Abstract
Objective
C57BL/6J mice infected with Theiler's murine encephalomyelitis virus (TMEV) develop acute behavioral seizures in the first week of infection and later develop chronic epilepsy. The TMEV model provides a useful platform to test novel antiseizure therapeutics. The present study was designed to test the efficacy of cannabidiol (CBD) in reducing acute seizures induced by viral infection.
Methods
C57BL/6J mice were infected intracortically with 2 × 105 plaque‐forming units of TMEV. Mice were divided into two treatment groups—1) CBD‐treated mice and 2) vehicle‐treated mice. Frequency and severity of acute seizures were evaluated by video‐monitoring the mice four times daily by the experimenter blinded to the treatment group.
Results
Cannabidiol (180 mg/kg; 360 mg/kg/day) decreased both the frequency and severity of acute behavioral seizures following TMEV infection, but 150 mg/kg of CBD did not improve overall seizure outcome. The time to peak effect (TPE) of CBD in the 6 Hz 32 mA psychomotor seizure test using C57BL/6J mice was observed at 2 hours post‐CBD treatment. Interestingly, CBD (150 mg/kg) significantly reduced frequency and severity of TMEV‐induced acute seizures at 2 hours post‐CBD treatment. These results suggest that CBD could be effective in decreasing TMEV‐induced acute seizures when the seizure test is conducted at the TPE of CBD.
Significance
Cannabinoids are increasingly studied for their potential antiseizure effects. Several preclinical and clinical studies provide evidence that CBD could be an effective therapy for intractable epilepsies. The present study corroborates those previous findings and provides an opportunity to investigate pharmacokinetics, pharmacodynamics, and mechanism(s) of antiseizure effects of CBD in the TMEV model, which may help to design future clinical studies more effectively.
Keywords: animal model, cannabidiol, infection, seizures, TMEV
Key Points.
High dose (180 mg/kg) of cannabidiol (CBD) is effective in reducing acute behavioral seizures induced by TMEV infection in C57BL/6J mice.
The time to peak effect of CBD is at 2 hours posttreatment in C57BL/6J mice based on the 6 Hz 32 mA psychomotor seizure test.
Lower dose of CBD (150 mg/kg) does not affect overall TMEV‐induced acute seizures; but decreases frequency and severity of seizures when evaluated at 2 hours post‐CBD treatment.
The TMEV model of infection‐induced epilepsy is a useful tool to test the efficacy of novel antiseizure treatments
1. INTRODUCTION
Mice infected with the Daniels strain of Theiler's murine encephalomyelitis virus (TMEV) represent a novel animal model of acquired limbic epilepsy caused by CNS infection. C57BL/6J mice infected intracortically with TMEV develop acute behavioral seizures between 3 and 7 days postinfection (dpi). These mice survive the infection, clear the virus by 14 dpi, show no behavioral seizures during the latent period, and, later on, develop chronic spontaneous seizures after about 2 months postinfection.1, 2, 3, 4 The histopathological and biochemical changes in the hippocampus, notably pyramidal cell loss in the cornu ammonis 1 (CA1) region and scar formation (while largely sparing the CA3 region and the dentate gyrus), microgliosis, astrogliosis, inflammation, and oxidative stress, are observed during the acute seizure period.2, 4, 5, 6, 7, 8 TMEV‐infected mice with seizures also have behavioral comorbidities such as impaired cognitive functions and anxiety‐like behavior.9 Thus, the pattern of acute seizures and epilepsy development, the pathological changes, and the inflammatory conditions in the TMEV‐infected mouse brain recapitulate clinical observations from patients suffering from infection‐induced temporal lobe epilepsy (TLE).10, 11 Therefore, the TMEV‐induced mouse model of TLE provides a unique opportunity to study the molecular changes occurring in the brain to better understand epileptogenesis and to design therapeutic interventions that potentially prevent the development of disease.
Epilepsy remains a serious neurological disorder despite the availability of many antiseizure drugs (ASDs), as about 30% of epilepsy patients are pharmacoresistant to treatment. This unmet medical need necessitates research in discovering novel treatment to control seizures. Cannabidiol (CBD), a nonpsychogenic phytoconstituent of the Cannabis sativa plant, has attracted clinical interest for the treatment of several treatment refractory epilepsies.12 The results from the phase 3 randomized double‐blinded placebo‐controlled clinical trials of CBD found significant reduction in convulsive seizure frequency in the patients with two rare and severe forms of epilepsy—Dravet syndrome and Lennox‐Gastaut syndrome.13, 14 Based on these results, the US Food and Drug Administration recently approved Epidiolex® (highly purified CBD oral solution) for the treatment of seizures associated with both of these syndromes. These results warrant further studies to test the efficacy of CBD in other types of genetic and acquired epilepsies.
The present studies were aimed to test the efficacy of CBD in preventing acute behavioral seizures in the TMEV model. CBD (180 mg/kg twice daily; 360 mg/kg/day) significantly reduced frequency and severity of TMEV‐induced acute seizures. Although a lower dose of CBD (150 mg/kg) had no effect on acute seizures overall, it decreased frequency and severity of seizures at 2 hours following CBD treatment, but not at 9 hours posttreatment. The time to peak effect (TPE) of CBD in the 6 Hz 32 mA psychomotor seizure test was observed at 2 hours post‐CBD treatment, which may explain the effectiveness of CBD (150 mg/kg) in suppressing acute seizures at 2 hours post‐CBD treatment. The results reported herein suggest that CBD may prove to be efficacious in this model of infection‐induced seizures.
2. METHODS
2.1. Animals
Male C57BL/6J mice (#006460) aged 5‐6 weeks were purchased from Jackson Laboratory. After arrival, mice were allowed to acclimatize for at least 3 days prior to the experiment. Mice were housed in groups, provided food and water ad libitum, and kept in a facility providing a 12 hours light/dark cycle starting at 6 am. All the procedures performed were in accordance with the guidelines provided and approved by the Institutional Animal Care and Use Committee of the University of Utah. The University of Utah is registered with the United States Department of Agriculture and the United States Public Health Service. In addition, the University of Utah animal care and use programs and facilities are accredited by Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
2.2. Method of TMEV infection and seizure monitoring
Mice are briefly anesthetized with isoflurane and injected with 20 µL of either phosphate‐buffered saline (PBS) or 2 × 105 PFU (plaque‐forming units) DA‐TMEV solution intracortically in the right hemisphere by inserting the needle at a 90° angle to the skull. The injection region is located slightly medial to the equidistant point on the imaginary line connecting the eye and the ear. A sterilized syringe containing a plastic jacket on the needle exposing 2.5 mm of needle is used for infection to restrict the injection site to the somatosensory cortex.
Acute behavioral seizures were induced by briefly agitating the mice by shaking their cages, and the seizures were video‐monitored four times daily between 2 and 8 days postinfection (dpi). Seizure intensity was graded using modified Racine scale as follows: Stage 1—mouth and facial movements; Stage 2—head nodding; Stage 3—forelimb clonus; Stage 4—forelimb clonus, rearing; Stage 5—forelimb clonus, rearing, and falling; and Stage 6—intense running, jumping, repeated falling, and severe clonus.15 Seizure scores were assigned after verifying seizure video recordings by an investigator blinded to the treatment groups. Seizure frequency was reported as the average number of seizures during the entire acute seizure period, whereas seizure severity/intensity was represented as the average cumulative seizure burden at each dpi during the acute seizure period. Cumulative seizure burden at each dpi for a mouse was calculated by summing all of the seizure scores up to that dpi.
2.3. CBD treatment
Cannabidiol was received from the National Institute on Drug Abuse (NIDA). Since CBD does not dissolve in aqueous solvents, it was formulated as an oil‐in‐water emulsion using a mixture of 100% Ethanol, Kolliphor®, and 0.9% saline (1:1:18). CBD was first dissolved in 100% ethanol, and equal amount of Kolliphor® (polyoxyl castor oil, BASF) was added. The mixture was vortexed, and a small amount of 0.9% saline was added gradually while vortexing the mixture vigorously after every addition to make an emulsion. The mixture of CBD in 100% ethanol and Kolliphor® appears transparent, and after the addition of small amount of 0.9% saline, the emulsion appears milky. The emulsion was made fresh just before each treatment time point, kept on ice, and used within 20 minutes of preparation. It was vortexed immediately before injecting each mouse i.p. at 10 mL/kg. Mice in the control group were treated with a vehicle solution prepared similarly by mixing 100% Ethanol, Kolliphor®, and 0.9% saline (1:1:18).
2.4. 6 Hz psychomotor seizure test
6 Hz psychomotor seizure test was conducted as described before16 to determine the TPE of CBD. C57BL/6J mice were treated with CBD (50 mg/kg, i.p.), and corneal stimulation was applied at 0.5, 1, 2, and 4 hours posttreatment in a different group of mice. An electrolyte solution containing 0.5% tetracaine as topical anesthetic agent was applied to the eyes before corneal stimulation (6 Hz, 0.2 ms rectangular pulse width, 3 seconds duration) at 32 mA current intensity (1.5× of current intensity that elicits convulsive seizures in 97% of mice). The seizure was characterized by an initial momentary stun followed immediately by forelimb clonus, twitching of the vibrissae, and Straub tail. Mice without seizure were counted as “protected” mice, and the time point post‐CBD treatment where maximum number of mice was found to be “protected” was considered the TPE of CBD.
2.5. Statistics
The dataset involving continuous variables is represented by mean and the standard error of the mean (SEM), and the dataset with ordinal variables is presented as a frequency distribution. Seizure frequency data are represented as box and whisker plots. The central lines and plus signs within the box and whisker plot represent medians and means, respectively; the two ends of the rectangles represent first and third quartiles. The upper and lower whiskers extend to the highest and lowest values in the dataset, respectively. Each filled circle in the box and whisker plot represents an individual mouse. Experimental design involving two groups with one continuous dependent variable was analyzed by unpaired two‐tailed t test or Welch's t test, whereas design involving two categorical independent variables and one continuous dependent variable was analyzed by repeated measures two‐way ANOVA. Cumulative seizure burden, which was calculated from a ranked dataset, was analyzed by the Scheirer‐Ray‐Hare test, which is an extension of Kruskal‐Wallis test for two randomized factorial designs.17 The statistical calculations were conducted using GraphPad Prism® 7 and Microsoft Excel.
3. RESULTS
3.1. CBD (180 mg/kg) effectively reduces TMEV‐induced acute seizure frequency and intensity
Previously published work from our group has demonstrated that the ED50 (95% confidence interval) for CBD in preventing 6 Hz psychomotor seizures induced at the 44 mA intensity in male CF1 mice was 164 mg/kg (124‐200).18 Therefore, we chose a slightly higher initial dose (180 mg/kg twice daily; 360 mg/kg/day) to determine the antiseizure potential of CBD in the TMEV infection–induced model of epilepsy.
We started to test whether CBD is efficacious in reducing TMEV‐induced seizures using a prophylactic approach. Male C57BL6/J mice were treated with 180 mg/kg CBD i.p. every 12 hours (7 am and 7 pm; 360 mg/kg/day) starting two days prior to TMEV infection for a total of 10 days (Figure 1A). Acute seizures were video‐monitored four times daily (twice during CBD treatment, and at 4 and 9 hours post‐CBD treatment), and the severity of seizures was assessed using a modified Racine seizure scale from stage 3 to 6 as described in Methods and represented as a heat map in Figure 1B. All TMEV‐infected mice in the vehicle treatment group developed acute seizures, whereas 3 mice in the CBD group did not have seizures. CBD‐treated mice had a dramatic reduction in the average number of seizures that occurred over the acute seizure period (Figure 1C, upper panel; CBD: 1.5 ± 0.40, Vehicle: 8.3 ± 0.73, n = 10, p < 0.0001) and seizure severity between 4 and 8 dpi compared to vehicle‐treated mice (Figure 1C, lower panel). Antiseizure effects of CBD were observed both at 4 hours (Figure 1D) and at 9 hours post‐CBD treatment (Figure 1E). Although 70% of CBD‐treated mice had at least one behavioral seizure, CBD treatment delayed the development of seizures and significantly prolonged the seizure freedom period (P < 0.0001, log‐rank test; Figure 1F).
Figure 1.
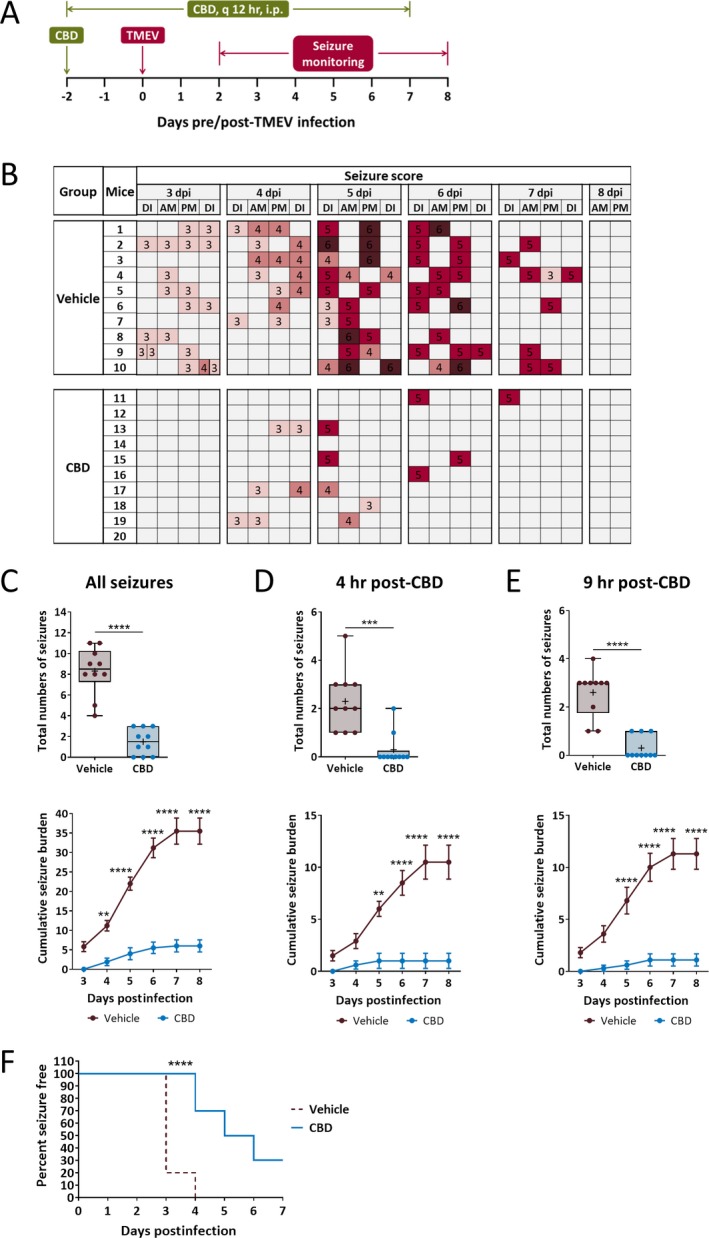
Prophylactic treatment with 180 mg/kg cannabidiol (CBD) reduces average frequency and severity of TMEV‐induced acute seizures. A, Outline of prophylactic CBD treatment regimen. B, Acute seizures were induced by handling the mice four times a day—during CBD/vehicle injections (7 am, 7 pm) and during seizure monitoring (11 am, 4 pm). Seizure severity was video‐monitored with the experimenter blinded to the treatment groups, scored based on a modified Racine scale ranging from stage 3 to 6, and depicted as a heat map (DI—during injection, dpi—days postinfection). C‐E, Prophylactic CBD treatment significantly reduces seizure frequency plotted as total numbers of seizures per mouse (upper panel) and seizure severity as measured by average cumulative seizure burden (lower panel) of all seizures observed during acute seizure period (3‐8 dpi) (C). Antiseizure effect of CBD was observed at 4 h (D) and also at 9 h (E) post‐CBD treatment. F, Development of the first acute seizure is significantly delayed in CBD‐treated mice compared to vehicle‐treated mice. Statistics: unpaired t test (seizure frequency), Scheirer‐Ray‐Hare test and Sidak's multiple comparison test (seizure severity), log‐rank test (% seizure‐free), ** P < 0.01, **** P < 0.0001, n = 10 mice per group.
After observing antiseizure effects of CBD under the prophylactic treatment regimen, we tested the efficacy of CBD in suppressing TMEV‐induced acute seizures under therapeutic treatment regimen. Since a significant portion of TMEV‐infected mice begins to develop acute seizures on 3 dpi, we started CBD treatment on 3 dpi and continued through 7 dpi (Figure 2A). As in the prophylactic study, CBD (180 mg/kg) was administered at 7 am and 7 pm and seizures were evaluated at 4 and 9 hours post‐CBD treatment in the morning. The seizure scores for all the mice are presented in the heat map (Figure 2B). In both treatment groups, mice had similar numbers of seizures on 3 dpi. However, the seizure frequency and severity were dramatically reduced in the CBD group at 4 dpi and these effects were continued during the remaining period of CBD treatment. Average number of seizures was reduced by 63% in CBD‐treated mice compared to vehicle‐treated mice (Figure 2C, upper panel; CBD: 2.5 ± 0.60, Vehicle: 6.7 ± 0.82, n = 10, P = 0.0006). In addition, seizure severity was also significantly decreased in the CBD‐treated mice between 5 and 8 dpi (Figure 2C, lower panel). Antiseizure effects of CBD were observed both at 4 hours (Figure 2D) and at 9 hours post‐CBD treatment (Figure 2E). In contrast to the prophylactic treatment regimen, analysis of seizure freedom over time postinfection found no difference between both the groups in this treatment regimen (P = 0.3751, log‐rank test) since the majority of the mice in both the groups developed seizures within 3‐4 dpi (Figure 2F).
Figure 2.
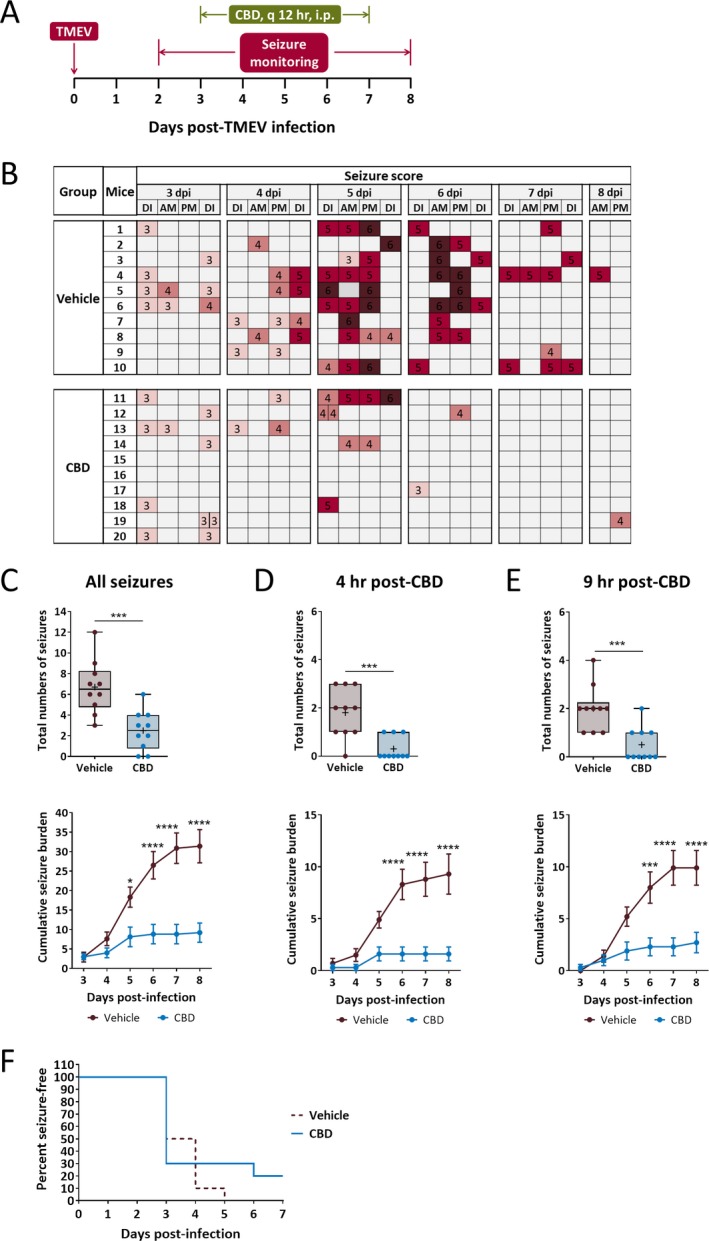
Therapeutic treatment with cannabidiol (CBD) (180 mg/kg) reduces average frequency and severity of TMEV‐induced acute seizures. A, Outline of therapeutic CBD treatment regimen. B, Acute seizures were induced by handling the mice four times a day—during CBD/vehicle injections (7 am, 7 pm) and during seizure monitoring (11 am, 4 pm). Seizure severity was video‐monitored with the experimenter blinded to the treatment groups, categorized based on modified Racine scale ranging from stage 3 to 6, and depicted as a heat map (DI—during injection, dpi—days postinfection). C‐E, Therapeutic CBD treatment significantly reduces seizure frequency plotted as total numbers of seizures per mouse (upper panel) and seizure severity as measured by average cumulative seizure burden (lower panel) of all seizures observed during acute seizure period (3‐8 dpi) (C). Antiseizure effect of CBD was observed both at 4 h (D) and at 9 h (E) post‐CBD treatment. F, Development of the first seizure is not significantly different between treatment groups. Statistics: unpaired t test (seizure frequency), Scheirer‐Ray‐Hare test and Sidak's multiple comparison test (seizure severity), log‐rank test (% seizure‐free), * P < 0.05, *** P < 0.001, **** P < 0.0001, n = 10 mice per group.
Although CBD (180 mg/kg) was efficacious in reducing TMEV‐induced seizures, mice treated with this dose of CBD experienced significant loss of weight starting from 4 dpi through 9 dpi compared to TMEV‐infected mice that received vehicle treatment (Figure 3A). The weight of TMEV‐infected mice during the acute seizure period is often significantly lower than the noninfected mice1 as also observed in this study (Figure 3A). However, CBD (180 mg/kg)‐treated mice lost a greater amount of weight than vehicle‐treated infected mice and the weight loss was coincident with the CBD treatment period, as mice started to recover after 8 dpi. CBD (180 mg/kg)‐treated mice were also observed to have hunched posture, hypolocomotion, piloerection, and ruffled fur, which may suggest a hypothermic effect of the treatment. Hypothermia can contribute to the reduction in seizures and has been used clinically to control seizures associated with refractory status epilepticus.19 CBD at high doses may cause hypothermia, and it is possible that CBD indirectly reduced TMEV‐induced seizures by causing hypothermia in mice. To test this idea, we measured body temperature of CBD (180 mg/kg)‐treated mice (same dosing regimen as used in Figures 1 and 2) up to 4 hours post‐CBD treatment in the morning from 3 to 7 dpi by a rectal temperature probe. As shown in Figure 3B, average body temperature measured immediately before CBD treatment (0 hour) ranged from 37.5 to 38.3°C. On 3 dpi, the average body temperature was reduced by 3.4°C from the baseline at 1 and 2 hours post‐CBD treatment (Figure 3C) and that could be considered mildly hypothermic; however, the body temperature quickly recovered partially by 4 hours post‐CBD treatment. While a reduction in average body temperature of 1.2‐2.1°C occurred at 2 hours post‐CBD treatment every day from 4 to 7 dpi (Figure 3C), it is not generally considered to be hypothermic.20 In contrast, seizure frequency and severity were similar between CBD (180 mg/kg)‐treated and vehicle‐treated mice on 3 dpi, but significantly reduced during 4‐7 dpi (Figure 2B). Thus, no obvious correlation between core body temperature and seizures in CBD‐treated mice was found, suggesting that mild hypothermia after the first dose of 180 mg/kg CBD may not be a major factor that likely contributed to the observed decrease in acute seizures.
Figure 3.
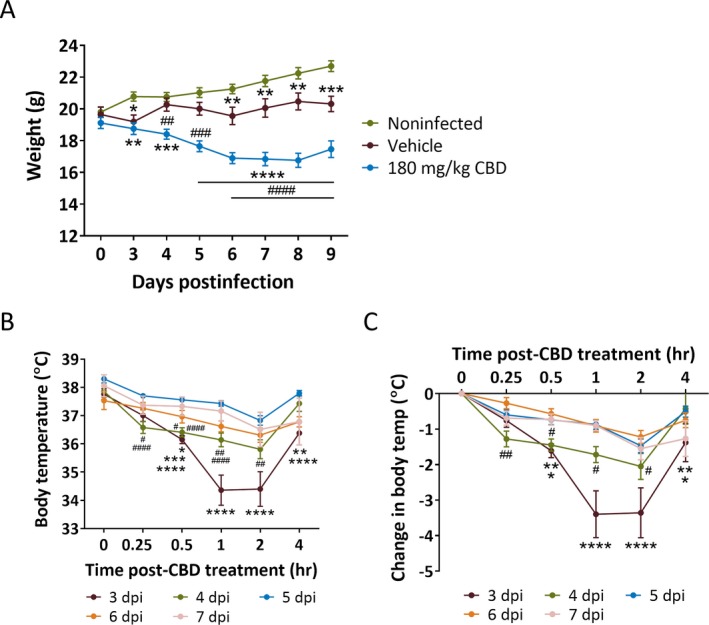
Significant weight loss during acute seizure period and moderate hypothermia at 3 d post‐TMEV infection in 180 mg/kg CBD‐treated mice. A, Average weight of mice each day during the acute infection period shows a significant reduction in TMEV‐infected mice (2 × 105 PFU/mouse) treated with 180 mg/kg CBD (i.p. every 12 h for 5 d starting at 3 dpi) compared to their vehicle‐treated counterparts between 4 and 9 dpi. TMEV‐infected mice in both CBD and vehicle treatment groups had significantly lower weight compared to noninfected mice. B, The body temperature of 180 mg/kg CBD‐treated mice as measured immediately before CBD treatment (0 h) and at 0.25, 0.5, 1, 2, and 4 h posttreatment each day in the morning during 3‐7 dpi shows a significant reduction on 3 dpi compared to other days. C, The change in body temperature in 180 mg/kg CBD‐treated mice shows a maximum decrease of 3.4 and 2.1°C at 2 h post‐CBD treatment on 3 and 4 dpi, respectively. Statistics: repeated measures two‐way ANOVA, Tukey's multiple comparison test, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, # P < 0.05, ## P < 0.01, ### P < 0.001, #### P < 0.0001, n = 10 mice per group. Asterisk and number signs indicate comparison between 180 mg/kg CBD group to noninfected group and to vehicle group, respectively, in (A), whereas in (B) and (C), asterisk signs indicate comparisons between 3 dpi and 4‐7 dpi, and the number signs indicate comparisons between 4 dpi and 5‐7 dpi.
3.2. CBD (150 mg/kg) reduces TMEV‐induced acute seizures at 2 and 4 hours, but not at 9 hours, posttreatment
Although CBD (180 mg/kg) was efficacious in reducing TMEV‐induced acute seizures, it caused significant weight loss and other adverse effects (hunched posture, hypolocomotion, piloerection, and ruffled fur), which may suggest that mice were overdosed by 180 mg/kg of CBD. This was unexpected because these adverse effects were not observed previously where CBD was tested at over 200 mg/kg dose effectively.18 However, that study screened efficacy of CBD in CF1 mice and Sprague Dawley rats in several other models of epilepsy and, importantly, only treated animals acutely. Also, the efficacy of CBD varied widely with median ED50 ranging from 83 to 164 mg/kg in the models tested. Therefore, it is quite possible that maximum tolerable dose of CBD in the TMEV model is lower than that in the other models. We tested lower doses of CBD (45‐150 mg/kg) in a pilot study to find the highest dose of CBD without adverse effects and 150 mg/kg of CBD was identified to be well tolerated by TMEV‐infected mice. It was surprising that lowering the dose only by 30 mg/kg avoided adverse effects associated with 180 mg/kg dose in this model. However, it is important to note that the percentage of protected mice increased from 50% to 100% in 6 Hz 44 mA seizure model in CF1 mice by only 20 mg/kg increase in CBD (from 180 to 200 mg/kg).18 This shows that many factors mainly strain of mice and type of animal model have significant impact on the dose‐response of CBD. When we tested the efficacy of 150 mg/kg of CBD in the TMEV model, average seizure frequency and severity were found to be similar between the CBD and vehicle groups (Figure 4A). However, mice treated with 150 mg/kg CBD had a significant reduction in the average number of seizures at 4 hours post‐CBD treatment during the acute seizure period (Figure 4B, upper panel; CBD: 0.5 ± 0.15, Vehicle: 1.45 ± 0.31, n = 20, P = 0.0108). In contrast to what was observed with the 180 mg/kg dose, 150 mg/kg did not affect average number of seizures evaluated at 9 hours post‐CBD treatment (Figure 4C, upper panel; CBD: 1.15 ± 0.25, Vehicle: 1.55 ± 0.26, n = 20, P = 0.2747). Likewise, the average cumulative seizure burden measured by analyzing seizures observed only at 4 hours post‐CBD time point was significantly reduced in 150 mg/kg CBD group at 6 and 7 dpi (Figure 4B, lower panel).
Figure 4.
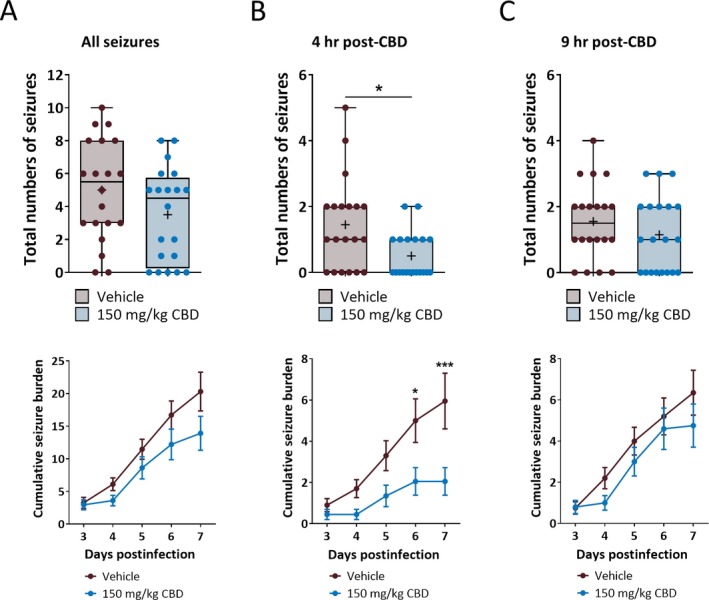
Cannabidiol (CBD) (150 mg/kg) administration decreases TMEV‐induced seizures observed at 4 h post‐CBD treatment but not at 9 h post‐CBD treatment. A, CBD (150 mg/kg) treatment (i.p. every 12 h for 5 d starting at 3 dpi) does not affect frequency (upper panel) and severity (lower panel) of all the behavioral seizures observed during 3‐7 dpi. B, Analysis of seizures observed at 4 h post‐CBD treatment shows a significant reduction in the average number of seizures (upper panel) and the average cumulative seizure burden at 6 and 7 dpi (lower panel) compared to the vehicle group. C, CBD (150 mg/kg) treatment does not affect seizure endpoints observed at 9 h posttreatment. Statistics: unpaired t test (upper panels in (A) and (C)), Welch's t test (upper panel in (B)), Scheirer‐Ray‐Hare test and Sidak’s multiple comparison test (all lower panels), * P < 0.05, *** P < 0.001, n = 20 mice per group.
These results suggest that the time to peak effect (TPE) of CBD is within 9 hours post‐CBD treatment. Since body temperature of CBD‐treated mice reached a minimum level at 2 hours post‐CBD (180 mg/kg) treatment and then partially recovered by 4 hours posttreatment, it was suspected that the time to peak effect (TPE) of CBD would be less than 4 hours in this model and CBD (150 mg/kg) would have been more effective if the seizures were evaluated at a time point less than 4 hours posttreatment. Therefore, we first investigated the TPE of CBD in male C57BL6/J mice using the 6 Hz psychomotor seizure test as described previously.16 The TPE for CBD was found to be 2 hours post‐CBD treatment, with a diminished efficacy at 4 hours (Table 1). To corroborate this finding, we tested the TMEV‐induced seizure activity at the TPE of CBD (150 mg/kg). The score of all the seizures observed during acute seizure period is shown as a heat map in Figure 5A. There was no difference in seizures immediately before CBD treatment everyday in the morning during acute seizure period (Figure 5B, upper panel; CBD: 1.6 ± 0.24, Vehicle: 1.6 ± 0.28, n = 20, P > 0.9999). However, mice treated with CBD had a significant reduction in the average number of acute seizures monitored at 2 hours posttreatment (Figure 5C, upper panel; CBD: 0.45 ± 0.14, Vehicle: 1.50 ± 0.29, n = 20, P = 0.0032), but not at 9 hours (Figure 5D, upper panel; CBD: 1.75 ± 0.35, Vehicle: 2.30 ± 0.42, n = 20, P = 0.3215). The average cumulative seizure burden at 2 hour post‐CBD treatment was also significantly reduced in 150 mg/kg CBD group at 5, 6, and 7 dpi (Figure 5C, lower panel). These results suggest that CBD could be effective in decreasing TMEV‐induced acute seizures when the seizure test is conducted at the TPE of CBD, and more frequent dosing regimen (for example, three times per day) could be more effective for a sustained therapeutic effect in this model.
Table 1.
The time to peak effect (TPE) of cannabidiol (CBD) (50 mg/kg, i.p.) in the 6 Hz 32 mA model was determined to be 2 h posttreatment in adult C57BL/6J male mice.
| Time post‐CBD treatment (h) | n (mice protected)/n (mice tested) |
|---|---|
| 0.25 | 0/4 |
| 0.5 | 4/8 |
| 1 | 7/12 |
| 2 | 8/12 |
| 4 | 4/8 |
Seizures were induced by 6 Hz 32 mA corneal stimulation at various time points following CBD treatment, and the number of mice protected from seizures was determined to measure the TPE of CBD.
Figure 5.
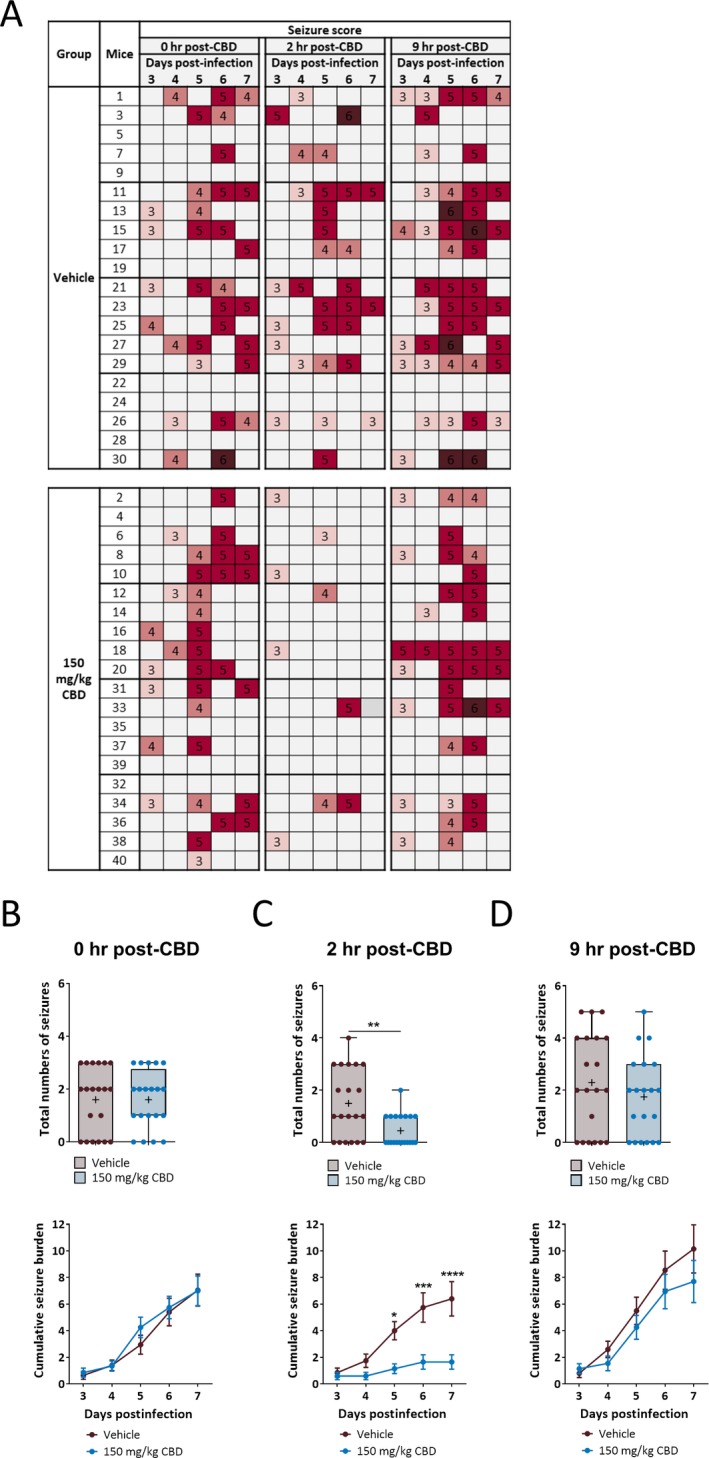
Cannabidiol (CBD) (150 mg/kg) treatment decreases TMEV‐induced acute seizures at the time to peak effect of CBD treatment (2 h). A, Mice were treated with either 150 mg/kg CBD (i.p. every 12 h for 5 d starting at 3 d post‐TMEV infection) or vehicle and the seizures were monitored as described in the methods just before CBD treatment in the morning (0 h post‐CBD) and at 2 and 9 h post‐CBD treatment. The heat map shows the seizure severity based on a modified Racine seizure scale ranging from stage 3 to 6. B, Both CBD and vehicle‐treated mice have similar seizure frequency and burden measured at 0 h post‐CBD treatment. C, Analysis of seizures monitored at 2 h post‐CBD treatment shows a significant reduction in the average number of seizures (upper panel) and the average cumulative seizure burden at 5, 6, and 7 dpi (lower panel) compared to the vehicle group. D, CBD (150 mg/kg) treatment does not affect seizure endpoints measured at 9 h posttreatment. Statistics: unpaired t test (upper panels in (B) and (D)), Welch's t test (upper panel in (C)), Scheirer‐Ray‐Hare test and Sidak’s multiple comparison test (lower panels in (B‐D)), * P < 0.05, ** P < 0.01, *** P < 0.001, *** P < 0.001, n = 20 mice per group.
4. DISCUSSION
The experiments conducted here demonstrate that CBD (180 mg/kg) consistently reduced both the frequency and severity of TMEV‐induced acute behavioral seizures. Although the lower dose of CBD (150 mg/kg; 300 mg/kg/day) did not improve the acute seizure condition overall using this treatment regimen, it significantly reduced frequency and severity of acute seizures monitored at either 2 or 4 hours post‐CBD treatment. Indeed, the beneficial effects of treatment were not observed when the seizures were monitored at 9 hours post‐CBD treatment. The TPE of CBD was 2 hours in the 6 Hz 32 mA psychomotor seizure test using C57BL/6J mice. These results suggest that CBD could be effective in decreasing TMEV‐induced acute seizures when the seizure test is conducted at the TPE of CBD. Future studies should conduct dose‐response study of CBD in this model by evaluating seizures at 2 hours post‐CBD treatment. In addition, more frequent dosing, perhaps in chow, may also confer greater protection at this lower dose. Finally, pharmacokinetic studies would aid in the understanding of the efficacy time course.
A majority of animal studies in various seizure models have reported antiseizure effects of CBD, whereas a few did not find any significant reduction in seizures.21 Importantly, CBD suppressed hind limb extension, an indicator of antiseizure effect, in maximum electroshock model of acute generalized seizures in CF1 mice when the seizure test was conducted 2 hours posttreatment (ie, the time of peak effect).22 Rats treated with 1‐100 mg/kg of CBD one hour prior to seizure evaluation significantly reduced seizure severity and mortality in the model of generalized seizures induced by pentylenetetrazole and in the model of limbic seizures induced by pilocarpine and penicillin.23, 24 Recently, a study conducted by the National Institute of Neurological Disorders and Stroke funded Epilepsy Therapy Screening Program found that CBD provided protection against seizures in several acute as well as chronic seizure models in rodents with the ED50 ranging from 83 to 164 mg/kg.18 TMEV infection–induced seizures represent an etiologically relevant model of acquired epilepsy that captures many pathophysiological changes occurring in the patients of TLE acquired following CNS infection and encephalitis. Therefore, the present study was aimed at testing efficacy of CBD in the TMEV model. Since many factors such as the dose of CBD (1‐400 mg/kg), dosing regimen, animal species, and model specific characteristics differ widely among previous animal studies testing effectiveness of CBD in epilepsy, it was difficult to design pharmacological studies of CBD in the TMEV model based on prior work. We determined to test the effects of 180 mg/kg of CBD on TMEV‐induced acute seizures based on a published study from our group in which the ED50 of CBD was calculated as 164 mg/kg in the 6 Hz 44 mA psychomotor seizure test using CF1 mice.18 Other pharmacokinetic parameters such as half‐life and bioavailability of CBD in TMEV‐infected mice are still unknown, and the detailed understanding of these parameters will be helpful in determining an optimum dosing paradigm of CBD in this model.
The mechanisms of how TMEV infection in the brain causes seizures in C57BL/6J mice are not completely understood. Excessive inflammation, oxidative stress, and parenchymal damage may contribute to the development of acute seizures.25, 26 Microgliosis, astrogliosis, and infiltration of peripheral macrophages occur in the hippocampus of TMEV‐infected mice during acute seizures and may contribute to excessive inflammatory response and oxidative stress.5, 27 Indeed, protein levels of several cytokines, chemokines, and oxidative stress markers are significantly increased in the hippocampus of TMEV‐infected mice during the acute seizure period.6, 7, 8, 27 Mice deficient in TNFR1, TNFα, or IL‐6 are much less susceptible to developing TMEV‐induced acute seizures compared to wild‐type mice.7, 8 Inhibiting infiltration of macrophages and microgliosis by minocycline or wogonin treatment has been shown to decrease the number of TMEV‐infected mice that developed acute seizures.27, 28 Taken together, these data support the hypothesis that inflammation likely plays a significant role in driving seizures in TMEV‐infected mice and antiinflammatory therapies, either as monotherapy and/or in combination with antiseizure drugs (ASDs), could be effective in reducing acute seizures or perhaps even prevent the development of epilepsy in TMEV‐infected mice. CBD exerts antioxidant and antiinflammatory effects.21, 29 CBD decreases the release of TNFα in lipopolysaccharide‐treated rats30, and CBD is also known to decrease the levels of other proinflammatory cytokines including IL‐2, IFN‐γ, IL‐6, IL‐12 (p‐40), and IL‐17 in mice.31 In addition, CBD can ameliorate behavioral symptoms in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis potentially by suppressing T‐cell proliferation and microglial activity in the spinal cord.32 CBD treatment also provides long‐term improvement in motor deficits in TMEV‐induced demyelinating disease, a model of multiple sclerosis in SJL/J mice, by decreasing infiltration of leukocytes, microglial activation, and proinflammatory cytokines IL‐1β and TNFα.33 Therefore, it is likely that CBD may provide antiseizure effects by controlling inflammation in the brain following TMEV infection. The changes in the levels of the proinflammatory cytokines and oxidative stress markers, coincident with the changes in acute seizures due to CBD treatment, should be measured in future studies to test this hypothesis. Additionally, CBD imparts antiseizure effects in several animal models through variety of other mechanisms.21 For example, CBD may modulate neuronal excitability by decreasing presynaptic release of glutamate by inhibiting G‐protein‐coupled receptor 55 (GRP55)‐mediated release of intracellular Ca2+.34 Furthermore, CBD can enhance adenosine signaling by inhibiting adenosine uptake mechanisms.30, 35 Adenosine is known to function as an endogenous immunosuppressant36 and can also directly inhibit seizures and confer neuroprotective effects.37 Further studies are required to elucidate the mechanisms by which CBD may confer antiseizure effects in the TMEV‐infected mice.
The present study highlights the importance of the TMEV model of limbic epilepsy as a drug screening tool for the development of novel therapeutic approaches for the treatment of acute seizures as well as for the prevention of epileptogenesis. The efficacy of carbamazepine and valproate has been assessed in this model.38 Valproate (200 mg/kg) and carbamazepine (20 mg/kg) given twice daily during the first week of TMEV infection did not decrease numbers of TMEV‐infected mice developing acute seizures; however, valproate reduced the seizure severity.38 In contrast, carbamazepine treatment increased the numbers of mice developing seizures and the concomitant seizure burden, and decreased the latency to first seizure.38 Thus, this model is refractory to at least one commonly used ASD. Given intense scientific and public interest in cannabinoid‐based therapies for intractable epilepsies, thorough animal studies are required to evaluate the safety and efficacy of CBD either alone or, even more importantly, in combination with available antiseizure drugs (ASDs) in a battery of epilepsy models.
In conclusion, CBD (180 mg/kg) given twice daily intraperitoneally consistently reduces acute behavioral seizures induced by TMEV infection. CBD (150 mg/kg) also decreased the incidence and severity of seizures when the seizure test was conducted at 2 hours post‐CBD treatment. The dose‐response study should be conducted by testing the seizures at 2 hours post‐CBD treatment to measure the ED50 of CBD in the TMEV model. Acute seizures due to CNS infection greatly increase the probability for the development of chronic spontaneous seizures.39, 40 Whether suppression of TMEV‐induced acute seizures by CBD treatment prevents the development of epilepsy in this model is an area of future investigation.
CONFLICT OF INTEREST
The authors report no relevant conflict of interest. The authors confirm that they have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
This research was supported by the Margolis Foundation (K.S.W.), the Epilepsy Foundation TRICE award (M.D.S.), the American Epilepsy Society predoctoral fellowship (D.C.P.), and the National Institutes of Health (NS065714, R.S.F.). Cannabidiol was provided by the National Institute on Drug Abuse (NIDA) drug supply program.KSW is a consultant for Xenon Pharmaceuticals and is on the Scientific Advisory Board of Blackfynn, Inc.
Patel DC, Wallis G, Fujinami RS, Wilcox KS, Smith MD. Cannabidiol reduces seizures following CNS infection with Theiler's murine encephalomyelitis virus. Epilepsia Open. 2019;4:431–442. 10.1002/epi4.12351
REFERENCES
- 1. Libbey JE, Kirkman NJ, Smith MCP, Tanaka T, Wilcox KS, White HS, et al. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–74. [DOI] [PubMed] [Google Scholar]
- 2. Stewart K‐A, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler's virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69:1210–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bröer S, Käufer C, Haist V, Li L, Gerhauser I, Anjum M, et al. Brain inflammation, neurodegeneration and seizure development following picornavirus infection markedly differ among virus and mouse strains and substrains. Exp Neurol. 2016;279:57–74. [DOI] [PubMed] [Google Scholar]
- 4. Patel DC, Wilcox KS. Postinfectious epilepsy Models of Seizures and Epilepsy. New York, NY: Elsevier; 2017: pp. 683–96. [Google Scholar]
- 5. Loewen JL, Barker‐Haliski ML, Dahle EJ, White HS, Wilcox KS. Neuronal injury, gliosis, and glial proliferation in two models of temporal lobe epilepsy. J Neuropathol Exp Neurol. 2016;75:366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhuyan P, Patel DC, Wilcox KS, Patel M. Oxidative stress in murine Theiler's virus‐induced temporal lobe epilepsy. Exp Neurol. 2015;271:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel DC, Wallis G, Dahle EJ, McElroy PB, Thomson KE, Tesi RJ, et al. Hippocampal TNFalpha signaling contributes to seizure generation in an infection‐induced mouse model of limbic epilepsy. eNeuro. 2017;4 10.1523/ENEURO.0105-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umpierre AD, Remigio GJ, Dahle EJ, Bradford K, Alex AB, Smith MD, et al. Impaired cognitive ability and anxiety‐like behavior following acute seizures in the Theiler's virus model of temporal lobe epilepsy. Neurobiol Dis. 2014;64:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das A, Wallace GC, Holmes C, McDowell Ml, Smith JA, Marshall JD, et al. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012;220:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Lanerolle NC, Lee TS. New facets of the neuropathology and molecular profile of human temporal lobe epilepsy. Epilepsy Behav. 2005;7:190–203. [DOI] [PubMed] [Google Scholar]
- 12. O'Connell BK, Gloss D, Devinsky O. Cannabinoids in treatment‐resistant epilepsy: a review. Epilepsy Behav. 2017;70:341–48. [DOI] [PubMed] [Google Scholar]
- 13. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet Syndrome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed] [Google Scholar]
- 14. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox‐Gastaut Syndrome. N Engl J Med. 2018;378:1888–97. [DOI] [PubMed] [Google Scholar]
- 15. Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. [DOI] [PubMed] [Google Scholar]
- 16. Barton ME, Klein BD, Wolf HH, Steve White H. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–27. [DOI] [PubMed] [Google Scholar]
- 17. Scheirer CJ, Ray WS, Hare N. The analysis of ranked data derived from completely randomized factorial designs. Biometrics. 1976;32:429–34. [PubMed] [Google Scholar]
- 18. Klein BD, Jacobson CA, Metcalf CS, Smith MD, Wilcox KS, Hampson AJ, et al. Evaluation of cannabidiol in animal seizure models by the epilepsy therapy screening program (ETSP). Neurochem Res. 2017;42:1939–48. [DOI] [PubMed] [Google Scholar]
- 19. Guilliams K, Rosen M, Buttram S, Zempel J, Pineda J, Miller B, et al. Hypothermia for pediatric refractory status epilepticus. Epilepsia. 2013;54:1586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong JP, Saravolac EG, Clement JG, Nagata LP. Development of a murine hypothermia model for study of respiratory tract influenza virus infection. Lab Anim Sci. 1997;47:143–47. [PubMed] [Google Scholar]
- 21. Rosenberg EC, Tsien RW, Whalley BJ, Devinsky O. Cannabinoids and epilepsy. Neurotherapeutics. 2015;12:747–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol. 2001;428:51–7. [DOI] [PubMed] [Google Scholar]
- 23. Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones NA, Glyn SE, Akiyama S, Hill TDM, Hill AJ, Weston SE, et al. Cannabidiol exerts anti‐convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344–52. [DOI] [PubMed] [Google Scholar]
- 25. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rowley S, Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. 2013;62:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol. 2013;87:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin‐6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011;85:6913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burstein S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23:1377–85. [DOI] [PubMed] [Google Scholar]
- 30. Liou GI, Auchampach JA, Hillard CJ,Zhu G, Yousufzai B, Mian S et al. Mediation of cannabidiol anti‐inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci. 2008;49:5526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hegde VL, Nagarkatti PS, Nagarkatti M. Role of myeloid‐derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6:e18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kozela E, Lev N, Kaushansky N, Eilam R, Rimmerman N, Levy R, et al. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis‐like disease in C57BL/6 mice. Br J Pharmacol. 2011;163:1507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mecha M, Feliú A, Iñigo PM, Mestre L, Carrillo‐Salinas FJ, Guaza C. Cannabidiol provides long‐lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013;59:141–50. [DOI] [PubMed] [Google Scholar]
- 34. Sylantyev S, Jensen TP, Ross RA, Rusakov DA. Cannabinoid‐ and lysophosphatidylinositol‐sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci USA. 2013;110:5193–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci USA. 2006;103:7895–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–9. [DOI] [PubMed] [Google Scholar]
- 37. Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60:1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barker‐Haliski Ml, Dahle EJ, Heck TD, Pruess TH, Vanegas F, Wilcox KS, et al. Evaluating an etiologically relevant platform for therapy development for temporal lobe epilepsy: effects of carbamazepine and valproic acid on acute seizures and chronic behavioral comorbidities in the Theiler's murine encephalomyelitis virus mouse model. J Pharmacol Exp Ther. 2015;353:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol. 1998;44:908–12. [DOI] [PubMed] [Google Scholar]
- 40. Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49(Suppl 1):13–8. [DOI] [PubMed] [Google Scholar]


