Abstract
In recent years, cancer treatment-induced bone loss (CTIBL) and increased risk of fracture has become an emerging problem as breast cancer (BC) survival has increased due to early diagnosis and improved treatments. In premenopausal women with BC, chemotherapy and tamoxifen are the treatments of choice in hormone receptor-negative and hormone receptor-positive BC respectively. Their effect on fracture risk has only been investigated in a few small-scale studies.
Therefore, we investigated the fracture risk in a cohort study based on data from the Disease Analyzer database (IQVIA) and included 1761 individuals with BC and 1761 healthy women for comparison. After applying similar inclusion criteria, patients with BC were matched 1:1 to those without BC with regard to age, index year, and physician. Within 10 years of the index date, 6.4% of healthy women and 14.2% with BC sustained a fracture (log-rank p-value < 0.001), showing a positive association between breast cancer and fractures (adjusted hazard ratio (HR)=2.39, p < 0.001). When analyzing women with BC with and without tamoxifen treatment, 14.7% with and 12.9% without tamoxifen sustained a fracture. However, after adjustment, the HR was 2.58 (p < 0.001) for women on tamoxifen versus healthy women and 1.63 (p = 0.181) for women with BC without tamoxifen treatment versus healthy women.
In conclusion, premenopausal women with BC with or without tamoxifen treatment had an increased incidence of fractures compared to healthy women, but this difference was only significant when comparing tamoxifen users versus healthy women. More studies are needed to identify the specific risk factors of women at high risk.
Keywords: Breast cancer, Fracture, Chemotherapy, Osteoporosis, Tamoxifen, Premenopausal women
1. Introduction
Breast cancer (BC) is the most common malignant disease in women and is also the second leading cause of cancer-related deaths among women aged 40–55 in the United States [1]. In the U.S., 40,000 female patients die each year as a result of this disease [2]. In the United Kingdom, BC has the fifth highest incidence rate in Europe and the seventh highest in the world (95.0 new cases per 100,000 people) [3]. Worldwide, BC currently affects more than one in ten women [1]. In recent years, the biologic characteristics of the primary tumor (breast cancer) have increasingly been classified. Especially the distinction in subtypes and whether a tumor is positive for hormone receptor expression or not has led to different treatment regimens (e.g., chemotherapy, endocrine treatment). According to the recommendation of current treatment guidelines, the hormone receptor-positive subtypes require adjuvant endocrine treatment. Such treatment may be a combination of tamoxifen and ovarian function suppression (OFS) with luteinizing hormone-releasing hormone (LHRH) agonists or tamoxifen alone, with or without adjuvant chemotherapy depending on the biology of the primary tumor, tumor size, and grading, as well as lymph node involvement [4], [5].
In the past, several studies have investigated the influence of OFS with LHRH on bone health, and a substantial annual decrease of bone mineral density (BMD) of 5–7% has been reported. However, the long-term influence of chemotherapy (CHT)-induced temporary or permanent secondary amenorrhea in hormone-sensitive BC on bone health has not been well examined. Short-term results indicate an equivalent loss of BMD compared to LHRH analogs.
Tamoxifen is a selective estrogen receptor modulator (SERM). Depending on the target organ, tamoxifen can have agonistic or antagonistic effects and may have a positive or negative impact on the skeleton. According to previous reports, the positive or negative skeletal effect of tamoxifen is dependent on a woman's menopausal status [6]. Recently, Kyvernitakis et al. reported that premenopausal women with BC treated with tamoxifen had a higher fracture risk, while postmenopausal women with BC showed no such increase of fracture risk compared to healthy controls [7], [8], [9].
According to the recommendation of current treatment guidelines, hormone receptor-negative BC in premenopausal women requires adjuvant CHT, which also includes trastuzumab if the primary tumor is HER2-positive. Hormone receptor-negative primary BC is more common in premenopausal women (37%) than in postmenopausal women (21%) [10]. CHT may induce temporary or permanent secondary amenorrhea based on the CHT used and the patient's age. The influence of secondary amenorrhea on bone loss has been previously studied, but data on fracture incidence are still lacking.
Regardless of the anti-cancer treatment chosen, the long-term effects in premenopausal women with BC lead to a clinically significant loss of BMD and perhaps an increased risk of fracture. Considering the young age and the continuing increase in life expectancy of premenopausal woman with BC, bone health is becoming a major concern.
The aim of our study was to investigate the fracture incidence in a large sample of premenopausal women with BC, taking into account the influence of endocrine treatment or chemotherapy.
2. Methods
2.1. Database
This study was based on data from the Disease Analyzer database (IQVIA), which compiles drug prescriptions, diagnoses, and basic medical and demographic data obtained directly and in anonymous format from computer systems used in the practices of general practitioners and specialists. Diagnoses (International Classification of Diseases, 10th revision [ICD-10]), prescriptions (Anatomical Therapeutic Chemical [ATC] Classification system), and the quality of reported data are monitored by IQVIA based on a number of criteria (e.g., completeness of documentation and linkage between diagnoses and prescriptions). In the UK, the sampling methods used to select physicians’ practices were appropriate for obtaining a representative database of outpatients [11]. The sampling method for the Disease Analyzer database is based on statistics from all doctors in the UK. These statistics are used to determine the panel composition according to the following strata: region, community size category, and physician age.
Finally, several studies using the UK Disease Analyzer database have already been published [3], [12].
2.2. Study population
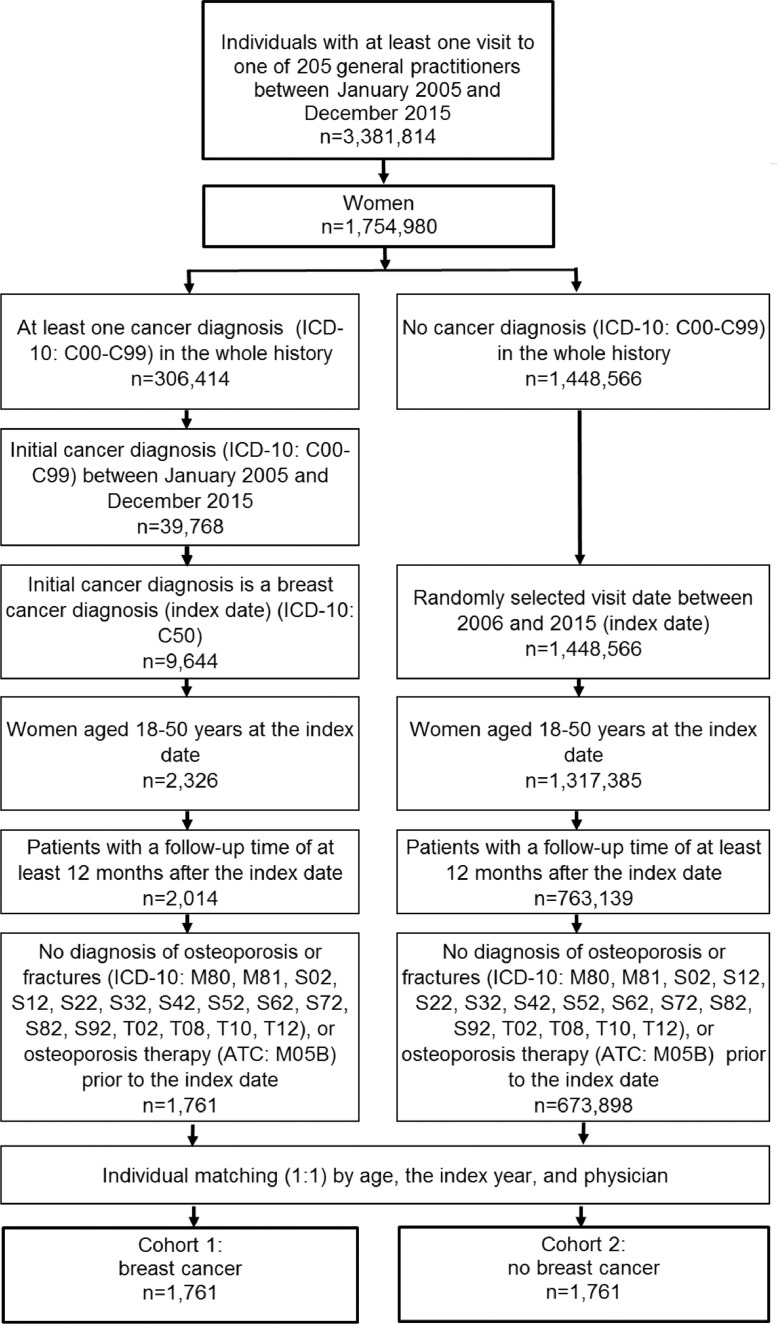
The current study sample included patients who received a BC diagnosis (ICD-10: C50) for the first time in one of 205 general practices in the UK between January 2005 and December 2015 (index date). Inclusion criteria were as follows: a follow-up time of at least 12 months after the index date; age between 18 and 50 years at the index date; and no diagnosis of osteoporosis (ICD-10: M80, M81) or fractures (S02, S12, S22, S32, S42, S52, S62, S72, S82, S92, T02, T08, T10, T12), or osteoporosis therapy (ATC: M05B) prior to or at the index date. After applying similar inclusion criteria, patients without a cancer diagnosis were matched 1:1 to patients with BC based on age, index year, and physician. The index date for participants without cancer was a randomly selected visit between January 2005 and December 2015. The present study included 1761 individuals with BC and 1761 individuals without cancer (Fig. 1).
Fig. 1.
Selection of study patients.
2.3. Study variables
Variables included age, index year, smoking behavior (current smokers, ex-smokers, and never smokers), body mass index (BMI), three comorbidities that can be associated with fracture risk and that were documented within 12 months prior to the index date (i.e. diabetes mellitus (ICD-10: E10-14), disorders of bone density and structure (M82-M85), and visual disturbances (H53, H54)), and prescriptions of corticosteroids (ATC: H02) documented within 12 months prior to the index date.
2.4. Study outcome
The main outcome of the study was the incidence of any fracture as a function of BC within 10 years of the index date.
2.5. Statistical analyses
Differences in the sample characteristics between women with BC and those without cancer were tested using chi-squared tests for categorical variables and Wilcoxon tests for continuous variables. We calculated the cumulative incidence of fractures in the BC and no-cancer groups for up to 10 years after the index date using Kaplan-Meier curves. Patients were censored at the time of their first fracture diagnosis or loss to follow-up, whichever occurred first. Since mortality data are not available in the Disease Analyzer database, dead participants were considered as lost to follow-up. We adopted multivariate Cox regression models to study the association between BC and fractures in the overall sample. In the second step, regression analyses were conducted separately in women with BC with and without tamoxifen therapy. Women treated with aromatase inhibitor therapy were excluded from the analysis. A p-value of <0.05 was considered statistically significant, and statistical analyses were performed using SAS 9.4.
3. Results
The present study included 3522 women aged between 18 and 50 years with and without breast cancer (Fig. 1). The mean age was 43.3 years (SD = 6.1 years); 72.8% were 41–50 years, 22.3% 31–40 years, and 4.9% 18–30 years old. We observed no significant difference in the proportions of women who never smoked (50.5% versus 50.3%), but small differences in current smokers (23.7% versus 27.6%, p = 0.038) and ex-smokers (24.0% versus 20.3%, p = 0.035). No statistical differences were found in body mass index, diabetes, disorders of bone density and structure, or visual disturbances (Table 1).
Table 1.
Baseline characteristics of women with and without breast cancer after (1:1) matching.
| Variable | Breast cancer (%) | No cancer (%) | p-value |
|---|---|---|---|
| N | 1761 | 1761 | |
| Age at baseline (Mean, SD) | 43.3 (6.1) | 43.3 (6.1) | 1.000 |
| Age 18–30 | 4.9 | 4.9 | 1.000 |
| Age 31–40 | 22.3 | 22.3 | |
| Age 41–50 | 72.8 | 72.8 | |
| Smoking behavior | |||
| Current smoker | 23.7 | 27.6 | 0.038 |
| Ex-smoker | 24.0 | 20.3 | 0.035 |
| Never smoked | 50.5 | 50.3 | 0.933 |
| Body mass index | |||
| ≤19.0 | 3.3 | 3.9 | 0.063 |
| >19.1–24.9 | 51.6 | 48.5 | |
| >25.0–29.9 | 26.1 | 24.3 | |
| ≥30.0 | 19.0 | 23.4 | |
| Diagnosis within 12 months prior to the index date | |||
| Diabetes mellitus (E10-14) | 2.2 | 2.1 | 0.817 |
| Disorders of bone density and structure (M82-M85)* | 0.3 | 0.5 | 0.284 |
| Visual disturbances (H53, H54) | 2.1 | 1.9 | 0.629 |
| Prescriptions within 12 months prior to the index date | |||
| Systemic corticosteroids (ATC: H02) | 2.9 | 3.9 | 0.124 |
Disorders of bone density and structure include adult osteomalacia, malunion of fracture, fibrous dysplasia, skeletal fluorosis, hyperostosis of skull, and osteitis condensans.
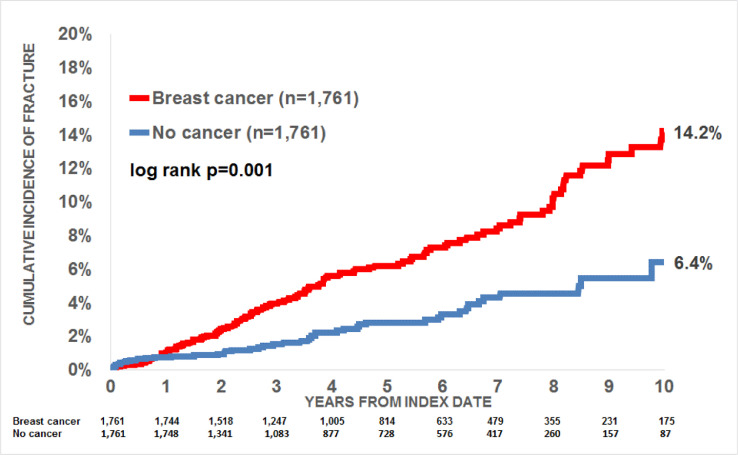
Within 10 years of the index date, 14.2% of women with breast cancer and 6.4% of those without cancer received a first fracture documentation (log-rank p-value< 0.001; Fig. 2).
Fig. 2.
Cumulative incidence of fractures in women aged 18–50 with breast cancer and non-cancer women.
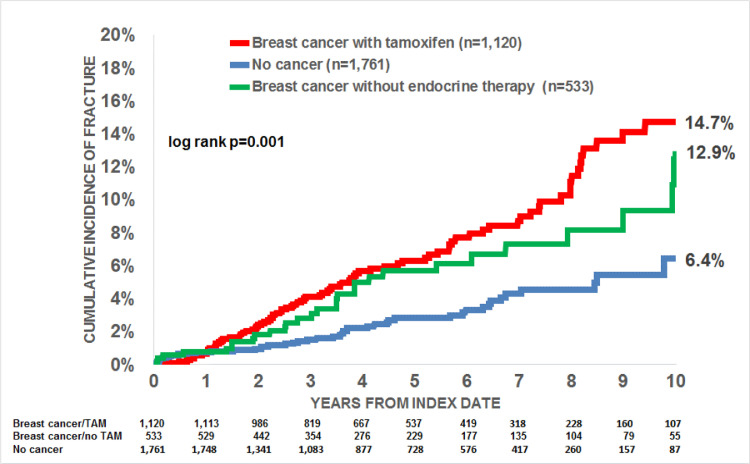
We observed a positive association between breast cancer and fractures (adjusted hazard ratio (HR)=2.39, p < 0.001) (Table 2). When separately analyzing women with and without tamoxifen therapy, 14.7% with and 12.9% without tamoxifen received a fracture diagnosis (Fig. 3). However, after adjusting for covariables (BMI, smoking behavior, comorbidities, and corticosteroid therapy), the HR was 2.58 (p < 0.001) for breast cancer with tamoxifen therapy versus no cancer and 1.63 (p = 0.181) for breast cancer without tamoxifen therapy versus no cancer (Table 2). The difference in incidence of fractures between women with and without breast cancer using tamoxifen was not significant.
Table 2.
Association between breast cancer and fracture incidence (multivariate Cox regression models).
| Age group | Breast cancer vs. no cancer | Breast cancer with tamoxifen vs. no cancer | Breast cancer without endocrine therapy vs. no cancer |
|---|---|---|---|
| OR (95% CI)* and p-values | OR (95% CI)* and p-values | OR (95% CI)* and p-values | |
| Total | 2.39 (1.70–3.34) | 2.67 (1.58–4.53) | 1.63 (0.80–3.33) |
| P <0.001 | P < 0.001 | p = 0.181 | |
| Age 18–30 | 1.06 (0.07–16.86) | 0.00 | 2.08 (0.13–33.22) |
| 0.970 | p = 0.605 | ||
| Age 31–40 | 3.98 (1.16–13.68) | 2.66 (1.18–5.98) | 2.12 (0.87–5.17) |
| p = 0.028 | p = 0.018 | p = 0.098 | |
| Age 41–50 | 2.25 (1.35–3.75) | 2.48 (1.64–3.76) | 2.02 (1.15–3.56) |
| p = 0.002 | P < 0.001 | p = 0.015 |
multivariable Cox regression adjusted for BMI, smoking behavior, comorbidities, and corticosteroid therapy.
Fig. 3.
Cumulative incidence of fractures in women aged 18–50 with breast cancer with and without tamoxifen therapy, and non-cancer women.
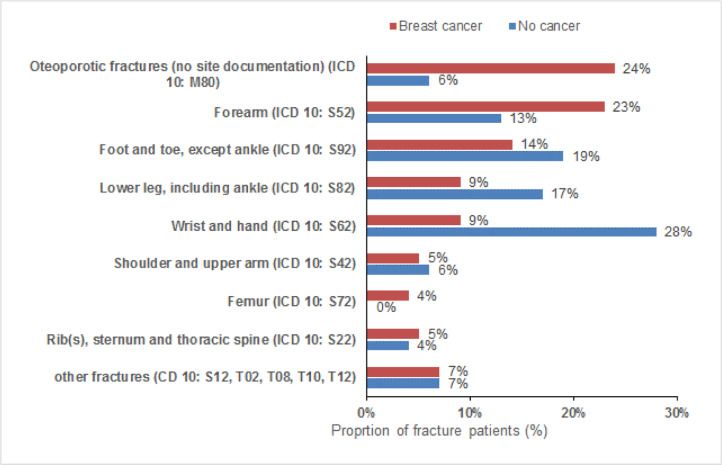
In women with a fracture diagnosis, osteoporotic fractures with no site information (ICD 10: M80) and forearm fractures (ICD 10: S52) were found more often in breast cancer than in non-cancer patients (24% versus 6%, and 23% versus 13%). In contrast, fractures of the wrist and hand (9% versus 28%), lower legs (9% versus 17%), and foot and toe (14% versus 19%), were less common in women with breast cancer compared to those without cancer (Fig. 4).
Fig. 4.
Fracture sites in women aged 18–50 with and without breast cancer.
4. Discussion
The present large-scale, population-based cohort study confirms the negative impact of tamoxifen treatment on the incidence of fractures in premenopausal women with BC. We observed a positive association between breast cancer and fractures: within 10 years of the index date, 14.2% of women with BC and 6.4% of those without cancer had received a first fracture documentation.
The analysis of the patients with or without tamoxifen showed a higher incidence of fractures (14.7%) in the group with tamoxifen compared to the group without tamoxifen therapy (13%). After adjusting for covariables, the difference remained significant (p < 0.001) for breast cancer with tamoxifen therapy versus no cancer and was not significant (p = 0.057) for breast cancer without tamoxifen therapy versus no cancer. This may be due to the different case numbers for the group with tamoxifen (n = 1120) and the group without tamoxifen (n = 641).
Assuming that the patients who did not receive tamoxifen and/or any endocrine treatment (aromatase inhibitor, OFS) received chemotherapy, we would expect a higher fracture rate in this group than in the tamoxifen group.
In the documentation of the fractures, a distinction could be made between osteoporotic fractures and traumatic fractures. However, no localization was documented for the osteoporotic fractures; only the osteoporotic fracture itself was documented. The distribution of fracture localities compared between no cancer and breast cancer patients is interesting. It is striking that the incidence of osteoporotic fractures in the group with breast cancer is significantly higher (24%) than in the group without breast cancer (6%). Furthermore, we found a similar ratio for fractures of the forearm and the distal radius: 23% incidence in the breast cancer group and 13% in the group without breast cancer. Thus, distal radius fractures (DRFs) that occur after a fall from body height should be considered an indicator for the presence of underlying osteoporosis, for which postmenopausal women and men >65 years old are at a particular risk [13,14]. Fractures of the distal radius (DRFs) are commonly one of the first fracture sites to indicate underlying osteoporosis. In a trial conducted by Sakuma et al. [13], who investigated the average age at the time of an osteoporotic fracture, patients suffering a distal radius fracture were shown to be almost 20 years younger than patients suffering a hip, vertebral or proximal humerus fracture (average age at the time of DRFs was 60.2 years vs. average age of the abovementioned fractures= 81.4; 77.7; 75.7 years) [13]. In our study, the patients were even younger, but due to the breast cancer diagnoses and the adjuvant therapies with or without tamoxifen, they had a significantly higher fracture rate compared to the healthy group.
Prior evidence has clearly shown that cancer treatment-induced bone loss (CTIBL) results in substantial loss of bone mineral density (BMD) [15]. This effect was particularly seen in premenopausal women with BC. As shown in a recent study with premenopausal women, those who received adjuvant chemotherapy for 6 months (4–6 cycles of chemotherapy) experienced a significant loss of 3.8% BMD in the lumbar spine [15]. Tamoxifen alone and/or in combination with ovarian function suppression (OFS) are standards of care for women with estrogen receptor-positive breast cancer [4].
Tamoxifen as a selective estrogen receptor modulator has been shown to have bone-protecting effects with regard to BMD in postmenopausal women [7]; in contrast, in premenopausal BC patients, a loss of BMD has been reported [10].
Powles at al. pointed out in a sub-study of a trial examining three years of tamoxifen treatment that premenopausal women receiving tamoxifen experienced a mean BMD loss at the lumbar spine of 3.26% versus the baseline, compared with no significant bone loss in the placebo group [16]. This finding is similar to the results of Kyvernitakis et al. (2018), who observed not only BMD loss but also increased fracture risk [6].
This loss of bone mineral density may lead to the increased risk of fractures that was apparent in our study. Our results show that the incidence of fractures for premenopausal BC patients receiving tamoxifen (14.7%) is not significantly higher than in patients receiving adjuvant chemotherapy (13.0%).”
To the best of our knowledge, this is the first real-world data evaluation of the incidence of fractures in premenopausal BC patients receiving tamoxifen or adjuvant chemotherapy.
The present study is subject to certain limitations. The incidence of fractures and the classification of osteoporotic fractures or the absence thereof relied on the documentation of ICD codes by general practitioners. No data were available regarding menopausal status, which means that age-matched women may not always have really entered menopause and vice versa. Information is lacking about chemotherapy and accompanying endocrine treatment. Therefore, we postulate with a high certainty that patients with diagnosed BC and without documented tamoxifen treatment received adjuvant chemotherapy and or endocrine treatment. Furthermore, no valid information on TNM status was documented in the database. The amount of substantial bone loss during treatment of BC in premenopausal women is a relevant unmet medical need that is currently inadequately addressed. Since CTIBL leads to increased fracture rates, preventive measures must be considered [10]. In particular, younger breast cancer patients, who have good long-term chances of survival, will be confronted later in life with a significantly increased risk of fracture or incidence of fractures. Furthermore, the adjuvant therapy recommendations tend to extend therapy with tamoxifen beyond the currently usual 5 years [17]. Persistence with this treatment is high [18]. On one hand, this leads to better survival rates; on the other hand, however, an increase in side effects must also be assumed. In tamoxifen therapy, premenopausal breast cancer patients would then have to expect a further increase in fracture rate.
The follow-up practices after the initial diagnosis of breast cancer are assumed to be variable in the UK; studies on long-term side effects are missing, and patients thus miss out on optimal treatment approaches [19].
An interesting outlook here involves studies with zoledronic acid treatment (given every 6 months during adjuvant therapy), which could effectively protect against such substantial loss of bone mass and may provide long-lasting benefits [20], [21], [22].
In conclusion, premenopausal women with BC undergoing therapy with tamoxifen are at a high risk. In our study, the incidence of fractures in this group was significantly higher than in the group without tamoxifen treatment. Further studies and clinical trials concerning the long-time side effects and possible preventive treatments are still needed.
Declaration of Competing Interest
All authors declare that they have no conflicts of interest with regard to this article.
Acknowledgments
Acknowledgments
Nothing to declare.
Funding
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100254.
Appendix. Supplementary materials
References
- 1.Yedjou C.G., Tchounwou P.B., Payton M., Miele L., Fonseca D.D., Lowe L., Alo R.A. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int. J. Environ. Res. Pub. Health. 2017;14(5) doi: 10.3390/ijerph14050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.C.E. DeSantis, J. Ma, A. Goding Sauer, L.A. Newman, A. Jemal, Breast cancer statistics, 2017, racial disparity in mortality by state, CA: Cancer J. Clin.67(6) (2017) 439–448. [DOI] [PubMed]
- 3.Schmidt N., Jacob L., Coleman R., Kostev K., Hadji P. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res. Treat. 2016;155(1):151–157. doi: 10.1007/s10549-015-3661-3. [DOI] [PubMed] [Google Scholar]
- 4.Aebi S., Davidson T., Gruber G., Cardoso F. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011;22(Suppl 6):vi12–vi24. doi: 10.1093/annonc/mdr371. [DOI] [PubMed] [Google Scholar]
- 5.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E., Zackrisson S., Cardoso F. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015;26(Suppl 5):v8–30. [Google Scholar]
- 6.Hadji P., Kauka A., Ziller M., Birkholz K., Baier M., Muth M., Bauer M. Effects of zoledronic acid on bone mineral density in premenopausal women receiving neoadjuvant or adjuvant therapies for HR+ breast cancer: the ProBONE II study. Osteoporos. Int.: A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(4):1369–1378. doi: 10.1007/s00198-013-2615-z. [DOI] [PubMed] [Google Scholar]
- 7.Mincey B.A., Duh M.S., Thomas S.K., Moyneur E., Marynchencko M., Boyce S.P., Mallett D., Perez E.A. Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clin. Breast Cancer. 2006;7(2):127–132. doi: 10.3816/CBC.2006.n.021. [DOI] [PubMed] [Google Scholar]
- 8.Hadji P., Gnant M., Body J.J., Bundred N.J., Brufsky A., Coleman R.E., Guise T.A., Lipton A., Aapro M.S. Cancer treatment-induced bone loss in premenopausal women: a need for therapeutic intervention? Cancer Treat. Rev. 2012;38(6):798–806. doi: 10.1016/j.ctrv.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J., Sestak I., Baum M., Buzdar A., Howell A., Dowsett M., Forbes J.F. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 10.Vehmanen L., Elomaa I., Blomqvist C., Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24(4):675–680. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 11.Vehmanen L.K., Elomaa I., Blomqvist C.P., Saarto T. The effect of ovarian dysfunction on bone mineral density in breast cancer patients 10 years after adjuvant chemotherapy. Acta Oncol. (Stockholm, Sweden) 2014;53(1):75–79. doi: 10.3109/0284186X.2013.792992. [DOI] [PubMed] [Google Scholar]
- 12.Saarto T., Vehmanen L., Blomqvist C., Elomaa I. Ten-year follow-up of 3 years of oral adjuvant clodronate therapy shows significant prevention of osteoporosis in early-stage breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008;26(26):4289–4295. doi: 10.1200/JCO.2007.15.4997. [DOI] [PubMed] [Google Scholar]
- 13.Kyvernitakis I., Kostev K., Hadji P. The tamoxifen paradox-influence of adjuvant tamoxifen on fracture risk in pre- and postmenopausal women with breast cancer. Osteoporos. Int.: A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2018;29(11):2557–2564. doi: 10.1007/s00198-018-4642-2. [DOI] [PubMed] [Google Scholar]
- 14.Love R.R., Mazess R.B., Barden H.S., Epstein S., Newcomb P.A., Jordan V.C., Carbone P.P., DeMets D.L. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N. Engl. J. Med. 1992;326(13):852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 15.Zidan J., Keidar Z., Basher W., Israel O. Effects of tamoxifen on bone mineral density and metabolism in postmenopausal women with early-stage breast cancer. Med. Oncol. (Northwood, London, England) 2004;21(2):117–121. doi: 10.1385/MO:21:2:117. [DOI] [PubMed] [Google Scholar]
- 16.Barni S., Lissoni P., Tancini G., Ardizzoia A., Cazzaniga M. Effects of one-year adjuvant treatment with tamoxifen on bone mineral density in postmenopausal breast cancer women. Tumori. 1996;82(1):65–67. doi: 10.1177/030089169608200114. [DOI] [PubMed] [Google Scholar]
- 17.Ogdie A L.S., Parkinson J., Dattani H., Kostev K., Gelfand J.M. Medical record databases. Pharmacoepidemiology. 2012:224–243. [Google Scholar]
- 18.Kyvernitakis I., Kostev K., Nassour T., Thomasius F., Hadji P. The impact of depot medroxyprogesterone acetate on fracture risk: a case-control study from the uK. Osteoporos. Int.: A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2017;28(1):291–297. doi: 10.1007/s00198-016-3714-4. [DOI] [PubMed] [Google Scholar]
- 19.Sakuma M., Endo N., Oinuma T., Endo E., Yazawa T., Watanabe K., Watanabe S. Incidence and outcome of osteoporotic fractures in 2004 in Sado City, Niigata Prefecture, Japan. J. Bone Min. Metab. 2008;26(4):373–378. doi: 10.1007/s00774-007-0841-1. [DOI] [PubMed] [Google Scholar]
- 20.Jerrhag D., Englund M., Karlsson M.K., Rosengren B.E. Epidemiology and time trends of distal forearm fractures in adults - a study of 11.2 million person-years in Sweden. BMC Musculoskelet. Disord. 2017;18(1):240. doi: 10.1186/s12891-017-1596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalder M., Kyvernitakis I., Albert U.S., Baier-Ebert M., Hadji P. Effects of zoledronic acid versus placebo on bone mineral density and bone texture analysis assessed by the trabecular bone score in premenopausal women with breast cancer treatment-induced bone loss: results of the ProBONE II substudy. Osteoporos. Int.: A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(1):353–360. doi: 10.1007/s00198-014-2955-3. [DOI] [PubMed] [Google Scholar]
- 22.Cameron D.A., Douglas S., Brown J.E., Anderson R.A. Bone mineral density loss during adjuvant chemotherapy in pre-menopausal women with early breast cancer: is it dependent on oestrogen deficiency? Breast Cancer Res. Treat. 2010;123(3):805–814. doi: 10.1007/s10549-010-0899-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.