Abstract
Ubiquitin-specific protease 26 (USP26) is an X-linked gene exclusively expressed in the testis and codes for the USP26, a peptidase enzyme that belongs to the deubiquitinating enzyme family. Recent studies have indicated that mutations in USP26 affect spermatogenesis and are associated with male infertility in humans and mice. However, the exact role of USP26 in spermatogenesis and how it affects male reproduction remains unknown. In this study, we generated a conventional Usp26 knockout mouse model and found that deletion of Usp26 in male mice (Usp26−/Y) leads to significantly reduced pup numbers per litter and significantly increased intervals between two consecutive offspring. We also found that the serum follicle stimulating hormone and testosterone levels of adult Usp26−/Y mice were significantly decreased compared to those of Usp26+/Y mice. Histological examination results showed that Usp26−/Y mice had significantly increased percentage of abnormal seminiferous tubules at different ages. Flow cytometry results exhibited that Usp26−/Y mice had significantly reduced percentage of mature haploid cells in the testes compared to Usp26+/Y mice. Sperm counts in epididymis were also significantly declined in Usp26−/Y mice compared to those in Usp26+/Y mice. Immunohistochemistry and immunofluorescence staining and immunoprecipitation analysis results showed that USP26 and androgen receptor were co-localized in mouse testicular cells at different ages and they both had physiological interactions. All these results demonstrated that the loss of Usp26 affects spermatogenesis and hormone secretion and causes male subfertility. Our study also provides the evidence on the interactions between USP26 and androgen receptor in mouse testis, whereby pointing to a potential mechanism.
Keywords: ubiquitin specific protease 26 (USP26), Usp26 gene, male subfertility, spermatogenesis, androgen receptor (AR), knockout (KO) mice
The phenotype of Usp26 knock out in male mice is subfertility, which may be related to the loss of regulation of androgen-AR signaling by USP26 in the testis.
Introduction
Infertility affects 15% of couples seeking children worldwide [1], and approximately 30%–55% of these couples involve male factor infertility [2, 3]. Many factors are thought to have an impact on male infertility including occupational and environmental exposures and particular genetic factors [4]. Known genetic disorders are responsible for 15% of overall male infertility cases [5–7], and genetic alterations yet to be discovered could well account for many “idiopathic infertility” cases [8]. Recent genetic studies in mice have shown that abundant X-linked genes play important roles in regulation of male fertility [9, 10]. The involvement of mutations in the X-linked gene, ubiquitin protease 26 (USP26) (located at Xq26.2), in male infertility was studied extensively, but has remained controversial [11–15].
The USP26 gene was first reported by Wang et al. [9], who isolated it from mouse spermatogonia. Its expression was reported to be testis-specific in mice and humans [9, 16–18]. USP26 belongs to a family of deubiquitination enzymes (DUBs), which play crucial roles in regulating protein stability and activity [19, 20]. Protein ubiquitination is a post-translational modification process that regulates diverse arrays of cellular and biological processes and intracellular signaling pathways [21]. It is understood that, where there is ubiquitination, deubiquitination by DUBs also exists to counterbalance the ubiquitination process in a reversible manner. DUBs are negative regulators of protein ubiquitination that play an important role in regulating ubiquitin-dependent processes, which play crucial roles in regulating protein stability and activity [19, 20]. Recent studies on DUBs have convincingly demonstrated that these enzymes play an important role in the process of spermatogenesis [22]. Spermatogenesis is a complex process, which includes three phases based on functional considerations: (1) the proliferative phase, in which spermatogonia undergo rapid successive divisions; (2) the meiotic phase, in which genetic material in spermatocytes is recombined and segregated; and (3) the spermiogenic phase, in which spermatids transform into spermatozoa [23]. Such a complex program requires the precise expression of enzymes and structural proteins, which is affected not only by regulation of gene transcription and translation, but also by targeted protein degradation. It has become apparent that spermatogenesis is dependent on the balanced actions of ubiquitination and deubiquitination [24]. Studies from our group and others have described specific mutations in USP26 that are associated with spermatogenesis [11, 12, 25, 26]. The exclusive expression and localization of USP26 in human and mouse testicular tissues have been reported [16, 18, 27]. USP26 has also been recognized as a regulator of androgen receptor (AR) signaling by reversing hormone-induced AR ubiquitination in an in vitro study [17]. However, the mechanism of how USP26 affects spermatogenesis remains unknown.
In this study, we report the first investigation of Usp26’s function in male reproduction by generating the Usp26 knockout (KO) mouse model. We found that mutant male mice (Usp26−/Y) exhibit subfertility with impaired testicular development and spermiogenesis, which may be related to the dysregulation of AR by lacking USP26 expression in the testis.
Materials and methods
Animals and ethics statement
This study was performed strictly in accordance with the Guidelines of the Institutional Animal Care and Use Committee (IACUC) of Xi’an Jiaotong University, Xi’an, P.R. China. The protocol was approved by the IACUC of Xi’an Jiaotong University (IACUC Approval #: No. XJTULAC2017-683). All animals were housed in a temperature- and humidity-controlled facility with 12-h light: 12-h darkness cycle and provided with food and water ad libitum. Euthanasia was performed by CO2 asphyxiation followed by cervical dislocation. All efforts were made to minimize suffering.
Generation of Usp26 knockout mice and genotyping
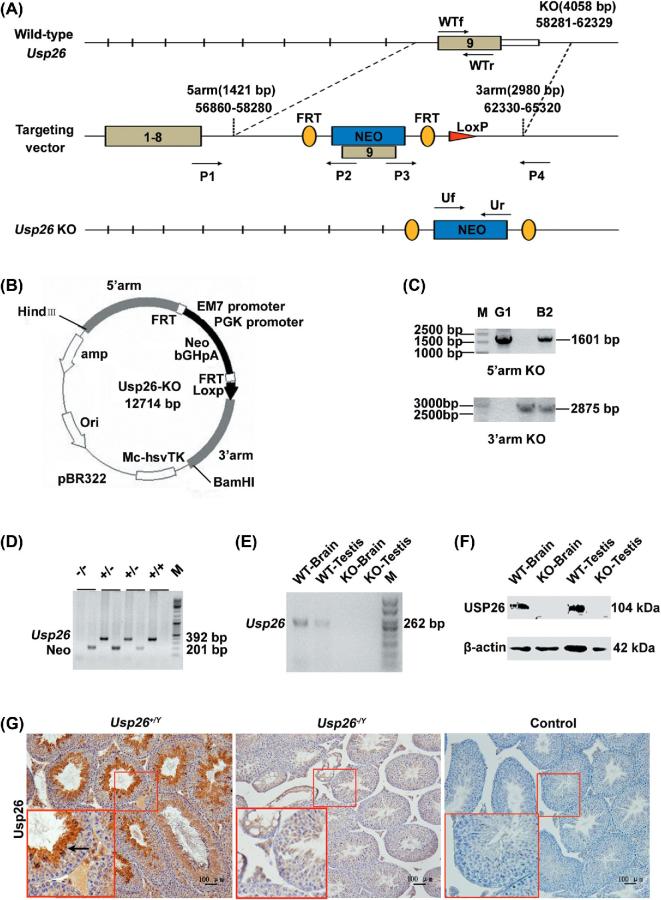
The Usp26 heterozygous mice were generated at the Shanghai Biomodel Organism Science & Technology Development Co., Ltd, Shanghai, China. The breeding of heterozygous mice to generate Usp26 homozygous KO mice was performed at the Xi’an Jiaotong University animal facility. The Usp26-targeting vector was constructed using a recombinant strategy [28–30]. The gene-targeting strategy was designed to remove exon 9 from Usp26. The targeting construct, which had 1421-bp and 2980-bp genomic sequences as the 5′ and 3′ homology regions, respectively, was inserted into the pBR322 vector (Figure 1A and B). To assemble the targeting construct, a neomycin gene driven by the phosphoglycerate kinase (PGK) promoter and a PGK poly (A) signal was cloned in a 5′-to-3′ orientation in a Not I site within exon 9 contained in a BamHI fragment. This insertion disrupts the reading frame. A flanking herpes simplex TK gene driven by the same promoter and enhancer was added to the 3′ end of the BamHI fragment. Thirty-five micrograms of the targeting vector was linearized by Not I and transfected into SCR012 (derived from 129SV/J) embryonic stem cells (ESCs) by electroporation. Ninety-six ESC clones were selected with G418 (Geneticin, Sigma-Aldrich). Among these neomycin-resistant cells, eight ESCs that had undergone homologous recombination were identified by polymerase chain reaction (PCR) analysis with two pairs of primers: Usp26-5P (P1), neo-R (P2) and neo-Lh (P3), Usp26-3P212 (P4) (see Figure 1A for the primer location, and Supplemental Table S1 for the PCR primer sequences, PCR product sizes, and PCR conditions). Two correctly targeted ESC clones (G1 and B2) (Figure 1C) were micro-injected into C57BL/6J blastocysts to generate more than 50% male chimeras, which were then crossed into female mice with a C57BL/6J genetic background. The offspring were selected by PCR analysis of their genomic DNA using P1/P2 and P3/P4 primers. A total of seven F1 mice (five male, two female) with both P1/P2 and P3/P4 positive were obtained.
Figure 1.
Targeted disruption of the Usp26 gene. A) The structure of the endogenous mouse Usp26 gene, the targeting construct, and the disrupted allele. The positions of a 1601-bp and a 2875-bp probe used for Southern blotting are shown. B) Mapping Usp26-KO plasmid. C) 0.7% agarose gel analysis on homologous recombination positive ESC clones (G1 and B2). M: MBI GeneRuler 1 kb DNA ladder. D) 1.8% agarose gel analysis on genotyping of mice. M: MBI GeneRuler DNA ladder. E) Usp26 mRNA expression in brain and testes of wild-type and homozygote mice. M: TIANGEN 50-bp DNA ladder. F) USP26 protein level in the testis and the brain of Usp26+/Y and Usp26−/Y mice analyzed by Western blotting. Beta-actin was applied as a loading control. G) USP26 protein expression and distribution in testis of Usp26+/Y and Usp26−/Y mice analysed by IHC staining. PBS served as a negative control. Bar = 100 μm.
The F1 Usp26 heterozygous mice were crossbred at least six generations before use in this study. The F2 offspring and subsequent generations were screened by genotyping PCR for the presence of Usp26 gene with primers WTf and WTr, and with primers Uf and Ur for the absence of the Usp26 gene (Supplemental Table S1 for the primer sequence, PCR product size, and PCR condition). The locations of the primers are shown in Figure 1A. For genotyping PCR, the genomic DNA was isolated from mouse tails by using the Tail DNA Isolation Kit (DP-304-02; Tiangen Biotech).
Fertility assay
Male wild-type mice have a Usp26+/Ygenotype, and KO mice have a Usp26−/Ygenotype. Female mice have three genotypes: Usp26+/+, Usp26+/−, and Usp26−/−. To examine fertility capacity, the male mice per genotype at 7 weeks of age were caged with normal sexually mature female mice (Usp26+/+) in 4-day estrum stage determined by vaginal epithelium smear at a male-to-female ratio of 1: 1 and examined each morning for a copulatory plug indicative of successful mating. The outcome of this successful mating is assessed by following the females to determine the successful establishment of pregnancy and the ability of the pregnancy to proceed to parturition over the normal period of gestation. At birth, the number of offspring was recorded. If a plug was not observed in the next morning after caging, further vaginal epithelial secretion smear was performed and examined whether sperm was present under microscope to prove copulation. The time from the first mating to the first litter (n = 21), the intervals between the two consecutive litters (n = 39), and the litter sizes (n = 21) were calculated for each male mouse. In addition, the number of males and females mated, the percentages of males producing plugs, pregnant females, and fertility rate were calculated and are given in Supplemental Table S2.
Serum hormones and general structural features of the Usp26−/Y mice
Sexually mature 12–14 mice per genotype at 7 weeks of age were euthanized, and blood samples were collected by cardiac puncture. Serum-luteinizing hormone (LH), follicle-stimulating hormone (FSH), estrogen hormone (E2), and testosterone (T) levels were measured using Enzyme-Linked Immunosorbent Assay (ELISA) kits (R&D Co.) following manufacturer's instructions.
Following euthanasia, the general features of the mice were recorded together with an assessment of organ weights. The in situ layout of the male reproductive tract was assessed. The gross morphological features of the testes, the vascular patterns on the testicular capsule, and the structures of the epididymis and vas deferens were noted together with their relationship with the seminal vesicles and bladder. The gross structures of the penis and the anogenital distance were noted.
Epididymal sperm count and sperm motility
To evaluate the sperm counts, viability, and motility, cauda epididymides from each genotype were dissected and immediately placed in Earle's balanced salts solution (pH 7.4) (Sigma-Aldrich Chemical). Sperm were squeezed out with fine forceps and allowed to disperse in Earle's solution (pH 7.4) at 37°C for 15 min in a CO2 incubator, followed by repeated pipetting. Thereafter, the sperm remaining as a mono-dispersed suspension were used to analyze sperm count and motility by computer-assisted sperm assay (CASA, S-QH-III-GK-9900, Qinghua). Sperm viability was counted by a hemocytometer after 0.5% eosin staining, and percentages of live sperm were recorded.
Flow cytometry of mouse testicular cells
Testicular germ cell suspensions were prepared as described previously [31–33]. Briefly, testicular seminiferous tubules from Usp26+/Y and Usp26−/Y mice at 7 weeks of age were treated with modified Roswell Park Memorial Institute 1640 Medium containing collagenase (600 U/mL), hyaluronidase (135 μg/mL), and DNase I (40 μg/mL), and then incubated with a mixture of trypsin (2.5 mg/mL) and DNase I (0.1 mg/mL) for 15 min. The germ cell suspensions were gently filtered through nylon gauze, centrifuged, and stained by acridine orange (AO, 50 μg/mL). All chemicals were purchased from Sigma-Aldrich Corporation, St., Louis, MO, USA. The samples were analyzed using a FACSCalibur Flow cytometer (Becton and Dickinson). The fluorescent signals were recorded, and the intensity of DNA histograms was used to compare various germ cell populations from Usp26+/Y and Usp26−/Y mice. The relative percentages of germ cell types were analyzed by FlowJo 7.6.1 Software.
Histological examination of the testis
Prior to tissue collection, all the mice were euthanized by CO2 asphyxiation, followed by decapitation (0–10 days postpartum (dpp)) or cervical dissociation (10 dpp—adult), and their testes and other tissues were dissected. Mice and testis weights were measured for every mouse in each age group. All tissue samples for morphological and immunohistochemical analyses were fixed in freshly prepared Bouin's solution or 4% paraformaldehyde (TIANLI, Chemical Reagents Ltd) in PBS (pH 7.4) for between 2 h and overnight, depending on the sample size, and then dehydrated through a graded ethanol series before being embedded in paraffin wax (Leica). Sections (5–6 μm) were placed on Superfrost Plus slides (Thermo, Pierce Biotechnology) for analysis. For hematoxylin & eosin staining, the slides were dewaxed in xylene and rehydrated through a decreasing graded ethanol series to distilled water, and then stained with Harris hematoxylin (Beijing Chemical Works) and eosin solution (Beijing Chemical Works). For Periodic acid Schiff (PAS) staining, dewaxed samples were oxidized with 1% periodic acid solution for 10 min, rinsed with distilled water, and stained with Schiff reagent for 30 min. Then, the slides were washed in bisulfite solution and counterstained with hematoxylin. All sections were examined in a blinded fashion according to the published method [25].
Immunohistochemistry and immunofluorescence staining
Immunohistochemistry (IHC) staining was performed as described in our previous study [16]. Briefly, testicular sections underwent rehydration via a graded ethanol series, beginning with xylene, and were subjected to an antigen retrieval step (10 min boiling in 0.01 M citric acid buffer, pH 6.0) before being exposed to 3% hydrogen peroxide in methanol for 20 min to block endogenous peroxidase activity in the tissue. The nonspecific binding sites were blocked with normal donkey serum. The sections were incubated overnight at 4°C with primary antibodies: goat anti-mouse USP26 (1:100; sc-51 013), rabbit anti-human AR (1:100; sc-816) (Santa Cruz Biotechnology), and rabbit anti-human AR (1:100; PG-21; Merckmillipore). Binding of primary antibodies was detected with biotinylated donkey anti-goat or anti-rabbit IgG. Streptavidin–avidin–horseradish peroxidase complex (ZB-2404; ZSGB) and Avidin-Biotin complex (PK-6101; Merckmillipore) were then applied for 25 min. Diaminobenzidine (DAB Kit, ZLI-9032; ZSGB) was used to visualize the immunohistochemical reaction. The sections were then counterstained with Harris hematoxylin. Sections were examined using a light microscope (Olympus BX 51).
Immunofluorescence staining was performed as described in our previous studies [16]. Briefly, all steps preceding the additions of the secondary antibodies were the same as those described above. At this point, the sections were incubated with donkey anti-goat IgG-FITC (1: 400; SC-2024) and donkey anti-rabbit IgG-TR (1: 400; SC-2784) (Santa Cruz Biotechnology), or donkey anti-goat IgG-TR (1: 400; PA1-28662) and donkey anti-rabbit IgG-Alexa Fluor 488 (1: 400; A-21206) (ThermoFisher) for 60 min at 37 °C. Negative controls with no primary antibodies were set up for each slide. Sections from at least three Usp26−/Y and Usp26+/Y mice were used for USP26 and AR co-localization studies.
RNA extraction and RT-PCR
To confirm the loss of Usp26 mRNA expression in the KO mice, total RNAs from testes of 8-week-old Usp26−/Y and Usp26+/Y mice were extracted and RT-PCR analysis was performed as previously described [16]. Briefly, RNA was isolated from testis and brain snap-frozen tissues using Trizol following the manufacturer's instructions (Invitrogen). Complementary DNA was synthesized via reverse transcription of 1 μg of RNA using the RevertAidTM First-strand cDNA Synthesis Kit (K1622; Fermentas Inc.). The set of primers used to amplify Usp26 from cDNA is shown in Table S1. Gapdh mRNA was measured in each tissue sample as an endogenous control in parallel with Usp26 mRNA and DEPC-treated water instead of a template as a negative control. The PCR reactions were carried out in triplicates to ensure consistent results. At least three independent tissue samples from Usp26−/Y and Usp26+/Ymice were examined.
Preparation of whole-cell extracts and Western blot analysis
To determine the expression of USP26 protein in Usp26−/Y and Usp26+/Y mice, testes and brains from 8-week-old mice were pulverized for protein extracts. To determine the alteration of AR during testis development in Usp26−/Y and Usp26+/Ymice, testes from 0, 10, 15, 20, 35, 45, 56, and 90 days postpartum (dpp or postnatal) were pulverized for protein extracts. Whole-cell lysates were prepared with radioimmunoprecipitation assay buffer (PE-02; Pioneer Biotechnology, Inc), separated on a 15% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis resolving gel, transferred to PVDF membrane (Roche Diagnostics), and blocked with 5% non-fat dried milk in Tris-buffered saline—Tween 20 for 1 h at room temperature. Western blot analysis was carried out overnight at 4°C for primary antibodies and at 37°C for 1 h for secondary antibodies followed by rinsing and chemiluminescent imaging using SuperSignal West ECL detection kit (34079; Thermo). The antibodies used were goat anti-USP26, rabbit anti-AR, and rabbit anti-β-actin.
Immunoprecipitation
For immunoprecipitation, the whole-cell lysates of testes from 8-week-old wild-type mice were prepared as described above. Lysates were incubated with 1 μg of antibodies (rabbit anti-human AR or goat anti-mouse USP26) overnight at 4°C, and antibodies were pulled down using 100 μl of the protein A/G Plus-Agarose immunoprecipitation reagent (sc-2003; Santa Cruz). The samples were washed three times with PBS containing 1% TritonX-100 (pH 7.4) and boiled in 50 μl of SDS sample buffer for Western blot analysis. All experiments were repeated at least three times.
Statistical analysis
Student t-test was used to compare data between wild-type and mutant mice. P < 0.05 indicated significance. All data analyses were performed using SPSS v17.0.
Results
Generation of Usp26-deficient mice
To determine the functional role of USP26 in male reproduction, we generated a Usp26 conventional KO mouse model using recombination strategy (Figure 1A). The mouse Usp26 gene is located on the X chromosome and is organized into nine exons [25]. A replacement vector was designed to delete the exon 9, which encodes the USP26 protease (913 amino acids). The mapping Usp26-KO vector is shown in Figure 1B. Two positive ES clones (G1 and B2) were identified by PCR (Figure 1C) and were injected into pre-prepared C57BL/6J blastocysts and four chimeric mice were obtained. Three chimeric males with >50% chimeric rate transmitted the targeted gene through their germ line to produce heterozygous male and female animals, and seven heterozygous mice were obtained. Heterozygous animals were then mated to produce homozygous animals. Figure 1D shows the genotyping PCR results. To access the efficiency of Usp26 gene deletion, RT-PCR was applied to examine Usp26 mRNA expression (Figure 1E). Western blot analysis (Figure 1F) and IHC staining (Figure 1G) confirmed that there is no USP26 protein expression in testis and brain of Usp26−/Ymice.
Usp26 deletion leads to subfertility in male mice
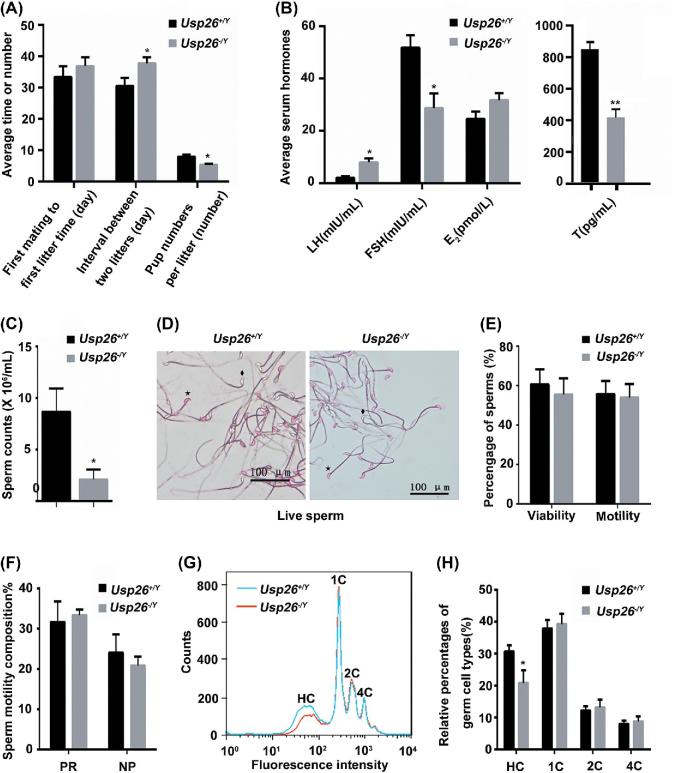
To determine whether the loss of USP26 expression affects mouse reproductive function, we performed a fertility test. Adult WT/Usp26−/Ymale mice were mated with WT/Usp26−/− female mice. Reproductive capacity was evaluated via the time intervals from the first cross to the first mating, the mating intervals between two consecutive mating, and the litter size. Although there were no differences on the time from the first mating to the first litter (Figure 2A), the intervals between the two consecutive litters were significantly increased (Figure 2A, P < 0.05), and the litter size (pups number) per litter was significantly reduced in Usp26−/Y mice compared to that in Usp26+/Y mice (Figure 2A, P < 0.05). Overall, the in vivo fertility rate of Usp26−/Y mice was reduced by about 18.72% compared to that of Usp26+/Y mice (Supplemental Table S2).
Figure 2.
Usp26 deletion leads to male mice subfertility. A) Reproductive capacity of Usp26+/Y and Usp26−/Y mice in vivo. n = 11 and 15; *P < 0.05. B) Sex hormone levels of Usp26+/Y and Usp26−/Y mice by ELISA (n = 10 per group). LH, luteinizing hormone; FSH, follicle-stimulating hormone; E2, estradiol; T, testosterone. *P < 0.05, **P < 0.001. C–F) Semen analysis results. All values are represented as means ± SD (n = 10). Statistical differences were determined by one-way ANOVA followed by Tukey's multiple-comparison test. C) Sperm counts of Usp26+/Y and Usp26−/Y mice conducted by Computer-aided sperm analysis (CASA). D) Counting the live spermatozoa by 0.5% eosin staining. ♦: live spermatozoa; ☆: dead spermatozoa. E) Sperm viability and motility of Usp26+/Y and Usp26−/Y mice conducted by CASA. F) Percentages of progressive and non-progressive spermatozoa of Usp26+/Y and Usp26−/Y mice counted by CASA. G–H) Flow-cytometry analysis of Usp26+/Y and Usp26−/Y mice testes (n = 10 per genotype): G) Representative DNA content distribution histogram of testicular cells. Flow-cytometry histogram reveals four main peaks in Usp26−/Y and Usp26+/Y mice: HC peak (the mature haploid cells or the elongated spermatids constitute), 1C peak (the immature haploid cells or round spermatids constitute), 2C peak (spermatogonia and the secondary spermatocytes constitute), and 4C peak (primary spermatocytes constitute); Blue line: wild-type mice; Red line: homozygous mice. H) Relative percentage of sub-haploid (HC), haploid (1C), diploid (2C) and tetraploid (4C) cells of Usp26−/Y and Usp26+/Y mouse testis, *P < 0.05.
The Usp26 KO mice had normal external genitals and no embryonic lethality. The in situ layout of the male reproductive tract is normal with normal gross morphological features of the testes, the vascular patterns on the testicular capsule, the epididymis, and the vas deferens (Supplemental Figure S1A). The gross structures of the penis and the anogenital distance were also normal. The body weights and the weights of the genitourinary blocs (GU-blocs), testes and epididymides, and other organs were normal in Usp26−/Y mice compared to those in Usp26+/Y mice (Supplemental Figure S1B). The major organs of Usp26−/Y mice were histologically normal (Supplemental Figure S1C).
Usp26 deletion leads to changes in serum hormone levels
To understand the reasons causing subfertility, we detected serum hormone levels in adult Usp26−/Y mice. Changes in serum levels of LH, FSH, E2, and testosterone (T) in Usp26−/Ymice are shown in Figure 2B. The serum T level of Usp26−/Ymice decreased dramatically to 49.1% of the Usp26+/Ymice (413.94 ± 57.82 vs. 842.76 ± 46.37, P < 0.001). The decrease in serum FSH was not as dramatic, reaching to 55.3% of Usp26+/Ymice (28.61 ± 5.71 vs. 51.78 ± 4.69, P < 0.05). In contrast to T and FSH, the serum LH levels of Usp26−/Ymice were dramatically increased to 387% of Usp26+/Ymice (7.90 ± 1.02 vs. 2.04 ± 0.52, P < 0.001).
Usp26 deletion leads to the reduction in sperm counts and the relative percentages of mature haploid germ cell population
The sperm parameters of Usp26−/Yand Usp26+/Y mice were analyzed as described in the Material and Methods section. Our results showed that the sperm counts of Usp26−/Ymice were significantly reduced compared to that of Usp26+/Ymice (Figure 2C, P < 0.05). The live sperm heads were stained red using the 0.5% eosin solution (Figure 2D). There is no significant difference in the sperm viability and motility between Usp26+/Y and Usp26−/Y mice (Figure 2E and F).
Testicular germ cell staining and fluorescence-activated cell sorting were performed to illustrate the changes in different germ cell populations in Usp26−/Y mice. Flow cytometric measurement of DNA content of testicular cells showed four main peaks in normal mice as described in previous studies [32, 34]. The mature haploid cells or elongated spermatids constitute the HC peak; 1C peak is composed of the immature haploid cells or round spermatids; diploid or mainly spermatogonia with a small number of non-germ cells like Sertoli cells and Leydig cells form 2C peak; and tetraploid primary spermatocytes constitute 4C peak. Compared to that of Usp26+/Y mice, the flow cytometric histogram showed a significant reduction of HC population (mature haploid germ cells) in Usp26−/Y mice. The immature haploid, diploid, and tetraploid cells had no difference between Usp26+/Y and Usp26−/Y mice (Figure 2G and H).
Usp26 deletion leads to abnormal testicular microstructures and defects in spermatogenesis
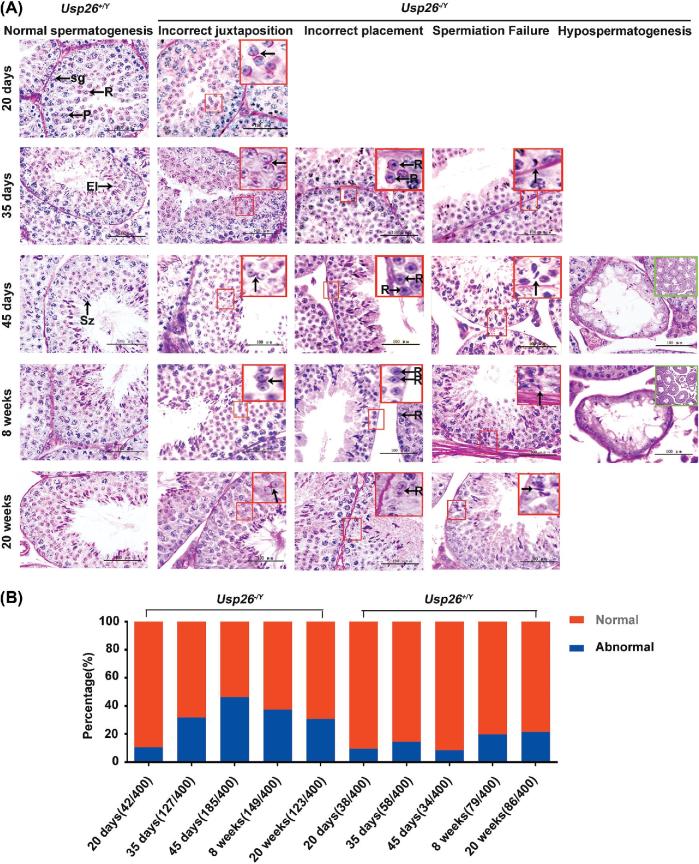
To determine the functional role of Usp26 in testis development and spermatogenesis, we compared the morphological changes of testis and spermatogenesis during post-natal development between Usp26−/Y and Usp26+/Ymice. Testis tissue sections of 0, 5, 10, 15, 20, 35, 45, 56, and 140 dpp were prepared. To reveal the dynamic changes during successive stages in the seminiferous epithelia, PAS staining was performed. We found that the testes of Usp26−/Y mice had normal development at 0, 5, 10, 15, and 20 dpp, but after 35 dpp, severe histological abnormalities in testes and spermatogenesis were found (Figure 3A). The most common spermatogenic defect was incorrect juxtaposition of cell types, on 35, 45, 56, and 140 dpp. Other spermatogenic defects include spermiation failure and hypospermatogenesis, which were found in the testes at 35, 56, and 140 dpp (Figure 3A). These histology observations confirmed the flow cytometric analysis results. In addition, the number of abnormal seminiferous tubules was significantly increased in Usp26−/Ymice compared to those in Usp26+/Ymice (Figure 3B).
Figure 3.
Histological analysis of mouse testes at different ages. A) Sections of the testes were stained with Periodic Acid Schiff (PAS). Day 20: Usp26+/Y, Round spermatids (R) exit meiosis ΙΙ towards the center of the tubule. Spermatogonia (sg) at the basement membrane and easily identifiable pachytene spermatocyte (P) close to the lumen. Usp26−/Y, incorrectly juxtaposed cell types, disorientation of round spermatids was observed. The purple-pink stained developing acrosomes are facing opposite directions (red box, black arrow). Day 35: Usp26+/Y, elongating spermatids (El), undergoing the striking morphological changes to become specific-shaped spermatozoa, located close to the lumen of the tubule, anchored by the high pyramidal Sertoli cells. Usp26−/Y, incorrectly juxtaposed cell types (red box, black arrow); incorrect placement cell types: round spermatids are seen adjacent to the basement membrane (red box, black arrows), whereas they should be seen approximately halfway towards the lumen; Spermiation failure tubules are found in homozygous testes, elongating spermatid is being drawn to the basement membrane (red box, black arrow); Day 45: Usp26+/Y, various spermatogenic cells are present in the seminiferous epithelia of testes and specifically arranged to form the recognizable cellular associations called “stage.” The tubule possesses typical II—III stage. Usp26−/Y, incorrectly juxtaposed cell types (red box, black arrows); incorrect placement cell types (red box, black arrows) Spermiation failure tubules (red box, black arrow); a hypospermatogenic testes of homozygote mice, wherein there are abnormal cross-sectioned tubules alongside normal cross-sections (Green box, X 100), in this case showing germ cell arrest (GCA, spermatocyte arrest, X 1000); Week 8: Usp26+/Y, the tubule represents typical V stage. Usp26−/Y, incorrectly juxtaposed cell types (red box, black arrow); incorrect placement cell types (red box, black arrows); Spermiation failure tubules (red box, black arrow); hypospermatogenic tubules are located among normal tubules (Green box, X 100). Week 20 (adult): Usp26+/Y, the tubule represents typical VII –VIII stage. Usp26−/Y, incorrectly juxtaposed cell types (red box, black arrow); incorrect placement cell types (red box, black arrow); Spermiation failure tubules (red box, black arrow). Scale bar = 100 μm. B) The percentages of the abnormal seminiferous tubules of Usp26+/Y and Usp26−/Y mouse testes at different ages.
Usp26 deletion leads to reduced androgen receptor expression in mouse testes at different ages
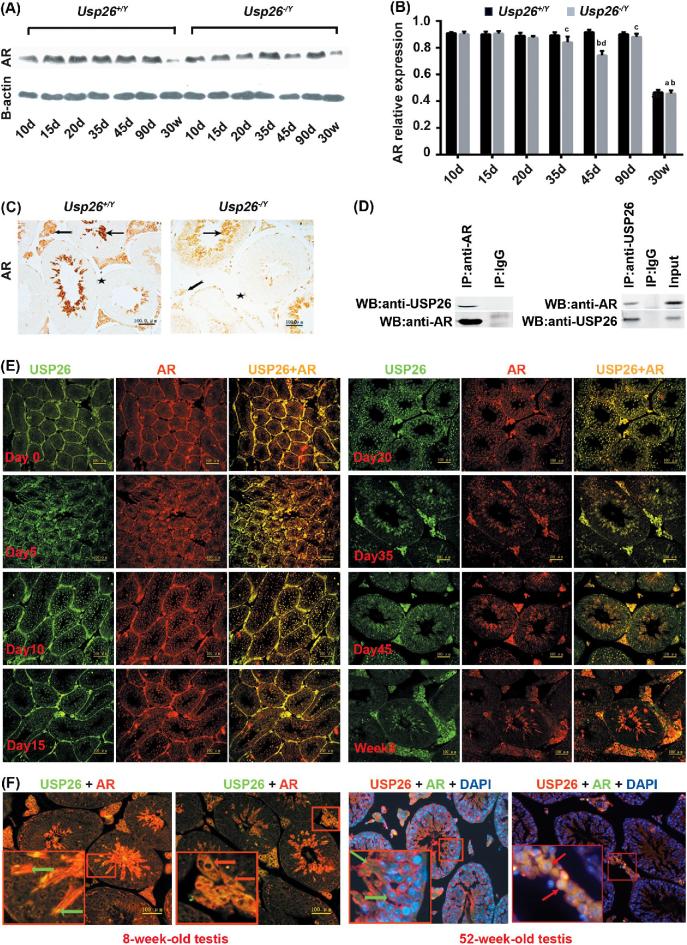
USP26 has been reported to be a regulator of AR signaling [17]. To understand the underlying mechanisms of how Usp26 affects AR signaling and male fertility, we studied the relationship of USP26 and AR in mouse testis. First, we compared the expression of AR in testis at different postnatal days (dpp). Western blotting results showed that AR protein levels had no obvious differences at 10, 15, and 20 dpp between Usp26+/Y and Usp26−/Y mice, but obviously declined after 20 dpp and were lower until adult in Usp26−/Y mice compared to that of the corresponding postnatal days in Usp26+/Y mice (Figure 4A and B). IHC staining results also showed reduced expression of AR in the testis of Usp26−/Ymice compared to that in Usp26+/Ymice (Figure 4C). To determine whether USP26 and AR physically interact with each other, we further conducted co-IP using adult mouse testis protein lysates as described in the Material and Methods section. We found that USP26 could pull down AR in the testicular protein lysates and vice versa (Figure 4D). To further understand the relationship between Usp26 and AR in the testis, we analyzed the expression pattern of Usp26 and AR in mouse testes of Usp26+/Y mice at different ages by performing IHC staining (Supplemental Figure S3) and IF staining (Figure 4E and F). We found that both Usp26 and AR were co-localized in the different stages of spermatogenic cells and Leydig cells; especially, the cytoplasm of 9–16 steps spermatids and Leydig cells have higher expression than other cell types. Some Leydig cell nuclei also have their co-localization (Figure 4E and F; Supplemental Figure S3).
Figure 4.
The expression of AR in testes of Usp26−/Y mice and their physical interaction. A) Western blotting results of AR protein in the testis of Usp26+/Y and Usp26−/Y mice at different ages. β-actin is used as loading control. B) Analysis of the Western blotting results of relative AR protein levels (AR/β-actin). All values were expressed as means ± SD (n = 10). a: P < 0.001, compared to other age groups in Usp26+/Y mice; b: P < 0.001, compared to other age groups in Usp26−/Y mice; c: P < 0.05, compared to the same age groups between Usp26+/Y and Usp26−/Y mice; d: P < 0.001, compared to the same age groups between Usp26+/Y and Usp26−/Y mice. C) IHC staining of AR in testis of Usp26+/Y and Usp26−/Y mice. Forked arrow indicates Leydig cells, ordinary arrow indicates germ cells, and asterisk indicates myoid cells. Scale bar = 100 μm. D) AR and USP26 protein-protein Co-IP in the testis of Usp26+/Y mice. E) USP26 and AR co-expressed in the testis of Usp26+/Y mice at different ages by IF staining. Scale bar = 100 μm. F) USP26 and AR co-expressed in 8- and 52-week-old testes of Usp26+/Y mice by IF staining. 8-week-old testis: USP26 and AR co-expressed in the cytoplasm of 9–16 steps spermatids (green arrows) and Leydig cells (red arrows). Scale bar = 100 μm. 52-week-old testis: USP26 and AR co-expressed in the cytoplasm of 9–16 steps spermatids (green arrows) and Leydig cells (red arrows). Amplification is the same as in 8-week-old testis.
Discussion
USP26 has been discussed by our group and several other groups to be one candidate gene with possible involvement in spermatogenesis and testicular development [11, 12, 14, 18, 25, 26, 35–37], since Wang et al. first identified USP26 as one of the X-linked genes expressed exclusively in the testis and spermatogonia [9]. However, there are no studies on the mechanisms whereby USP26 affects male reproduction in vivo. In this study, we reported the first investigation on the role of Usp26 in male reproduction by generating a Usp26 KO mouse model. The mouse Usp26 gene is located at Xq26.2 with a single exon 9 encoding a 93 kDa protein [25]. We generated the Usp26 KO mouse model by designing a targeting vector to delete the exon 9. Results from this study suggest that Usp26−/Ymice can be used as a model to study detailed molecular mechanisms of spermatogenesis and male subfertility.
Our previous studies had examined the spatial and temporal expression of USP26 in various mouse tissues [16]. We found that USP26 is not testis-specific as previously reported [9], but has predominantly testicular expression in mice. The distribution of USP26 in mouse testis exhibits a spatial and temporal pattern. USP26 protein was undetectable on 20 dpp, but by 30 dpp, it was found in step 9–16 spermatids and Leydig cells, and its expression becomes very strong by 35 and 45 dpp [16]. Lin et al.’s study reported that USP26 had a very low expression on day 10 and had an increased expression on day 12 [18]. USP26 is highly expressed early during murine spermatogenesis, in round spermatids, and at the blood–testis barrier [16, 18, 27]. In adult mice, USP26 is predominantly expressed in Leydig cells, spermatogonia, and Sertoli cells [9]. In this study, we found that Usp26−/Ymice had no obvious spermatogenic defects before 15 dpp, but occurred from 20 dpp, and became severe by 35 and 45 dpp and up to adulthood. The spermatogenic defect pattern was basically consistent with the expression pattern of USP26 in mouse testis. Therefore, USP26 plays a key role in mouse testicular development and spermiogenesis.
Spermatogenic abnormalities can be caused by many reasons, such as genetic factors and endocrine abnormalities such as T or LH deficiency. Testosterone is the main testicular steroid hormone and the major circulating androgen. In some cells, T is metabolized by the converting enzyme 5α-reductase to dihydrotestosterone (DHT) [38]. Both T and DHT bind to the same AR, which is encoded by a gene on the X chromosome and belongs to a superfamily of nuclear receptors [31]. Androgen plays a critical role in the virilization of the male urogenital system. The temporal and spatial characteristics of AR expression are among the major factors defining androgenic function. In testosterone-deficient mice, spermatogenesis does not progress beyond the pachytene stage [32]. LH deficiency also leads to the failure in the proper development of testes in males and female reproductive organs [39]. In Usp26 mutant mice, we found that the serum level of T in Usp26−/Y mice was reduced sharply. However, LH levels were increased and T/LH varied, which may be related to sperm counts and spermatogenesis; additionally, the expression of AR in testis was also reduced. Therefore, the reduced level of testosterone maybe related to the downregulation of AR in testicular tissues. By examining the AR expression in Usp26−/Y mice, we did not found obvious difference between WT and Usp26−/Y mice before postnatal day 15, but found obviously reduced expression after day 20. Interestingly, the defects of spermatogenesis were also found after day 20. Studies using genetically modified mice have played an important role in enhancing our understanding of spermatogenesis [40, 41]. These studies have confirmed that androgen and AR signaling pathways play a critical role in the differentiation and development of male internal and external genitalia, and AR has a role in the structural and functional differentiation of cells responsible for spermatogenesis, male fertility, and prostate development [42]. In Usp26−/Y mice, although we did not find obvious difference in the development of male internal and external genitalia from WT mice, we found that Usp26−/Y mice have retarded sexual behavior compared to WT mice. We also found that Usp26−/Y mice had significantly increased intervals between two consecutive litters compared to WT mice; thus, Usp26−/Y mice had reduced fertility compared to WT mice. This may be caused by reduced serum level of T that is related to the reduced expression of AR. The elevated levels of LH may be the feedback result of the downregulated T and FSH [37]. In addition, Usp26−/Y mice had significantly decreased sperm counts, which may be supported by Lin et al.’s study. They reported that USP26 was localized at blood–testis barrier and near Sertoli cell–germ cell interface in mouse testes, as well as in the acrosome of spermatids. The localization of USP26 in post-meiotic germ cells suggests a possible role in the process of spermiogenesis. The reduced sperm number in Usp26−/Y mice may be mainly caused by the spermiogenesis and spermiation failure [18].
The classic AR signaling pathway is modulated at the post-translational level via phosphorylation, acetylation, methylation, and ubiquitination [43]. It has been reported that USP26 could modulate this signaling pathway in HEK293 cells via reversing the ubiquitination of AR [17]. Whether USP26 also modulates the activity of AR in a similar way during spermatogenesis in testes remains to be established. USP26 is a member of the DUB family of enzymes. Although the substrates of USP26 in the testes remain unknown, a recent study reported that USP26 possesses deubiquitinating activity in mouse testis [18]. In this study, we found that USP26 and AR are co-localized in the cytoplasm of different spermatogenic cells, especially the step 9–16 spermatids and Leydig cells. Furthermore, USP26 and AR have physical interaction in testicular tissues. Our results indicate that AR is one of the substrates of USP26 in testes. Thus, the abnormalities in spermatogenesis and disturbed steroidogenic function in Usp26−/Y mice may be related to the dysregulated AR signaling. The interaction between USP26 and AR in mouse testis and the reduced AR expression in Usp26−/Ymice indicated a possible enhanced proteasome degradation of AR in Usp26−/Y mice as the deletion of Usp26 disrupted the delicate balance between ubiquitination and deubiquitination. However, further studies are needed to elucidate the precise mechanism, which could provide new insights into the prevention and treatment of male subfertility.
In conclusion, we comprehensively investigated the role of Usp26 in mouse spermatogenesis by generating a Usp26−/Y mouse model, providing new information on the male reproductive research. Notably, our study suggests that USP26 is required for maintaining normal testicular development and spermatogenesis, potentially acting as a regulator of AR in the testis. This is the first in vivo functional study highlighting the role of the DUB enzyme USP26 in male reproduction. Our discovery provides a framework for future studies designed to address the detailed mechanisms of USP26 during male germ cell development and spermatogenesis.
Supplemental data
Supplemental Figure S1. Characteristics of reproductive system in Usp26+/Y and Usp26−/Y male mice. A) Genitourinary bloc (GU-bloc, genital-urinary system, it includes bladder, prostate, seminal vesicle, urethra, and Cowper's gland) of 9-week-old Usp26+/Y and Usp26−/Y mice. B) Body weight, weight of brain, and reproductive organs of Usp26+/Y and Usp26−/Y mice. Data were presented as mean ± SD. C) Microstructures of different organs of Usp26+/Y and Usp26−/Y mice were assessed with H&E staining. Scale bar = 200 μm.
Supplemental Figure S2. Histological analysis of testes from Usp26+/Y and Usp26−/Y mice at certain postnatal days. Sections of the testes were stained with Periodic Acid Schiff (PAS). From Day 0 to Day 15, testis of Usp26+/Y and Usp26−/Y mice developed normally. Day 0: Sertoli cells (SC) surround gonocytes (g). Day 5: Spermatogonia (sg) occur at the basement membrane. Day 10: the differentiation of spermatogonia begins, and pre-leptotene and leptotene could be found close to the lumen. Day 15: Meiosis I begins with pachytene (P).
Supplemental Figure S3. AR and USP26 are co-expressed in the testis of 52-week-old Usp26+/Y mice by IHC staining in consecutive sections. A, B) AR and USP26 are co-expressed in the cytoplasm of Leydig cells partially (red box). Red arrow: Leydig cell. Scale bar = 20 μm and 100 μm. C, D) AR and USP26 are co-expressed in the cytoplasm of steps 9–16 spermatids and some round spermatids (red box). Red arrow: spermatid. Scale bar = 20 μm and 100 μm.
Supplemental Table S1. PCR primer sequences.
Supplemental Table S2. The effect of Usp26 deficiency on male fertility.
Supplemental Table S3. Antibodies.
Acknowledgements
We thank Shanghai Biomodel Organism Science & Technology Development Co., Ltd for generating the Usp26 heterozygous mice. Statistical analysis were completed in the Genomics and Biostatistics Core at the Tulane Center for Aging, which is supported by National Institute of General Medical Sciences Grant P20GM103629. The authors would like to acknowledge David Cunningham for assistance in manuscript preparation.
Footnotes
Grant support: This work was supported, in part, by the National Natural Science Foundation of China (30972990 and 81772725), the National Institutes of Health-National Institute of General Medical Sciences (P20GM103629), and the 2 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Natural Science Foundation of China and the National Institutes of Health.
References
- 1. Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013; 99(5):1324–1331e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bablok L, Dziadecki W, Szymusik I, Wolczynski S, Kurzawa R, Pawelczyk L, Jedrzejczak P, Hanke W, Kaminski P, Wielgos M. Patterns of infertility in Poland - multicenter study. Neuro Endocrinol Lett 2011; 32(6):799–804. [PubMed] [Google Scholar]
- 3. Jarow JP, Sharlip ID, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, Schlegel PN, Howards SS, Nehra A, Damewood MD, Overstreet JW, Sadovsky R. Best practice policies for male infertility. J Urol 2002; 167(5):2138–2144. [PubMed] [Google Scholar]
- 4. Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod 1998; 13(suppl 1):33–44. [DOI] [PubMed] [Google Scholar]
- 5. Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod BioMed Online 2007; 14(6):734–745. [DOI] [PubMed] [Google Scholar]
- 6. Aston KI. Genetic susceptibility to male infertility: news from genome-wide association studies. Andrology 2014; 2(3):315–321. [DOI] [PubMed] [Google Scholar]
- 7. Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med 2008; 14(11):1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neto FT, Bach PV, Najari BB, Li PS, Goldstein M. Genetics of male infertility. Curr Urol Rep 2016; 17(10):70. [DOI] [PubMed] [Google Scholar]
- 9. Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet 2001; 27(4):422–426. [DOI] [PubMed] [Google Scholar]
- 10. Nishimune Y, Tanaka H. Infertility caused by polymorphisms or mutations in spermatogenesis-specific genes. J Androl 2006; 27(3):326–334. [DOI] [PubMed] [Google Scholar]
- 11. Paduch DA, Mielnik A, Schlegel PN. Novel mutations in testis-specific ubiquitin protease 26 gene may cause male infertility and hypogonadism. Reprod BioMed Online 2005; 10(6):747–754. [DOI] [PubMed] [Google Scholar]
- 12. Stouffs K, Lissens W, Tournaye H, Van Steirteghem A, Liebaers I. Possible role of USP26 in patients with severely impaired spermatogenesis. Eur J Hum Genet 2005; 13(3):336–340. [DOI] [PubMed] [Google Scholar]
- 13. Ravel C, El Houate B, Chantot S, Lourenco D, Dumaine A, Rouba H, Bandyopadahyay A, Radhakrishna U, Das B, Sengupta S, Mandelbaum J, Siffroi JP. Haplotypes, mutations and male fertility: the story of the testis-specific ubiquitin protease USP26. Mol Hum Reprod 2006; 12(10):643–646. [DOI] [PubMed] [Google Scholar]
- 14. Ribarski I, Lehavi O, Yogev L, Hauser R, Bar-Shira Maymon B, Botchan A, Paz G, Yavetz H, Kleiman SE. USP26 gene variations in fertile and infertile men. Hum Reprod 2009; 24(2):477–484. [DOI] [PubMed] [Google Scholar]
- 15. Zhang W, Liu T, Mi YJ, Yue LD, Wang JM, Liu DW, Yan J, Tian QB. Evidence from enzymatic and meta-analyses does not support a direct association between USP26 gene variants and male infertility. Andrology 2015; 3(2):271–279. [DOI] [PubMed] [Google Scholar]
- 16. Zhang J, Tian H, Huo YW, Zhou DX, Wang HX, Wang LR, Zhang QY, Qiu SD. The expression of Usp26 gene in mouse testis and brain. Asian J Androl 2009; 11(4):478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dirac AM, Bernards R. The deubiquitinating enzyme USP26 is a regulator of androgen receptor signaling. Mol Cancer Res 2010; 8(6):844–854. [DOI] [PubMed] [Google Scholar]
- 18. Lin YW, Hsu TH, Yen PH. Localization of ubiquitin specific protease 26 at blood-testis barrier and near Sertoli cell-germ cell interface in mouse testes. Int J Androl 2011; 34(5pt2):e368–e377. [DOI] [PubMed] [Google Scholar]
- 19. Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell 2005; 123(5):773–786. [DOI] [PubMed] [Google Scholar]
- 20. Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J 1997; 11(14):1245–1256. [DOI] [PubMed] [Google Scholar]
- 21. Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 2004; 1695(1-3):189–207. [DOI] [PubMed] [Google Scholar]
- 22. Suresh B, Lee J, Hong SH, Kim KS, Ramakrishna S. The role of deubiquitinating enzymes in spermatogenesis. Cell Mol Life Sci 2015; 72(24):4711–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chocu S, Calvel P, Rolland AD, Pineau C. Spermatogenesis in mammals: proteomic insights. Syst Biol Reprod Med 2012; 58(4):179–190. [DOI] [PubMed] [Google Scholar]
- 24. Bose R, Manku G, Culty M, Wing SS. Ubiquitin-proteasome system in spermatogenesis. Adv Exp Med Biol 2014; 759:181–213. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J, Qiu SD, Li SB, Zhou DX, Tian H, Huo YW, Ge L, Zhang QY. Novel mutations in ubiquitin-specific protease 26 gene might cause spermatogenesis impairment and male infertility. Asian J Andrology 2007; 9(6):809–814. [DOI] [PubMed] [Google Scholar]
- 26. Asadpor U, Totonchi M, Sabbaghian M, Hoseinifar H, Akhound MR, Zari Moradi S, Haratian K, Sadighi Gilani MA, Gourabi H, Mohseni Meybodi A. Ubiquitin-specific protease (USP26) gene alterations associated with male infertility and recurrent pregnancy loss (RPL) in Iranian infertile patients. J Assist Reprod Genet 2013; 30(7):923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wosnitzer MS, Mielnik A, Dabaja A, Robinson B, Schlegel PN, Paduch DA. Ubiquitin Specific Protease 26 (USP26) expression analysis in human testicular and extragonadal tissues indicates diverse action of USP26 in cell differentiation and tumorigenesis. PLoS ONE 2014; 9(6):e98638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang P, Li MZ, Elledge SJ. Towards genetic genome projects: genomic library screening and gene-targeting vector construction in a single step. Nat Genet 2002; 30(1):31–39. [DOI] [PubMed] [Google Scholar]
- 29. Chan W, Costantino N, Li R, Lee SC, Su Q, Melvin D, Court DL, Liu P. A recombineering based approach for high-throughput conditional knockout targeting vector construction. Nucleic Acids Res 2007; 35(8):e64–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 2003; 13(3):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evans RM. The steroid and thyroid hormone receptor superfamily. Science 1988; 240(4854):889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reproduction 2010; 139(1):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boucheron C, Baxendale V. Isolation and purification of murine male germ cells. Methods Mol Biol 2012; 825:59–66. [DOI] [PubMed] [Google Scholar]
- 34. Jagetia GC, Jyothi P, Krishnamurthy H. Flow cytometric evaluation of the effect of various doses of vindesine sulphate on mouse spermatogenesis. Reprod Toxicol 1997; 11(6):867–874. [DOI] [PubMed] [Google Scholar]
- 35. Stouffs K, Lissens W, Tournaye H, Van Steirteghem A, Liebaers I. Alterations of the USP26 gene in Caucasian men. Int J Androl 2006; 29(6):614–617. [DOI] [PubMed] [Google Scholar]
- 36. Christensen GL, Griffin J, Carrell DT. Sequence analysis of the X-linked USP26 gene in severe male factor infertility patients and fertile controls. Fertil Steril 2008; 90(3):851–852. [DOI] [PubMed] [Google Scholar]
- 37. Lee IW, Kuan LC, Lin CH, Pan HA, Hsu CC, Tsai YC, Kuo PL, Teng YN. Association of USP26 haplotypes in men in Taiwan, China with severe spermatogenic defect. Asian J Androl 2008; 10(6):896–904. [DOI] [PubMed] [Google Scholar]
- 38. You L, Sar M. Androgen receptor expression in the testes and epididymides of prenatal and postnatal Sprague-Dawley rats. Endocrine 1998; 9(3):253–262. [DOI] [PubMed] [Google Scholar]
- 39. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci 2004; 101(49):17294–17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cooke HJ, Saunders PT. Mouse models of male infertility. Nat Rev Genet 2002; 3(10):790–801. [DOI] [PubMed] [Google Scholar]
- 41. de Rooij DG, de Boer P. Specific arrests of spermatogenesis in genetically modified and mutant mice. Cytogenet Genome Res 2003; 103(3-4):267–276. [DOI] [PubMed] [Google Scholar]
- 42. Zhou X. Roles of androgen receptor in male and female reproduction: lessons from global and cell-specific androgen receptor knockout (ARKO) mice. J Androl 2010; 31(3):235–243. [DOI] [PubMed] [Google Scholar]
- 43. Chang C, Lee SO, Wang RS, Yeh S, Chang TM. Androgen receptor (AR) physiological roles in male and female reproductive systems: lessons learned from AR-knockout mice lacking AR in selective cells. Biol Reprod 2013; 89(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.