Highlights
-
•
The hepatoprotective activity of silymarin against CCl4-induced liver injury was achieved.
-
•
Silymarin has potential to alleviate the side effects of CCl4-induced oxidative stress.
-
•
Silymarin enhanced oxidative stability by modulation antioxidant related genes.
Keywords: Silymarin, Oxidative stress, Gene expression, Broiler chickens, CCl4
Abstract
This study was conducted to investigate the hepatoprotective effects of silymarin on CCl4-induced oxidative stress in broiler chickens model. A total of 240 day-old broilers were divided into 4 equal groups (n = 60) composed of a control group (receiving 1 mL/Kg BW saline) and 3 groups treated with silymarin (receiving 100 mg/Kg BW silymarin), CCl4 (receiving 1 mL/Kg BW CCl4), and combination of silymarin + CCl4. Results indicated that silymarin has potential to mitigate the deleterious effects of CCl4 on protein and lipid metabolism. The protective activity of silymarin against CCl4-mediated lipid peroxidation was demonstrated by the lower serum content of MDA, as lipid peroxidation marker. CCl4-induced hepatotoxicity was demonstrated by the elevation of serum contents of ALP, AST, ALT, and GGT enzymes, whereas silymarin decreased serum activity of ALP and AST hepatic enzymes. The CCl4-challenged birds revealed considerable hepatic injures characterized by moderate to severe hepatocellular degeneration around the portal vein, aggregation of inflammatory cells, granulomatosis, cytolytic necrosis, periportal space fibrosis, and sinusoidal dilatation. However, liver damages were amended by the silymarin. In line with molecular study, a remarkable down-regulation was detected in the expression of CAT, GPx, and Mn-SOD hepatic genes in CCl4-challenged birds, whereas silymarin significantly up-regulated aforementioned genes. In general, current study showed that silymarin has potential to alleviate the adverse effects of oxidative stress in poultry farms.
1. Introduction
Modern commercial broilers that are genetically selected for rapid growth are prone to oxidative stress [1]. Oxidative stress is the loss of redox homeostasis by the excess production of reactive oxygen species (ROS); which exceed the scavenging activity of the antioxidant defense systems [2]. Hence, oxidative stress results in the initiation and progression of hepatic damage. Carbon tetrachloride (CCl4) intoxication is a broadly used experimental model to mimic oxidative stress in both in vitro and in vivo studies [3]. It has been reported that CCl4 undergoes metabolic activation through hepatic microsomal cytochrome P450 enzymes to form toxic trichloromethyl radical with the potential of oxidative stress which results in hepatocytes destruction and ultimately disturbance of hepatic functions [4]. In addition, deleterious effects of CCl4 on the hepatic antioxidant enzymes such as catalyze (CAT), glutathione peroxidase (GPx), and super oxide dismutase (SOD) have been reported [5].
The bioactive components of plants have gained considerable attention to alleviate the adverse effects of oxidative stresses. Milk thistle (Silybum marianum L.), as an important hepatoprotective plant, has been used for centuries as a natural remedy for the liver and biliary tract. Dried extracts of milk thistle seeds contain approximately 60% silymarin. Typically, silymarin consists of several flavonogligans including silibinin (50–60%), isosilibinin (5%), silychristin, (20%) and silydianin (10%) and a flavonoid (taxifolin) [6]. The beneficial effects of silymarin have been reported in broilers [7], Japanese quail [8], and mice [9]. Silybin, as the main bioactive of silymarin, is believed to have antioxidant activity and potential to prevent lipid oxidation in human and some animal models [10]. In accord with this, it has been demonstrated that silybin protects the structure and function of hepatocytes through scavenging free radicals, activating related antioxidant genes, restoring damaged tissues, and producing new hepatocytes [11], leading to this suggestion that silymarin could be used as an ideal agent for the comparison of hepatoprotective bioactive components [12]. At the molecular level, it prevents synthesis of the chemical mediators involved in inflammation such as tumor necrosis factor-alpha (TNF-α), leukotriene B4 (LTB4), and prostaglandin E2 (PGE2) and also interferes with the liver fibrosis process [13]. However, the precise mechanism of its protective effects has not been precisely explored. Hence, the main objective of the present study was to investigate the potential protective effects of silymarin on oxidative status and hepatic damage in broilers subjected to oxidative stress induced by CCl4.
2. Materials and methods
2.1. Plant preparation and extraction
Silybum marianum used in this experiment was collected in the summer from the heights of Ravansar (34° 52′ N 46° 27′ E; Altitude: 1650 m), Kermanshah province, Iran. Dried seeds of Silybum marianum were ground and sieved through a 60-meth size screen to get fine powder from which the ethanol extract (silymarin) was prepared. In brief, 20 g of the powder was defatted using 300 mL petroleum ether by soxhletion for 16 h. Defatted powder was then dissolved in ethanol (300 mL) for 10 h, followed by evaporating ethanol using vacuum drying oven (Binder, Germany) at 39 °C.
2.2. HPLC analysis
Standard of silymarin was provided commercially (Sigma-Aldrich Co, St. Louis, MO, US) and contains (silybin A, silybin B, isosilybin A, isosilybin B, silychristin, silydianin, and flavonoid of taxifolin). Solvents and other reagents were purchased from commercial source (Merck, Germany). Silymarin stock solution was prepared at 1 mg/ml concentration in methanol (Sigma-Aldrich). The standard working solutions used to build calibration curve were prepared by serial dilutions of stock solutions with methanol. Chromatography was performed using a SY-8100 HPLC (BFRL, China), equipped with a C18 column (25 cm ×4.6 mm). A mixture of methanol and water (50:50, v/v) was used as the mobile phase. The elution has been made in an isocratic mode at a flow rate of 1 mL/min and the detection made at 288 nm.
2.3. Birds, diets and experimental design
All experimental protocols were approved by the Gorgan University of Agricultural Sciences and Natural Resources, Animal Care and Use Committee (Iran) and were according to the European recommendations (Directive 2010/63/EU), which consider welfare standards for keeping chickens for meat production. A total of 240 male broiler chicks (Ross, 308) were obtained from a commercial hatchery and housed in an environmentally controlled poultry house with wood shavings as litter. Birds were divided into 4 equal groups (n = 60) composed of a control group (receiving 1 mL/Kg BW saline) and 3 groups treated with silymarin (receiving 100 mg/Kg BW silymarin), CCl4 (receiving 1 mL/Kg BW CCl4), and combination of silymarin + CCl4. Silymarin dosage was chosen based on a previous study [14]. Moreover, CCl4 was used based on our precious study [8] to induce oxidative stress. All the categories fed by a basal diet based on the Nutrient Requirements of Domestic Animals, Nutrient Requirements of Poultry [15] recommendations throughout the study. From day 21 of experiment, birds of control group received isotonic saline (i.p.), whilst experimental groups challenged by silymarin (orally), and solution of CCl4 in olive oil at the ratio of 1:1 (i.p.). Silymarin was fed directly into crop via a syringe equipped with a plastic nozzle and feeding tube (Nova Cath®, No. 10). Intraperitoneal injection of CCl4 was performed beginning on day 22, and again every three days [16]. In order to homogenize the stress, the control group received 1 mL/kg BW 0.9% sodium chloride solution via intraperitoneal injection [17] and also 1 mL/Kg BW of distilled water by oral gavage. Birds had free access to feed and water throughout the experiment. A light regimen of 23L: 1D was implemented throughout the trial.
2.4. Sampling procedures
CCl4 is one of the main hepatotoxins, hence, the attenuated effects of silymarin on CCl4-mediated hepatic damage was evaluated by assessing biochemical, histopathological, and oxidative related genes expression parameters. At the end of trial (42 d of old), the chicks of each experimental group were weighed individually. Two birds per replicate pen (8 birds per each experimental group; n = 8) with average body weight were randomly selected and euthanized by cervical dislocation for blood and tissue sampling. Blood samples (5 mL) obtained through cardiac puncture were poured into a 10-mL anticoagulant-free vacutainer tube and then centrifuged (3000× g, 15 min, 4 ºC) to obtain serum. The obtained sera were kept on ice and protected from light to avoid oxidation during sampling and then stored at -20 °C for further serological analysis. For histopathology assessment, liver tissue samples were excised immediately after sacrifice from 8 birds per treatment. Liver specimens were fixed in neutral-buffered formalin (10%) and then kept at −4 °C until histological examination. Moreover, in order to molecular evaluation, other portion of liver tissues was immediately snap-frozen in liquid nitrogen to prevent RNA degradation, and kept at −80 °C for total RNA extraction and real-time PCR analysis.
2.5. Sampling processing
An Olympus (AU2700) clinical chemistry analyzer (Olympus Inc., Japan) was applied to assay serum total protein, albumin, Total bilirubin, total cholesterol, triglyceride and high density lipoprotein cholesterol (HDL-c). LDL-c was calculated as follows [18]:
| LDL-c = Total cholesterol-[HDL+ (TG/5)] |
The enzyme activities of lactate dehydrogenase (LDH), total antioxidant capacity (TAC), and also the serum contents of malondialdehyde (MDA) as antioxidant biomarkers were determined spectrophotometrically (Alcyton 300, USA), using commercial kits according to the manufacturer’s instructions. The LDH activity was measured at 365 nm wave length using automated spectrophotometric chemistry analyzer (Alcyton 300, USA). The TAC was determined based on the method of ferric reducing-antioxidant power assay [19], using a spectrophotometer (Alcyton 300, USA) with 600 nm wave length. The serum content of MDA, an index of lipid peroxidation, was measured via the colorimetric method based on the reaction 2-thiobarbituric acid (2-TBA) at a wave length of 532 nm (Mihara and Uchiyama, 1978). The serum activities of hepatic alkaline phosphatase (ALP), aspartate aminotransaminase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT) as indicators of hepatic injury were evaluated by an auto analyzer, according to the instructions of the manufacturer.
For histopathology, fixed liver specimens were dehydrated through an ascending series of ethanol (70, 90, 96, and 100%), cleared via xylene, embedded in paraffin, and followed by slicing at 5–6 μm thick using microtome (Microm HM 335E, USA). The sectioned samples were then stained with hematoxylin-eosin (H &E). According to Benli et al. [20], an Olympus microscope (Olympus BX51, Japan) was used to evaluate the histopathological changes for lymphocytes, myeloid cells and also hyperplasia and necrosis.
For molecular studies, Biozol reagent (BioFLUX, Japan) was used to extract the total RNA from the frozen liver samples according to the directions of manufacturer, and cDNA was then synthesized by reverse transcriptase according to the instructions of the manufacturer (Fermentase, France) following treatment by DNase I. The wavelength of 260/280 nm of the spectrophotometer was chosen to quantify the total RNA and integrity was verified by gel electrophoresis. The primer sequences used for the target and reference genes of catalase (CAT), glutathione peroxidase (GPx), manganese dependent-superoxide dismutase (Mn-SOD), and β-actin as internal control are shown in Table 2. A gradient PCR was run to find the optimum annealing temperature. The reaction conditions were as follows: denaturation at 95 °C for 30 s, 40 cycles of 95 °C for 10 s, based on connection temperature for 30 s, and 72 °C for 20 s, and finally 1 cycle of 78 °C for 20 s as a final extension. The final product was checked by gel electrophoresis. The amplification process (Real-time PCR) were performed in a 20 μl reaction buffer consisting of SYBR Green Mix (BioPars-Gorgan University of Agricultural Sciences and Natural Resources, Iran) (9.9 μl), forward and reverse primers, 1 μl; 1 μl, cDNA, 3 μl, ddH2O, up to volume 20 μl, Dimethyl sulfoxide (DMSO), 1 μl; Taq, 0.2 μl. Comparative CT method was applied as a means to determine the relative gene expression levels. β-actin was used as internal reference for the standardization of the RT-PCR calculation. Real-time PCR tests were performed in four biological repeats including three technical replications for each sample. Mean values were used for statistical analysis.
Table 2.
Primers of CAT, Mn-SOD, GPx, and β-actin for real-time PCR1.
| Objective gene |
Accession number |
Primer sequence (5′ to 3′) | Annealing temperature (°C) | Amplicon length |
|---|---|---|---|---|
| CAT | NM_001031215.2 | Forward: CTTTCCCTCTTCCCTTACCA | 58.24 | 182 |
| Reverse: GGTAGTAGCTGGGCTCTGAAA | 58.62 | |||
| Mn-SOD | AF299388.1 | Forward: ACAGATAGCAGCCTGTGCAA | 59.62 | 163 |
| Reverse: CGCGTTCTCCCAGTTGAT | 59.79 | |||
| GPX | U22046.1 | Forward: GACGGCTGAGAGTTGATCCT | 59.41 | 176 |
| Reverse: CTGCGGGTATTTGATGTCC | 58.94 | |||
| β-actin | NM_205518.1 | Forward: AGTTACTCGCCTCTGTGAAGG | 94.59 | 198 |
| Reverse: CACATCTATCACTGGGGAACAC | 33.59 |
1CAT: Catalase; Mn-SOD: Manganese super oxide dismutase; GPX: Glutathione peroxide1; β-actin: Internal control.
2.6. Statistical analysis
The data on blood serum biochemical, oxidative biomarkers, and hepatic enzymes were subjected to the analysis of variance (ANOVA) using the PROC GLM procedure of SAS 9.1 [21]. Differences among experimental groups were separated by Tukey’s post hoc test. Considering the gene expression information, normalized data were used as a ratio of target gene mRNA to β-actin mRNA quantities and these data were transformed before statistical analysis. Statistical analysis of gene expression data was conducted by the REST software [22].
3. Results
3.1. Bioactive ingredients of silymarin
The quantitative data obtained from the HPLC analysis showed 6 detectable flavonolignans, including taxifolin (only flavonoid), silychristin, silydianin, silybin A, silybin B, and isosilybin A (Table 1). Quantitative data indicated that silibinin (51.53%), a mixture of two diastereomers of silybin A and silybin B is the major constituent, followed by the silychristin (24.51%). However, quantities of bioactive constituents of silymarin could be varied according to environmental conditions, including rainfall, temperature, soil texture, and altitude from the sea.
Table 1.
Results of HPLC analysis of silymarin.
| Item | Content (%) |
|---|---|
| Taxifolin | 6.44 |
| Silychristin | 24.51 |
| Silydianin | 7.60 |
| Silybin A1 | 24.81 |
| Silybin B1 | 26.72 |
| Isosilybin A | 4.21 |
| Isosilybin B | ND2 |
1Silybins A and B are two diastereomers of silibinin (silybin).
2Not detectable.
3.2. Blood biochemistry
The analyzed blood serum metabolites are presented in Table 3. In the CCl4 treated broilers, the serum content of the total protein and albumin were decreased by 28% and 18%, respectively compared with the control birds. Interestingly, combination of silymarin with CCl4 improved the protein metabolism, suggesting that silymarin has potential to mitigate the deleterious effects of CCl4 on protein metabolism. CCl4 treated broilers showed higher levels of the total cholesterol, triglyceride and LDL-c than birds in control group, demonstrating oxidative effects of CCl4 on lipid metabolism. Whereas, silymarin + CCl4 treated birds showed lower levels of triglyceride and LDL-c than CCl4-exposed birds, suggesting a positive alteration in lipid metabolism.
Table 3.
Effects of experimental treatments on blood serum biochemical at 42 d of age in broiler chickens.
| Experimental treatments |
Total protein (g/dl) |
Albumin (g/dl) |
Glucose (mg/dl) |
Total cholesterol (mg/dl) |
Triglyceride (mg/dl) |
LDL-c1 (mg/dl) |
HDL-c2 (mg/dl) |
|---|---|---|---|---|---|---|---|
| Control | 3.49a | 1.71a | 226.06 | 113.16b | 36.95b | 17.22b | 88.51 |
| Silymarin | 3.36a | 1.76a | 221.21 | 111.48b | 33.51b | 14.15b | 90.72 |
| CCl4 | 2.51b | 1.40b | 213.28 | 119.96a | 44.12a | 26.97a | 83.54 |
| Silymarin × CCl4 | 3.19a | 1.62a | 208.90 | 122.05a | 35.59b | 19.51b | 95.52 |
| SEM | 0.15 | 0.07 | 3.40 | 2.68 | 2.42 | 2.25 | 2.57 |
a-c means within a column with different superscript differ significantly (P < 0.05).
1LDL-c, low density lipoprotein cholesterol; 2HDL-c, high density lipoprotein cholesterol.
3.3. Oxidative status
The data of blood serum oxidative biomarkers are reported in Table 4. MDA of CCl4-treated broiler chickens was greater than in control birds, showing oxidative effects of CCl4. Combination effect of silymarin with CCl4 showed protective activity of silymarin against CCl4-mediated lipid peroxidation. CCl4-treated broilers showed lower TAC and higher LDH serum contents than control group, indicating antioxidant suppressive effect of CCl4.
Table 4.
Effects of experimental treatments on blood serum oxidative biomarkers (MDA, TAC and LDH) at 42 d of age in broiler chickens.
| Experimental treatments |
MDA1 (nmol/mL) | TAC2 (U/mL) | LDH3 (U/L) |
|---|---|---|---|
| Control | 1.37c | 1.95a | 93.42b |
| Silymarin | 1.62ac | 1.86a | 100.36ab |
| CCl4 | 2.23a | 1.20b | 108.54a |
| Silymarin × CCl4 | 1.87ab | 1.35b | 109.45a |
| SEM | 0.12 | 0.12 | 4.31 |
a-c means within a column with different superscript differ significantly (P < 0.05).
1MDA, Malondialdehyde; 2TAC, Total antioxidant capacity; 3LDH, Lactate dehydrogenase.
3.4. Hepatic and GGT enzymes activity
CCl4-treated broilers showed higher serum activity of ALP, AST, ALT, and GGT enzymes compared with birds in control group, indicating CCl4-induced hepatotoxicity. Whereas silymarin + CCl4 treated birds showed lower level of ALP and tended to decrease serum content of AST, suggesting hepatoprotective effect of silymarin against CCl4-induced hepatic damage (Table 5).
Table 5.
Effects of experimental treatments on the blood serum activity of hepatic enzymes (ALP, AST, ALT, and GGT) at 42 d of age in broiler chickens.
| Experimental treatments | ALP1 (U/L) |
AST2 (U/L) |
ALT3 (U/L) |
GGT4 (U/L) |
|---|---|---|---|---|
| Control | 203.41c | 221.25b | 6.65b | 10.22b |
| Silymarin | 225.18b | 225.91b | 7.28ab | 10.85b |
| CCl4 | 262.28a | 236.56a | 8.39a | 12.61a |
| Silymarin × CCl4 | 232.65b | 227.03ab | 8.15a | 11.46ab |
| SEM | 14.19 | 2.44 | 0.34 | 0.41 |
a-c means within a column with different superscript differ significantly (P<0.05).
1ALP, Alkaline phosphatase; 2AST, Aspartate aminotransferase; 3ALT, Alanine aminotransferease; 4GGT, Gamma-glutamyl transferase.
3.5. Histopathology of liver
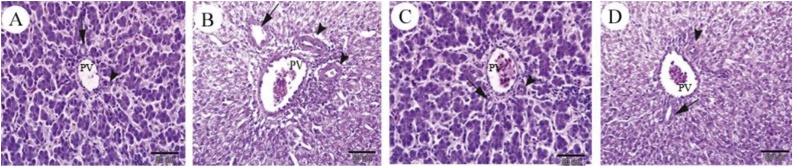
The observations of hepatic histopathology of broiler chickens are reported in Table 6, with representative images of the liver portal triad displayed in Fig. 1. Compared with the control group, liver tissue in CCl4-challenged birds revealed considerable injures characterized by moderate to severe hepatocellular degeneration around the portal vein, aggregation of inflammatory cells, granulomatosis, cytolytic necrosis, periportal space fibrosis, and sinusoidal dilatation.
Table 6.
Hepatic histopathological changes in broiler chickens treated by experimental treatments.
| Items | Control | CCl4 | Silymarin | CCl4 × Silymarin |
|---|---|---|---|---|
| Inflammation | – | +++ | – | – |
| Granulomatosis | – | + | + | + |
| Cytolytic necrosis | – | +++ | – | + |
| Periportal space fibrosis | – | ++ | + | + |
| Cellular degeneration | – | +++ | – | + |
| Sinusoidal dilatation | – | +++ | – | – |
(-) No histopathologic change, (+) histopathology in <20% of fields, (++) histopathology in 20–60% of fields, and (+++) histopathology in >60% of fields.
Fig. 1.
Photomicrographs of the liver portal triad histopathology in broiler chicks administrated by experimental treatments. A: Control, B: CCl4, C: Silymarin, D: Silymarin + CCl4. Branch of portal vein (PV), branch of portal artery (arrowheads) and branch of bile duct (arrow). Magnification of 20×.
3.6. Hepatic genes expression of oxidative stress
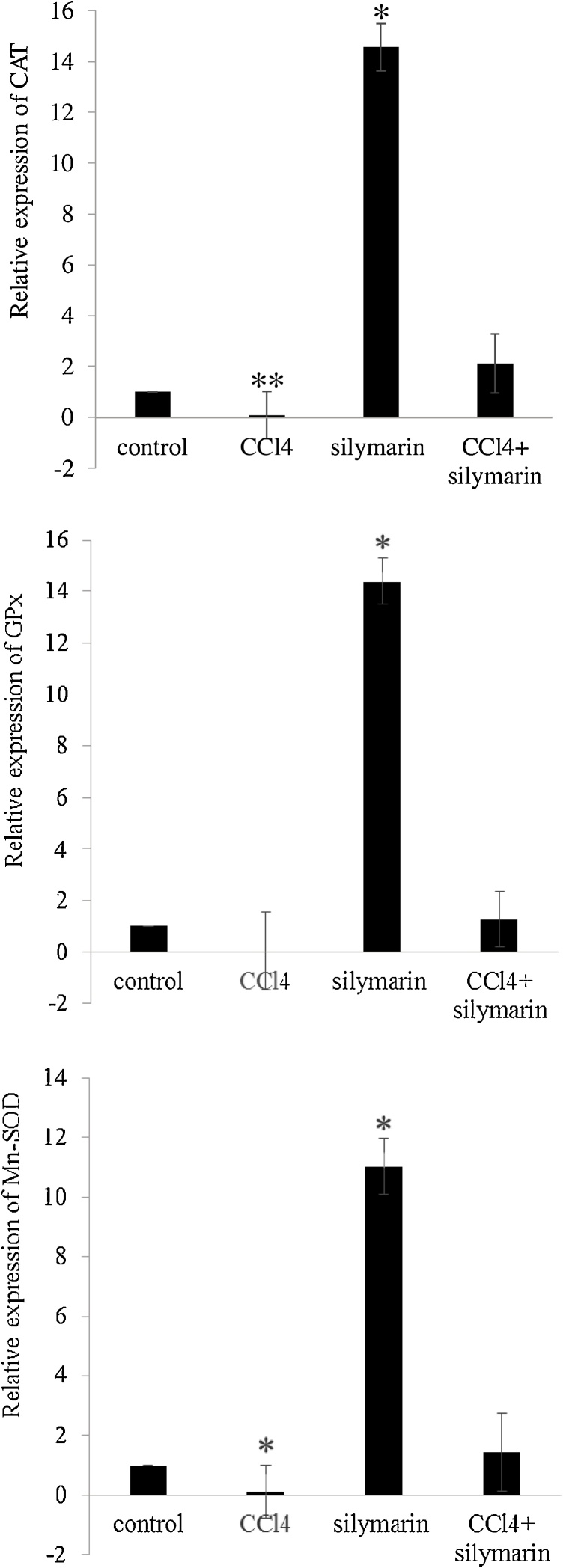
The relative hepatic mRNA expression of CAT, GPx, and Mn-SOD are depicted in Fig. 2. Results indicated a remarkable down-regulation in the expression of CATand Mn-SOD hepatic genes in CCl4-challenged birds, whereas silymarin significantly up-regulated aforementioned genes along with GPx, implying hepatoproductive effect of silymarin against CCl4-induced hepatotoxicity.
Fig. 2.
The catalase (CAT), glutathione peroxidase (GPx), and manganese-superoxide dismutase (Mn-SOD) relative mRNA expression in the liver of broiler chickens. * P < 0.05, ** P < 0.01 when comparing with the control.
4. Discussion
Modern commercial broilers that are genetically selected for rapid growth, are prone to oxidative stress with potential adverse effects on productive performance. The main effects of oxidative stress largely come from tissue damage and homeostasis disruption which are reflected by the changes in the blood serum contents, oxidative status and also alteration in the oxidative stress related genes expression [23]. The liver as the main target tissue is involved in responding to different sorts of oxidative stresses, therefore any disruption in hepatocyte function results in changes in blood serum contents and enzymatic functions. CCl4 is converted to its toxic trichloromethyl free radical through hepatic microsomal cytochrome P450 [3], with the potential oxidative stress which results in DNA destruction, cellular structure devastation, and finally disturbance of hepatic cellular metabolism [24]. Our findings were in line with reports showing hypoproteinaemia and albuminuria in CCl4 intoxicated broilers [16,25], rats [[26], [27], [28]], and Japanese quail [8]. In contrast, it has been commented that silymarin stimulates protein synthesis through activating the RNA synthesis of ribosomes [29]. Generally, it is believed that stress is accompanied by catabolic profile, because it promotes proteolysis, lipolysis, and glycogenolysis [30]. The elevated serum levels of total cholesterol and triglyceride in CCl4 intoxicated birds agree with previous reports indicating that oxidative stress reduces the rate of lipolysis and the activity of lipolytic enzymes [27,31]. In contradict with a previous study [27], birds subjected to CCl4 tended to show a lower blood serum content of glucose. Consistent to our results, it has been documented that glycolysis is the main metabolic route for glucose utilization by skeletal muscle under stress conditions, and this can be reflected by the increased activity of LDH at this condition [32].
The serum content of MDA along with TAC, ALP, AST, ALT, and GGT activities are broadly used as indicators of the oxidative stress and tissue damage. MDA as an indicator of lipid peroxidation and oxidative stress is widely used to assess the extent of oxidative deterioration of lipids. In accord with some previous findings [27,33], CCl4-challenged birds had a higher serum MDA content, implying a greater extent of lipid peroxidation. Binding of toxic free radical of CCl4 (trichloromethyl radical) to macromolecules induces peroxidative degradation of polyunsaturated fatty acids. This leads to the formation of lipid peroxides, which in turn produce MDA with potential deleterious effects [3]. However, our results differ from a study showing no significant effect of CCl4 on lipid peroxidation in Japanese quail [8]. This contradiction could be attributed to the sensitivity of experimental species. In modern poultry genotypes the rapid growth rate might be responsible for the higher sensitivity against oxidative stress conditions.
Obtained results were in accord with the reports demonstrating greater blood serum function of AST and ALT in CCl4 intoxicated broilers [16,34] and Wistar rats [28], AST in Japanese quail [35], and ALP in Japanese quail [36].
The protective and antioxidant activities of silymarin on the cellular mitochondrial have been well documented [37]. For instance, it has been documented that silibinin has potential to optimize electron-transport chain and decrease ROS production by influencing on the ROS-producing enzymes in the mitochondria [38]. In contrast, CCl4 may be responsible for its adverse oxidative effects by converting to its toxic free radical via hepatic microsomal cytochrome P450 system [24,39]. Our results were in accord with numerous studies indicating hepatotoxicity of CCl4 in rats [26,28,40,41], broiler chickens [16,34], and Japanese quail [8,36]. Similarly, CCl4-mediated hepatic damage was reported in male hamster [33]. In this study, the elevated serum ALP and GGT activity with each other can be attributed to the damaged hepatic and its biliary system. According to our results, hepatoprotective effects of silymarin was demonstrated by the attenuation of CCl4-induced hepatic lesions. Similarly, Pradeep et al. [37] reported hepatoprotective and antioxidant potential of silymarin (50 mg/Kg body weight) in rats challenged with diethylnitrosamine. A previous study indicated that silymarin can inhibit CCl4 activation through inhibition of cytochrome P450 system [42]. Similarly, it has been commented that silymarin as a potent antioxidant can influence enzyme systems associated with glutathione and superoxide dismutase via removing free radicals [43,44]. Either the action on cytochrome P450 or the inhibition of oxidative damage could be responsible for the protective effect against oxidative stress-induced alteration in liver, such as that observed in the present study. In accord to our results, it has been highlighted that the adverse effects of CCl4 could be mitigated by Zingiber officinale [28] and green tea [33], and Tanacetum parthenium [27].
In terms of mRNA expression, this study indicated that CCl4 down-regulated the expression of oxidative related CAT and Mn-SOD genes, whilst silymarin up-regulated the expression of aforementioned gens along with the GPx. The enzymatic antioxidant system including SOD, CAT, and GPX is the first line of antioxidant defense which converts harmful molecules including hydrogen peroxides and hydroperoxides to harmless molecules [45]. In line with this, SOD catalyses dismutation of superoxide radicals to hydrogen peroxide and oxygen; CAT catalyses the breakdown of hydrogen peroxide to water and molecular oxygen, and GPX decomposes peroxides through other mechanisms [45,46]. Obtained results were in accord with reports indicating down-regulation of SOD [47,48] gene in heat-stressed broilers and in challenged breeder roosters with dexamethasone-induced oxidative stress [49]. Similarly, Mujahid et al. [50] highlighted that oxidative stress-mediated down-regulation of SOD gene expression could be due to inhibitory effect of MDA, a final yield of oxidative deterioration of lipids, on the antioxidant enzymes and also its acceleratory effects on the oxidative damage to biomolecules. However, our results were inconsistent with Rimoldi et al. [51] who showed an increased expression of CAT gene in broilers subjected to four weeks of chorionic heat stress. This contradiction might be due to the involvement of a non-enzymatic defense system. For example, it has been highlighted that uric acid can be considered a potent scavenger of free radicals in mammals and particularly in birds [52]. In addition, it has been commented that mild heat stress activates the expression of SOD and GPX genes via stimulation of mitochondrial biogenesis [23,53], whilst long-term heat stress results in down-regulation of oxidative related genes expression by impairing mitochondrial biogenesis [54]. In accord to our results, it has been reported that mitochondrial function can be improved by an antioxidant [55]. It has been remarked that up-regulation of genes related to oxidative stress can protect cells against oxidative damage [45]. Such an increase in transcripts levels probably implies an increase in antioxidant enzymes activity since the activity of these enzymes is often paralleled by the increase in mRNA copy number.
5. Conclusion
This study demonstrated that silymarin has potential to mitigate the oxidative stress-induced homeostasis disruption in broilers through modulation of oxidative stress biomarkers and hepatic oxidative genes expression.
Declaration of Competing Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors acknowledge the Gorgan University of Agricultural Sciences and Natural Resources (Iran) for its assistance.
References
- 1.Zheng X.C., Wu Q.J., Song Z.H., Zhang H., Zhang J.F., Zhang L.L., Zhang T.Y., Wang C., Wang T. Effects of Oridonin on growth performance and oxidative stress in broilers challenged with lipopolysaccharide. Poult. Sci. 2016;95:2281–2289. doi: 10.3382/ps/pew161. [DOI] [PubMed] [Google Scholar]
- 2.Celi P. The role of oxidative stress in small ruminants’ healthand production. R. Bras. Zootec. 2010;39:348–363. [Google Scholar]
- 3.Manibusan M.K., Odin M., Eastmond D.A. Postulated carbon tetrachloride mode of action: a review. J. Environ. Sci. Health C. 2007;25:185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- 4.Khan T.H., Sultana S. Antioxidant and hepatoprotective potential of Aegle marmelos Correa. against CCl4-induced oxidative stress and early tumor events. J. Enzyme Inhib. Med. Chem. 2009;24:320–327. doi: 10.1080/14756360802167754. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava A., Shivanandappa T. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2010;118:411–417. [Google Scholar]
- 6.Burgess C.A. Silybum marianum (Milk Thistle) J. Pharm. Soc. Wincons. 2003:38–40. [Google Scholar]
- 7.Blevins S., Siegel P.B., Blodgett D.J., Ehrich M., Saunders G.K., Lewis R.M. Effects of silymarin on gossypol toxicosis in divergent lines of chickens. Poult. Sci. 2010;89:1878–1886. doi: 10.3382/ps.2010-00768. [DOI] [PubMed] [Google Scholar]
- 8.Moradi F., Samadi F., Dastar B., Samadi S. The effects of silymarin on oxidative status and bone characteristics in Japanese quail subjected to oxidative stress induced by carbon tetrachloride. Poult. Sci. J. 2017;5:97–104. [Google Scholar]
- 9.Zhu S.Y., Jiang N., Yang J., J Tu, Zhou Y., Xiao X., Dong Y. Silybum marianum oil attenuates hepatic steatosis and oxidative stress in high fat diet-fed mice. Biomed. Pharmacother. 2018;100:191–197. doi: 10.1016/j.biopha.2018.01.144. [DOI] [PubMed] [Google Scholar]
- 10.Madrigal-Santillán E., Madrigal-Bujaidar E., Álvarez-González I., Sumaya-Martínez M.T., Gutiérrez-Salinas J., Bautista M., Morales-González A., González-Rubio M.G.L., Aguilar-Faisal J.L., Morales-González J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. WJG. 2014;20:14787. doi: 10.3748/wjg.v20.i40.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anthony K., Saleh M. Free radical scavenging and antioxidant activities of silymarin components. Antioxidants. 2013;2:398–407. doi: 10.3390/antiox2040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhiman R.K., Chawla Y.K. Herbal medicines for liver diseases. Dig. Dis. Sci. 2005;50:1807–1812. doi: 10.1007/s10620-005-2942-9. [DOI] [PubMed] [Google Scholar]
- 13.Polyak S.J., Morishima C., Lohmann V., Pal S., Lee D.Y., Liu Y., Graf T.N., Oberlies N.H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5995–5999. doi: 10.1073/pnas.0914009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cengiz M., Mehtap Kutlu H., Burukoglu D.D., Ayhancı A. A comparative study on the therapeutic effects of silymarin and silymarin-loaded solid lipid nanoparticles on D-GaIN/TNF-α-induced liver damage in Balb/c mice. Food Chem. Toxicol. 2015;77:93–100. doi: 10.1016/j.fct.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 15.NRC . 9th edn. National Research Council. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- 16.Sonkusale P., Bhandarker A.G., Kurkare N.V., Ravikanth K., Maini S., Sood D. Hepatoprotective activity of superliv liquid and repchol in CCl4 induced FLKS syndrome in broilers. Int. J. Poult. Sci. 2011;10:49–55. [Google Scholar]
- 17.Sharma A., Sharma M.K., Kumar M. Protective effect of Mentha piperita against arsenic-induced toxicity in liver of swiss albino mice. Basic Clin. Pharmacol. Toxicol. 2006;100:249–257. doi: 10.1111/j.1742-7843.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald W., Levy R., Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 20.Benli A.C.K., Köksal G., Özkul A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): effects on gill, liver and kidney histology. Chemosphere. 2008;72:1355–1358. doi: 10.1016/j.chemosphere.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 21.SAS Institute . SAS Institute Inc; Cary, NC. USA: 2002. SAS/STAT® 9. User’s Guide. [Google Scholar]
- 22.Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C.T., George A., Brooks G.A. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J. Appl. Physiol. 2011;112:354–361. doi: 10.1152/japplphysiol.00989.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhadauria M., Nirala K.S., Shukla S. Multiple treatment of Propolis ameliorates carbon tetrachloide induced liver injuries in rats. Food Chem. Toxicol. 2008;46:2703–2712. doi: 10.1016/j.fct.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Khodadust M.R., Samadi F., Ganji F., Jafari Ahangari Y., Asadi Gh. Effects of peppermint (Mentha piperita L.) alcoholic extract on carbon tetrachloride-induced hepatotoxicity in broiler chickens under heat stress condition. Poult. Sci. J. 2015;1:1–16. [Google Scholar]
- 26.Samudram P., Rajeshwari H., Vasuki R., Geetha A., Sathiya P. Hepatoprotective activity of Bi-herbal ethanolic extraction CCl4 induced hepatic damage in rats. Afr. J. Biochem. Res. 2008;2:061–065. [Google Scholar]
- 27.Mahmoodzadeh Y., Mazani M., Rezagholizadeh L. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol. Rep. 2017;4:455–462. doi: 10.1016/j.toxrep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oke G.O., Abiodun A.A., Imafidon C.E., Monsi B.F. Zingiber officinale (Roscoe) mitigates CCl4-induced liver histopathology and biochemical derangements through antioxidant, membrane-stabilizing and tissue-regenerating potentials. Toxicol. Rep. 2019;6:416–425. doi: 10.1016/j.toxrep.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vargas-Mendoza N., Madrigal-Santillan E., Morales-Gonzalez A., Esquivel-Soto J., Esquivel-Chirino C., Garcıa-Luna Y., Gonzalez- Rubio M., Gayosso-de-Lucio J., Morales-Gonzalez J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014;6:144–149. doi: 10.4254/wjh.v6.i3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donkoh A. Ambient temperature: a factor affecting performance and physiological response of broiler chickens. Int. J. Biometeorol. 1989;33:259–265. doi: 10.1007/BF01051087. [DOI] [PubMed] [Google Scholar]
- 31.Geraert P.A., Padilha J.C., Guillaumin S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br. J. Nutr. 1996;75:195–204. doi: 10.1079/bjn19960124. [DOI] [PubMed] [Google Scholar]
- 32.Rhoads R.P., Baumgard L.H., Suagee J.K. 2011 and 2012 early careers achievement awards: metabolic priorities during heat stress with an emphasis on skeletal muscle. J. Anim. Sci. 2013;91:2492–2503. doi: 10.2527/jas.2012-6120. [DOI] [PubMed] [Google Scholar]
- 33.Elgawish R.A.R., Rahman H.G.A., Abdelrazek H.M. Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediated apoptosis. Toxicol. Rep. 2015;2:149–1156. doi: 10.1016/j.toxrep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C., Zhang T., Cui X., Li S., Zhao X., Zhong X. Hepatoprotective effects of a Chinese herbal formula, Longyin Decoction, on carbon - tetrachloride-induced liver injury in chickens. Evid. Complement. Alternat. Med. 2013;10:392–401. doi: 10.1155/2013/392743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khorramshahi M., Samadi F., Ganji F. The effects of Cynara scolymus L. on carbon tetracholoride induced liver toxicity in Japanese quail. Int. J. Agric. Sci. 2014;4:362–369. [Google Scholar]
- 36.Behboodi H.R., Samadi F., Shams Shargh M., Ganji F., Samadi S. Effects of silymarin on growth performance, internal organs and some blood parameters in Japanese quail subjected to oxidative stress induced by carbon tetrachloride. Poult. Sci. J. 2017;5:31–40. [Google Scholar]
- 37.Pradeep K., Mohan C.V.R., Gobianand K., Karthikeyan S. Silymarin modulates the oxidant–antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2007;560:110–116. doi: 10.1016/j.ejphar.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Rolo A.P., Oliveira P.J., Moreno A.J., Palmeira C.M. Protection against post-ischemic mitochondrial injury in rat liver by silymarin or TUDC. Hepatol. Res. 2003;26:217–224. doi: 10.1016/s1386-6346(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 39.Preethi K.C., Kuttan R. Hepato and reno protective action of Calendula officinalis L. flower extract. Exp. Biol. 2009;47:163–168. [PubMed] [Google Scholar]
- 40.Hayes J.R., Condie L.W., Borzelleca J.F. Acute, 14-day repeated dosing, and 90-day subchronic toxicity studies of carbon tetrachloride in CD-1 mice. Toxicol. Sci. 1986;7:454–463. doi: 10.1016/0272-0590(86)90095-3. [DOI] [PubMed] [Google Scholar]
- 41.Muriel P., Mourelle M. Prevention by silymarin of membrane alterations in acute CCl4 liver damage. J. Appl. Toxicol. 1990;10:275–279. doi: 10.1002/jat.2550100408. [DOI] [PubMed] [Google Scholar]
- 42.Mikstacka R., Gnojkowski J., Baer-Dubowska W. Effect of natural phenols on the catalytic activity of cytochrome P450 2E1. Acta Biochim. Pol. 2002;49(2002):917–925. [PubMed] [Google Scholar]
- 43.Mira L., Silva M., Manso C.F. Scavenging of reactive oxygen species by silybin dihemisuccinate. Biochem. Pharmacol. 1994;48:753–759. doi: 10.1016/0006-2952(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 44.Valenzuela A., Aspillaga M., Vial S., Guerra R. Selectivity of silymarin on the increase of the glutathione content in different tissues of the rat. Planta Med. 1989;55:420–422. doi: 10.1055/s-2006-962056. [DOI] [PubMed] [Google Scholar]
- 45.Ighodaro O.M., Akinloye O.A. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defense grid. Alexandria J. Med. 2018;54:287–293. [Google Scholar]
- 46.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- 48.Al-Zghoul M.B., Sukker H., Ababneh M.M. Effect of thermal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult. Sci. 2018;0:1–11. doi: 10.3382/ps/pey379. [DOI] [PubMed] [Google Scholar]
- 49.Min Y.N., Niu Z.Y., Sun T.T., Wang Z.P., Jiao P.X., Zi B.B., Chen P.P., Tian D.L., Liu F.Z. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poult. Sci. 2018;97:1238–1244. doi: 10.3382/ps/pex417. [DOI] [PubMed] [Google Scholar]
- 50.Mujahid A., Akiba Y., Warden C.H., Toyomizu M. Sequential changes in superoxide production, anion carriers and substrate oxidation in skeletal muscle mitochondria of heat stressed chickens. FEBS Lett. 2007;581:3461–3467. doi: 10.1016/j.febslet.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 51.Rimoldi S., Lasagna E., Sarti F.M., Marelli S.P., Cozzi M.C., Bernardini G., Terova G. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene. 2015;6:17–25. doi: 10.1016/j.mgene.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simoyi M.F., Van Dyke K., Klandorf H. Manipulation of plasma uric acid in broiler chicks and its effect on leukocyte oxidative activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:791–796. doi: 10.1152/ajpregu.00437.2001. [DOI] [PubMed] [Google Scholar]
- 53.Wenz T. Regulation of mitochondrial biogenesis and PGC-1α under cellular stress. Mitochondrion. 2013;13:134–142. doi: 10.1016/j.mito.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y., Song J., Fu R.Q., Sun Y.F., Wen J. Expression of adenosine monophosphate-activated protein kinase subunit related to the rate of intramuscular lipogenesis in fast and slow-growing chicken strains. Avian Biol. Res. 2015;8:138–144. [Google Scholar]
- 55.Oliveira M.R., Nabavi S.F., Daglia M., Rastrelli L., Nabavi S.M. Epigallocatechin gallate and mitochondria—a story of life and death. Pharmacol. Res. 2016;104:70–85. doi: 10.1016/j.phrs.2015.12.027. [DOI] [PubMed] [Google Scholar]