Abstract
The prevalence and diagnosis of nonalcoholic fatty liver disease (NAFLD) is on the rise worldwide and currently has no FDA-approved pharmacotherapy. The increase in disease burden of NAFLD and a more severe form of this progressive liver disease, nonalcoholic steatohepatitis (NASH), largely mirrors the increase in obesity and type 2 diabetes (T2D) and reflects the hepatic manifestation of an altered metabolic state. Indeed, metabolic syndrome, defined as a constellation of obesity, insulin resistance, hyperglycemia, dyslipidemia and hypertension, is the major risk factor predisposing the NAFLD and NASH. There are multiple potential pharmacologic strategies to rebalance aspects of disordered metabolism in NAFLD. These include therapies aimed at reducing hepatic steatosis by directly modulating lipid metabolism within the liver, inhibiting fructose metabolism, altering delivery of free fatty acids from the adipose to the liver by targeting insulin resistance and/or adipose metabolism, modulating glycemia, and altering pleiotropic metabolic pathways simultaneously. Emerging data from human genetics also supports a role for metabolic drivers in NAFLD and risk for progression to NASH. In this review, we highlight the prominent metabolic drivers of NAFLD pathogenesis and discuss the major metabolic targets of NASH pharmacotherapy.
Abbreviations used in this paper: ACC, acetyl-CoA carboxylase; ALT, alanine aminotransferase; ASO, anti-sense oligonucleotide; AST, aspartate aminotransferase; ChREBP, carbohydrate response element binding protein; CI, confidence interval; DGAT, diacylglycerol O-acyltransferase; DNL, de novo lipogenesis; FAS, fatty acid synthase; FFA, free fatty acid; FGF, fibroblast growth factor; FXR, Farnesoid X receptor; GLP-1, glucagon-like peptide-1; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; NAS, Nonalcoholic Fatty Liver Disease Activity Score; NASH, nonalcoholic steatohepatitis; OR, odds ratio; PDFF, proton density fat fraction; PPAR, peroxisome proliferator-activated receptor; SGLT2, sodium glucose co-transporter 2; SREBP-1c, sterol regulatory element binding protein-1c; T2D, type 2 diabetes; T2DM, type 2 diabetes mellitus; TG, triglyceride; TH, thyroid hormone; THR, thyroid hormone receptor; Treg, regulatory T cells; TZD, thiazolidinedione; VLDL, very low-density lipoprotein
Summary.
This review highlights the major metabolic drivers of nonalcoholic fatty liver disease pathogenesis and gives a current overview of the treatment landscape for nonalcoholic steatohepatitis.
Nonalcoholic fatty liver disease (NAFLD) is a constellation of conditions that originates with excess accumulation of fat within the liver (defined as >5%). Nonalcoholic steatohepatitis (NASH) is a clinical and histological subset of NAFLD that is associated with increased all-cause mortality, cirrhosis and end-stage liver disease, increased cardiovascular mortality, and increased incidence of both liver-related and non–liver-related cancers.1 NASH is diagnosed clinically by liver biopsy demonstrating steatosis, inflammation, and cytological ballooning of liver hepatocytes, often with varying degrees of fibrosis. NASH progresses with increasing degrees of fibrosis, with cirrhosis developing in a subset of patients,1 with the most common complication of cirrhosis being hepatocellular carcinoma.2
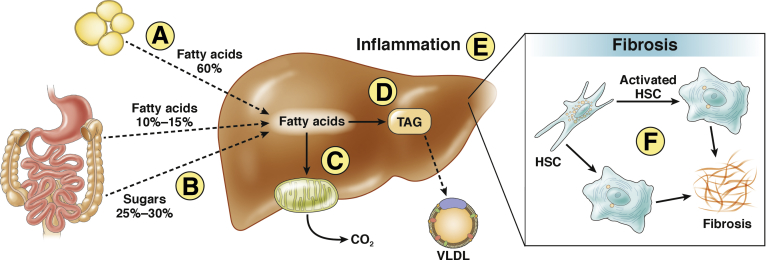
Metabolic perturbations, including insulin resistance, impaired glycemic control, and altered lipid metabolism, have been hypothesized to contribute to the molecular pathogenesis of NAFLD and NASH (Figure 1). Steatosis is a necessary but not sufficient component of the pathogenesis of NASH.3 Consistent with this, multiple studies have demonstrated that the severity of steatosis predicts the risk of concomitant steatohepatitis as well as the risk of progression to cirrhosis.4, 5, 6 Genetic polymorphisms in humans, including the I148M polymorphism in the PNPLA3 gene, have also been shown to increase risk of steatosis and in turn increase risk for NASH.7, 8 These observations taken together provide molecular evidence that steatosis is a key pathogenic factor in NASH.
Figure 1.
Model of hepatic lipid metabolism, inflammation, and fibrosis. Hepatic lipid content is a balance between uptake and synthesis of lipids and lipid disposal/export. The primary source of hepatic fat is the liberation of free fatty acids from adipose/fat tissue via lipolysis (A),27, 106, 107 with additional uptake of fatty acids from the circulation (B) and de novo lipogenesis (D). Lipids are cleared from the liver by beta-oxidation of fatty acids in the mitochondria (C) and through export as very low-density lipoprotein (VLDL) particles. Excess hepatic lipid accumulation, on its own or in combination with additional environmental insults, can trigger an inflammatory response in the liver characterized histologically as hepatocyte ballooning (E). Ultimately, the hepatic inflammatory response drives NASH disease progression as it promotes activation of hepatic stellate cells (HSC) and fibrosis (F).
Hepatic steatosis is a consequence of an imbalance in triglyceride (TG) production or uptake into the liver and clearance or removal (Figure 1).9 Elevated body mass index is a significant risk factor for steatosis10 suggesting that excess caloric intake and obesity contribute to the development of NAFLD. Altering the balance of hepatic TG accumulation and removal by either (or both) reducing fat production or promoting fat clearance is likewise expected to reduce steatosis. Amelioration of steatosis in turn is hypothesized to reduce buildup of lipotoxic lipid species, suppress hepatic inflammation, and subsequently reduce fibrogenesis (Figure 1). Indeed, studies in animal models have demonstrated that multiple modalities which reduce steatosis result in downstream improvements in hepatic inflammation and fibrosis.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
Perhaps the most compelling data in support of targeting metabolic pathways in NASH comes from the bariatric surgery literature. Bariatric surgery leads to restoration of energy balance and improvements in metabolic homeostasis resulting in amelioration of steatosis and marked downstream improvements in, or resolution of, NASH in people. Meta-analysis of multiple bariatric surgery studies demonstrates that this improvement in steatosis observed in 92% of patients is also accompanied by improved steatohepatitis in 81%, lower fibrosis in 66%, and complete resolution in 70% of patients with NASH.22 Similarly, weight loss in patients with NASH produced by intense nutritional counseling has also been reported to reduce steatosis leading to improvements in NASH resolution.23 Emerging data also suggest that at least some pharmacological agents that principally target metabolic drivers of steatosis lead to downstream improvements in NASH resolution or fibrosis when administered to patients with NASH with fibrosis, making this an attractive therapeutic angle (vide infra and see Table 1).
Table 1.
Mechanisms of Action and Associated Pharmacotherapies With Clinical Data in NAFLD/NASH
| Mechanism of action | Drug name | Current development status | Key clinical data summary | ClinicalTrials.Gov identifier for key studies |
|---|---|---|---|---|
| Lipid metabolism pathway modulators | ||||
| ACC inhibitor | PF-05175157 (SM) | Ph2—discontinued | Systemically distributed, DNL inhibition, and stimulation of fatty acid oxidation in healthy volunteers. Discontinued due to undisclosed safety concern | NCT01792635 |
| MK-4074 (SM) | Ph1—discontinued | Liver directed exposure, Hepatic DNL inhibition and steatosis lowering in NAFLD subjects; discontinued due to TG elevations | NCT01431521 | |
| GS-0976 (SM) | Ph2 | Liver directed exposure, DNL inhibition, and steatosis lowering in NAFLD patients | NCT02856555 | |
| PF-05221304 (SM) | Ph2 | Liver directed exposure, DNL inhibition, no TG increase in healthy subjects at doses which inhibit DNL ≤80% |
NCT03448172 NCT03248882 |
|
| FAS inhibitor | TVB-2640 (SM) | Ph2 | DNL inhibition in healthy subjects | None listed |
| DGAT2 inhibitor | IONIS-DGAT2rx (ASO) | Ph2 | Ph1 dose-escalation study completed in healthy overweight subjects | NCT03334214 |
| PF-06427878 (SM) | Ph1 -discontinued | Steatosis lowering in overweight subjects—discontinued due to human PK profile | NCT02855177 | |
| PF-06865571 (SM) | Ph1b/Ph2 | None disclosed |
NCT03513588 NCT03776175 |
|
| Nuclear hormone receptor agonists | ||||
| PPARγ agonist | Pioglitazone (SM) | Approved T2D, exploratory studies for NASH | Improvements in steatosis, NAS, NASH resolution, and fibrosis |
NCT00063622 NCT00994682 |
| PPARα agonist | Fenofibrate (SM) | Approved for dyslipidemia, exploratory studies in NAFLD/NASH | No apparent direct improvements in NASH endpoints or steatosis in small exploratory studies |
NCT00262964 NCT02354976 |
| PPARδ agonist | GW501516 (SM) | Ph2—discontinued | Steatosis lowering in subjects with NAFLD | NA |
| PPARα/δ agonist | Elafibranor (SM) | Ph3 | Failed to meet protocol specified primary endpoint in Ph2, modest improvement relative to placebo in post hoc definition of NASH resolution, improvement in cardio metabolic parameters noted |
NCT01694849 NCT02704403 |
| PPARα/γ agonist | Saroglitazar (SM) | Approved for dyslipidemia in T2D patients in India, Ph2 for NASH | NAFLD/NASH data pending | NCT03061721 |
| FXR | Obeticholic acid (SM) | Conditional approval for PBC, Ph3 for NASH and cirrhosis as a result of NASH | Improvements in all components of NAS, NASH resolution and fibrosis, mild elevations in LDL, reductions in HDL, increased incidence of pruritus |
NCT01265498 NCT02548351 NCT03439254 |
| GS-9674 (SM) | Ph2 | Reduction in steatosis and increased incidence of pruritus at 100-mg dose; 30-mg dose (currently being evaluated in ongoing Ph2 monotherapy and combination Ph2 biopsy study) produced very modest relative liver fat reduction (3.7%, placebo adjusted) |
NCT02854605 NCT03449446 |
|
| Tropifexor (LJN-452) (SM) | Ph2 | Interim results of an ongoing Ph2 study were presented noting ALT reductions and modest liver fat content reduction at 60- and 90-μg doses, mild increase in LDL, and decrease in HDL. Increased pruritus incidence relative to placebo noted at 90-μg dose | NCT02855164 | |
| EDP-305 (SM) | Ph2 | FGF19 increases and C4 decreases noted in Ph1. Increased incidence in Pruritus and decreased HDL (but no LDL increase) at 20-mg dose relative to placebo |
NCT02918929 NCT03421431 |
|
| MET409 (SM) | Ph1 | Ph1 ongoing | Not posted | |
| THR-β agonist | MGL-3196 (SM) | Ph2 | Reduction in liver fat content and ALT noted following 12 and 36 wk of administration. NASH w and ≥1-point improvements in fibrosis noted after 36 wk administration. | NCT02912260 |
| VK2809 (SM) | Ph2 | >50% relative reduction (placebo adjusted) in liver fat content noted at 10-mg daily dose. Increases in ALT noted in Ph1 and at early time points in Ph2, though ALT was not different from placebo after 12 wk administration. ALT reduction would have been expected given robust liver fat reductions. | NCT02927184 | |
| FGF peptide mimetics | ||||
| FGF19 | NGM 282 (P) | Ph2 | 57% and 45% relative reductions (placebo adjusted) in hepatic steatosis noted at 6 and 3-mg doses accompanied by improvements in ALT, AST, and C4. Increased incidence of injection site reactions and GI AEs noted in subjects who received MGM 282 vs placebo. Improvements in all components of NAS as well as fibrosis noted in non–placebo-controlled 12-wk exploratory study. |
NCT01943045 NCT02443116 |
| FGF21 | BMS-986036 (P) | Ph2 | Reductions in liver fat content accompanied by improvements in ALT, AST, Pro-C3, and MRE noted in 16 Ph2 studies. Improvements in cardio metabolic parameters (TG, LDL, HDL) also noted. | NCT02413372 |
| β-Klotho/FGFR1c receptor agonist | NGM313 (MK-3655) | Ph1b proof of concept study in normal healthy overweight/obese shows reduction in liver fat content |
NCT02708576 NCT03298464 |
|
| Incretin therapies | ||||
| GLP-1 mimetics | Liraglutide (P) | Approved for T2D, exploratory studies for NASH | Improvements in NASH resolution and fibrosis noted in NASH patients administered liraglutide vs placebo for 48 wk. | NCT01237119 |
| Semaglutide (P) | Approved for T2D, in Ph2 for NASH | Robust improvements in glycemic control and body weight loss noted in both once-weekly and once-daily administered semaglutide. Ph2 biopsy study underway | NCT02970942 | |
| DPP4 inhibitor | Sitagliptin (SM) | Approved for T2D, exploratory studies for NASH | Sitagliptin showed no improvements in liver fat content, ALT, AST, and MRE vs placebo in a double-blinded, placebo-controlled study, though some earlier, small, open-label, or retrospective studies showed apparent improvements in liver enzymes and reduction in NAS | NCT01963845 |
| SGLT2 inhibitors | ||||
| SGLT1/2 inhibitor | LIK066 | Ph2 | Ph2 study in obese patients with NASH ongoing | NCT03205150 |
| SGLT2 inhibitor | dapagliflozin | Ph3 | Ph3 planned to study efficacy and safety of dapagliflozin in NASH | NCT03723252 |
| Other mechanisms of action | ||||
| MPC inhibitor | MSDC-0602K (SM) | Ph2 | A press release from Cirius reported that statically significant reductions in ALT and AST were observed in an interim analysis on an ongoing Ph2b trial | NCT02784444 |
| PXL065 (DRX-065) (SM) | Ph1 | Ph1 study in healthy subjects ongoing | Not posted | |
ACC, acetyl-CoA carboxylase; ALT, alanine aminotransferase; ASO, antisense oligonucleotide; AST, aspartate aminotransferase; DGAT, diacylglycerol O-acyltransferase; DNL, de novo lipogenesis; DPP4, •••; FAS, fatty acid synthase; FGF, fibroblast growth factor; FXR, Farnesoid X receptor; GLP-1, glucagon-like peptide-1; HDL, high-density lipoprotein; MPC, mitochondrial pyruvate carrier; MRE, •••; NAFLD, nonalcoholic fatty liver disease; NAS, Nonalcoholic Fatty Liver Disease Activity Score; NASH, nonalcoholic steatohepatitis; P, peptide or modified peptide; PBC, primary biliary cholangitis; PPAR, peroxisome proliferator-activated receptor; SGLT2, sodium glucose co-transporter 2; SM, small molecule; T2D, type 2 diabetes; TG, triglyceride; THR, thyroid hormone receptor.
Regulators of Hepatic Lipid Metabolism
Over nutrition, accompanied by hyperinsulinemia and hyperglycemia, drives steatosis by promoting de novo lipogenesis (DNL). Lipogenic transcription factors including carbohydrate response element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c) are upregulated in rodent models, leading to increased expression of lipogenic genes and subsequent increased flux through acetyl-CoA carboxylase (ACC) and elevations in hepatic malonyl-CoA.24 This leads to increased hepatic DNL and suppressed hepatic fatty acid oxidation which together promote steatosis (Figure 1).24 Hepatic SREBP1c has also been reported to be upregulated in patients with NAFLD25, 26 and elevated rates of hepatic DNL have been found to be a distinctive characteristic of NAFLD.27 Humans with elevated liver fat showed a >3-fold increase in hepatic DNL relative to subjects with normal liver fat, but differences between the groups were not detected in adipose free fatty acid (FFA) flux or in production of very low-density lipoprotein (VLDL) from FFAs.27 The observation that fructose consumption, which strongly promotes hepatic DNL,28 is a risk factor for NAFLD29, 30 may further underscore the importance of DNL in this disease.
Fructose-induced hepatic lipogenesis also likely contributes to the rise in rates of NAFLD. Fructose is a simple sugar found naturally in fruit and is a major component of sucrose (table sugar) and high-fructose corn syrup. Dietary trends in recent decades demonstrate a sharp increase in consumption of fructose and epidemiological studies have shown a strong correlation between consumption of fructose-sweetened beverages and risk for fatty liver.30, 31, 32, 33 Unlike glucose, whose metabolism by the liver is tightly regulated, fructose is rapidly phosphorylated by ketohexokinase to fructose-1-phosphate without feedback inhibition, and is metabolized further in the cell to generate substrates for gluconeogenesis and de novo lipogenesis. Fructose is a more potent inducer of DNL than glucose34 and chronic fructose consumption induces activation of SREBP1c and ChREBP and downstream lipogenic gene transcription.31 Thus, strategies aimed at directly inhibiting fructose metabolism (ie, ketohexokinase inhibition35 or limiting fructose consumption in the diet) represent attractive therapeutic approaches for NAFLD and NASH.
Emerging data also indicate that DNL may be important in regulating some inflammatory pathways in NASH. Berod et al36 reported that proinflammatory interleukin-17 secreting T cells of the T helper 17 lineage show increased dependency on DNL to produce phospholipids for cellular membranes. In contrast, anti-inflammatory regulatory T cells (Treg) utilize exogenous fatty acids. Inhibition of DNL in primary T cells blunts formation of proinflammatory interleukin-17 secreting T cells of the T helper 17 lineage cells and promotes formation of anti-inflammatory Treg. Further, a higher frequency of Th17 cells in liver and an increase in the ratio of Th17 cells to Treg in peripheral blood and in liver marks progression from NAFLD to NASH in humans.37 Pharmacologic agents that inhibit DNL have potential to reduce steatosis and lipotoxicity. Inhibitors of the lipogenic enzymes ACC, fatty acid synthase (FAS), and diacylglycerol O-acyltransferase (DGAT) are in clinical development for NASH (Table 1).
ACC Inhibitors
ACC catalyzes the first committed step in DNL, the production of malonyl-CoA though the adenosine triphosphate–dependent condensation of acetyl-CoA with carbonate.38 Malonyl-CoA is also an allosteric inhibitor of carnitine palmitoyltransferase 1, which is limiting for transport of long-chain fatty acyl-CoAs into the mitochondria for beta-oxidation.38 As a result, ACC acts as a key switch regulating the transition from fatty acid synthesis to fatty acid oxidation. There are 2 closely related isoforms of ACC, ACC1 and ACC2, encoded by separate gene products. In general, ACC2 is the major isoform in oxidative tissues such as muscle and heart, while ACC1 predominates in lipogenic tissues such as liver and adipose; however, ACC2 may be the major ACC isoform in human liver.39 The first potent and selective small molecule dual ACC1/2 inhibitor, CP-640186, was reported in 2003.40 Pharmacologic ACC inhibition was shown to inhibit formation of malonyl-CoA, stimulate fatty acid oxidation and block DNL both in vitro and in vivo.40, 41, 42, 43, 44, 45 This compound was the starting point for optimization that ultimately led to the discovery of PF-05175157,44 a systemically distributed, CNS excluded, dual ACC1/2 inhibitor, which was progressed into clinical development in 2010 (NCT01274663). In Phase 1 studies, PF-05175157 inhibited hepatic DNL and stimulated fatty acid oxidation (as assessed using indirect calorimetry) in human subjects.44 Clinical development of PF-05175157 was subsequently terminated due to an undisclosed safety finding (NCT01792635). Liver-directed ACC inhibitors presently in development are anticipated to have an improved safety profile relative to the first-generation systemically distributed ACC inhibitor PF-05175157 . In 2017, Kim et al46 reported that a liver directed ACC inhibitor MK-4074, at doses that fully inhibited hepatic DNL, reduced hepatic steatosis by 44.6% from baseline (placebo adjusted) after 4 weeks of administration. However, MK-4074 unexpectedly increased circulating TG levels 200%.46 Mechanistic studies in rodents demonstrated that decreases in polyunsaturated fatty acids increased SREBP-1C activation, hepatic GPAT1 expression, and VLDL secretion.46 Subsequent studies47 suggest that decreased TG clearance may also contribute to increased circulating TG levels.
A second-generation liver-directed ACC inhibitor, PF-05221304, was also reported to increase serum TG levels in healthy human subjects at doses that inhibited hepatic DNL by ≥90%.48 However, no increases in serum TGs were observed at doses that partially inhibited hepatic DNL by ≤80%.48 These data suggest that at doses that partially inhibit DNL, rather than ablate ACC activity, compensatory increases in circulating TG levels may not be observed. Importantly, 50 to 70% inhibition of DNL is expected to fully normalize the 2- to 3-fold increase in hepatic DNL flux observed in patients with NAFLD.27 Additional studies are needed to determine if this degree of DNL inhibition can be achieved without circulating TG increases in NASH patients. GS-09674 (formerly NDI-010976) and PF-05221304 are liver directed ACC inhibitors in Phase 2 development for NASH (Table 1). In an open-label non–placebo-controlled study, GS-09674 (20 mg daily) inhibited hepatic DNL by 23% from baseline and reduce steatosis from baseline by 43% after 12 weeks of administration.49 In a larger randomized, placebo-controlled study, 21% relative liver fat reduction (placebo adjusted, P = .002), was observed at the 20-mg daily dose after 12 weeks of administration.50
FAS Inhibitors
FAS, a multidomain enzyme complex, catalyzes the production of palmitate using malonyl-CoA, acetyl-CoA, and nicotinamide adenine dinucleotide phosphate as an electron donor. FAS inhibitors have been of interest for oncology as many tumor types depend on increased DNL flux for membrane production and to maintain redox balance by regenerating NADP+.51, 52 Early generation FAS inhibitors suffered from 1 or more liabilities including poor potency, off target activity, or suboptimal physiochemical or pharmacokinetic properties.52, 53 While early FAS inhibitors functioned as substrate competitors, more recently inhibitors targeting co-factor binding sites have been identified. TVB-2640 is the first FAS inhibitor to enter clinical development. TVB-2640 inhibited DNL in human subjects with metabolic syndrome.54 TVB-2640 is in Phase 1 development for NASH (Table 1) and Phase 2 development for oncology.
DGAT1 and DGAT2 Inhibitors
DGATs catalyze the esterification of a fatty acid with diacylglycerol to form TG.55 DGAT activity contributes to intestinal fat absorption, control of circulating lipid concentrations, flux of lipoproteins between adipose and liver, and muscle energy metabolism.56 In mammals, 2 DGAT enzymes have been characterized; DGAT1 is highly expressed in intestine and plays a central role in fat absorption57 while DGAT2 is highly expressed in liver and adipose tissue.58 Within hepatocytes, DGAT1 primarily esterifies exogenous fatty acids while DGAT2 primarily incorporates DNL-derived fatty acids into TG.59 Pharmacologic inhibitors of DGAT1 and DGAT2 have been evaluated in nonclinical models and progressed into clinical development. Small molecule DGAT1 inhibitors of multiple structural classes block postprandial TG excursion following lipid challenge in nonclinical models.60 Pronounced and dose-limiting GI side effects were observed with DGAT1 inhibitors in human subjects leading to compound discontinuation.60, 61, 62, 63 In contrast, DGAT2 is not expressed at high levels in the intestine58 and does not play a major role in intestinal lipid absorption.64 Anti-sense oligonucleotide (ASO) knock down and pharmacological inhibition of DGAT2 suppresses TG production, but also induces adaptive downregulation of SREBP1, leading to decreased lipogenic gene expression and induction of oxidative pathways.65, 66 Thus, DGAT2 inhibition exerts both direct inhibition of hepatic TG synthesis and adaptive suppression of DNL and stimulation of hepatic fatty acid oxidation. The net result of these changes is to reduce hepatic diacylglycerol and TG which, in turn, lowers hepatocyte lipid and VLDL-TG secretion in rodents.65, 66 Similarly, oral administration of a small molecule DGAT2 inhibitor PF-06427878 for 2 weeks reduced steatosis, as assessed using magnetic resonance imaging proton density fat fraction (PDFF), by ∼40% from baseline (placebo adjusted) in human NAFLD subjects.67 This was accompanied by improvements in liver enzymes.67 Yamaguchi et al68 reported, however, that DGAT2 ASO administration to db/db mice fed a methionine- and choline-deficient diet reduced steatosis but increased fibrosis relative to mice treated with saline. However, in more recent studies, small molecule DGAT2 inhibitors improved fibrosis in the STAM mouse model,69, 70 the choline deficient high fat fed rat model71 and the LDLr–/– Leiden NASH rat model.72 Additional experiments are needed to determine if these differences are attributed to the use of ASO vs small molecule inhibitors, the specific ASO used by Yamaguchi et al,68 direct effects on DGAT2 inhibition or differences in animal models. Currently, a DGAT2 ASO (IONIS-DGAT2Rx) is reported to be in Phase 2 (NCT03334214) and the small molecule DGAT2 inhibitor PF-06865571 is in Phase 2 (NCT03776175) development for NASH as monotherapy and in combination with PF-05221304 (Table 1).
Nuclear Hormone Receptors
Nuclear hormone receptors act as ligand-inducible transcription factors to exert pleiotropic effects on metabolism73, 74 and thus may exert pleiotropic effects. Nuclear hormone receptor modulators are of interest for NASH. Specifically, peroxisome proliferator-activated receptor (PPAR) modulators, Farnesoid X receptor (FXR) agonists, and thyroid hormone receptor β (THR-β) agonists have been evaluated in NAFLD or NASH trials or are presently in development for NASH (Table 1). Two investigational agents claimed to exert the NASH benefits of pioglitazone independent of activity on PPARγ, are also in clinical development for NASH (Table 1).
Peroxisome Proliferator-Activated Receptors
PPARs are members of the nuclear hormone receptor subfamily that act as ligand-activated transcription factors to regulate metabolic and energy homeostasis. There are 3 PPAR subtypes with distinct tissue distribution and physiological function: PPARα, PPARγ, and PPARβ/δ. PPARα has relatively high expression in the liver and activation reduces hepatic triglyceride levels. Activation of PPARγ improves insulin sensitivity and enhances glucose metabolism, while activation of PPARβ/δ promotes fatty acid metabolism and suppresses macrophage-mediated inflammation.75 Pioglitazone (Table 1) is a thiazolidinedione (TZD) insulin sensitizing PPARγ agonist approved for glycemic control in T2D patients. Multiple studies have evaluated the effects of pioglitazone on NASH biopsy endpoints in patients with T2D and, in some cases, those without.76 In the Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial, pioglitazone (30 mg daily) produced reductions in steatosis (P < .001), lobular inflammation (P = .004), but not fibrosis score (P = .12) relative to placebo in patients with NASH without T2D.77 However, the primary outcome, which assessed a standardized composite score for steatosis, lobular inflammation, hepatocyte ballooning, and fibrosis was not met (P = .04 vs target value of P < .025).77 Cusi et al78 evaluated administration of pioglitazone (45 mg daily) or placebo along with a hypocaloric diet for 18 months in NASH patients with prediabetes or T2D. 58% of subjects whom received pioglitazone, vs 17% for placebo, achieved the primary endpoint goal (2-point reduction in NAFLD Activity Score [NAS]), and 51%, vs 19% for placebo, demonstrated NASH resolution. Pioglitazone administration was associated with an improvement in fibrosis score. Additionally, a meta-analysis of randomized clinical trials in which patients were treated with pioglitazone or rosiglitazone concluded that TZD administration was associated with improved advanced fibrosis (odds ratio [OR], 3.15; 95% confidence interval [CI], 1.25–7.93; P = .01; I2 = 0%), improved fibrosis of any stage (OR, 1.66; 95% CI, 1.12–2.47; P = .01; I2 = 0%), and NASH resolution (OR, 3.22; 95% CI, 2.17–4.79; P < .001; I2 = 0%).76 The observation that TZDs, and notably pioglitazone, led to improvements in multiple dimensions of NASH supports that hypothesis that agents that address the metabolic underpinnings of NASH can lead to downstream improvements in NASH resolution and fibrosis.
While pioglitazone is traditionally thought to act as an agonist of PPARγ, others have hypothesized that the benefits of pioglitazone, but not the side effects of the drug, can be separated from PPAR activity.79, 80 These investigators postulated that TZDs attenuate mitochondrial pyruvate carrier activity and that new selective mitochondrial pyruvate carrier modulators will share the therapeutic benefits of TZDs but not the side effects that limit TZD use.79, 80 Two such agents, MSDC-0602K (Cirius Therapeutics, San Diego, CA), and PXL065 (formerly DRX-065 from DeuteRx) (Poxel, Lyon, France) are presently in development for NASH (Table 1). The data generated in these trials will be helpful in testing this hypothesis.
Elafibranor is a dual PPARβ/δ agonist in Phase 3 development for NASH (Table 1). PPARα regulates lipid flux into the liver, regulating hepatic fatty acid uptake and oxidation, resulting in circulating TG reductions and high-density lipoprotein (HDL) increases. Fibrates are PPARα agonists indicted as adjunctive therapy to diet to reduce elevated LDL, total cholesterol, TG and apolipoprotein B, and to increase HDL in adult patients with primary hypercholesterolemia. A small pilot study in patients with biopsy confirmed NASH did not demonstrate significant improvements in NAFLD activity score, steatosis, or fibrosis from baseline measures following 48 weeks administration of fenofibrate (200 mg daily).81 Additionally, fenofibrate (200 mg daily) administration did not reduce steatosis in overweight or obese human volunteers with NAFLD, though serum TG reductions were observed.82, 83 These observations suggest that selective PPARα agonists may not contribute directly to NASH efficacy. PPARδ regulates fatty acid uptake and β-oxidation as well as insulin sensitivity.84 Administration of the PPARδ agonist GW501516 (Table 1) (10 mg daily) to healthy moderately overweight subjects lowered liver fat content by 20% relative to placebo (baseline adjusted) (P < .05).85 GW501516 administration also produced significant reductions in fasting TG (–30%), LDL (–23%), and insulin (–11%).85
In the Phase IIb Study to Evaluate the Efficacy and Safety of GFT505 Versus Placebo in Patients With Non-Alcoholic Steatohepatitis (NASH) (GOLDEN-505) Phase 2 trial, elafibranor (Table 1) (80 mg and 120 mg daily) or placebo was administered daily to patients with NASH without cirrhosis for 52 weeks. Elafibranor failed to meet the intent to treat protocol-defined primary outcome, resolution of NASH without fibrosis worsening.86 However, a modestly higher proportion of patients in the 120 mg elafibranor group (19%) vs the placebo group (12%) achieved a post hoc modified definition of NASH resolution without fibrosis.86 Elafibranor administration was also associated with improvement in cardiometabolic risk factors including a 0.46% (placebo adjusted) reduction in hemoglobin A1C, and reductions in fasting plasma glucose, homeostatic model assessment of insulin resistance (HOMA-IR), TG, LDL, FFA, fructosamine, and C-peptide.86 The Phase 3 Study to Evaluate the Efficacy and Safety of Elafibranor Versus Placebo in Patients With Nonalcoholic Steatohepatitis (NASH) (RESOLVE-IT) Phase 3 study [Phase 3 Study to Evaluate the Efficacy and Safety of Elafibranor Versus Placebo in Patients With Nonalcoholic Steatohepatitis (NASH)] is presently underway to evaluate the efficacy and safety of elafibranor versus placebo in patients with NASH (NCT02704403). Saroglitazar (Table 1) is a dual PPARα/γ agonist that is approved in India for treatment of diabetic dyslipidemia and hypertriglyceridemia not controlled by statin therapy.87 Saroglitazar is also presently in Phase 2 development for NASH (NCT03061721).
FXR Agonists
FXR is a nuclear hormone receptor activated by bile acids.88 FXR forms a heterodimer with the retinoid X receptor to regulate expression of target genes, generally through binding to inverted repeat elements separated by a single nucleotide.89 FXR is expressed in the ileum and in hepatic parenchymal and nonparenchymal cells such as Kupffer and stellate cells. FXR has multiple functions including feedback regulation of bile acid synthesis, regulation of glucose and lipid metabolism and regulation of hepatic inflammation and fibrosis.88 Multiple FXR agonists are in clinical development for NASH (Table 1). These agents are thought to drive efficacy through a combination of intestinal action leading to increased secretion of fibroblast growth factor 19 (FGF19) (or FGF15 in rodents) as well as direct action on the liver.
The most advanced FXR agonist is obeticholic acid, INT-747 (Table 1), a bile acid derivative presently approved for the treatment of primary biliary cholangitis and recently completed a Phase 3 trial (Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment [REGENERATE]) for the treatment of NASH with fibrosis. In the Phase 2 Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment (FLINT trial), 45% of subjects administered obeticholic acid (25 mg daily) for 72 weeks versus 21% of subjects administered placebo (P = .0002) had improved liver histology.90 Multiple dimensions of NASH pathogenesis were improved with 22% vs 13% (P = .08) showing NASH resolution, 35% vs 19% (P = .004) showing improvements in fibrosis, 45% vs 31% (P = .03) showing improvements in hepatocyte ballooning, 53% vs 35% (P = .006) showing improvements in lobular inflammation, and 61% vs 38% (P = .001) showing improvements in steatosis, in subjects administered obeticholic acid or placebo, respectively.90 Administration of obeticholic acid vs placebo was also associated with worsening in multiple cardiometabolic risk factors including significant increases in total cholesterol (P = .009), LDL cholesterol (P < .0001), alkaline phosphatase (P < .0001), HOMA-IR (P < .01), and decreased HDL (P = .01).90 In addition, administration of obeticholic acid was associated with increased incidence of pruritus, occurring in 23% of subjects who received obeticholic acid vs 6% of subjects who received placebo.90 In a subsequent study in Japanese subjects with NASH, significant improvements in the NAS were not observed in subjects administered obeticholic acid for 72 weeks at 10 mg (P = .807) or 20 mg (P = .3378).91 The 40-mg dose group achieved statistical significance (P = .0496) vs subjects who received placebo with 38% vs 20% of subjects, respectively, showing a 2-point improvement in NASH resolution.91 Significant differences were not observed in other histology endpoints including the proportion of patients with steatosis and inflammation improvement, ballooning resolution, and NASH resolution. No differences in improvements in fibrosis were observed between subjects who received obeticholic acid or placebo though dose-responsive increases in pruritus and changes in LDL, HDL, and cholesterol were consistent with data reported in the FLINT trial.91 Reasons for the apparent efficacy differences between efficacy responses in the FLINT trial conducted in the United States and the Japanese Phase 2 study are not clear. In the Phase 3 REGENERATE trial (NCT02548351), 23.1% of patients with NASH and F2 or F3 fibrosis who received 25-mg obeticholic acid daily vs 11.9% of subjects who received placebo (P = .0002) demonstrated a ≥1 stage improvement in fibrosis without worsening of NASH.92 There was no significant difference in the proportion of subjects who achieved ≥1 stage improvement in fibrosis without worsening of NASH who received 10-mg obeticholic acid daily vs placebo. Additionally, neither the 25-mg dose nor the 10-mg dose achieved significance vs placebo in the proportion of subjects with NASH and F2 or F3 fibrosis that achieved resolution of NASH without worsening of fibrosis.92 Pruritus was observed in 51% of subjects who received 25-mg obeticholic acid vs 18% of subjects who received placebo and pruritus associated treatment discontinuation was higher in the 25-mg obeticholic acid treatment group (9%) than placebo (<1%). More patients (3%) in the 25-mg obeticholic acid treatment group experienced gallstones or cholecystitis compared with the placebo group (<1%), and consistent with the results of the Phase 2 FLINT study, obeticholic acid treatment was associated with an increase in LDL cholesterol.92
The Food and Drug Administration identified 19 cases of death and 11 cases of serious liver injury in patients being treated obeticholic acid (Ocaliva) for primary biliary cholangitis, resulting in issuance of a Food and Drug Administration warning on September 21, 2017, and an update to this warning on February 1, 2018.93 Most of these cases were in subjects with moderate-to-severe decreases in liver function who received excessive dosing relative to the doses recommended for patients with impaired liver function. The warning noted that Ocaliva may also be associated with liver injury in some patients with mild disease who are receiving the correct dose, as 5 cases of serious liver injury were reported in patients with no or mild decreases in liver function before initiating Ocaliva.93 The impact of this warning for the NASH indication is unclear. Phase 3 trials evaluating the efficacy of obeticholic acid (10 and 25 mg daily) in patients with compensated cirrhosis as a consequence of NASH (REVERSE trial; NCT03439254) is ongoing.
While obeticholic acid is a steroidal bile acid analog, other FXR agonists in development are steroidal or nonsteroidal non–bile acid derivatives. These second generation FXR agonists were initially hypothesized to have an improved safety profile relative to obeticholic acid, though emerging clinical data suggest that the pruritus and worsened cardiometabolic risk factor response associated with obeticholic acid administration may be a consequence of FXR agonism rather than off-target effects resulting from the bile acid or steroidal structure of the compound. Administration of the non–bile acid, nonsteroidal FXR agonist GS-9674 (Table 1) produced a 24.6% relative median reduction in liver fat (placebo adjusted) at the 100-mg dose (P < .001), but only a 3.7% relative median reduction in liver fat (placebo adjusted) at the 30-mg dose (P = .029) in a Phase 2 study in NASH patients.94 These reductions in steatosis were associated with trends toward reductions in alanine aminotransferase (ALT), though these did not reach statistical significance (P = .11 and P = .41 for 100 mg and 30 mg, respectively).94 Trends toward increased total cholesterol (5% [P = .14] and 4.4% [P = .48] for 100 mg and 30 mg, respectively, placebo adjusted) and LDL cholesterol (10.2% [P = .13] and 9.8% [P = .28] for 100 mg and 30 mg, respectively) were also observed. Moderate to severe pruritus was observed in 14.3% of subjects who received 100 mg GS-9674, compared with 3.6% of subjects who received 30 mg GS-9674 or placebo.94 At the time of writing, only the 30-mg dose of GS-9674, which produced only a modest 3.7% placebo-adjusted reduction in steatosis, is being evaluated in the ongoing Phase 2 Safety and Efficacy of Selonsertib, Firsocostat, Cilofexor, and Combinations in Participants With Bridging Fibrosis or Compensated Cirrhosis Due to Nonalcoholic Steatohepatitis (NASH) (ATLAS) trial investigating the safety, tolerability, and efficacy of selonsertib, GS-0976, and GS-9674 as monotherapy or in combination (co-administration) in NASH patients (NCT02781584).
Interim results were presented from the Study of Safety and Efficacy of Tropifexor (LJN452) in Patients With Non-alcoholic Steatohepatitis (NASH) FLIGHT-FXR Phase 2 study (NCT02855164) evaluating safety and efficacy of tropifexor (LJN-452) in NASH patients.95 Liver fat content was reduced by 5.4% and 10.7% (relative reduction from baseline, placebo adjusted) at the 60-μg and 90-μg doses, respectively. Similarly, ALT was reduced from baseline by 8.2% and 11.4% (placebo adjusted) at the 60-μg and 90-μg doses, respectively. Tropifexor administration was associated with a mild increase in LDL and decrease in HDL and pruritus was reported in 14% and 8% of subjects at the 60-μg and 90-μg doses, respectively, relative to 7% of subjects that received placebo.
EDP-305 (Table 1) a steroidal, non–bile acid, FXR agonist is being evaluated in a Phase 2 study to assess the safety, tolerability, pharmacokinetics and efficacy in NASH patients (NCT03421431). In a Phase 1 multiple ascending dose study, robust FXR target engagement was demonstrated for EDP-305 with increases in FGF19 and decreases in C4.96 Increased incidence of pruritus was observed in subjects who received the 20-mg dose of EDP-305 relative to placebo.96 This dose was also associated with reductions in HDL and total cholesterol, but not increases in LDL, in subjects with presumptive NASH.96
THR-β Agonists
The thyroid hormone (TH) triiodothyronine and its prohormone thyroxine exert beneficial metabolic effects on TG and cholesterol through action on the THR-β in the liver. However, TH also exerts adverse cardiac and bone effects primarily through action on THR-α. First-generation THR-β–selective agonists sobetirome (GC-1) and eprotirome (KB2115) demonstrated marked reductions in circulating lipids, but were terminated for poor toleration (sobetirome) or significant elevation in transaminases in humans and deleterious cartilage findings in nonclinical toxicology studies (eprotirome).97
MGL-3196 is liver directed THR-β selective agonist designed to minimize action outside the liver by improved selectivity vs THR-α and selective uptake into the liver.98 MGL-3196 administration to healthy volunteers with mildly elevated cholesterol was well tolerated with reductions in total cholesterol, LDL and TG levels observed at doses >50 mg.99 A phase 2 study was conducted evaluating MGL-3196 in NASH patients utilizing an atypical dosing regimen where MGL-3196 was administered starting at 80 mg with a subject by subject ±20-mg dose adjustment possible at week 4 based on individual subject exposure.100 This atypical dosing regime was likely utilized because MGL-3196 demonstrated variability in exposure at higher doses and a ∼3-fold increase in exposure in healthy subjects at 100 mg relative to exposures observed at 80 mg after 14 days of dosing.99 After 12 weeks of administration, MGL-3196 produced a 26.7% relative reduction in liver fat from baseline (placebo adjusted) and a 5.8% relative reduction in ALT from baseline (placebo adjusted).100 After 36 weeks of administration, the reduction in liver fat was sustained (29% relative reduction in liver fat from baseline, placebo adjusted).101 In addition, NASH resolution was observed in 27% of subjects administered MGL-3196 vs 6% of subjects who received placebo (P = .02).101 Resolution in NASH was principally observed in those MGL-3196 treated subjects who achieved ≥30% relative reduction from baseline of liver fat content. 37% of the patients who received MGL-3196 and showed ≥30% relative reduction from baseline in liver fat content achieved NASH resolution, while only 4% of patients who received MGL-3196 and did not achieve this level of liver fat reduction demonstrated NASH resolution. Further, ≥1-point reduction in fibrosis was observed in 32% of subjects who received MGL-3196 vs 12% of subjects who received placebo (P = .03).101 A second THR-β selective agonist VK2809 (formerly MB 07811) is also in Phase 2 development for NASH. In a 12-week Phase 2 study in patients with NAFLD, subjects who received 10-mg daily VK2809 administration showed a 50.8% (P < .01) reduction from baseline in liver fat content (placebo adjusted).102 ALT levels increased, mostly during early treatment, but after 12 weeks of administration, ALT levels were not different in subjects that received VK2809 or placebo. ALT increases were also observed with VK2809 in some subjects in Phase 1.103
Adipose Tissue Metabolism in Nafld
Obesity and systemic insulin resistance are major comorbidities associated with NAFLD104, 105 and human studies demonstrate that circulating FFAs derived from adipose tissue lipolysis are the principal supplier of fat to the liver and the single biggest contributor to fasting VLDL-TG in human NAFLD.27, 106, 107 An important study in humans by Lambert et al27 used stable isotopes to compare fatty acid flux in subjects with NAFLD compared with controls matched for other metabolic parameters, and demonstrated that NAFLD subjects not only have elevated hepatic de novo lipogenesis, but also have significantly elevated nocturnal plasma FFA levels. Given that most of the fat in the liver is derived from adipose tissue lipolysis, this could be an important target for NAFLD pharmacotherapy. In healthy lean people, insulin normally inhibits the breakdown and release of fatty acids from adipocytes. As insulin resistance develops in the context of metabolic disease, however, there is a failure of insulin to suppress hormone sensitive lipase, leading to a largely unrestrained release of FFAs from adipose tissue into the blood. The majority of these circulating FFAs are subsequently taken up by the liver resulting in hepatic steatosis. In addition, hypertrophy of expanding adipocytes leads to recruitment of adipose tissue macrophages and release of inflammatory cytokines including tumor necrosis factor alpha. Tumor necrosis factor alpha further exacerbates adipose insulin resistance via direct effects on the insulin receptor signaling cascade, leading to additional effects on lipolysis.108 The relative contribution of distinct adipose depots to NAFLD pathogenesis in humans is largely unknown. Visceral adipose tissue lies anatomically adjacent to the portal vein, is a major source of inflammatory cytokines and is closely linked to systemic insulin resistance yet contributes a relatively small percentage to the overall nonesterified fatty acid pool. Recent studies have implicated abdominal subcutaneous adipose tissue dysfunction in NASH lipotoxicity, pointing to likely contributions from multiple adipose depots in linking systemic insulin resistance to NAFLD.109 Overall there is a compelling body of evidence linking dysfunctional adipose tissue metabolism to NASH subjects,110 suggesting a path forward for NASH treatments that improve adipose tissue health, restore adipose insulin sensitivity and inhibit adipose tissue lipolysis.
Glycemic Modulators
Incretin Therapies
Glucagon-like peptide-1 (GLP-1) is a gut-derived incretin hormone with pleiotropic beneficial metabolic effects, including stimulation of insulin secretion, inhibition of glucagon secretion, and weight loss.111 Incretin-based therapies are increasingly being used to treat T2D and obesity. Given the efficacy of GLP-1R agonists and DPP-4 inhibitors in treating insulin-resistant metabolic dysfunction, it is no surprise that these mechanisms are also being investigated as potential NASH therapies. Numerous GLP-1 analogs, GLP-1R agonists, and DPP-4 inhibitors have shown promising efficacy in many preclinical models of NAFLD and NASH (reviewed in Bifari et al112). Human subjects with insulin resistance and NAFLD have been shown to have increased hepatic messenger RNA expression of DPP-4113 and slight elevations in plasma DPP-4 activity compared with healthy control subjects.114 However, the DPP-4 inhibitor sitagliptin showed no beneficial effect on insulin resistance or liver steatosis/function in small clinical trials.115, 116 The most compelling evidence to date for the potential of the GLP-1 mechanism in NASH arises from the LEAN (Liraglutide Efficacy and Action in NASH) trial. The GLP-1R agonist liraglutide (dose = 1.8 mg given once daily for 48 weeks to overweight and obese subjects with a diagnosis of NASH) resulted in increased resolution of NASH and importantly, reduced progression of fibrosis, compared with the placebo group.117 The longer acting GLP-1 agonist semaglutide is currently being evaluated in a Phase 2 trial (NCT02970942) for subjects with biopsy-confirmed NASH.
Agonism of the glucagon receptor is also being evaluated for efficacy in NAFLD/NASH, primarily in combination with GLP-1 agonists, due to expected effects on reducing food intake and increasing energy expenditure. Additionally, dual glucose-dependent insulinotropic polypeptide/GLP-1 receptor agonists are also under development for treating metabolic dysfunction; this dual incretin approach appears to be more efficacious on metabolic endpoints preclinically when compared with single agonists (reviewed in Seghieri et al118). In support of this concept, recent results published from a randomized, placebo-controlled Phase 2 trial of the dual glucose-dependent insulinotropic polypeptide/GLP-1R agonist LY3298176 in patients with T2D demonstrated statistically significant HbA1c lowering with greater weight loss than the GLP-1R agonist dulaglutide alone.119 Furthermore, the polypeptide SAR425899, which acts as an agonist at both the GLP-1R and the glucagon receptor, reduced body weight and lowered glycemia in type 2 diabetes mellitus (T2DM) subjects,120 and is currently being investigated for efficacy in T2DM patients with NASH (NCT03437720). Numerous agents are currently under development in this area for their promise to reduce body weight and subsequently impact insulin resistance and liver health (summarized in Brandt et al121).
The mechanism(s) by which GLP1 improves hepatic steatosis, liver inflammation, and injury remain unclear. Although somewhat controversial, the liver is not likely a direct target of GLP1’s actions because most studies have not been able to detect expression of the canonical GLP1 receptors in hepatocytes.122, 123 It is possible that improved steatosis seen with GLP1-based therapies is secondary to weight loss, improved glycemic control and effects on inflammation or the gut microbiota. Regardless, a treatment that targets multiple components of metabolic derangement present in NASH, including body weight, glycemia, hepatic steatosis and inflammation, is highly desirable. Furthermore, the reduced cardiovascular risk shown in the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial provides additional support for this mechanism in treating NASH patients who are at higher risk of cardiovascular disease.124 Although incretin-based therapies for NASH appear promising and will continue to be vetted in the clinic, more studies are needed to gain an understanding of the relevant site(s) of action and a clear mechanistic link to disease resolution.
Sodium Glucose Co-transporter 2 Inhibitors
Insulin resistance and NAFLD are tightly linked as evidenced by the significantly increased risk of NAFLD in patients with T2D.125 Inhibitors of the renal sodium glucose co-transporter 2 (SGLT2) are a growing class of T2DM agents which improve blood glucose levels by increasing urinary output of excess glucose (reviewed in Vivian126). Treatment with SGLT2 inhibitors leads to multiple beneficial effects, including improved glycemia without risk of hypoglycemia, improved cardiovascular risk factors, and slight reductions in body weight. The class in general is well-tolerated, with urinary tract and genital infections being the most common side effects, although more serious adverse events including euglycemic diabetic ketoacidosis127 and increased risk of lower limb amputation have been reported for at least 1 member of the SGLT2 inhibitor class.128 Notably, results of the EMPA-REG clinical trial (NCT01131676) demonstrated that administration of once-daily empagliflozin in patients with T2D at high cardiovascular risk resulted in a reduction in cardiovascular mortality compared with placebo.129 Given that NASH patients are at an increased risk of cardiovascular mortality,125 this is an attractive feature for potential NASH pharmacotherapy.
Numerous preclinical studies have demonstrated benefit of SGLT2 inhibition in NASH models.11, 130, 131, 132, 133, 134, 135 To date, however, only a few relatively small clinical trials have been conducted with SGLT2 inhibitors in NAFLD or NASH patients (summarized in Khan et al125). It is difficult to draw robust conclusions from these studies due to the small sample size and mild effects on most endpoints examined. In a small study on the effects of canagliflozin on hepatic function in T2DM patients with biopsy-confirmed NASH (n = 10), subjects received 100-mg canagliflozin once a day for 12 weeks. A mild degree of ALT lowering was reported: the change in ALT from baseline to week 12 was –23.9 U/L (95% CI, –48.1 to 0.3; P = .0526).136 Empagliflozin was found to lower ALT/aspartate aminotransferase (AST) levels in individuals with T2D assessed by analysis of several placebo-controlled randomized trials including the EMPA-REG OUTCOME trial.137 In the E-LIFT trial, 50 patients with T2DM and NAFLD were randomly assigned to the control group or to receive 10-mg daily empagliflozin for 20 weeks with a change in liver fat measured by magnetic resonance imaging PDFF. When compared with baseline measurements, the empagliflozin group showed a statistically significant reduction in magnetic resonance imaging PDFF (16.2% to 11.3%; P < .0001) while the control group did not show a statistically significant reduction (16.4% to 15.5%; P < .057).138 Several open-label pilot studies are listed in ClinicalTrials.gov investigating SGLT2 inhibitors in NAFLD patients (NCT02696941; NCT02875821; NCT02964715; NCT02649465; NCT03646292), and a prospective study examining the effects on empagliflozin on liver fat and energy metabolism in patients with newly diagnosed T2DM recently completed in August 2018 (NCT02637973). Currently, a combined SGLT1/2 inhibitor (LIK066) is being investigated in a 12-week randomized, placebo-controlled study in obese subjects with NASH (NCT03205150). Finally, the SGLT2 inhibitor dapagliflozin will be evaluated in a randomized, placebo-controlled phase 3 interventional trial (NCT03723252) and is expected to be completed in 2021.
The precise mechanism for improvements in NAFLD or NASH pathogenesis with the SGLT2 inhibitor class is likely multifactorial. SGLT2 inhibition leads to a small amount of weight loss, improvements in glycemic control, reduced insulin levels and a reduction in inflammation. Lower circulating glucose and insulin levels result in decreased activation of ChREBP and SREBP1c in the liver and subsequently reduced fatty acid synthesis. Regardless of the mechanism, initial small trials hint at possible efficacy for this class of drugs in improving NAFLD, an established comorbidity in diabetic patients. Larger clinical trials with hepatic histopathological endpoints are needed to establish efficacy in treating NASH.
Fibroblast Growth Factors
FGFs comprise a large, pleiotropic family of growth factors implicated in a broad range of physiological processes including cell growth and differentiation and metabolic control. FGFs can act in an autocrine, paracrine, or endocrine manner, and signaling is initiated by binding to an FGF receptor and co-receptor (ie, β-klotho).139 FGF21 is produced in the liver and other metabolically active tissues, and in rodents is nutritionally regulated as a target of PPARα.140, 141, 142 Depending on the species, pharmacological administration of FGF21 has been shown to improve dyslipidemia, reduce body weight and improve insulin sensitivity, making this a potentially attractive therapy for treatment of metabolic disorders (reviewed in Gimeno and Moller143, Reitman,144 and Zhang and Li145). The precise mechanism(s) by which FGF21 may improve metabolic dysfunction are largely unknown and are likely pleiotropic, involving multiple cell types or sites of action and downstream metabolic pathways including stimulation of hepatic fatty acid oxidation, glucose uptake into adipose, autonomic regulation in the brain and anti-inflammatory effects.146, 147, 148
FGF21 has received significant attention as a possible therapeutic modality for NASH due to its demonstrated beneficial effects on liver metabolism in rodents, primates and humans. Increased fructose consumption is associated with fatty liver disease and leads to an elevation of circulating FGF21 levels149 via a ChREBP-mediated mechanism.150 In this context, FGF21 is an important component of the protective, homeostatic response to fructose consumption and the absence of FGF21 in mice on a high-fructose diet exacerbates liver disease.150 Consistent with this notion, a recent study demonstrated that FGF21 is required to blunt progression from fatty liver to hepatocellular carcinoma upon prolonged consumption of a high-fat, high-sucrose diet.151 Mice deficient in FGF21 develop significant hepatic steatosis, worsening of fibrosis and inflammation and apoptosis on a methionine- and choline- deficient diet which could be improved via FGF21 infusion.152 Treatment of obese mice with FGF21 leads to weight loss, reduction of hepatic steatosis and improved liver metabolic function153, 154, 155, 156, 157, 158, 159; importantly, FGF21 administration in NASH rodent models also has body weight-independent anti-inflammatory and antifibrotic effects in the liver.152 In Zucker rats, FGF21 improved glucose tolerance and liver insulin sensitivity without significant effects on body weight.160 Consistently, in diabetic rhesus monkeys FGF21 administration led to improvements in fasting plasma glucose and insulin levels, reduced triglycerides with only a minor reduction in body weight,161 and injection of a FGF21 mimetic antibody activating the β-klotho/FGFR1c receptor in obese cynomolgus monkeys induced reductions in plasma insulin, triglycerides, glucose, and body weight.162
Numerous FGF21 analogs and mimetics have been developed and are in various stages of clinical development (Table 1 and summarized in Gimeno and Moller143). Phase 1 clinical trials show improvements in dyslipidemia, insulin sensitivity, and body weight in obese/T2D subjects.163, 164, 165 Numerous Phase 2 clinical trials are being conducted with a pegylated form of FGF21 for the treatment of NASH (BMS-986036; A Study of Experimental Medication BMS-986036 in Adults With Nonalcoholic Steatohepatitis (NASH) and Stage 3 Liver Fibrosis (FALCON 1) trials; NCT02413372),166 and an integrated safety analysis presented at the International Liver Congress (European Association for the Study of the Liver) meeting in April 2018 concluded that daily or weekly subcutaneous dosing of BMS-986036 for up to 16 weeks was generally well tolerated in obese or T2D subjects.167 Potential safety signals have been reported in some clinical trials using a long-acting FGF21 molecule, PF-05231023, including modulation of markers of bone formation/turnover and increases in blood pressure and pulse rate not predicted in nonhuman primates.164, 165 It is unclear whether these are mechanistic effects of FGF21 agonism; thus, it will be important to do a comprehensive safety assessment of current and future clinical stage FGF21 assets. The interest in this mechanism remains robust as evidenced by recent optioning of the β-klotho/FGFR1c receptor agonist NGM313 (renamed MK-3655168), which showed reductions in steatosis in a Phase 1 open label single dose study without a placebo control (NCT03298464),169 and interest in another FGF21 analog (AKR-001).
Rodent FGF15 and the human analog FGF19 are expressed in enterocytes of the small intestine and secreted into portal circulation. FGF15/FGF19 transcription is regulated by FXR binding to a response element on the FGF15/19 gene and circulating FGF15/19 levels are increased as a consequence of intestinal FXR agonist activity. FGF15/19 functions to regulate bile acid synthesis as well as carbohydrate metabolism and energy expenditure.170 FGF15/19 inhibits bile acid synthesis in the liver by repressing transcription of cholesterol 7α-hydroxylase, the rate-limiting enzyme in the bile acid synthetic pathway, and stimulates the gallbladder to fill with bile. FGF15/19 promotes hepatic glycogen and protein synthesis through action on the FGFR4/β-klotho receptor complex in the liver and suppresses hepatic gluconeogenesis. Mice expressing FGF19 have higher metabolic rate, improved glycemic control, and reduced body weight and liver fat content despite increased food consumption. Additionally, however, FGF15/19 also promotes liver growth and has also been implicated in liver tumorigenesis.170 NGM282 is a modified analog of FGF19 designed to be a nontumorigenic variant that maintains the positive metabolic functions of native FGF19.171 Once-daily subcutaneous administration of NGM282 for 12 weeks to patients with NASH produced marked reductions in steatosis with a 57% and 45% (P < .0001) relative reduction from baseline (placebo adjusted) observed at the 6- and 3-mg doses, respectively.172 Improvements in ALT (P < .0001), AST (P < .0001), and C4 (P < .0001) were also noted, though improvements in glycemic control were not observed. Injection site reactions were observed in 54% and 41% of subjects in the 6- and 3-mg dose groups relative to 7% in the placebo group.172 Additionally, 32% and 19% of subjects in the 6- and 3-mg dose groups experience at least 1 adverse event leading to temporary interruption or discontinuation of study drug relative to 4% of subjects administered placebo. Incidence of diarrhea were also higher in treated subjects (36% and 41% at the 6- and 3-mg dose levels, respectively) relative to placebo (22%), as were abdominal pain, nausea and abdominal distension.172 In an exploratory, non–placebo-controlled, 12-week study, 67% (1 mg) and 74% (3 mg) of subjects showed an improvement in steatosis, 33% (1 mg), and 42% (3 mg) showed improvement in inflammation, 42% (1 mg) and 53% (3 mg) showed improvement in ballooning, and 25% (1 mg) and 42% (3 mg) showed improvement in fibrosis, as assessed by liver histology.173 Overall, initial clinical results suggest FGF19 agonism may be a viable treatment for NASH, but as with all current NASH assets, more studies need to be conducted in humans to assess the side effect profile and tolerability of this mechanism for longer term chronic treatment.174
Conclusions
Novel therapies are being evaluated and developed for NASH at a rapid rate, with most agents targeting at least some aspect of metabolic dysfunction. While many of these established mechanisms are covered in this review, there are additional emerging areas under investigation for effects on hepatic metabolism including AMPK activation,175, 176 sirtuins,177, 178 and the gut microbiome.179 It should be noted, however, that metformin, which activates AMPK, has not impacted histological endpoints in NAFLD or NASH despite showing improvements on HOMA-IR and liver function.180 Disruption in the gut-liver barrier (aka “leaky gut”) is common in NASH and may be an important link between the transition from simple steatosis to a more severe form of the disease.181 Although NASH is well accepted as a “metabolic” disease, with insulin resistance, obesity, and hepatic steatosis as major risk factors predisposing to disease pathogenesis, there is heterogeneous susceptibility in the progression from NAFLD to NASH. Part of this variability may be explained by genetic predisposition as emerging human genetics has revealed several genetic risk variants for NAFLD or NASH, including mutations in PNPLA3 (148M), TM6SF2 (E167K), GCKR, and hydroxysteroid dehydrogenase 13 (reviewed in182, 183, 184, 185, 186). Emerging analysis of the liver transplant literature suggests that NAFLD, and even NASH, will recur in the new liver at a greater rate if underlying metabolic dysfunction is not “cured,” providing support for the notion that NASH is a metabolically driven disease.187, 188 Indeed, lifestyle modification through diet and physical activity remains at the forefront of NASH management and has the potential to improve overall metabolic health and predisposing NASH risk factors. However, long-term compliance to lifestyle changes is a challenge for most patients, underscoring the urgent need for effective pharmacotherapies for patients with NASH.
Acknowledgements
The authors thank Dave Beebe for his critical review of this manuscript.
Footnotes
Conflicts of interest The authors declare the following: William P. Esler and Kendra K. Bence are employees of Pfizer, Inc and may be shareholders of Pfizer, Inc.
References
- 1.Sanyal A.J., Friedman S.L., McCullough A.J., Dimick-Santos L. American Association for the Study of Liver D, United States F, Drug A. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392–1405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan F.Z., Perumpail R.B., Wong R.J., Ahmed A. Advances in hepatocellular carcinoma: Nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol. 2015;7:2155–2161. doi: 10.4254/wjh.v7.i18.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day C.P., James O.F. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 4.Reeves H.L., Burt A.D., Wood S., Day C.P. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol. 1996;25:677–683. doi: 10.1016/s0168-8278(96)80238-8. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen T.I., Orholm M., Bentsen K.D., Hoybye G., Eghoje K., Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet. 1984;2:241–244. doi: 10.1016/s0140-6736(84)90295-2. [DOI] [PubMed] [Google Scholar]
- 6.Wanless I.R., Lentz J.S. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 7.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A., Boerwinkle E., Cohen J.C., Hobbs H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rotman Y., Koh C., Zmuda J.M., Kleiner D.E., Liang T.J., Nash C.R.N. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C., Grundy S.M., Hobbs H.H. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 11.Honda Y., Imajo K., Kato T., Kessoku T., Ogawa Y., Tomeno W., Kato S., Mawatari H., Fujita K., Yoneda M., Saito S., Nakajima A. The selective SGLT2 inhibitor ipragliflozin has a therapeutic effect on nonalcoholic steatohepatitis in mice. PLoS One. 2016;11:e0146337. doi: 10.1371/journal.pone.0146337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji G., Wang Y., Deng Y., Li X., Jiang Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health Dis. 2015;14:134. doi: 10.1186/s12944-015-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kita Y., Takamura T., Misu H., Ota T., Kurita S., Takeshita Y., Uno M., Matsuzawa-Nagata N., Kato K., Ando H., Fujimura A., Hayashi K., Kimura T., Ni Y., Otoda T., Miyamoto K., Zen Y., Nakanuma Y., Kaneko S. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PLoS One. 2012;7:e43056. doi: 10.1371/journal.pone.0043056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein T., Fujii M., Sandel J., Shibazaki Y., Wakamatsu K., Mark M., Yoneyama H. Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med Mol Morphol. 2014;47:137–149. doi: 10.1007/s00795-013-0053-9. [DOI] [PubMed] [Google Scholar]
- 15.Liu W., Struik D., Nies V.J., Jurdzinski A., Harkema L., de Bruin A., Verkade H.J., Downes M., Evans R.M., van Zutphen T., Jonker J.W. Effective treatment of steatosis and steatohepatitis by fibroblast growth factor 1 in mouse models of nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2016;113:2288–2293. doi: 10.1073/pnas.1525093113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison M.C., Liang W., Mulder P., Verschuren L., Pieterman E., Toet K., Heeringa P., Wielinga P.Y., Kooistra T., Kleemann R. Mirtoselect, an anthocyanin-rich bilberry extract, attenuates non-alcoholic steatohepatitis and associated fibrosis in ApoE( *)3Leiden mice. J Hepatol. 2015;62:1180–1186. doi: 10.1016/j.jhep.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Qiang X., Xu L., Zhang M., Zhang P., Wang Y., Wang Y., Zhao Z., Chen H., Liu X., Zhang Y. Demethyleneberberine attenuates non-alcoholic fatty liver disease with activation of AMPK and inhibition of oxidative stress. Biochem Biophys Res Commun. 2016;472:603–609. doi: 10.1016/j.bbrc.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Soares e Silva A.K., de Oliveira Cipriano Torres D., dos Santos Gomes F.O., dos Santos Silva B., Lima Ribeiro E., Costa Oliveira A., dos Santos L.A., de Lima Mdo C., Pitta Ida R., Peixoto C.A. LPSF/GQ-02 inhibits the development of hepatic steatosis and inflammation in a mouse model of non-alcoholic fatty liver disease (NAFLD) PLoS One. 2015;10:e0123787. doi: 10.1371/journal.pone.0123787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staels B., Rubenstrunk A., Noel B., Rigou G., Delataille P., Millatt L.J., Baron M., Lucas A., Tailleux A., Hum D.W., Ratziu V., Cariou B., Hanf R. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 20.Verbeek J., Lannoo M., Pirinen E., Ryu D., Spincemaille P., Vander Elst I., Windmolders P., Thevissen K., Cammue B.P., van Pelt J., Fransis S., Van Eyken P., Ceuterick-De Groote C., Van Veldhoven P.P., Bedossa P., Nevens F., Auwerx J., Cassiman D. Roux-en-y gastric bypass attenuates hepatic mitochondrial dysfunction in mice with non-alcoholic steatohepatitis. Gut. 2015;64:673–683. doi: 10.1136/gutjnl-2014-306748. [DOI] [PubMed] [Google Scholar]
- 21.Wada T., Miyashita Y., Sasaki M., Aruga Y., Nakamura Y., Ishii Y., Sasahara M., Kanasaki K., Kitada M., Koya D., Shimano H., Tsuneki H., Sasaoka T. Eplerenone ameliorates the phenotypes of metabolic syndrome with NASH in liver-specific SREBP-1c Tg mice fed high-fat and high-fructose diet. Am J Physiol Endocrinol Metab. 2013;305:E1415–E1425. doi: 10.1152/ajpendo.00419.2013. [DOI] [PubMed] [Google Scholar]
- 22.Mummadi R.R., Kasturi K.S., Chennareddygari S., Sood G.K. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–1402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Huang M.A., Greenson J.K., Chao C., Anderson L., Peterman D., Jacobson J., Emick D., Lok A.S., Conjeevaram H.S. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 24.Hooper A.J., Adams L.A., Burnett J.R. Genetic determinants of hepatic steatosis in man. J Lipid Res. 2011;52:593–617. doi: 10.1194/jlr.R008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohjima M., Enjoji M., Higuchi N., Kato M., Kotoh K., Yoshimoto T., Fujino T., Yada M., Yada R., Harada N., Takayanagi R., Nakamuta M. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351–358. [PubMed] [Google Scholar]
- 26.Pettinelli P., Del Pozo T., Araya J., Rodrigo R., Araya A.V., Smok G., Csendes A., Gutierrez L., Rojas J., Korn O., Maluenda F., Diaz J.C., Rencoret G., Braghetto I., Castillo J., Poniachik J., Videla L.A. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta. 2009;1792:1080–1086. doi: 10.1016/j.bbadis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudgins L.C., Parker T.S., Levine D.M., Hellerstein M.K. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab. 2011;96:861–868. doi: 10.1210/jc.2010-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abid A., Taha O., Nseir W., Farah R., Grosovski M., Assy N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol. 2009;51:918–924. doi: 10.1016/j.jhep.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Ouyang X., Cirillo P., Sautin Y., McCall S., Bruchette J.L., Diehl A.M., Johnson R.J., Abdelmalek M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman M.A., Samuel V.T. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab. 2016;27:719–730. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.M., Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 33.Stanhope K.L., Schwarz J.M., Havel P.J. Adverse metabolic effects of dietary fructose: results from the recent epidemiological, clinical, and mechanistic studies. Curr Opin Lipidol. 2013;24:198–206. doi: 10.1097/MOL.0b013e3283613bca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann S.M., Tschop M.H. Dietary sugars: a fat difference. J Clin Invest. 2009;119:1089–1092. doi: 10.1172/JCI39332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huard K., Ahn K., Amor P., Beebe D.A., Borzilleri K.A., Chrunyk B.A., Coffey S.B., Cong Y., Conn E.L., Culp J.S., Dowling M.S., Gorgoglione M.F., Gutierrez J.A., Knafels J.D., Lachapelle E.A., Pandit J., Parris K.D., Perez S., Pfefferkorn J.A., Price D.A., Raymer B., Ross T.T., Shavnya A., Smith A.C., Subashi T.A., Tesz G.J., Thuma B.A., Tu M., Weaver J.D., Weng Y., Withka J.M., Xing G., Magee T.V. Discovery of fragment-derived small molecules for in vivo inhibition of ketohexokinase (KHK) J Med Chem. 2017;60:7835–7849. doi: 10.1021/acs.jmedchem.7b00947. [DOI] [PubMed] [Google Scholar]
- 36.Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C.N., Bahre H., Tschirner S.K., Gorinski N., Gohmert M., Mayer C.T., Huehn J., Ponimaskin E., Abraham W.R., Muller R., Lochner M., Sparwasser T. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 37.Rau M., Schilling A.K., Meertens J., Hering I., Weiss J., Jurowich C., Kudlich T., Hermanns H.M., Bantel H., Beyersdorf N., Geier A. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of Th17 cells in the liver and an increased Th17/resting regulatory T cell ratio in peripheral blood and in the liver. J Immunol. 2016;196:97–105. doi: 10.4049/jimmunol.1501175. [DOI] [PubMed] [Google Scholar]
- 38.Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr. 2008;28:253–272. doi: 10.1146/annurev.nutr.28.061807.155434. [DOI] [PubMed] [Google Scholar]
- 39.Kreuz S., Schoelch C., Thomas L., Rist W., Rippmann J.F., Neubauer H. Acetyl-CoA carboxylases 1 and 2 show distinct expression patterns in rats and humans and alterations in obesity and diabetes. Diabetes Metab Res Rev. 2009;25:577–586. doi: 10.1002/dmrr.997. [DOI] [PubMed] [Google Scholar]
- 40.Harwood H.J., Jr., Petras S.F., Shelly L.D., Zaccaro L.M., Perry D.A., Makowski M.R., Hargrove D.M., Martin K.A., Tracey W.R., Chapman J.G., Magee W.P., Dalvie D.K., Soliman V.F., Martin W.H., Mularski C.J., Eisenbeis S.A. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. J Biol Chem. 2003;278:37099–37111. doi: 10.1074/jbc.M304481200. [DOI] [PubMed] [Google Scholar]
- 41.Corbett J.W., Freeman-Cook K.D., Elliott R., Vajdos F., Rajamohan F., Kohls D., Marr E., Zhang H., Tong L., Tu M., Murdande S., Doran S.D., Houser J.A., Song W., Jones C.J., Coffey S.B., Buzon L., Minich M.L., Dirico K.J., Tapley S., McPherson R.K., Sugarman E., Harwood H.J., Jr., Esler W. Discovery of small molecule isozyme non-specific inhibitors of mammalian acetyl-CoA carboxylase 1 and 2. Bioorg Med Chem Lett. 2010;20:2383–2388. doi: 10.1016/j.bmcl.2009.04.091. [DOI] [PubMed] [Google Scholar]
- 42.Freeman-Cook K.D., Amor P., Bader S., Buzon L.M., Coffey S.B., Corbett J.W., Dirico K.J., Doran S.D., Elliott R.L., Esler W., Guzman-Perez A., Henegar K.E., Houser J.A., Jones C.S., Limberakis C., Loomis K., McPherson K., Murdande S., Nelson K.L., Phillion D., Pierce B.S., Song W., Sugarman E., Tapley S., Tu M., Zhao Z. Maximizing lipophilic efficiency: the use of Free-Wilson analysis in the design of inhibitors of acetyl-CoA carboxylase. J Med Chem. 2012;55:935–942. doi: 10.1021/jm201503u. [DOI] [PubMed] [Google Scholar]
- 43.Griffith D.A., Dow R.L., Huard K., Edmonds D.J., Bagley S.W., Polivkova J., Zeng D., Garcia-Irizarry C.N., Southers J.A., Esler W., Amor P., Loomis K., McPherson K., Bahnck K.B., Preville C., Banks T., Moore D.E., Mathiowetz A.M., Menhaji-Klotz E., Smith A.C., Doran S.D., Beebe D.A., Dunn M.F. Spirolactam-based acetyl-CoA carboxylase inhibitors: toward improved metabolic stability of a chromanone lead structure. J Med Chem. 2013;56:7110–7119. doi: 10.1021/jm401033t. [DOI] [PubMed] [Google Scholar]
- 44.Griffith D.A., Kung D.W., Esler W.P., Amor P.A., Bagley S.W., Beysen C., Carvajal-Gonzalez S., Doran S.D., Limberakis C., Mathiowetz A.M., McPherson K., Price D.A., Ravussin E., Sonnenberg G.E., Southers J.A., Sweet L.J., Turner S.M., Vajdos F.F. Decreasing the rate of metabolic ketone reduction in the discovery of a clinical acetyl-CoA carboxylase inhibitor for the treatment of diabetes. J Med Chem. 2014;57:10512–10526. doi: 10.1021/jm5016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kung D.W., Griffith D.A., Esler W.P., Vajdos F.F., Mathiowetz A.M., Doran S.D., Amor P.A., Bagley S.W., Banks T., Cabral S., Ford K., Garcia-Irizarry C.N., Landis M.S., Loomis K., McPherson K., Niosi M., Rockwell K.L., Rose C., Smith A.C., Southers J.A., Tapley S., Tu M., Valentine J.J. Discovery of spirocyclic-diamine inhibitors of mammalian acetyl CoA-carboxylase. Bioorg Med Chem Lett. 2015;25:5352–5356. doi: 10.1016/j.bmcl.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 46.Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X., Burgess S.C., Li C., Ruddy M., Chakravarthy M., Previs S., Milstein S., Fitzgerald K., Kelley D.E., Horton J.D. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26:576. doi: 10.1016/j.cmet.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Goedeke L., Bates J., Vatner D.F., Perry R.J., Wang T., Ramirez R., Li L., Ellis M.W., Zhang D., Wong K.E., Beysen C., Cline G.W., Ray A.S., Shulman G.I. Acetyl-CoA carboxylase inhibition reverses NAFLD and hepatic insulin resistance but promotes hypertriglyceridemia in rodents. Hepatology. 2018;68:2197–2211. doi: 10.1002/hep.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergman A., Gonzalez S.C., Tarabar S., Saxena A., Esler W., Amin N. Safety, tolerability, pharmacokinetics and pharmacodynamics of a liver-targeting ACC inhibitor (PF-05221304) following single and multiple oral doses. J Hepatol. 2018;68(Suppl 1):S582. [Google Scholar]
- 49.Lawitz E.J., Coste A., Poordad F., Alkhouri N., Loo N., McColgan B.J., Tarrant J.M., Nguyen T., Han L., Chung C., Ray A.S., McHutchison J.G., Subramanian G.M., Myers R.P., Middleton M.S., Sirlin C., Loomba R., Nyangau E., Fitch M., Li K., Hellerstein M. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2018;16:1983–1991. doi: 10.1016/j.cgh.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 50.Loomba R., Kayali Z., Noureddin M., Ruane P., Lawitz E.J., Bennett M., Wang L., Harting E., Tarrant J.M., McColgan B.J., Chung C., Ray A.S., Subramanian G.M., Myers R.P., Middleton M.S., Lai M., Charlton M., Harrison S.A. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155:1463–1473.e6. doi: 10.1053/j.gastro.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menendez J.A., Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 52.Menendez J.A., Lupu R. Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin Ther Targets. 2017;21:1001–1016. doi: 10.1080/14728222.2017.1381087. [DOI] [PubMed] [Google Scholar]
- 53.Angeles T.S., Hudkins R.L. Recent advances in targeting the fatty acid biosynthetic pathway using fatty acid synthase inhibitors. Expert Opin Drug Discov. 2016;11:1187–1199. doi: 10.1080/17460441.2016.1245286. [DOI] [PubMed] [Google Scholar]
- 54.Syed-Abdul M.M., Parks E.J., Bingham K., Le N., Hammoud G.M., Gaballah A.H., Kemble G., Buckley D., McCulloch W., Manrique C.M. Progressive reductions in hepatic DNL with increasing doses of TVB-2640, a first-in-class pharmacologic inhibitor of FASN. Organ Crosstalk in Obesity and NAFLD, Keystone, Coloradoeee, 2018. http://3vbio.com/wp-content/uploads/2018/02/Keystone-2018-poster-SyedAbdul_letter.pdf Available at: Accessed March 20, 2019.
- 55.Yen C.L., Stone S.J., Koliwad S., Harris C., Farese R.V., Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhatt-Wessel B., Jordan T.W., Miller J.H., Peng L. Role of DGAT enzymes in triacylglycerol metabolism. Arch Biochem Biophys. 2018;655:1–11. doi: 10.1016/j.abb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Buhman K.K., Smith S.J., Stone S.J., Repa J.J., Wong J.S., Knapp F.F., Jr., Burri B.J., Hamilton R.L., Abumrad N.A., Farese R.V., Jr. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem. 2002;277:25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 58.Cases S., Stone S.J., Zhou P., Yen E., Tow B., Lardizabal K.D., Voelker T., Farese R.V., Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 59.Qi J., Lang W., Geisler J.G., Wang P., Petrounia I., Mai S., Smith C., Askari H., Struble G.T., Williams R., Bhanot S., Monia B.P., Bayoumy S., Grant E., Caldwell G.W., Todd M.J., Liang Y., Gaul M.D., Demarest K.T., Connelly M.A. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J Lipid Res. 2012;53:1106–1116. doi: 10.1194/jlr.M020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeVita R.J., Pinto S. Current status of the research and development of diacylglycerol O-acyltransferase 1 (DGAT1) inhibitors. J Med Chem. 2013;56:9820–9825. doi: 10.1021/jm4007033. [DOI] [PubMed] [Google Scholar]
- 61.Denison H., Nilsson C., Kujacic M., Lofgren L., Karlsson C., Knutsson M., Eriksson J.W. Proof of mechanism for the DGAT1 inhibitor AZD7687: results from a first-time-in-human single-dose study. Diabetes Obes Metab. 2013;15:136–143. doi: 10.1111/dom.12002. [DOI] [PubMed] [Google Scholar]
- 62.Denison H., Nilsson C., Lofgren L., Himmelmann A., Martensson G., Knutsson M., Al-Shurbaji A., Tornqvist H., Eriksson J.W. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes Metab. 2014;16:334–343. doi: 10.1111/dom.12221. [DOI] [PubMed] [Google Scholar]
- 63.Maciejewski B.S., LaPerle J.L., Chen D., Ghosh A., Zavadoski W.J., McDonald T.S., Manion T.B., Mather D., Patterson T.A., Hanna M., Watkins S., Gibbs E.M., Calle R.A., Steppan C.M. Pharmacological inhibition to examine the role of DGAT1 in dietary lipid absorption in rodents and humans. Am J Physiol Gastrointest Liver Physiol. 2013;304:G958–G969. doi: 10.1152/ajpgi.00384.2012. [DOI] [PubMed] [Google Scholar]
- 64.Hung Y.H., Carreiro A.L., Buhman K.K. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:600–614. doi: 10.1016/j.bbalip.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi C.S., Savage D.B., Kulkarni A., Yu X.X., Liu Z.X., Morino K., Kim S., Distefano A., Samuel V.T., Neschen S., Zhang D., Wang A., Zhang X.M., Kahn M., Cline G.W., Pandey S.K., Geisler J.G., Bhanot S., Monia B.P., Shulman G.I. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 66.Yu X.X., Murray S.F., Pandey S.K., Booten S.L., Bao D., Song X.Z., Kelly S., Chen S., McKay R., Monia B.P., Bhanot S. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 67.Birnbaum M. Global Engage; London, UK: 2018. The Etiology and Treatment of Fatty Liver Disease. Global NASH Congress 2018. [Google Scholar]
- 68.Yamaguchi K., Yang L., McCall S., Huang J., Yu X.X., Pandey S.K., Bhanot S., Monia B.P., Li Y.X., Diehl A.M. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology. 2008;47:625–635. doi: 10.1002/hep.21988. [DOI] [PubMed] [Google Scholar]
- 69.Perez S., Purkal J.J., Clark R., Kou K., Vera N.B., Lanba A., Futatsugi K., Gosset J.R., Opsahl A., Okerberg C., Pfefferkorn J.A., Erion D.M., Goodwin B. American Society for Biochemistry and Molecular Biology; Monterey, CA: 2015. Characterization of the Effects of a Novel DGAT2 Inhibitor of Hepatic Lipid Metabolism. DEUEL Conference, Abstract 21. [Google Scholar]
- 70.Erion D, Purkal JJ, Pfefferkorn JA, Goodwin B. DGAT2 inhibition prevents NAFLD and fibrosis in the STAM model of NASH. Presented at: Liver Metabolism and Nonalcoholic Fatty Liver Disease (NAFLD) Keystone Symposium, Whistler, Canada, March 25, 2015.
- 71.Ross T.T., Crowley C., Beebe D.A., Kou K., Kelly K., Calle R.A., Tesz G.J., Bence K., Dullea R., Esler W. European Association for the Study of Liver; Geneva, Switzerland: 2018. ACCi/DGAT2i combination therapy for the treatment of NASH. EASL NAFLD Summit; pp. P01–P09. [Google Scholar]
- 72.Salic K., Morrison M.C., Brosnan J., Gart E., Bence K., Kleemann R., Tesz G.J. European Association for the Study of Liver; Geneva, Switzerland: 2018. Inhibition of DGAT2 improves hepatic steatosis, inflammation and cardiovascular risk factors in the LDLr-/-.Leiden mouse. EASL NAFLD Summit; pp. P06–P09. [Google Scholar]
- 73.Aranda A., Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 74.Mangelsdorf D.J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R.M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanaka N., Aoyama T., Kimura S., Gonzalez F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142–157. doi: 10.1016/j.pharmthera.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Musso G., Cassader M., Paschetta E., Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177:633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A., Lavine J.E., Tonascia J., Unalp A., Van Natta M., Clark J., Brunt E.M., Kleiner D.E., Hoofnagle J.H., Robuck P.R., Nash C.R.N. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C., Tio F., Hardies J., Darland C., Musi N., Webb A., Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 79.Colca J.R., McDonald W.G., Cavey G.S., Cole S.L., Holewa D.D., Brightwell-Conrad A.S., Wolfe C.L., Wheeler J.S., Coulter K.R., Kilkuskie P.M., Gracheva E., Korshunova Y., Trusgnich M., Karr R., Wiley S.E., Divakaruni A.S., Murphy A.N., Vigueira P.A., Finck B.N., Kletzien R.F. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers (mTOT)--relationship to newly identified mitochondrial pyruvate carrier proteins. PLoS One. 2013;8:e61551. doi: 10.1371/journal.pone.0061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colca J.R., McDonald W.G., McCommis K.S., Finck B.N. Treating fatty liver disease by modulating mitochondrial pyruvate metabolism. Hepatol Commun. 2017;1:193–197. doi: 10.1002/hep4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fernandez-Miranda C., Perez-Carreras M., Colina F., Lopez-Alonso G., Vargas C., Solis-Herruzo J.A. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Fabbrini E., Mohammed B.S., Korenblat K.M., Magkos F., McCrea J., Patterson B.W., Klein S. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:2727–2735. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oscarsson J., Onnerhag K., Riserus U., Sunden M., Johansson L., Jansson P.A., Moris L., Nilsson P.M., Eriksson J.W., Lind L. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: a double-blind, randomized, placebo-controlled study. J Clin Lipidol. 2018;12:1390–1403.e4. doi: 10.1016/j.jacl.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y., Colby J.K., Zuo X., Jaoude J., Wei D., Shureiqi I. The Role of PPAR-delta in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int J Mol Sci. 2018;19:E3339. doi: 10.3390/ijms19113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riserus U., Sprecher D., Johnson T., Olson E., Hirschberg S., Liu A., Fang Z., Hegde P., Richards D., Sarov-Blat L., Strum J.C., Basu S., Cheeseman J., Fielding B.A., Humphreys S.M., Danoff T., Moore N.R., Murgatroyd P., O'Rahilly S., Sutton P., Willson T., Hassall D., Frayn K.N., Karpe F. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 86.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L., Romero-Gomez M., Boursier J., Abdelmalek M., Caldwell S., Drenth J., Anstee Q.M., Hum D., Hanf R., Roudot A., Megnien S., Staels B., Sanyal A., Group G.-I.S. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159.e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 87.Joshi S.R. Saroglitazar for the treatment of dyslipidemia in diabetic patients. Expert Opin Pharmacother. 2015;16:597–606. doi: 10.1517/14656566.2015.1009894. [DOI] [PubMed] [Google Scholar]
- 88.Yuan L., Bambha K. Bile acid receptors and nonalcoholic fatty liver disease. World J Hepatol. 2015;7:2811–2818. doi: 10.4254/wjh.v7.i28.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calkin A.C., Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F., Chalasani N., Dasarathy S., Diehl A.M., Hameed B., Kowdley K.V., McCullough A., Terrault N., Clark J.M., Tonascia J., Brunt E.M., Kleiner D.E., Doo E., Network N.C.R. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Intercept Pharmaceuticals Press Release Intercept pharmaceuticals announces results of phase 2 trial of OCA in NASH patients in Japan. GLOBE NEWSWIRE. October 28, 2015 [Google Scholar]
- 92.Younossi Z, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome P, Sheridan D, Trotter J, Knapple W, Lawitz E, Kowdley K, Montano-Loza A, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-PintoH, Graupera I, Orr D, Gluud LL, Dufour J-F, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal A. Positive results from REGENERATE: A phase 3 international, randomized, placebo-controlled study evaluating obeticholic acid treatment for NASH. EASL International Liver Congress, Vienna, Austria, GS-06, April 11, 2019.
- 93.FDA Safety Announcement: “FDA adds Boxed Warning to highlight correct dosing of Ocaliva (obeticholic acid) for patients with a rare chronic liver disease” released on 1 Feb, 2018. https://www.fda.gov/Drugs/DrugSafety/ucm594941.htm Available at: Accessed March 1, 2019.
- 94.Patel K., Harrison S.A., Trotter J.F., Herring R., Rojter S.E., Kayali Z., Shiffman M.L., Freilich B.L., Lawitz E.J., Harting E., Nguyen T., Chung C., Subramanian M., Myers R.P., Middleton M.S., Rinella M., Noureddin M. The non-steroidal FXR agonist GS–9674 leads to significant reductions in hepatic steatosis, serum bile acids, and liver biochemistry in a phase 2, randomized, placebo-controlled trial of patients with NASH. Hepatology. 2018;68:1460A–1461A. [Google Scholar]
- 95.Sanyal A., lopez P., Lawitz E.J., Kim W., Huang J.-F., Andreone P., Bee B., Goh G., Chen Y.-C., Ratziu V., Kim Y.J., Ryan M., Weltman M., Geier A., Loeffler J., Schaefer F., Vaidyanathan S., Brass C. Tropifexor (TXR), an FXR agonist for the treatment of NASH—interim results from first two parts of phase 2b study Flight-FXR. Hepatology. 2018;68 LB–23. [Google Scholar]
- 96.Ahmed A., Sanderson K., Dickerson D., Adda N. Pharmacokinetics, pharmacodynamics, and safety of EDP-305, in healthy and presumptive NAFLD subjects. J Hepatol. 2018;68(Suppl 1):S584. [Google Scholar]
- 97.Elbers L.P., Kastelein J.J., Sjouke B. Thyroid hormone mimetics: the past, current status and future challenges. Curr Atheroscler Rep. 2016;18:14. doi: 10.1007/s11883-016-0564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelly M.J., Pietranico-Cole S., Larigan J.D., Haynes N.E., Reynolds C.H., Scott N., Vermeulen J., Dvorozniak M., Conde-Knape K., Huang K.S., So S.S., Thakkar K., Qian Y., Banner B., Mennona F., Danzi S., Klein I., Taub R., Tilley J. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dio xo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor beta agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014;57:3912–3923. doi: 10.1021/jm4019299. [DOI] [PubMed] [Google Scholar]
- 99.Taub R., Chiang E., Chabot-Blanchet M., Kelly M.J., Reeves R.A., Guertin M.C., Tardif J.C. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-beta agonist. Atherosclerosis. 2013;230:373–380. doi: 10.1016/j.atherosclerosis.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 100.Harrison S., Moussa S., Bashir M., Alkhouri N., Frias J., Baum S., Tetri B., Bansal M., Taub R. MGL-3196, a selective thyroid hormone receptor-beta agonist significantly decreases hepatic fat in NASH patients at 12 weeks,the primary endpoint in a 36 week serial liver biopsy study, In EASL International Liver Congress, Paris, France. Journal of Hepatology. April 7th, 2018;68:S37–S64. [Google Scholar]
- 101.Harrison S., Guy C., Bashir M., Frias J., Alkhouri N., Baum S., Taub R., Moylan C., Bansal M., Neuschwander-Tetri B.A., Moussa S. In a placebo-controlled 36-week phase 2 trial, treatment with Mgl-3196 compared with placebo results in significant reductions in hepatic fat (MRI-PDFF), liver enzymes, fibrosis biomarkers, atherogenic lipids, and improvement in NASH on serial liver biopsy. Hepatology. 2018;68:9A–10A. [Google Scholar]
- 102.Loomba R., Neutel J., Bernard D., Severance R., Mohseni R., Dao M., Saini S., Margaritescu, Homer K., Tran B., Mancini M., Masamune H., Lian B. VK2809, a novel liver-directed thyroid receptor beta agonist, significantly reduces liver fat in patients with non-alcoholic fatty liver disease: a phase 2 randomized, placebo-controlled trial. Hepatology. 2018;68:1447A. [Google Scholar]
- 103.Lian B., Hanley R., Schoenfeld S. A phase 1 randomized, double-blind, placebo-controlled, multiple ascending dose study to evaluate safety, tolerability and pharmacokinetics of the liver-selective TR-beta agonist Vk2809 (Mb07811) in hypercholesterolemic subjects. J Am Coll Cardiol. 2016;67:1932. [Google Scholar]
- 104.Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A.J., Natale S., Forlani G., Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 105.Kotronen A., Juurinen L., Tiikkainen M., Vehkavaara S., Yki-Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–130. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 106.Barrows B.R., Parks E.J. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 107.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hotamisligil G.S. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 109.Armstrong M.J., Hazlehurst J.M., Hull D., Guo K., Borrows S., Yu J., Gough S.C., Newsome P.N., Tomlinson J.W. Abdominal subcutaneous adipose tissue insulin resistance and lipolysis in patients with non-alcoholic steatohepatitis. Diabetes Obes Metab. 2014;16:651–660. doi: 10.1111/dom.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725.e6. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 111.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 112.Bifari F., Manfrini R., Dei Cas M., Berra C., Siano M., Zuin M., Paroni R., Folli F. Multiple target tissue effects of GLP-1 analogues on non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) Pharmacol Res. 2018;137:219–229. doi: 10.1016/j.phrs.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 113.Miyazaki M., Kato M., Tanaka K., Tanaka M., Kohjima M., Nakamura K., Enjoji M., Nakamuta M., Kotoh K., Takayanagi R. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729–733. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 114.Baumeier C., Schluter L., Saussenthaler S., Laeger T., Rodiger M., Alaze S.A., Fritsche L., Haring H.U., Stefan N., Fritsche A., Schwenk R.W., Schurmann A. Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol Metab. 2017;6:1254–1263. doi: 10.1016/j.molmet.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cui J., Philo L., Nguyen P., Hofflich H., Hernandez C., Bettencourt R., Richards L., Salotti J., Bhatt A., Hooker J., Haufe W., Hooker C., Brenner D.A., Sirlin C.B., Loomba R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Joy T.R., McKenzie C.A., Tirona R.G., Summers K., Seney S., Chakrabarti S., Malhotra N., Beaton M.D. Sitagliptin in patients with non-alcoholic steatohepatitis: a randomized, placebo-controlled trial. World J Gastroenterol. 2017;23:141–150. doi: 10.3748/wjg.v23.i1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R., Hazlehurst J.M., Guo K., team Lt. Abouda G., Aldersley M.A., Stocken D., Gough S.C., Tomlinson J.W., Brown R.M., Hubscher S.G., Newsome P.N. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 118.Seghieri M., Christensen A.S., Andersen A., Solini A., Knop F.K., Vilsboll T. Future perspectives on GLP-1 receptor agonists and GLP-1/glucagon receptor Co-agonists in the treatment of NAFLD. Front Endocrinol (Lausanne) 2018;9:649. doi: 10.3389/fendo.2018.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C., Urva S., Gimeno R.E., Milicevic Z., Robins D., Haupt A. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 120.Tillner J., Posch M.G., Wagner F., Teichert L., Hijazi Y., Einig C., Keil S., Haack T., Wagner M., Bossart M., Larsen P.J. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab. 2019;21:120–128. doi: 10.1111/dom.13494. [DOI] [PubMed] [Google Scholar]
- 121.Brandt S.J., Kleinert M., Tschop M.H., Muller T.D. Are peptide conjugates the golden therapy against obesity? J Endocrinol. 2018;238:R109–R119. doi: 10.1530/JOE-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Drucker D.J. Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes. 2015;64:317–326. doi: 10.2337/db14-1514. [DOI] [PubMed] [Google Scholar]
- 123.Dhir G., Cusi K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-alcoholic fatty liver disease: a novel therapeutic option. J Investig Med. 2018;66:7–10. doi: 10.1136/jim-2017-000554. [DOI] [PubMed] [Google Scholar]
- 124.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., Steinberg W.M., Stockner M., Zinman B., Bergenstal R.M., Buse J.B., Committee L.S., Investigators L.T. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khan R., Bril F., Cusi K., Newsome P.N. Modulation of insulin resistance in NAFLD. Hepatology. 2018 Dec 16 doi: 10.1002/hep.30429. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 126.Vivian E.M. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context. 2014;3:212264. doi: 10.7573/dic.212264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qiu H., Novikov A., Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev. 2017;33:dmrr2886. doi: 10.1002/dmrr.2886. [DOI] [PubMed] [Google Scholar]
- 128.Rastogi A., Bhansali A. SGLT2 inhibitors through the windows of EMPA-REG and CANVAS trials: a review. Diabetes Ther. 2017;8:1245–1251. doi: 10.1007/s13300-017-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., Broedl U.C., Inzucchi S.E., Investigators E.-R.O. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 130.Jojima T., Tomotsune T., Iijima T., Akimoto K., Suzuki K., Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr. 2016;8:45. doi: 10.1186/s13098-016-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kabil S.L., Mahmoud N.M. Canagliflozin protects against non-alcoholic steatohepatitis in type-2 diabetic rats through zinc alpha-2 glycoprotein up-regulation. Eur J Pharmacol. 2018;828:135–145. doi: 10.1016/j.ejphar.2018.03.043. [DOI] [PubMed] [Google Scholar]
- 132.Komiya C., Tsuchiya K., Shiba K., Miyachi Y., Furuke S., Shimazu N., Yamaguchi S., Kanno K., Ogawa Y. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS One. 2016;11:e0151511. doi: 10.1371/journal.pone.0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qiang S., Nakatsu Y., Seno Y., Fujishiro M., Sakoda H., Kushiyama A., Mori K., Matsunaga Y., Yamamotoya T., Kamata H., Asano T. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr. 2015;7:104. doi: 10.1186/s13098-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shiba K., Tsuchiya K., Komiya C., Miyachi Y., Mori K., Shimazu N., Yamaguchi S., Ogasawara N., Katoh M., Itoh M., Suganami T., Ogawa Y. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8:2362. doi: 10.1038/s41598-018-19658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tahara A., Kurosaki E., Yokono M., Yamajuku D., Kihara R., Hayashizaki Y., Takasu T., Imamura M., Li Q., Tomiyama H., Kobayashi Y., Noda A., Sasamata M., Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 136.Seko Y., Nishikawa T., Umemura A., Yamaguchi K., Moriguchi M., Yasui K., Kimura M., Iijima H., Hashimoto T., Sumida Y., Okanoue T., Itoh Y. Efficacy and safety of canagliflozin in type 2 diabetes mellitus patients with biopsy-proven nonalcoholic steatohepatitis classified as stage 1-3 fibrosis. Diabetes Metab Syndr Obes. 2018;11:835–843. doi: 10.2147/DMSO.S184767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sattar N., Fitchett D., Hantel S., George J.T., Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME(R) trial. Diabetologia. 2018;61:2155–2163. doi: 10.1007/s00125-018-4702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kuchay M.S., Krishan S., Mishra S.K., Farooqui K.J., Singh M.K., Wasir J.S., Bansal B., Kaur P., Jevalikar G., Gill H.K., Choudhary N.S., Mithal A. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 139.Kuro O.M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15:27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 140.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 141.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., Elmquist J.K., Gerard R.D., Burgess S.C., Hammer R.E., Mangelsdorf D.J., Kliewer S.A. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 142.Maratos-Flier E. Fatty liver and FGF21 physiology. Exp Cell Res. 2017;360:2–5. doi: 10.1016/j.yexcr.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 143.Gimeno R.E., Moller D.E. FGF21-based pharmacotherapy--potential utility for metabolic disorders. Trends Endocrinol Metab. 2014;25:303–311. doi: 10.1016/j.tem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 144.Reitman M.L. FGF21 mimetic shows therapeutic promise. Cell Metab. 2013;18:307–309. doi: 10.1016/j.cmet.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang J., Li Y. Fibroblast growth factor 21, the endocrine FGF pathway and novel treatments for metabolic syndrome. Drug Discov Today. 2014;19:579–589. doi: 10.1016/j.drudis.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 146.Inagaki T. Research perspectives on the regulation and physiological functions of FGF21 and its association with NAFLD. Front Endocrinol (Lausanne) 2015;6:147. doi: 10.3389/fendo.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 148.Lin X., Liu Y.B., Hu H. Metabolic role of fibroblast growth factor 21 in liver, adipose and nervous system tissues. Biomed Rep. 2017;6:495–502. doi: 10.3892/br.2017.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dushay J.R., Toschi E., Mitten E.K., Fisher F.M., Herman M.A., Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab. 2015;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fisher F.M., Kim M., Doridot L., Cunniff J.C., Parker T.S., Levine D.M., Hellerstein M.K., Hudgins L.C., Maratos-Flier E., Herman M.A. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab. 2017;6:14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singhal G., Kumar G., Chan S., Fisher F.M., Ma Y., Vardeh H.G., Nasser I.A., Flier J.S., Maratos-Flier E. Deficiency of fibroblast growth factor 21 (FGF21) promotes hepatocellular carcinoma (HCC) in mice on a long term obesogenic diet. Mol Metab. 2018;13:56–66. doi: 10.1016/j.molmet.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fisher F.M., Chui P.C., Nasser I.A., Popov Y., Cunniff J.C., Lundasen T., Kharitonenkov A., Schuppan D., Flier J.S., Maratos-Flier E. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology. 2014;147:1073–1083.e6. doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y., Moller D.E., Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 154.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J., Sandusky G.E., Hammond L.J., Moyers J.S., Owens R.A., Gromada J., Brozinick J.T., Hawkins E.D., Wroblewski V.J., Li D.S., Mehrbod F., Jaskunas S.R., Shanafelt A.B. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Laeger T., Baumeier C., Wilhelmi I., Wurfel J., Kamitz A., Schurmann A. FGF21 improves glucose homeostasis in an obese diabetes-prone mouse model independent of body fat changes. Diabetologia. 2017;60:2274–2284. doi: 10.1007/s00125-017-4389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu J., Stanislaus S., Chinookoswong N., Lau Y.Y., Hager T., Patel J., Ge H., Weiszmann J., Lu S.C., Graham M., Busby J., Hecht R., Li Y.S., Li Y., Lindberg R., Veniant M.M. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009;297:E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 157.Lee J.H., Kang Y.E., Chang J.Y., Park K.C., Kim H.W., Kim J.T., Kim H.J., Yi H.S., Shong M., Chung H.K., Kim K.S. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res. 2016;8:4750–4763. [PMC free article] [PubMed] [Google Scholar]
- 158.Bao L., Yin J., Gao W., Wang Q., Yao W., Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. 2018;175:3379–3393. doi: 10.1111/bph.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jimenez V., Jambrina C., Casana E., Sacristan V., Munoz S., Darriba S., Rodo J., Mallol C., Garcia M., Leon X., Marco S., Ribera A., Elias I., Casellas A., Grass I., Elias G., Ferre T., Motas S., Franckhauser S., Mulero F., Navarro M., Haurigot V., Ruberte J., Bosch F. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol Med. 2018;10:e8791. doi: 10.15252/emmm.201708791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bernardo B., Lu M., Bandyopadhyay G., Li P., Zhou Y., Huang J., Levin N., Tomas E.M., Calle R.A., Erion D.M., Rolph T.P., Brenner M., Talukdar S. FGF21 does not require interscapular brown adipose tissue and improves liver metabolic profile in animal models of obesity and insulin-resistance. Sci Rep. 2015;5:11382. doi: 10.1038/srep11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kharitonenkov A., Wroblewski V.J., Koester A., Chen Y.F., Clutinger C.K., Tigno X.T., Hansen B.C., Shanafelt A.B., Etgen G.J. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 162.Foltz I.N., Hu S., King C., Wu X., Yang C., Wang W., Weiszmann J., Stevens J., Chen J.S., Nuanmanee N., Gupte J., Komorowski R., Sekirov L., Hager T., Arora T., Ge H., Baribault H., Wang F., Sheng J., Karow M., Wang M., Luo Y., McKeehan W., Wang Z., Veniant M.M., Li Y. Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Sci Transl Med. 2012;4:162ra153. doi: 10.1126/scitranslmed.3004690. [DOI] [PubMed] [Google Scholar]
- 163.Gaich G., Chien J.Y., Fu H., Glass L.C., Deeg M.A., Holland W.L., Kharitonenkov A., Bumol T., Schilske H.K., Moller D.E. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 164.Kim A.M., Somayaji V.R., Dong J.Q., Rolph T.P., Weng Y., Chabot J.R., Gropp K.E., Talukdar S., Calle R.A. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes Metab. 2017;19:1762–1772. doi: 10.1111/dom.13023. [DOI] [PubMed] [Google Scholar]
- 165.Talukdar S., Zhou Y., Li D., Rossulek M., Dong J., Somayaji V., Weng Y., Clark R., Lanba A., Owen B.M., Brenner M.B., Trimmer J.K., Gropp K.E., Chabot J.R., Erion D.M., Rolph T.P., Goodwin B., Calle R.A. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 2016;23:427–440. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 166.Bristol-Myers-Squibb’s BMS-986036 (Pegylated FGF21) shows consistent improvement in liver fat, liver injury, and fibrosis in patients with nonalcoholic steatohepatitis (NASH) in phase 2 trial. April 22, 2017. https://news.bms.com/press-release/bmy/bristol-myers-squibbs-bms-986036-pegylated-fgf21-shows-consistent-improvement-live Available at:
- 167.Halegoua-Demarzio D., Charles E., Tetri B., Kundu S., Tirucherai G., Christian R. An integrated safety analysis of phase 2 studies of BMS-986036, a PEGylated fibroblast growth factor 21 analogue for the treatment of non-alcoholic steatohepatitis. J Hepatol. 2018;68(Suppl 1):S584–S585. [Google Scholar]
- 168.Merck Exercises Option for NGM Bio’s Investigational Insulin Sensitizer, NGM313, for the Treatment of NASH and Type 2 Diabetes. NGM-Biopharmaceuticals Newsroom. https://ir.ngmbio.com/news-releases/news-release-details/merck-exercises-option-ngm-bios-investigational-insulin Available at:
- 169.Shankar S., Bashir M., Baxter B., Phung V., Yan A.Z., Rossi S.J.R., DePaoli A.M. LB-21: NGM313, a novel once-monthly activator of β-Klotho/FGFR1c, significantly reduces hepatic steatosis and key biomarkers of nonalcoholic steatohepatitis: results of a randomized, active-controlled clamp study in obese insulin resistant patients with NAFLD. Hepatology. 2018;68:1459A. [Google Scholar]
- 170.Kliewer S.A., Mangelsdorf D.J. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 2015;33:327–331. doi: 10.1159/000371670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou M., Wang X., Phung V., Lindhout D.A., Mondal K., Hsu J.Y., Yang H., Humphrey M., Ding X., Arora T., Learned R.M., DePaoli A.M., Tian H., Ling L. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res. 2014;74:3306–3316. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]
- 172.Harrison S.A., Rinella M.E., Abdelmalek M.F., Trotter J.F., Paredes A.H., Arnold H.L., Kugelmas M., Bashir M.R., Jaros M.J., Ling L., Rossi S.J., DePaoli A.M., Loomba R. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 173.Harrison S, Trotter JF, Paredes AH, Guy C, Bashir M, Jaros M, Banerjee R, Baxter B, ling L, Rossi S, Somaratne RA, DePaoli AM. NGM282 rapidly improves NAFLD activity score (NAS) and fibrosis in 12 weeks in patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH): results of a phase 2 multi-center dose finding study. Presented at: AASLD Liver Meeting, San Francisco, California, November 9, 2018.
- 174.Charlton M. FGF-19 agonism for NASH: a short study of a long disease. Lancet. 2018;391:1124–1126. doi: 10.1016/S0140-6736(18)30425-2. [DOI] [PubMed] [Google Scholar]
- 175.Smith B.K., Marcinko K., Desjardins E.M., Lally J.S., Ford R.J., Steinberg G.R. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730–E740. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- 176.Garcia D., Hellberg K., Chaix A., Wallace M., Herzig S., Badur M.G., Lin T., Shokhirev M.N., Pinto A.F.M., Ross D.S., Saghatelian A., Panda S., Dow L.E., Metallo C.M., Shaw R.J. Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD. Cell Rep. 2019;26:192–208.e6. doi: 10.1016/j.celrep.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Colak Y., Yesil A., Mutlu H.H., Caklili O.T., Ulasoglu C., Senates E., Takir M., Kostek O., Yilmaz Y., Yilmaz Enc F., Tasan G., Tuncer I. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. J Gastrointestin Liver Dis. 2014;23:311–3119. doi: 10.15403/jgld.2014.1121.233.yck. [DOI] [PubMed] [Google Scholar]
- 178.Nassir F., Ibdah J.A. Sirtuins and nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:10084–10092. doi: 10.3748/wjg.v22.i46.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Banini B.A., Sanyal A.J. Current and future pharmacologic treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2017;33:134–141. doi: 10.1097/MOG.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li Y., Liu L., Wang B., Wang J., Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kolodziejczyk A.A., Zheng D., Shibolet O., Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11:e9302. doi: 10.15252/emmm.201809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stender S., Kozlitina J., Nordestgaard B.G., Tybjaerg-Hansen A., Hobbs H.H., Cohen J.C. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49:842–847. doi: 10.1038/ng.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Anstee Q.M., Day C.P. The genetics of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:645–655. doi: 10.1038/nrgastro.2013.182. [DOI] [PubMed] [Google Scholar]
- 184.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 185.Abul-Husn N.S., Cheng X., Li A.H., Xin Y., Schurmann C., Stevis P., Liu Y., Kozlitina J., Stender S., Wood G.C., Stepanchick A.N., Still M.D., McCarthy S., O'Dushlaine C., Packer J.S., Balasubramanian S., Gosalia N., Esopi D., Kim S.Y., Mukherjee S., Lopez A.E., Fuller E.D., Penn J., Chu X., Luo J.Z., Mirshahi U.L., Carey D.J., Still C.D., Feldman M.D., Small A., Damrauer S.M., Rader D.J., Zambrowicz B., Olson W., Murphy A.J., Borecki I.B., Shuldiner A.R., Reid J.G., Overton J.D., Yancopoulos G.D., Hobbs H.H., Cohen J.C., Gottesman O., Teslovich T.M., Baras A., Mirshahi T., Gromada J., Dewey F.E. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pirola C.J., Garaycoechea M., Flichman D., Arrese M., San Martino J., Gazzi C., Castano G.O., Sookoian S. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. J Lipid Res. 2019;60:176–185. doi: 10.1194/jlr.P089953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Newsome P.N. Recurrence of nonalcoholic fatty liver disease after liver transplantation: it is common, but does it affect outcome? Liver Transpl. 2010;16:420–422. doi: 10.1002/lt.22038. [DOI] [PubMed] [Google Scholar]
- 188.Yalamanchili K., Saadeh S., Klintmalm G.B., Jennings L.W., Davis G.L. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]