independent mechanism such as the non-canonical pathway of the inflammasome. In this pathway, cytoplasmic CASP-4/5 (human) or CASP-11 (mouse) directly recognizes microbial products (e.g. LPS) in the intracellular space which can initiate activation of the inflammasome in a CASP-1-independent manner [118, 151, 152]. Alternatively, decidual IL1β could have been processed by inflammasome-independent mechanisms such as neutrophil elastase, cathepsin G, collagenase [153], mast cell chymase [154], matrix metalloproteinases (MMP) 2, 3, and 9 [155], neutrophil proteinase 3 [156], and cathepsin C [157]. The presence of additional caspase-1-independent mechanisms for IL1β cleavage was confirmed by a study in which CASP-1 and ASC-deficient mice had unimpaired IL1β production [158].
In this study, we observed that the decidua basalis contained high amounts of the NLRP3 protein, but this was not accompanied by an increase in the active form of CASP-1. These data suggest that, in the decidua basalis, the NLRP3 inflammasome may be implicated in different processes that are not related to pyroptosis (i.e. processing of CASP-1). Recent reports have shown that the NLRP3 inflammasome is expressed in neutrophils and involved in the generation of neutrophil extracellular traps (NETs) [159, 160]. In line with this concept, we have shown that NETs are formed in the chorioamniotic membranes with acute histologic chorioamnionitis [161], suggesting that the NLRP3 inflammasome is expressed by decidual neutrophils infiltrating the chorioamniotic space during intra-amniotic infection and participates in NET formation.
A novel strategy to prevent intra-amniotic inflammation-induced preterm birth
The NLRP3 inflammasome has previously been proposed as a potential therapeutic target to treat multiple inflammatory diseases [162–169]. Several synthetic [162–165, 167] and natural [166, 168] compounds with varying specificity for the NLRP3 pathway have been tested. One promising treatment is MCC950, a specific small-molecule inhibitor of the NLRP3 inflammasome [95]. The exact mechanisms whereby MCC950 prevents NLRP3 inflammasome activation are unknown; however, it was established that this compound does not target other inflammasome sensor molecules such as NLRP1, AIM2, or NLRC4 [95]. This specific inhibitor was used in multiple mouse models of inflammatory disease [170–179]; moreover, a preliminary study provided evidence that MCC950 could be safe for clinical use in humans [180]. In addition, several recent studies have indicated that MCC950 can be used to treat neonatal [95, 181] and infant [182] mice in models of cryopyrin-associated periodic syndrome (CAPS) [95], Muckle–Wells syndrome [181], and influenza A viral infection [182]. In this study, we demonstrated that inhibition of the NLRP3 inflammasome via MCC950 extends gestational length and can not only reduce the rate of IAI-induced preterm birth by 30%, but can significantly improve neonatal survival as well. It is worth mentioning that treatment with MCC950 did not completely prevent preterm birth and adverse neonatal outcomes, suggesting that LPS triggered NLRP3 inflammasome-independent mechanisms (e.g. ras/raf-1/MEK/MAPK [183] and p38 MAPK [184]) that were not blocked by this inhibitor. Together with the above evidence, our results support a role for the NLRP3 inflammasome in the inflammatory mechanisms leading to preterm labor/birth and adverse neonatal outcomes.
Conclusion
This study provides evidence showing that intra-amniotic inflammation triggered by a microbial product (e.g. LPS) induced the canonical activation of the NLRP3 inflammasome in the fetal membranes, as evidenced by the high amounts of the NLRP3 protein, active CASP-1, and released IL1β (Figure 8). In the decidua basalis, however, the increased amounts of the NLRP3 protein did not coincide with an increase in active form of CASP-1, suggesting that CASP-1-independent mechanisms (e.g. non-canonical activation of the NLRP3 inflammasome) participate in the processing of IL1β in this compartment (Figure 8).
Figure 8.
Conceptual framework. Intra-amniotic inflammation triggered by a microbial product (e.g. LPS) induced the canonical activation of the NLRP3 inflammasome in the fetal membranes, as evidenced by the high amounts of the NLRP3 protein, active CASP-1, and released IL1β. In the decidua basalis, however, the increased amounts of the NLRP3 protein did not coincide with an increase in active form of CASP-1, suggesting that CASP-1-independent mechanisms (e.g. non-canonical activation of the NLRP3 inflammasome) participate in the processing of IL1β in this compartment. Other pathways implicated in the processing of IL1β in the decidual tissues are also displayed.
Supplementary Material
Acknowledgements
We thank the research assistants from the PRB Perinatal Translational Science Laboratory for their help in carrying out some of the assays. We also thank Drs. Adi Tarca and Bogdan Done for their help with the mRNA expression data analysis.
Notes
Edited by Dr. Romana Nowak
Footnotes
Grant support: This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No.HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
Supplementary data
Supplemental Figure 1. Concentrations of IL1β in the amniotic fluid of mice intra-amniotically injected with LPS [94]. Dams were intra-amniotically injected with either LPS or PBS (n = 5–6 each) on 16.5 dpc. Mice were euthanized on 17.5 dpc and the amniotic fluid was collected. The ProcartaPlex Mouse Cytokine & Chemokine Panel 1A 36-plex (Invitrogen) was used to measure the concentrations of IL1β in amniotic fluid samples, according to the manufacturer's instructions. Plates were read using the Luminex 100 SystemFill (Luminex, Austin, TX, USA), and analyte concentrations were calculated with ProcartaPlex Analyst 1.0 Software from Affymetrix, San Diego, CA, USA. The sensitivity of the assay was 0.14 pg/mL (IL1β). The inter- and intra-assay coefficients of variation were less than 10%.
Supplementary Table S1. TaqMan® gene expression assays.
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1. Ferguson MG, Rhodes PG, Morrison JC, Puckett CM. Clinical amniotic fluid infection and its effect on the neonate. Am J Obstet Gynecol 1985; 151:1058–1061. [DOI] [PubMed] [Google Scholar]
- 2. Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988; 12:262–279. [PubMed] [Google Scholar]
- 3. Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am 1997; 11:135–176. [DOI] [PubMed] [Google Scholar]
- 4. Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001; 185:1130–1136. [DOI] [PubMed] [Google Scholar]
- 5. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nut Rev 2007; 65:194–202. [DOI] [PubMed] [Google Scholar]
- 6. Kemp MW. Preterm birth, intrauterine infection, and fetal inflammation. Front Immunol 2014; 5:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edey LF, O’Dea KP, Herbert BR, Hua R, Waddington SN, MacIntyre DA, Bennett PR, Takata M, Johnson MR. The local and systemic immune response to intrauterine LPS in the prepartum mouse. Biol Reprod 2016; 95:125–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keelan JA. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol 2018; 125:89–99. [DOI] [PubMed] [Google Scholar]
- 9. Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med 2010; 362:529–535. [DOI] [PubMed] [Google Scholar]
- 10. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet North Am Ed 2012; 379:2162–2172. [DOI] [PubMed] [Google Scholar]
- 11. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014; 345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430–440. [DOI] [PubMed] [Google Scholar]
- 13. Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014; 71:330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014; 72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L et al.. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2014; 24:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ et al.. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015; 28:1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 1991; 165:821–830. [DOI] [PubMed] [Google Scholar]
- 18. Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol 1994; 32:200–210. [DOI] [PubMed] [Google Scholar]
- 19. Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol 1996; 87:231–237. [DOI] [PubMed] [Google Scholar]
- 20. Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, Chaiyasit N, Yeo L, Shaman M, Lannaman K, Cher B, Hassan SS et al.. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med 2017; 45:523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H et al.. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017; 217:693.e1–693.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, Silva P, Faro J, Alhousseini A, Gill N, Hassan SS, Hsu CD. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol 2018; 79:e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990; 85:1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992; 27:117–123. [DOI] [PubMed] [Google Scholar]
- 25. Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993; 30:167–183. [DOI] [PubMed] [Google Scholar]
- 26. Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993; 81:941–948. [PubMed] [Google Scholar]
- 27. Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997; 177:19–26. [DOI] [PubMed] [Google Scholar]
- 28. Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997; 177:825–830. [DOI] [PubMed] [Google Scholar]
- 29. Arntzen KJ, Kjollesdal AM, Halgunset J, Vatten L, Austgulen R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med 1998; 26:17–26. [DOI] [PubMed] [Google Scholar]
- 30. Figueroa R, Garry D, Elimian A, Patel K, Sehgal PB, Tejani N. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med 2005; 18:241–247. [DOI] [PubMed] [Google Scholar]
- 31. Holst RM, Laurini R, Jacobsson B, Samuelsson E, Savman K, Doverhag C, Wennerholm UB, Hagberg H. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med 2007; 20:885–893. [DOI] [PubMed] [Google Scholar]
- 32. Kacerovsky M, Celec P, Vlkova B, Skogstrand K, Hougaard DM, Cobo T, Jacobsson B. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One 2013; 8:e60399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol 2015; 213:836.e1–836.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kunze M, Klar M, Morfeld CA, Thorns B, Schild RL, Markfeld-Erol F, Rasenack R, Proempeler H, Hentschel R, Schaefer WR. Cytokines in noninvasively obtained amniotic fluid as predictors of fetal inflammatory response syndrome. Am J Obstet Gynecol 2016; 215:96.e1–96.e8. [DOI] [PubMed] [Google Scholar]
- 35. Yoneda N, Yoneda S, Niimi H, Ueno T, Hayashi S, Ito M, Shiozaki A, Urushiyama D, Hata K, Suda W, Hattori M, Kigawa M et al.. Polymicrobial amniotic fluid infection with Mycoplasma/Ureaplasma and other bacteria induces severe intra-amniotic inflammation associated with poor perinatal prognosis in preterm labor. Am J Reprod Immunol 2016; 75:112–125. [DOI] [PubMed] [Google Scholar]
- 36. Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet 1986; 1:1380. [DOI] [PubMed] [Google Scholar]
- 37. Bry K, Hallman M. Prostaglandins, inflammation, and preterm labor. J Perinatol 1989; 9:60–65. [PubMed] [Google Scholar]
- 38. Mazor M, Wiznitzer A, Maymon E, Leiberman JR, Cohen A. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci 1990; 26:425–428. [PubMed] [Google Scholar]
- 39. Hsu CD, Meaddough E, Aversa K, Hong SF, Lee IS, Bahodo-Singh RO, Lu LC, Copel JA. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am J Perinatol 1998; 15:683–687. [DOI] [PubMed] [Google Scholar]
- 40. Lee SE, Park IS, Romero R, Yoon BH. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2009; 22:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maddipati KR, Romero R, Chaiworapongsa T, Zhou SL, Xu Z, Tarca AL, Kusanovic JP, Munoz H, Honn KV. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J 2014; 28:4835–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maddipati KR, Romero R, Chaiworapongsa T, Chaemsaithong P, Zhou SL, Xu Z, Tarca AL, Kusanovic JP, Gomez R, Chaiyasit N, Honn KV. Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4. FASEB J 2016; 30:3296–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park JY, Romero R, Lee J, Chaemsaithong P, Chaiyasit N, Yoon BH. An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2016; 29:2563–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997; 42:1–8. [DOI] [PubMed] [Google Scholar]
- 45. Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998; 179:194–202. [DOI] [PubMed] [Google Scholar]
- 46. Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007; 50:652–683. [DOI] [PubMed] [Google Scholar]
- 47. Lee J, Romero R, Lee KA, Kim EN, Korzeniewski SJ, Chaemsaithong P, Yoon BH. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol 2016; 214:366.e1–366.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. von Chamier M, Reyes L, Hayward LF, Brown MB. Impact of gestational nicotine exposure on intrauterine and fetal infection in a rodent model. Biol Reprod 2017; 96:1071–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Panaitescu B, Romero R, Gomez-Lopez N, Yeo L, Gotsch F. Intrauterine infection, preterm parturition, and the fetal inflammatory response syndrome. In: James D, Steer PJ, Weiner CP, Gonik B, Robson SC (eds.), High-Risk Pregnancy: Management Options. Cambridge, UK: Cambridge University Press; 2018: 579–603. [Google Scholar]
- 50. Keelan JA, Newnham JP. Editorial: Advances in the Prevention and Treatment of Inflammation-Associated Preterm Birth. Front Immunol 2016; 7:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, Hsu CD, Panaitescu B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth and adverse neonatal outcomes. Biol Reprod 2018Dec 28. doi: 10.1093/biolre/ioy264. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, Tarca AL, Abrahams VM et al.. A role for the inflammasome in spontaneous preterm labor with acute histologic chorioamnionitis. Reprod Sci 2017; 24:1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Modi BP, Teves ME, Pearson LN, Parikh HI, Haymond-Thornburg H, Tucker JL, Chaemsaithong P, Gomez-Lopez N, York TP, Romero R, Strauss JF 3rd. Mutations in fetal genes involved in innate immunity and host defense against microbes increase risk of preterm premature rupture of membranes (PPROM). Mol Genet Genomic Med 2017; 5:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Strauss JF, 3rd Romero R, Gomez-Lopez N, Haymond-Thornburg H, Modi BP, Teves ME, Pearson LN, York TP, Schenkein HA. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol 2018; 218:294–314.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS, Hsu CD. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol 2018:e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002; 10:417–426. [DOI] [PubMed] [Google Scholar]
- 57. Petrilli V, Papin S, Tschopp J. The inflammasome. Curr Biol 2005; 15:R581. [DOI] [PubMed] [Google Scholar]
- 58. Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell 2006; 126:659–662. [DOI] [PubMed] [Google Scholar]
- 59. Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol 2007; 82:259–264. [DOI] [PubMed] [Google Scholar]
- 60. Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 2007; 7:31–40. [DOI] [PubMed] [Google Scholar]
- 61. Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol 2009; 183:7623–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 2010; 22:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140:821–832. [DOI] [PubMed] [Google Scholar]
- 65. Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G. Inflammasomes as microbial sensors. Eur J Immunol 2010; 40:611–615. [DOI] [PubMed] [Google Scholar]
- 66. Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol 2011; 187:597–602. [DOI] [PubMed] [Google Scholar]
- 67. Horvath GL, Schrum JE, De Nardo CM, Latz E. Intracellular sensing of microbes and danger signals by the inflammasomes. Immunol Rev 2011; 243:119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012; 13:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol 2012; 13:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Franchi L, Nunez G. Immunology. Orchestrating inflammasomes. Science 2012; 337:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes.Nat Rev Immunol 2013; 13:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol 2015; 25:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease and therapeutics. Nat Med 2015; 21:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sharma D, Kanneganti TD. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J Cell Biol 2016; 213:617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF- B activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009; 183:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann NY Acad Sci 2014; 1319:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Franchi L, Eigenbrod T, Nunez G. Cutting Edge: TNF- Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. J Immunol 2009; 183:792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J et al.. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 1992; 356:768–774. [DOI] [PubMed] [Google Scholar]
- 79. Black RA, Kronheim SR, Merriam JE, March CJ, Hopp TP. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem 1989; 264:5323–5326. [PubMed] [Google Scholar]
- 80. Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci 1989; 86:5227–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA et al.. Molecular cloning of the interleukin-1 beta converting enzyme.Science 1992; 256:97–100. [DOI] [PubMed] [Google Scholar]
- 82. Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T et al.. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 1997; 275:206–209. [DOI] [PubMed] [Google Scholar]
- 83. Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R et al.. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997; 386:619–623. [DOI] [PubMed] [Google Scholar]
- 84. Dinarello CA. Interleukin-1beta, interleukin-18, and the interleukin-1beta converting Enzymea. Annals NY Acad Sci 1998; 856:1–11. [DOI] [PubMed] [Google Scholar]
- 85. Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol 1999; 19:1–11. [DOI] [PubMed] [Google Scholar]
- 86. Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri–induced inflammation. Immunity 2000; 12:581–590. [DOI] [PubMed] [Google Scholar]
- 87. Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF- B-driven protein synthesis. J Immunol 2005; 175:7611–7622. [DOI] [PubMed] [Google Scholar]
- 88. Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol 2015; 33:49–77. [DOI] [PubMed] [Google Scholar]
- 89. Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, Tarca AL, Abrahams VM et al.. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol 2018; 79:e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gomez-Lopez N, Romero R, Xu Y, Garcia-Flores V, Leng Y, Panaitescu B, Miller D, Abrahams VM, Hassan SS. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol 2017; 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Panaitescu B, Romero R, Gomez-Lopez N, Xu Y, Leng Y, Maymon E, Pacora P, Erez O, Yeo L, Hassan SS, Hsu CD. In vivo evidence of inflammasome activation during spontaneous labor at term. J Matern Fetal Neonatal Med 2018; 17:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG, Chaemsaithong P, Chaiworapongsa T, Dong Z, Tarca AL, Abrahams VM, Yeo L et al.. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci 2017; 24:934–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gomez-Lopez N, Romero R, Arenas-Hernandez M, Panaitescu B, Garcia-Flores V, Mial TN, Sahi A, Hassan SS. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med 2018; 31:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, Hassan SS, Alvarez-Salas LM et al.. Inflammation-induced adverse pregnancy and neonatal outcomes can be improved by the immunomodulatory peptide exendin-4. Front Immunol 2018; 9:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS et al.. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015; 21:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008; 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 97. van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol 2011; 32:110–116. [DOI] [PubMed] [Google Scholar]
- 98. Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003; 6:435–448. [DOI] [PubMed] [Google Scholar]
- 99. Heller DS, Rimpel LH, Skurnick JH. Does histologic chorioamnionitis correspond to clinical chorioamnionitis? J Reprod Med 2008; 53:25–28. [PubMed] [Google Scholar]
- 100. Toti P, Arcuri F, Tang Z, Schatz F, Zambrano E, Mor G, Niven-Fairchild T, Abrahams VM, Krikun G, Lockwood CJ, Guller S. Focal increases of fetal macrophages in placentas from pregnancies with histological chorioamnionitis: potential role of fibroblast monocyte chemotactic protein-1. Am J Reprod Immunol 2011; 65:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Benirschke K, Burton G, Baergen R. Infectious diseases. In: Pathology of the Human Placenta. Berlin: Springer Berlin Heidelberg; 2012: 557–655. [Google Scholar]
- 102. Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med 2012; 17:20–25. [DOI] [PubMed] [Google Scholar]
- 103. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015; 213:S29–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Redline RW. Classification of placental lesions. Am J Obstet Gynecol 2015; 213:S21–S28. [DOI] [PubMed] [Google Scholar]
- 105. Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S et al.. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008; 21:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989; 160:1117–1123. [DOI] [PubMed] [Google Scholar]
- 107. Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol 2000; 183:1138–1143. [DOI] [PubMed] [Google Scholar]
- 108. Jacobsson B, Holst RM, Mattsby-Baltzer I, Nikolaitchouk N, Wennerholm UB, Hagberg H. Interleukin-18 in cervical mucus and amniotic fluid: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation and preterm delivery. BJOG 2003; 110:598–603. [PubMed] [Google Scholar]
- 109. Bakele M, Joos M, Burdi S, Allgaier N, Poschel S, Fehrenbacher B, Schaller M, Marcos V, Kummerle-Deschner J, Rieber N, Borregaard N, Yazdi A et al.. Localization and functionality of the inflammasome in neutrophils. J Biol Chem 2014; 289:5320–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004; 20:319–325. [DOI] [PubMed] [Google Scholar]
- 111. Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 2014; 505:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 2006; 281:36560–36568. [DOI] [PubMed] [Google Scholar]
- 113. Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS, Brown EJ. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol 2008; 10:1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 2008; 452:103–107. [DOI] [PubMed] [Google Scholar]
- 115. Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009; 30:556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol 2009; 182:6460–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 2009; 183:3578–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 2012; 150:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Clay GM, Sutterwala FS, Wilson ME. NLR proteins and parasitic disease.Immunol Res 2014; 59:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237–241. [DOI] [PubMed] [Google Scholar]
- 121. Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006; 440:228–232. [DOI] [PubMed] [Google Scholar]
- 122. Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008; 9:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008; 320:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci 2008; 105:9035–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yamasaki K, Muto J, Taylor KR, Cogen AL, Audish D, Bertin J, Grant EP, Coyle AJ, Misaghi A, Hoffman HM, Gallo RL. NLRP3/Cryopyrin is necessary for interleukin-1β (IL-1β) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem 2009; 284:12762–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cassel SL, Joly S, Sutterwala FS. The NLRP3 inflammasome: a sensor of immune danger signals. Semin Immunol 2009; 21:194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cassel SL, Sutterwala FS. Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol 2010; 40:607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev 2011; 243:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lappas M. Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1β secretion. Am J Reprod Immunol 2014; 71:189–201. [DOI] [PubMed] [Google Scholar]
- 130. Brickle A, Tran HT, Lim R, Liong S, Lappas M. Autophagy, which is decreased in labouring fetal membranes, regulates IL-1β production via the inflammasome. Placenta 2015; 36:1393–1404. [DOI] [PubMed] [Google Scholar]
- 131. Cross SN, Potter JA, Aldo P, Kwon JY, Pitruzzello M, Tong M, Guller S, Rothlin CV, Mor G, Abrahams VM. Viral infection sensitizes human fetal membranes to bacterial lipopolysaccharide by MERTK inhibition and inflammasome activation. J Immunol 2017; 199:2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Willcockson AR, Nandu T, Liu CL, Nallasamy S, Kraus WL, Mahendroo M. Transcriptome signature identifies distinct cervical pathways induced in lipopolysaccharide-mediated preterm birth. Biol Reprod 2018; 98:408–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, Dong Z, Hassan SS, Gomez-Lopez N. HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the inflammasome. Biol Reprod 2016; 95:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Marques LM, Rezende IS, Barbosa MS, Guimaraes AM, Martins HB, Campos GB, do Nascimento NC, Dos Santos AP, Amorim AT, Santos VM, Farias ST, Barrence FA et al.. Ureaplasma diversum genome provides new insights about the interaction of the surface molecules of this bacterium with the host. PLoS One 2016; 11:e0161926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Vick EJ, Park HS, Huff KA, Brooks KM, Farone AL, Farone MB. Gardnerella vaginalis triggers NLRP3 inflammasome recruitment in THP-1 monocytes. J Reprod Immunol 2014; 106:67–75. [DOI] [PubMed] [Google Scholar]
- 136. Whidbey C, Vornhagen J, Gendrin C, Boldenow E, Samson JM, Doering K, Ngo L, Ezekwe EA Jr., Gundlach JH, Elovitz MA, Liggitt D, Duncan JA et al.. A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO Molecular Medicine 2015; 7:488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 1982; 145:1–8. [DOI] [PubMed] [Google Scholar]
- 138. Gibbs RS, Weiner MH, Walmer K, St Clair PJ. Microbiologic and serologic studies of Gardnerella vaginalis in intra-amniotic infection. Obstet Gynecol 1987; 70:187–190. [PubMed] [Google Scholar]
- 139. Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989; 161:817–824. [DOI] [PubMed] [Google Scholar]
- 140. Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH et al.. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015; 43:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Potts LC, Feng L, Seed PC, Jayes FL, Kuchibhatla M, Antczak B, Nazzal MK, Murtha AP. Inflammatory response of human gestational membranes to ureaplasma parvum using a novel dual-chamber tissue explant system1. Biol Reprod 2016; 94:119. [DOI] [PubMed] [Google Scholar]
- 142. Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B, Gomez-Lopez N, Romero R, Yoon BH. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol 2017; 216:604.e1–604.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet Gynecol 1989; 73:31–34. [PubMed] [Google Scholar]
- 144. Liang L, Kover K, Dey SK, Andrews GK. Regulation of interleukin-6 and interleukin-1β gene expression in the mouse deciduum. J Reprod Immunol 1996; 30:29–52. [DOI] [PubMed] [Google Scholar]
- 145. Ammala M, Nyman T, Salmi A, Rutanen EM. The interleukin-1 system in gestational tissues at term: effect of labour. Placenta 1997; 18:717–723. [DOI] [PubMed] [Google Scholar]
- 146. Ibrahim SA, Ackerman WEt, Summerfield TL, Lockwood CJ, Schatz F, Kniss DA. Inflammatory gene networks in term human decidual cells define a potential signature for cytokine-mediated parturition. Am J Obstet Gynecol 2016; 214:284.e1–284.e47. [DOI] [PubMed] [Google Scholar]
- 147. Pontillo A, Girardelli M, Agostinis C, Masat E, Bulla R, Crovella S. Bacterial LPS differently modulates inflammasome gene expression and IL-1β secretion in trophoblast cells, decidual stromal cells, and decidual endothelial cells. Reprod Sci 2013; 20:563–566. [DOI] [PubMed] [Google Scholar]
- 148. Masumoto J, Taniguchi S, Ayukawa K, Sarvotham H, Kishino T, Niikawa N, Hidaka E, Katsuyama T, Higuchi T, Sagara J. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem 1999; 274:33835–33838. [DOI] [PubMed] [Google Scholar]
- 149. Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 2007; 14:1590–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gomez-Lopez N, Romero R, Xu Y, Garcia-Flores V, Leng Y, Panaitescu B, Miller D, Abrahams VM, Hassan SS. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol 2017; 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP et al.. Non-canonical inflammasome activation targets caspase-11. Nature 2011; 479:117–121. [DOI] [PubMed] [Google Scholar]
- 152. Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 2012; 490:288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem 1990; 265:6318–6322. [PubMed] [Google Scholar]
- 154. Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med 1991; 174:821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 1998; 161:3340–3346. [PubMed] [Google Scholar]
- 156. Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A 1999; 96:6261–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kono H, Orlowski GM, Patel Z, Rock KL. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J immunol 2012; 189:3734–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA et al.. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J immunol 2010; 184:3326–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K. Noncanonical inflammasome signaling elicits gasdermin D–dependent neutrophil extracellular traps. Sci Immunol 2018; 3. [DOI] [PubMed] [Google Scholar]
- 160. Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Kruger R, Herzig A et al.. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 2018; 3. [DOI] [PubMed] [Google Scholar]
- 161. Gomez-Lopez N, Romero R, Leng Y, Garcia-Flores V, Xu Y, Miller D, Hassan SS. Neutrophil extracellular traps in acute chorioamnionitis: A mechanism of host defense. Am J Reprod Immunol 2017; 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Marchetti C, Chojnacki J, Toldo S, Mezzaroma E, Tranchida N, Rose SW, Federici M, Van Tassell BW, Zhang S, Abbate A. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia–reperfusion in the mouse. J Cardiovasc Pharmacol 2014; 63:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zhang Y, Liu L, Peng YL, Liu YZ, Wu TY, Shen XL, Zhou JR, Sun DY, Huang AJ, Wang X, Wang YX, Jiang CL. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther 2014; 20:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Song C, He L, Zhang J, Ma H, Yuan X, Hu G, Tao L, Meng J. Fluorofenidone attenuates pulmonary inflammation and fibrosis via inhibiting the activation of NALP3 inflammasome and IL-1beta/IL-1R1/MyD88/NF-kappaB pathway. J Cell Mol Med 2016; 20:2064–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Primiano MJ, Lefker BA, Bowman MR, Bree AG, Hubeau C, Bonin PD, Mangan M, Dower K, Monks BG, Cushing L, Wang S, Guzova J et al.. Efficacy and pharmacology of the NLRP3 inflammasome inhibitor CP-456,773 (CRID3) in murine models of dermal and pulmonary inflammation. J Immunol 2016; 197:2421–2433. [DOI] [PubMed] [Google Scholar]
- 166. Wang Y, Yan X, Mi S, Li Z, Zhu H, Sun X, Zhao B, Zhao C, Zou Y, Hu K, Ding X, Sun A et al.. Naoxintong attenuates Ischaemia/reperfusion Injury through inhibiting NLRP3 inflammasome activation. J Cell Mol Med 2017; 21:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Fusco R, Gugliandolo E, Biundo F, Campolo M, Di Paola R, Cuzzocrea S. Inhibition of inflammasome activation improves lung acute injury induced by carrageenan in a mouse model of pleurisy. FASEB J 2017; 31:3497–3511. [DOI] [PubMed] [Google Scholar]
- 168. Zou P, Liu X, Li G, Wang Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Molecular medicine reports 2018; 17:3212–3217. [DOI] [PubMed] [Google Scholar]
- 169. Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 2018; 17:688–688. [DOI] [PubMed] [Google Scholar]
- 170. van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slutter B, Foks AC, Bot I, Kuiper J. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E–deficient mice—brief report. Arterioscler Thromb Vasc Biol 2017; 37:1457–1461. [DOI] [PubMed] [Google Scholar]
- 171. Dolunay A, Senol SP, Temiz-Resitoglu M, Guden DS, Sari AN, Sahan-Firat S, Tunctan B. Inhibition of NLRP3 inflammasome prevents LPS-induced inflammatory hyperalgesia in mice: contribution of NF-κB, caspase-1/11, ASC, NOX, and NOS isoforms. Inflammation 2017; 40:366–386. [DOI] [PubMed] [Google Scholar]
- 172. van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SC, Robertson AA, Pasterkamp G, Hoefer IE. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J 2017; 38:828–836. [DOI] [PubMed] [Google Scholar]
- 173. Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson AAB, Schroder K, Kunde D, Eri R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep 2018; 8:8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zhai Y, Meng X, Ye T, Xie W, Sun G, Sun X. Inhibiting the NLRP3 inflammasome activation with MCC950 ameliorates diabetic encephalopathy in db/db mice. Molecules 2018; 23:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ismael S, Nasoohi S, Ishrat T. MCC950, the selective inhibitor of nucleotide oligomerization domain-like receptor protein-3 inflammasome, protects mice against traumatic brain injury. J Neurotrauma 2018; 35:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Xu X, Yin D, Ren H, Gao W, Li F, Sun D, Wu Y, Zhou S, Lyu L, Yang M, Xiong J, Han L et al.. Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol Dis 2018; 117:15–27. [DOI] [PubMed] [Google Scholar]
- 177. Ismael S, Zhao L, Nasoohi S, Ishrat T. Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep 2018; 8:5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ren H, Kong Y, Liu Z, Zang D, Yang X, Wood K, Li M, Liu Q. Selective NLRP3 (Pyrin Domain–Containing Protein 3) inflammasome inhibitor reduces brain injury after intracerebral hemorrhage. Stroke 2018; 49:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Xu KY, Wu CY, Tong S, Xiong P, Wang SH. The selective Nlrp3 inflammasome inhibitor Mcc950 attenuates lung ischemia-reperfusion injury. Biochem Biophys Res Commun 2018; 503:3031–3037. [DOI] [PubMed] [Google Scholar]
- 180. Marchetti C, Swartzwelter B, Gamboni F, Neff CP, Richter K, Azam T, Carta S, Tengesdal I, Nemkov T, D’Alessandro A, Henry C, Jones GS et al.. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci USA 2018; 115:E1530–E1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W et al.. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med 2017; 214:3219–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Coates BM, Staricha KL, Ravindran N, Koch CM, Cheng Y, Davis JM, Shumaker DK, Ridge KM. Inhibition of the NOD-like receptor protein 3 inflammasome is protective in juvenile influenza A virus infection. Front Immunol 2017; 8:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Geppert TD, Whitehurst CE, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med 1994; 1:93–103. [PMC free article] [PubMed] [Google Scholar]
- 184. Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krull M, Eichel-Streiber C, Goebeler M, Ludwig S, Suttorp N. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood 2000; 95:3044–3051. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.