Abstract
Anti-beta 2 glycoprotein 1 (anti-β2GP1) antibodies are commonly found in patients with autoimmune diseases such as the antiphospholipid syndrome (APS) and systemic lupus erythematosus (SLE). Their presence is highly associated with increased risk of vascular thrombosis and/or recurrent pregnancy-related complications. Although they are a subtype of anti-phospholipid (APL) antibody, anti-β2GP1 antibodies form complexes with β2GP1 before binding to different receptors associated with anionic phospholipids on structures such as platelets and endothelial cells. β2GP1 consists of five short consensus repeat termed “sushi” domains. It has three interchangeable conformations with a cryptic epitope at domain 1 within the molecule. Anti-β2GP1 antibodies against this cryptic epitope are referred to as ‘type A’ antibodies, and have been suggested to be more strongly associated with both vascular and obstetric complications. In contrast, ‘type B’ antibodies, directed against other domains of β2GP1, are more likely to be benign antibodies found in asymptomatic patients and healthy individuals. Although the interactions between anti-β2GP1 antibodies, β2GP1, and platelets have been investigated, the actual targeted metabolic pathway(s) and/or receptor(s) involved remain to be clearly elucidated. This review will discuss the current understanding of the interaction between anti-β2GP1 antibodies and β2GP1, with platelet receptors and associated signalling pathways.
Keywords: anti-beta 2 glycoprotein 1 antibodies, beta 2 glycoprotein 1, platelet, antiphospholipid antibody, antiphospholipid syndrome, systemic lupus erythematosus
1. Introduction
Anti-phospholipid (APL) antibodies are a heterogeneous group of autoantibodies targeting different phospholipid binding protein antigens. These autoantibodies include lupus anti-coagulant (LAC), anti-cardiolipin (aCL), anti-beta 2 glycoprotein 1 (anti-β2GP1), and anti-prothrombin antibodies [1]. APL antibodies dysregulate normal cellular activities and are associated with recurrent thrombosis (venous, arterial, and microvascular), pregnancy complications (e.g., obstetric failure, pre-eclampsia and eclampsia), and non-specific manifestations (e.g., thrombocytopenia, heart valve disease, chorea, livedo reticularis/racemosa, and nephropathy) [2]. APL antibodies are also present in 1%–5% of healthy populations, including children [3,4]. These populations appear to be asymptomatic, since their autoantibodies are associated with low reactivity [4].
Persistently high levels of APL antibodies, together with specific clinical manifestations, are required for the diagnosis of antiphospholipid syndrome (APS) [1]. APS can occur in isolation or in association with underlying autoimmune diseases such as systemic lupus erythematosus (SLE). The Sydney criteria for the diagnosis of APS recommend that three standard diagnostic assays are used to detect APL antibodies [5]. These diagnostic assays include two enzyme-linked immunosorbent assays (ELISA) that directly detect APL antibodies binding to cardiolipin-β2GP1 complexes, or β2GP1 only. The third is a clotting assay which indirectly detects APL antibodies by measuring their functional effects on the coagulation system (LAC activity, Table 1) [1,3,6]. Although these assays detect overlapping subpopulations of autoantibodies, their correlation with the clinical manifestations of APS can be varied. LAC assays are superior for detecting pathological subpopulations of APL antibodies when the quality of plasma is maintained [7]. ELISAs for aCL and anti-β2GP1 antibodies, however, are weakly associated with thrombotic complications. This may be due to poor standardisation of assays, variable sources and the integrity of β2GPI, the secondary calibration process, and/or the assessment and derivation of cut-off values [8]. Consequently, a combination of these tests is used to determine the clinical risk. Patients with persistently high APL antibodies titres (positive in ELISA) and positive LAC activities on at least two occasions, 12 weeks apart, are at higher risk of thrombosis and/or pregnancy complications [1].
Table 1.
Detection of anti-phospholipid antibodies and their clinical significance.
| Assays | Principle of Detection | Antibodies Detected | Clinical Significance [5] |
|---|---|---|---|
| LAC | Clotting assay | LAC (mainly against β2GP1 and prothrombin) | |
| aCL antibody | Immunological assay | aCL antibody (IgG, IgM, IgA) |
|
| Anti-β2GP1 antibody | Immunological assay | Anti-β2GP1 antibody (IgG, IgM, IgA) |
|
| Anti-prothrombin antibody | Immunological assay | Anti-prothrombin and anti-phosphatidylserine-prothrombin complex |
Information collated from Miyakis et al. (2006) [5]. Abbreviations: LAC, lupus anti-coagulant; aCL antibody, anti-cardiolipin antibody; Ig, Immunoglobulin; anti-β2GP1 antibody, anti-beta 2 glycoprotein 1 antibody.
The criteria for the diagnosis of APS are well established, yet the interactions between APL antibodies, targeted antigens, and receptors remain unclear. Anti-β2GP1 antibodies and their target, β2GP1, have become a focus of research for their potential role in thrombosis and pregnancy complications [9]. β2GP1-dependent LAC antibodies demonstrate a stronger correlation with thrombosis compared to β2GP1-independent LAC antibodies [10,11]. Similarly, β2GP1-dependent aCL antibodies are more highly associated with APL antibodies-related complications compared to transient β2GP1-independent aCL antibodies induced by infections [12]. Many potential mechanisms of interaction between anti-β2GP1 antibodies, β2GP1, and cells—e.g., platelets, endothelial cells and monocytes—have been suggested [13]. However, studies investigating the effects of anti-β2GP1 antibodies and β2GP1 on platelets [14,15,16] may help lead to an improved understanding of their interactions, and consequently, their impact on the haemostatic system [17]. Activation of platelet receptor(s)/metabolic pathway(s) by anti-β2GP1 antibodies and β2GP1 may result in excessive clot formation and potentially initiate thrombosis and/or pregnancy complications [14,15,16]. Therefore, this review discusses the current understanding of the characteristics and interactions between β2GP1 and anti-β2GP1 antibodies in relation to platelet receptors and function.
2. β2GP1
APL antibodies were originally thought to bind directly to phospholipids [26]. In the 1990s, three independent groups demonstrated that APL antibodies actually interacted with phospholipids via β2GP1 [27,28,29], significantly raising the interest in this protein. β2GP1 had been discovered earlier in 1961 [30], and its amino acid sequence determined in 1984 [31]. It was misnamed apolipoprotein H [32], since it is not an integral part of lipoproteins. Once synthesised in the liver and placenta, β2GP1 circulates in blood at a concentration of approximately 4–5 μM. Blood levels of β2GP1 are higher in older individuals and in patients with APS, but are lower in pregnant women and patients with stroke and myocardial infarction [33].
β2GP1 is an evolutionarily conserved single chain anionic phospholipid-binding glycoprotein, with a molecular weight of approximately 43 kDa [34,35,36]. It belongs to the complement control protein superfamily [37] and consists of 326 amino acids that are arranged in five short consensus repeat, termed “sushi” domains [31,38,39]. The first four domains, each comprising approximately 60 amino acids, are conserved sequences linked together by two disulfide bridges. The fifth domain (DV), however, is a modified form with 82 amino acids. It contains a six residue insertion, a 19-amino acid C-terminal extension and an additional disulfide bond that includes a C-terminal cysteine. These positively charged lysine-rich amino acids (282–287) determine the affinity of β2GP1 for anionic phospholipids and negatively charged molecules. DV also adopts a flexible hydrophobic loop (amino acids 311–317), containing a Trp-Lys sequence which is potentially able to insert into membranes. β2GP1 has four N-glycosylation sites (Arg143, Arg 164, Arg 174, and Arg 234) located in third domain (DIII) and fourth domain (DIV). There is also one O-linked sugar on Thr130 in β2GP1 that accounts for approximately 20% w/w of the total molecular mass [40].
2.1. Conformations of β2GP1
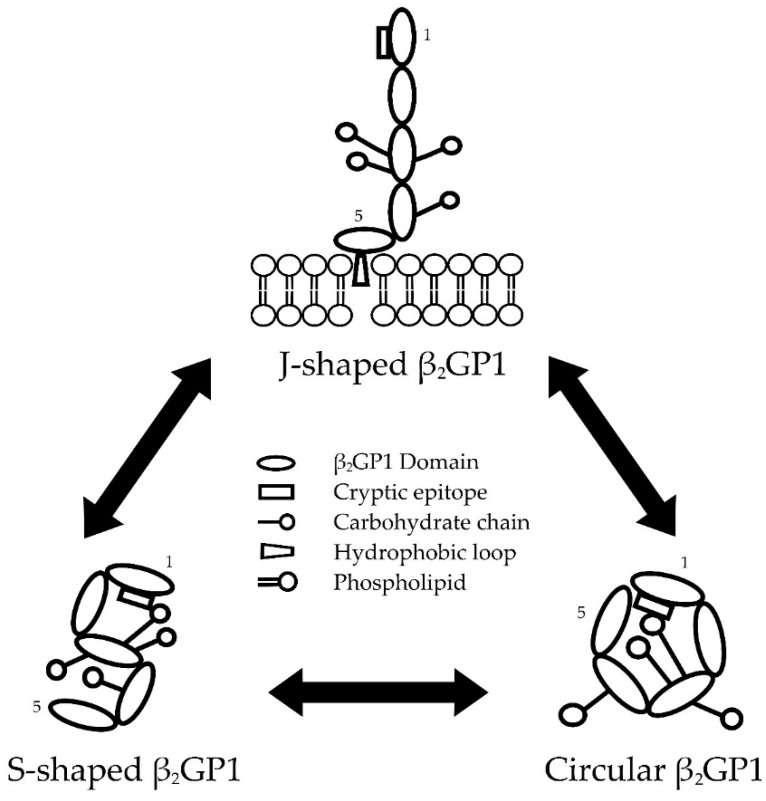
β2GP1 adopts many post-translational modifications which alter the structure and function of the molecule and the exposure of the cryptic epitope [41]. Among them, three interchangeable conformations are more commonly reported (Figure 1). The first conformation was reported by two groups [38,42] based on the crystal structure of the protein. In this conformation, first four domains are stretched with DV at a right angle to the other domains, resembling a J-shape, fish-hook or ‘hockey stick’ conformation. The second reported conformation is S-shaped, as demonstrated using small-angle X-ray scattering [43]. This conformation contains carbohydrate chains from DIII–IV that are twisted and positioned on DI. The third conformation is a common ‘closed’ circular formation present in plasma where DI interacts with DV. This circular formation was initially proposed by Koike et al. in 1998 [44], and later directly visualised by Agar et al. (2010) using electron microscopy [41].
Figure 1.
The interchangeable conformations of beta-2-glycoprotein 1 (β2GP1). β2GP1 is able to transform between three conformations: J-shaped, S-shaped, and circular β2GP1. Cryptic epitopes in S-shaped are shielded by carbohydrate chains [43]. Whereas, cryptic epitopes in circular β2GP1 are shielded by both carbohydrate chains and domain V [41,44]. Binding of domain V positively charged amino acids and hydrophobic loop to phospholipid membrane breaks the shield on domain I [41]. This exposes the cryptic epitope and allows the binding of clinically significant anti-domain-I-β2GP1 antibody.
2.1.1. Transformation between β2GP1 Conformations
The discovery of three interchangeable β2GP1 structures led to increased understanding of the interaction between anti-β2GP1 antibodies and β2GP1. These conformational alterations determine the exposure of the cryptic epitope which includes arginine 39–arginine 43 (R39–R43), DI–II interlinker, and possibly aspartic acid residues at positions 8 and 9 [45]. Anti-domain-I-β2GP1 (anti-DI-β2GP1) antibodies targeting this discontinuous epitope are highly associated with APL antibodies-related clinical manifestations [46,47].
β2GP1 is suggested to circulate in an S-shaped or a circular conformation, with less than 0.1% of β2GP1 in circulation present in the J-shaped conformation [41,47]. The cryptic epitope in both S-shaped and circular β2GP1 is shielded by carbohydrate chains positioned on top of DI [43,48]. In circular β2GP1, these negatively-charged carbohydrate chains are also proposed to neutralise the positively-charged DI, allowing the binding of DV [47]. Therefore, S-shaped β2GP1 may represent an intermediate form of the molecule as it transforms from a circular to J-shaped conformation [47]. When positively charged amino acids and hydrophobic loop in DV interact with anionic surfaces, β2GP1 opens out to the J-shaped conformation, breaking the shield on DI and exposing the cryptic epitope [41].
2.1.2. Factors Affecting β2GP1 Conformation
The conformation of β2GP1 is dependent on its interaction with anionic surfaces. Its affinity decreases in the presence of ethylene-diamine-tetra-acetic acid (EDTA) [49], and high concentrations of bivalent cations—e.g., calcium and magnesium ions [50]. β2GP1 that has been cleaved at DV is also known to have lower affinity [51]. Conversely, dimerisation [52] and increasing β2GP1 concentration [50] elevate its affinity. Besides exposure to anionic surfaces, alternations to pH and salt concentration in vitro allow structural transformation of β2GP1 [41]. High pH and salt concentrations convert circular β2GP1 into the J-shaped conformation, and vice versa at a low pH and salt concentration. It has also been speculated that these alterations in pH and salt concentration possibly affect the hydrophilic interaction that may be present between DI and DV [41].
APS patients have been proposed to have higher oxidative stress compared to healthy individuals [53]. Oxidative stress favours disulfide bonding between Cys32 and Cys60 (located at DI) and within Cys288 and Cys326 (located at DV) of β2GP1. These bonds potentially encourage the binding of anti-β2GP1 antibodies to β2GP1, and might lead to thrombus formation. Oxidation and biotinylation of β2GP1 glycan chains also induce β2GP1 dimerisation, which raises β2GP1 affinity [54]. Additionally, it is speculated that the intramolecular interaction and conformation of β2GP1 can be affected by increased sialylation of β2GP1 glycan structures [55].
Lastly, the structure of β2GP1 can be inherently diverse. Among the four allelic variants, β2GP1 Val/Val genotypes were frequently found to co-exist with anti-β2GP1 antibodies [56]. It has also been proposed that the Val247 variant of circular-β2GP1 is easier to transform into J-shaped β2GP1 after losing the electrostatic interaction between Glu228 (located in DIV) and Lys308 (located in DV) [57]. Thus, this transformation exposes the cryptic epitope for antibody binding and raises the risk of thrombosis.
2.2. Physiological Role(s) of β2GP1
The precise physiological role of β2GP1 is unknown. β2GP1-deficient individuals appear to be healthy, suggesting that β2GP1 function might not be essential for life [58]. However, the disulphide bonds and phospholipid binding sites in β2GP1 are highly conserved across the animal kingdom [36]. Therefore, it is very unlikely that this abundant and well-conserved molecule exists without a function.
Although β2GP1-deficient individuals do not have an associated haemostatic abnormality, many functions in the regulation of haemostasis have been attributed to β2GP1. First, β2GP1 has been demonstrated to inhibit adenosine diphosphate (ADP)-mediated platelet aggregation and serotonin secretion [59,60]. Second, β2GP1 might be a mediator for von Willebrand factor (vWF) activation and clearance. β2GP1 has been reported to bind to the A1-domain of vWF, preferably vWF in a glycoprotein (GP) Ib-binding conformation. This low affinity binding allows the formation of disulfide bridges between β2GP1 and vWF. Thus, the disulfide bridges prevent vWF-mediated platelet activation [15] and potentially protect the cleavage of vWF by the vWF protease, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) [61]. Thirdly, β2GP1 has also been demonstrated to be involved in several coagulation pathways, yet these effects remain to be elucidated [60].
β2GP1 has been suggested to be a general scavenger in circulation [62,63]. During apoptosis or cellular activation, the reorganisation of the plasma membrane exposes phosphatidylserine on the cell surface. β2GP1 binds to phosphatidylserine expressed on these apoptotic cells [62], as well as platelet microparticles [63], to assist their phagocytosis by macrophages. In addition, β2GP1 is also involved in innate immunity as demonstrated by the insertion of DV of β2GP1 into bacterial membranes that can lead to cytosol leakage and death of bacteria [64]. β2GP1 also changes its conformation while binding to lipopolysaccharide on Gram-negative bacteria, forming a complex which allows recognition and clearance by monocytes [65]. Finally, β2GP1 might be important in embryonic development, as the percentage of null offspring born in β2GP1 knock-out mice is lower than expected [66].
In summary, β2GP1 has been proposed to be involved in a range of physiological processes, including clot formation, fibrinolysis, cell activation, immune responses, atherosclerosis, apoptosis, angiogenesis, and fetal loss [60]. Further research is clearly warranted to determine the precise physiological role(s) of β2GP1.
3. Anti-β2GP1 Antibodies
By itself, β2GP1 has no deleterious effect on normal cellular function, but rather interferes with the physiological function of cells following binding with anti-β2GP1 antibodies. Therefore, it has been proposed that anti-β2GP1 antibodies induce a new function for β2GP1 [67]. The affinity of β2GP1 is low and only binds to anionic phospholipids below a certain concentration [41,48]. Upon binding with anionic phospholipids, it transforms into the J-shaped conformation and exposes the cryptic epitope located at DI which enables antibodies to bind. When the amount of β2GP1 bound to anionic phospholipid membrane reaches a certain density, antibodies dimerise the adjacent β2GP1 molecules [48]. This dimerisation forms a high affinity anti-β2GP1-β2GP1 complex, activating targeted cells and causing APL antibodies-related manifestations.
3.1. Clinical Significance of Anti-β2GP1 Antibodies
The presence of anti-β2GP1 antibodies, especially those with LAC activity, is highly associated with increased thrombotic risk compared to other APL antibody subgroups [10,11]. APS patients have higher levels of platelet activation as reflected by raised urinary thromboxane metabolites [68]. Moreover, the co-existence of J-shaped β2GP1 and anti-β2GP1 antibodies prolongs the activated partial thromboplastin time of normal plasma, compared to J-shaped β2GP1 alone [41], suggesting that anti-β2GP1 antibodies also affect secondary haemostasis. Conversely, 40% of APS patients have a prolonged bleeding time without an accompanying bleeding tendency [69]. Although there is no clear explanation for these contradictory findings, it suggests that anti-β2GP1 antibodies affect normal haemostatic function.
The contribution of anti-β2GP1 antibodies to placental-related pregnancy complications remains controversial. A systematic review and meta-analysis reported that there were insufficient data to support an association between anti-β2GP1 antibodies and pregnancy complications [70]. However, an in vitro study demonstrated that anti-β2GP1 antibodies stimulate trophoblasts to increase secretion of vascular endothelial growth factor, placental growth factor, and soluble endoglin, leading to a higher risk of obstetrical complication [71]. Furthermore, anti-β2GP1-β2GP1 complexes have been suggested to disrupt the anticoagulant shield formed by annexin A5 on vascular cells [72]. Thus, patients could be predisposed to placental thrombosis that may result in fetal growth restriction and/or pregnancy loss.
3.2. Etiology of Anti-β2GP1 Antibodies
The etiology of anti-β2GP1 antibodies remains unclear. Both genetic and environmental factors may contribute to their production [2,73]. Various animal models and family/population studies have indicated that several human leukocyte antigen genes are associated with the occurrence of APL antibodies and the development of thrombosis [74,75,76]. These pathogenic antibodies are thought to be produced by activated auto-reactive T and B cells due to the similarity between foreign and self-protein/peptide sequences (molecular mimicry) [77]. Viruses, bacteria, mycoplasma and parasites with the same amino acid sequences can also initiate antibody production [78]. However, this theory is unable to clearly explain the etiology, as antibodies are also produced by injecting anionic phospholipids such as cardiolipin, phosphatidylserine, or lipopolysaccharide into animals [79,80].
Anti-β2GP1 antibodies might be naturally occurring antibodies, as benign and low affinity APL antibodies are found in 1%–5% of healthy individuals [3,81]. The mechanism(s) of transition of anti-β2GP1 antibody from benign to pathogenic are unknown, however there is evidence to suggest that this may be induced by infection. β2GP1 binds to pathogenic phospholipids such as protein H from Streptococcus pyogenes [82], causing conformational change, exposure of the cryptic epitope, and inducing production of pathogenic anti-DI-β2GP1 antibodies. The conformation of β2GP1 is also susceptible to many factors and may trigger the synthesis of antibodies. Similarly, antibody production can be prompted by ageing, vaccination, drugs, and malignancies. Their association with clinical manifestations, however, requires further investigation [2,73].
3.3. The Two Hit Hypothesis
The detection of anti-β2GP1 antibodies in healthy individuals [3,4], APS, and SLE patients without complications [83] indicates that the antibody alone is insufficient for the pathogenesis of APS. It is proposed that a “first-hit” injury primes the endothelium, and a “second-hit” injury triggers thrombus formation. Studies have shown that anti-β2GP1 antibodies infused into mice only initiate thrombus formation following vessel-wall injury [84,85]. Endothelium priming involves vessel-wall injury, infection, recent surgery [86], and rarely, the disturbance of redox balance in the vascular milieu [53]. Once primed, the “second-hit” injury, such as smoking, immobilisation, pregnancy, malignancy, etc., stimulates the development of thrombosis [87].
3.4. Types of Anti-β2GP1 Antibodies
The two hit hypothesis has been proposed to be a good model for the pathogenesis of APS [4]. Yet, it cannot clarify why APL antibodies present in healthy individuals are not pathogenic. Some studies suggest that this could be due to differences in the targeted epitope [10,48] and the structure of anti-β2GP1 antibodies [4]. Anti-β2GP1 antibodies isolated from primary APS patients are considered to be poly-reactive, as they have been found to react against several domains of β2GP1, such as DV (52.9%–64.6%), DIV (45.8%), DI–II (33.1%), and DIII (20.5%) [88]. Anti-DI-β2GP1 antibodies recognising the cryptic epitope of DI (Type A) in symptomatic APS patients are strongly associated with thrombotic history and positive LAC activity [10]. Conversely, antibodies that are directed against other domains (Type B) in healthy populations are weakly correlated with thrombosis. These more benign type B antibodies also have lower avidity compared to those pathogenic type A antibodies [89].
Besides binding epitopes, anti-β2GP1 antibodies can be classified according to immunoglobulin (Ig) isotype; i.e., IgG, IgM, and IgA. Among these, anti-β2GP1 IgG antibodies are more strongly associated with the manifestations of APS [1]. Furthermore, different subclasses of anti-β2GP1 IgG antibodies, predominantly IgG2 and IgG3, have also been identified in APS patients and healthy children, respectively [4]. IgG3 is the most effective activator for the classical complement pathway, hence leading to increased C3c (a complement component) activation and binding to anti-β2GP1 IgG3 antibodies in healthy children [4]. Complement activation normally triggers platelet activation, which is related to the pathogenesis of APS [90,91]. However, C3c is an opsonin to improve the clearance of the bound target [92]. Instead of activating platelets, C3c binding enhances the clearance of pathogenic anti-β2GP1 immune complexes and protects healthy children from complications. Moreover, anti-β2GP1 antibodies in healthy and asymptomatic individuals are highly sialylated compared to symptomatic patients [4]. These sialylated anti-β2GP1 antibodies have been found to have protective roles for healthy individuals because of their inability to bind and activate platelets.
3.5. Anti-DI-β2GP1 Antibodies as a Diagnostic Tool
Anti-DI-β2GP1 antibodies are highly associated with both vascular and obstetric complications, compared to antibodies against other domains of β2GP1 [10]. Anti-DI-β2GP1 antibodies are regularly isolated from APS patients compared to those with infection-induced transient APL antibody positivity. APS patients at higher risk of complications (triple APL positivity) also have higher titres of anti-DI-β2GP1 antibodies [93], suggesting that the specificity of diagnosis of APS may increase when anti-DI-β2GP1 antibodies are included. However, assays that detect anti-DI-β2GP1 antibodies have lower sensitivity compared to those that detect the whole β2GP1 molecule, as patients might produce clinically significant antibodies against other epitopes [46]. Currently, commercially available kits are not available for the detection of anti-DI-β2GP1 antibodies. Instead, research assays with different sensitivities have been reported, such as ELISAs that use N-terminally biotinylated DI on streptavidin plates [94] and a β2GP1-DI chemiluminescence immunoassay (CIA, INOVA Diagnostic, San Diego, CA, US) [95]. Further studies are warranted to determine the diagnostic and prognostic value of assays that detect anti-DI-β2GP1 antibodies.
4. Anti-β2GP1-β2GP1 Complexes and Platelets
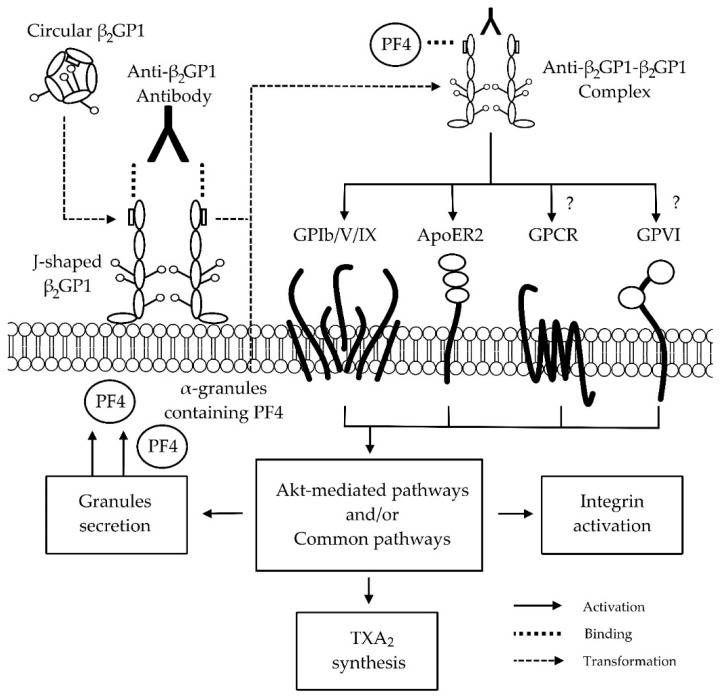
Although there is consensus that β2GP1 interacts with anti-β2GP1 antibodies to form anti-β2GP1-β2GP1 complexes with high affinity to anionic phospholipids [41,48], the affected pathway(s) remains unclear. Potential mechanisms by which APL antibodies might increase the risk of vascular and obstetric complications are reviewed elsewhere [13]. In this review, we have only focused on the effects of anti-β2GP1 antibodies and β2GP1 on platelets (Figure 2).
Figure 2.
Proposed mechanisms of interaction between anti-beta-2-glycoprotein 1 (β2GP1)-β2GP1 complex and platelet receptors. Circular β2GP1 binds to the anionic phospholipid platelet membrane and transforms into J-shaped β2GP1. This allows the anti-domain 1-β2GP1 antibody to bind and to form the anti-β2GP1-β2GP1 complex. The anti-β2GP1-β2GP1 complex has been proposed to interact with glycoprotein (GP) Ib of GPIb/V/IX [14] and apolipoprotein E receptor 2 (ApoER2) [96,97,98]. In our group, we propose that the complex might trigger adenosine diphosphate (ADP) and collagen-mediated pathways via guanine nucleotide-binding protein coupled receptor (GPCR) and GPVI, respectively [99,100]. Yet, further studies are needed to clarify the variability of results. The binding of the complex with receptors leads to the activation of protein kinase B (Akt)-mediated and/or common pathways, causing granules secretion, thromboxane A2 (TXA2) synthesis, integrin activation, and subsequently, clot formation. The platelet factor 4 (PF4) from secreted α-granules have also been showed to interact with the anti-β2GP1-β2GP1 complex [101] Abbreviations: β2GP1, beta-2-glycoprotein 1; GP, glycoprotein; ApoER2, apolipoprotein E receptor 2; ADP, adenosine diphosphate; GPCR, guanine nucleotide-binding protein coupled receptor; TXA2, thromboxane A2; PF4, platelet factor 4; Akt, protein kinase B.
Platelets are a crucial component of haemostasis, a physiological process that forms a localised clot at the vessel injury site to limit blood loss while maintaining normal blood circulation [17,102]. Activation of platelet receptors leads to platelet adhesion, aggregation, activation of the protein kinase B-mediated and/or common pathways, secretion of granules, integrin activation, synthesis of thromboxane A2, and finally, clot formation [17,103]. In the patients with autoimmune diseases, circular β2GP1 transforms into the J-shaped conformation after binding to the phospholipid membrane of platelets, allowing anti-β2GP1 antibodies to bind and form anti-β2GP1-β2GP1 complexes [48] (Figure 2). In turn, these complexes are proposed to activate platelet receptor(s)—e.g., glycoprotein (GP) Ib [14], apolipoprotein E receptor 2 (ApoER2) [16], guanine nucleotide-binding protein-coupled receptors-(GPCR) [100], and GPVI [99]. Furthermore, these complexes have also been suggested to affect other pathway(s) by inhibiting β2GP1 binding to vWF [15] and by interacting with platelet factor 4 (PF4) secreted from platelets [101]. The activation of platelet receptor(s) by these mechanisms potentially results in excessive clot formation and/or pregnancy complications [14,15,16]. Therefore, understanding the effects of anti-β2GP1-β2GP1 complexes on platelets is important not only to determine the mechanism(s) of interaction, but to also potentially assist in the development of novel or improved treatments for patients with autoimmune diseases.
It has been reported that β2GP1 directly binds to GPIb of the GPIb/V/IX receptor via DII–V [14]. The presence of anti-DI-β2GP1 antibodies potentially dimerises β2GP1 and inappropriately initiates GPIb-mediated platelet adhesion and aggregation [14,104]. This activation by anti-β2GP1-β2GP1 complexes may explain the increased thrombotic risk in APS patients [14].
Besides the GPIb receptor, DV of β2GP1 has been shown to dimerise and interact with the A1 portion of ApoER2 [96,97,98]. ApoER2, also known as low-density lipoprotein receptor-related protein 8, is the only low-density lipoprotein family receptor found on platelets [96]. This receptor is recognised to be targeted by the anti-β2GP1-β2GP1 complex, as the blockage of ApoER2 by its antagonist diminishes the effect of the anti-β2GP1-β2GP1 complex to increase the adhesion of platelets to collagen [105]. It has also been established that the interaction of anti-β2GP1-β2GP1 complexes with ApoER2 activates platelet analogously to GPIb-mediated platelet activation [16]. Recently, a dimer composed of two A1 portions of ApoER2 joined by a flexible link has been created [98]. This dimer is able to inhibit anti-β2GP1-β2GP1 complexes from binding to negatively-charged phospholipids and ApoER2 [98], reflecting another possible treatment option for patients with APS.
Anti-β2GP1-β2GP1 complexes may also affect GPCR and GPVI-mediated platelet activation pathways. Anti-β2GP1 antibodies from different origins have recently been reported to exhibit diverse effects on in vitro platelet aggregation. Affinity purified rabbit [99] and SLE patient-derived anti-β2GP1 antibodies [100] demonstrated inhibitory and enhancement effects, respectively, on ADP-induced platelet aggregation. When collagen was used, affinity purified rabbit anti-β2GP1 antibodies [99] enhanced platelet aggregation. However, no effect was demonstrated using patient-derived IgG fractions (containing aCL and anti-β2GP1 antibodies) [106] and affinity-purified goat anti-β2GP1 antibodies [107]. Based on these results, it is difficult to arrive at a consensus due to the variable effects possibly caused by anti-β2GP1 antibodies with different structure and binding specificities. Thus, further research is needed to elucidate the variable effects of anti-β2GP1-β2GP1 complexes on GPCR- and GPVI-mediated pathways.
As described above, β2GP1 binds with vWF to prevent platelet activation. It has been suggested that anti-β2GP1 antibodies in APS patients can neutralise this inhibitory effect, potentially leading to thrombosis and consumptive thrombocytopenia [15]. Furthermore, PF4, a pro-coagulant factor secreted from the α granules of platelets, has also been demonstrated to interact with β2GP1 [101]. PF4 is proposed to dimerise and stabilise β2GP1 on phospholipids, ensuring that β2GP1 is easily recognised by anti-β2GP1 antibodies. The formation of anti-β2GP1-β2GP1-PF4 complexes may activate platelets, leading to the development of thrombosis in APS patients [101].
5. Conclusion and Further Research
There is substantial literature available on the interaction between three interchangeable β2GP1 structures and anti-β2GP1 antibodies. The transformation of S-shaped or circular β2GP1 to J-shaped β2GP1 exposes the cryptic epitope in DI, enabling the binding of anti-β2GP1 antibodies, particularly those to DI of β2GP1. The formation of the anti-β2GP1-β2GP1 complex is thought to be responsible for the increased risk of thrombosis and/or pregnancy complications in patients with autoimmune diseases. Although numerous mechanisms of interaction between anti-β2GP1-β2GP1 complex and receptors/components have been proposed, the actual affected physiological pathway(s) remain unclear. One of the possible explanations for these ambiguities is the use of anti-β2GP1 antibodies with different structures and binding specificities from patient- and animal-derived origins across different studies. Therefore, further research is required to better clarify and categorise the type of antibodies used. This approach will in turn facilitate studies that will lead to increased understanding of the interactions between these antibodies and platelets.
In conclusion, the standardisation and development of methods, such as anti-DI-β2GP1 antibody ELISAs, are required to differentiate between the types and pathogenicity of anti-β2GP1 antibodies. This will allow more meaningful interpretation of laboratory- and clinic-based findings, which will potentially lead to the elucidation of the mechanism(s) of interaction between β2GP1, anti-β2GP1 antibodies and platelets. In combination, these further developments can help to improve the diagnostic and therapeutic techniques for patients with APS, and perhaps more widely, autoimmune diseases.
Acknowledgments
The authors acknowledge the support of the Lupus Association of Tasmania and the Clifford Craig Research Trust.
Abbreviations
The following abbreviations are used in this manuscript:
| APL | Anti-phospholipid |
| LAC | Lupus anti-coagulant |
| aCL | Anti-cardiolipin |
| Anti-β2GP1 | Anti-beta 2 glycoprotein 1 |
| APS | Antiphospholipid syndrome |
| SLE | Systemic lupus erythematosus |
| ELISA | Enzyme-linked immunosorbent assays |
| D | Domain |
| Anti-DI-β2GP1 | Anti-domain I-beta 2 glycoprotein 1 |
| EDTA | Ethylenediaminetetraacetic acid |
| ADP | Adenosine diphosphate |
| VWF | Von Willebrand factor |
| GP | Glycoprotein |
| ADAMTS13 | vWF protease |
| Ig | Immunoglobulin |
| ApoER2 | Apolipoprotein E receptor 2 |
| GPCR | Guanine nucleotide-binding protein-coupled receptors |
| PF4 | Platelet factor 4 |
Author Contributions
Yik C. Ho, Kiran D. K. Ahuja, Heinrich Körner and Murray J. Adams wrote the review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Keeling D., Mackie I., Moore G.W., Greer I.A., Greaves M. Guidelines on the investigation and management of antiphospholipid syndrome. Br. J. Haematol. 2012;157:47–58. doi: 10.1111/j.1365-2141.2012.09037.x. [DOI] [PubMed] [Google Scholar]
- 2.Biggioggero M., Meroni P.L. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun. Rev. 2010;9:A299–A304. doi: 10.1016/j.autrev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 3.De Groot P.G., Urbanus R.T. The significance of autoantibodies against β2-glycoprotein I. Blood. 2012;120:266–274. doi: 10.1182/blood-2012-03-378646. [DOI] [PubMed] [Google Scholar]
- 4.Fickentscher C., Magorivska I., Janko C., Biermann M., Bilyy R., Nalli C., Tincani A., Medeghini V., Meini A., Nimmerjahn F. The pathogenicity of anti-β2GP1-IgG autoantibodies depends on Fc glycosylation. J. Immunol. Res. 2015;2015:1–15. doi: 10.1155/2015/638129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyakis S., Lockshin M., Atsumi T., Branch D., Brey R., Cervera R., Derksen R., De Groot P., Koike T., Meroni P. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J. Thromb. Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 6.Urbanus R.T., Derksen R.H., De Groot P.G. Current insight into diagnostics and pathophysiology of the antiphospolipid syndrome. Blood Rev. 2008;22:93–105. doi: 10.1016/j.blre.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.De Groot P.G., Urbanus R.T. The future of antiphospholipid antibody testing. Semin. Thromb. Hemost. 2012;38:412–420. doi: 10.1055/s-0032-1304715. [DOI] [PubMed] [Google Scholar]
- 8.Reber G., Boehlen F., De Moerloose P. Technical aspects in laboratory testing for antiphospholipid antibodies: Is standardization an impossible dream? Semin. Thromb. Hemost. 2008;34:340–346. doi: 10.1055/s-0028-1085476. [DOI] [PubMed] [Google Scholar]
- 9.Du V.X., Kelchtermans H., De Groot P.G., De Laat B. From antibody to clinical phenotype, the black box of the antiphospholipid syndrome: Pathogenic mechanisms of the antiphospholipid syndrome. Thromb. Res. 2013;132:319–326. doi: 10.1016/j.thromres.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 10.De Laat B., Derksen R.H., Urbanus R.T., De Groot P.G. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of β2–glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105:1540–1545. doi: 10.1182/blood-2004-09-3387. [DOI] [PubMed] [Google Scholar]
- 11.De Laat B., Derksen R., Reber G., Musial J., Swadzba J., Bozic B., Cucnik S., Regnault V., Forastiero R., Woodhams B. An international multicentre-laboratory evaluation of a new assay to detect specifically lupus anticoagulants dependent on the presence of anti-beta2-glycoprotein I autoantibodies. J. Thromb. Haemost. 2011;9:149–153. doi: 10.1111/j.1538-7836.2010.04068.x. [DOI] [PubMed] [Google Scholar]
- 12.Chamley L., McKay E., Pattison N. Cofactor dependent and cofactor independent anticardiolipin antibodies. Thromb. Res. 1991;61:291–299. doi: 10.1016/0049-3848(91)90106-7. [DOI] [PubMed] [Google Scholar]
- 13.Tripodi A., De Groot P.G., Pengo V. Antiphospholipid syndrome: Laboratory detection, mechanisms of action and treatment. J. Intern. Med. 2011;270:110–122. doi: 10.1111/j.1365-2796.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- 14.Shi T., Giannakopoulos B., Yan X., Yu P., Berndt M.C., Andrews R.K., Rivera J., Iverson G.M., Cockerill K.A., Linnik M.D., et al. Anti-β2-glycoprotein I antibodies in complex with β2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54:2558–2567. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 15.Hulstein J.J., Lenting P.J., De Laat B., Derksen R.H., Fijnheer R., De Groot P.G. β2-glycoprotein I inhibits von Willebrand factor–dependent platelet adhesion and aggregation. Blood. 2007;110:1483–1491. doi: 10.1182/blood-2006-10-053199. [DOI] [PubMed] [Google Scholar]
- 16.Korporaal S.J., Relou I.A., Van Eck M., Strasser V., Bezemer M., Gorter G., Van Berkel T.J., Nimpf J., Akkerman J.-W.N., Lenting P.J. Binding of low density lipoprotein to platelet apolipoprotein E receptor 2' results in phosphorylation of p38MAPK. J. Biol. Chem. 2004;279:52526–52534. doi: 10.1074/jbc.M407407200. [DOI] [PubMed] [Google Scholar]
- 17.Jurk K., Kehrel B.E. Platelets: Physiology and biochemistry. Semin. Thromb. Hemost. 2005;31:381–392. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- 18.Galli M., Luciani D., Bertolini G., Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: A systematic review of the literature. Blood. 2003;101:1827–1832. doi: 10.1182/blood-2002-02-0441. [DOI] [PubMed] [Google Scholar]
- 19.Galli M., Barbui T. Antiphospholipid antibodies and pregnancy. Best Pract. Res. Clin. Haematol. 2003;16:211–225. doi: 10.1016/S1521-6926(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 20.Favaloro E.J., Silvestrini R. Assessing the usefulness of anticardiolipin antibody assays. Am. J. Clin. Pathol. 2002;118:548–557. doi: 10.1309/JAMH-GDQ6-6BYK-DW6J. [DOI] [PubMed] [Google Scholar]
- 21.Bahar A., Kwak J., Beer A., Kim J., Nelson L., Beaman K., Gilman-Sachs A. Antibodies to phospholipids and nuclear antigens in non-pregnant women with unexplained spontaneous recurrent abortions. J. Reprod. Immunol. 1993;24:213–222. doi: 10.1016/0165-0378(93)90076-T. [DOI] [PubMed] [Google Scholar]
- 22.Spadaro A., Riccieri V., Terracina S., Rinaldi T., Taccari E., Zoppinia A. Class specific rheumatoid factors and antiphospholipid syndrome in systemic lupus erythematosus. Lupus. 2000;9:56–60. doi: 10.1177/096120330000900110. [DOI] [PubMed] [Google Scholar]
- 23.Reber G., de Moerloose P. Anti-β2-glycoprotein I antibodies—When and how should they be measured? Thromb. Res. 2004;114:527–531. doi: 10.1016/j.thromres.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Faden D., Tincani A., Tanzi P., Spatola L., Lojacono A., Tarantini M., Balestrieri G. Anti-beta 2 glycoprotein I antibodies in a general obstetric population: Preliminary results on the prevalence and correlation with pregnancy outcome. Anti-beta2 glycoprotein I antibodies are associated with some obstetrical complications, mainly preeclampsia-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997;73:37–42. doi: 10.1016/s0301-2115(97)02736-x. [DOI] [PubMed] [Google Scholar]
- 25.Atsumi T., Ieko M., Bertolaccini M.L., Ichikawa K., Tsutsumi A., Matsuura E., Koike T. Association of autoantibodies against the phosphatidylserine-prothrombin complex with manifestations of the antiphospholipid syndrome and with the presence of lupus anticoagulant. Arthritis Rheum. 2000;43:1982–1993. doi: 10.1002/1529-0131(200009)43:9<1982::AID-ANR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.McIntyre J.A., Wagenknecht D.R., Sugi T. Phospholipid binding plasma proteins required for antiphospholipid antibody detection—An overview. Am. J. Reprod. Immunol. 1997;37:101–110. doi: 10.1111/j.1600-0897.1997.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura E., Igarashi Y., Fujimoto M., Ichikawa K., Koike T. Anticardiolipin cofactor(s) and differential diagnosis of autoimmune disease. Lancet. 1990;336:177–178. doi: 10.1016/0140-6736(90)91697-9. [DOI] [PubMed] [Google Scholar]
- 28.McNeil H.P., Simpson R.J., Chesterman C.N., Krilis S.A. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: Beta 2-glycoprotein I (apolipoprotein H) Proc. Natl. Acad. Sci. USA. 1990;87:4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galli M., Comfurius P., Maassen C., Hemker H.C., de Baets M.H., van Breda-Vriesman P.J., Barbui T., Zwaal R.F., Bevers E.M. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–1547. doi: 10.1016/0140-6736(90)91374-J. [DOI] [PubMed] [Google Scholar]
- 30.Schultze H. Glycoproteins of human plasma. Bull. Schweiz. Akad. Med. Wiss. 1961;17:77–91. [PubMed] [Google Scholar]
- 31.Lozier J., Takahashi N., Putnam F.W. Complete amino acid sequence of human plasma beta 2-glycoprotein I. Proc. Natl. Acad. Sci. USA. 1984;81:3640–3644. doi: 10.1073/pnas.81.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polz E., Kostner G.M. The binding of β2-glycoprotein I to human serum lipoproteins: Distribution among density fractions. FEBS Lett. 1979;102:183–186. doi: 10.1016/0014-5793(79)80955-2. [DOI] [PubMed] [Google Scholar]
- 33.Vlachoyiannopoulos P., Krilis S., Hunt J., Manoussakis M., Moutsopoulos H. Patients with anticardiolipin antibodies with and without antiphospholipid syndrome: Their clinical features and β2-glycoprotein I plasma levels. Eur. J. Clin. Invest. 1992;22:482–487. doi: 10.1111/j.1365-2362.1992.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 34.Miyakis S., Giannakopoulos B., Krilis S.A. Beta 2 glycoprotein I-function in health and disease. Thromb. Res. 2004;114:335–346. doi: 10.1016/j.thromres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Ioannou Y., Rahman A. Domain I of β2-glycoprotein I: Its role as an epitope and the potential to be developed as a specific target for the treatment of the antiphospholipid syndrome. Lupus. 2010;19:400–405. doi: 10.1177/0961203309360544. [DOI] [PubMed] [Google Scholar]
- 36.Agar C., De Groot P.G., Marquart J.A., Meijers J. Evolutionary conservation of the lipopolysaccharide binding site of β2-glycoprotein I. Thromb. Haemost. 2011;106:1069–1075. doi: 10.1160/TH11-05-0333. [DOI] [PubMed] [Google Scholar]
- 37.Steinkasserer A., Estaller C., Weiss E.H., Sim R.B., Day A.J. Complete nucleotide and deduced amino acid sequence of human beta 2-glycoprotein I. Biochem. J. 1991;277:387–391. doi: 10.1042/bj2770387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouma B., De Groot P.G., Van Den Elsen J.M., Ravelli R.B., Schouten A., Simmelink M.J., Derksen R.H., Kroon J., Gros P. Adhesion mechanism of human β2-glycoprotein I to phospholipids based on its crystal structure. EMBO J. 1999;18:5166–5174. doi: 10.1093/emboj/18.19.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelkmans L., De Laat B. Antibodies against domain I of β2-glycoprotein I: The one and only? Lupus. 2012;21:769–772. doi: 10.1177/0961203312437439. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen T., Schousboe I., Boel E., Mulvihill E.M., Hansen R.R., Moller K.B., Moller N.P.H., Sottrup-Jensen L. Molecular cloning and mammalian expression of human β2-glycoprotein I cDNA. FEBS Lett. 1991;289:183–186. doi: 10.1016/0014-5793(91)81065-G. [DOI] [PubMed] [Google Scholar]
- 41.Agar C., Van Os G.M., Morgelin M., Sprenger R.R., Marquart J.A., Urbanus R.T., Derksen R.H., Meijers J.C., De Groot P.G. β2-Glycoprotein I can exist in two conformations: Implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–1343. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 42.Schwarzenbacher R., Zeth K., Diederichs K., Gries A., Kostner G.M., Laggner P., Prassl R. Crystal structure of human β2-glycoprotein I: Implications for phospholipid binding and the antiphospholipid syndrome. EMBO J. 1999;18:6228–6239. doi: 10.1093/emboj/18.22.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammel M., Kriechbaum M., Gries A., Kostner G.M., Laggner P., Prassl R. Solution structure of human and bovine β2-glycoprotein I revealed by small-angle X-ray scattering. J. Mol. Biol. 2002;321:85–97. doi: 10.1016/S0022-2836(02)00621-6. [DOI] [PubMed] [Google Scholar]
- 44.Koike T., Ichikawa K., Kasahara H., Atsumi T., Tsutsumi A., Matsuura E. Epitopes on β2GPI recognized by anticardiolipin antibodies. Lupus. 1998;7:14–17. doi: 10.1177/096120339800700204. [DOI] [PubMed] [Google Scholar]
- 45.Ioannou Y., Pericleous C., Giles I., Latchman D.S., Isenberg D.A., Rahman A. Binding of antiphospholipid antibodies to discontinuous epitopes on domain I of human β2-glycoprotein I: Mutation studies including residues R39 to R43. Arthritis Rheum. 2007;56:280–290. doi: 10.1002/art.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chighizola C.B., Gerosa M., Meroni P.L. New tests to detect antiphospholipid antibodies: Anti-domain I beta-2-glycoprotein-I antibodies. Curr. Rheumatol. Rep. 2014;16:1–9. doi: 10.1007/s11926-013-0402-7. [DOI] [PubMed] [Google Scholar]
- 47.De Laat B., De Groot P.G. Autoantibodies directed against domain I of beta2-glycoprotein I. Curr. Rheumatol. Rep. 2011;13:70–76. doi: 10.1007/s11926-010-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Laat B., Derksen R.H.W.M., Van Lummel M., Pennings M.T.T., De Groot P.G. Pathogenic anti-β2-glycoprotein I antibodies recognize domain I of β2-glycoprotein I only after a conformational change. Blood. 2006;107:1916–1924. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 49.Wurm H. β2-Glycoprotein-I (apolipoprotein H) interactions with phospholipid vesicles. Int. J. Biochem. 1984;16:511–515. doi: 10.1016/0020-711X(84)90168-X. [DOI] [PubMed] [Google Scholar]
- 50.Perutková Š., Frank-Bertoncelj M., Rozman B., Kralj-Iglič V., Iglič A. Influence of ionic strength and beta2-glycoprotein I concentration on agglutination of like-charged phospholipid membranes. Coll. Surf. B. 2013;111:699–706. doi: 10.1016/j.colsurfb.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 51.Hoshino M., Hagihara Y., Nishii I., Yamazaki T., Kato H., Goto Y. Identification of the phospholipid-binding site of human β2-glycoprotein I domain V by heteronuclear magnetic resonance. J. Mol. Biol. 2000;304:927–939. doi: 10.1006/jmbi.2000.4243. [DOI] [PubMed] [Google Scholar]
- 52.Jankowski M., Vreys I., Wittevrongel C., Boon D., Vermylen J., Hoylaerts M.F., Arnout J. Thrombogenicity of β2-glycoprotein I–dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003;101:157–162. doi: 10.1182/blood-2002-05-1310. [DOI] [PubMed] [Google Scholar]
- 53.Giannakopoulos B., Krilis S.A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013;368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 54.Dupuy d'Angeac A., Stefas I., Graafland H., de Lamotte F., Rucheton M., Palais C., Eriksson A.-K., Bosc P., Rosé C., Chicheportiche R. Biotinylation of glycan chains in β2 glycoprotein I induces dimerization of the molecule and its detection by the human autoimmune anti-cardiolipin antibody EY2C9. Biochem. J. 2006;393:117–127. doi: 10.1042/BJ20050932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kondo A., Miyamoto T., Yonekawa O., Giessing A.M., Østerlund E.C., Jensen O.N. Glycopeptide profiling of beta-2-glycoprotein I by mass spectrometry reveals attenuated sialylation in patients with antiphospholipid syndrome. J. Proteomics. 2009;73:123–133. doi: 10.1016/j.jprot.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Chamorro A.-J., Marcos M., Mirón-Canelo J.-A., Cervera R., Espinosa G. Val247Leu beta2-glycoprotein-I allelic variant is associated with antiphospholipid syndrome: Systematic review and meta-analysis. Autoimmun. Rev. 2012;11:705–712. doi: 10.1016/j.autrev.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Yasuda S., Atsumi T., Matsuura E., Kaihara K., Yamamoto D., Ichikawa K., Koike T. Significance of valine/leucine247 polymorphism of β2-glycoprotein I in antiphospholipid syndrome: Increased reactivity of anti-β2-glycoprotein I autoantibodies to the valine247 β2-glycoprotein I variant. Arthritis Rheum. 2005;52:212–218. doi: 10.1002/art.20741. [DOI] [PubMed] [Google Scholar]
- 58.Haupt H., Schwick H.G., Storiko K. On a hereditary beta-2-glycoprotein I deficiency. Humangenetik. 1968;5:291–293. doi: 10.1007/BF00291636. [DOI] [PubMed] [Google Scholar]
- 59.Nimpf J., Wurm H., Kostner G. Interaction of beta 2-glycoprotein-I with human blood platelets: Influence upon the ADP-induced aggregation. Thromb. Haemost. 1985;54:397–401. [PubMed] [Google Scholar]
- 60.Rahgozar S. Revisiting Beta 2 glycoprotein I, the major autoantigen in the antiphospholipid syndrome. Iran. J. Immunol. 2012;9:73–85. [PubMed] [Google Scholar]
- 61.Passam F., Rahgozar S., Qi M., Raftery M., Wong J., Tanaka K., Ioannou Y., Zhang J., Gemmell R., Qi J. Redox control of β2-glycoprotein I–von Willebrand factor interaction by thioredoxin-1. J. Thromb. Haemost. 2010;8:1754–1762. doi: 10.1111/j.1538-7836.2010.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maiti S.N., Balasubramanian K., Ramoth J.A., Schroit A.J. Beta-2-glycoprotein 1-dependent macrophage uptake of apoptotic cells. Binding to lipoprotein receptor-related protein receptor family members. J. Biol. Chem. 2008;283:3761–3766. doi: 10.1074/jbc.M704990200. [DOI] [PubMed] [Google Scholar]
- 63.Abdel-Monem H., Dasgupta S.K., Le A., Prakasam A., Thiagarajan P. Phagocytosis of platelet microvesicles and β2–glycoprotein I. Thromb. Haemost. 2010;104:335–341. doi: 10.1160/TH09-12-0849. [DOI] [PubMed] [Google Scholar]
- 64.Nilsson M., Wasylik S., Morgelin M., Olin A.I., Meijers J., Derksen R.H., De Groot P.G., Herwald H. The antibacterial activity of peptides derived from human β2 glycoprotein I is inhibited by protein H and M1 protein from Streptococcus pyogenes. Mol. Microbiol. 2008;67:482–492. doi: 10.1111/j.1365-2958.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- 65.Agar C., De Groot P.G., Morgelin M., Monk S.D., Van Os G., Levels J.H., De Laat B., Urbanus R.T., Herwald H., Van der Poll T., et al. β2-glycoprotein I: A novel component of innate immunity. Blood. 2011;117:6939–6947. doi: 10.1182/blood-2010-12-325951. [DOI] [PubMed] [Google Scholar]
- 66.Sheng Y., Reddel S.W., Herzog H., Wang Y.X., Brighton T., France M.P., Robertson S.A., Krilis S.A. Impaired thrombin generation in β2-glycoprotein I null mice. J. Biol. Chem. 2001;276:13817–13821. doi: 10.1074/jbc.M010990200. [DOI] [PubMed] [Google Scholar]
- 67.De Groot P.G., Derksen R. Pathophysiology of antiphospholipid antibodies. Neth. J. Med. 2004;62:267–272. [PubMed] [Google Scholar]
- 68.Forastiero R., Martinuzzo M., Carreras L.O., Maclouf J. Anti-β2 glycoprotein I antibodies and platelet activation in patients with antiphospholipid antibodies: Association with increased excretion of platelet-derived thromboxane urinary metabolites. Thromb. Haemost. 1998;79:42–45. [PubMed] [Google Scholar]
- 69.Urbanus R.T., De Laat H.B., De Groot P.G., Derksen R.H. Prolonged bleeding time and lupus anticoagulant: A second paradox in the antiphospholipid syndrome. Arthritis Rheum. 2004;50:3605–3609. doi: 10.1002/art.20586. [DOI] [PubMed] [Google Scholar]
- 70.Abou-Nassar K., Carrier M., Ramsay T., Rodger M.A. The association between antiphospholipid antibodies and placenta mediated complications: A systematic review and meta-analysis. Thromb. Res. 2011;128:77–85. doi: 10.1016/j.thromres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 71.Carroll T.Y., Mulla M.J., Han C.S., Brosens J.J., Chamley L.W., Giles I., Pericleous C., Rahman A., Sfakianaki A.K., Paidas M.J. Modulation of trophoblast angiogenic factor secretion by antiphospholipid antibodies is not reversed by heparin. Am. J. Reprod. Immunol. 2011;66:286–296. doi: 10.1111/j.1600-0897.2011.01007.x. [DOI] [PubMed] [Google Scholar]
- 72.Rand J., Wu X., Quinn A., Taatjes D. The annexin A5-mediated pathogenic mechanism in the antiphospholipid syndrome: Role in pregnancy losses and thrombosis. Lupus. 2010;19:460–469. doi: 10.1177/0961203310361485. [DOI] [PubMed] [Google Scholar]
- 73.Willis R., Shoenfeld Y., Pierangeli S.S., Blank M. What is the origin of antiphospholipid antibodies? In Antiphospholipid Syndrome. Springer; New York, NY, USA: 2012. pp. 23–39. [Google Scholar]
- 74.Hashimoto Y., Kawamura M., Ichikawa K., Suzuki T., Sumida T., Yoshida S., Matsuura E., Ikehara S., Koike T. Anticardiolipin antibodies in NZW × BXSB F1 mice. A model of antiphospholipid syndrome. J. Immunol. 1992;149:1063–1068. [PubMed] [Google Scholar]
- 75.Ida A., Hirose S., Hamano Y., Kodera S., Jiang Y., Abe M., Zhang D., Nishimura H., Shirai T. Multigenic control of lupus-associated antiphospholipid syndrome in a model of (NZW × BXSB) F1 mice. Eur. J. Immunol. 1998;28:2694–2703. doi: 10.1002/(SICI)1521-4141(199809)28:09<2694::AID-IMMU2694>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 76.Castro-Marrero J., Balada E., Vilardell-Tarres M., Ordi-Ros J. Genetic risk factors of thrombosis in the antiphospholipid syndrome. Br. J. Haematol. 2009;147:289–296. doi: 10.1111/j.1365-2141.2009.07831.x. [DOI] [PubMed] [Google Scholar]
- 77.Epstein F.H., Albert L.J., Inman R.D. Molecular mimicry and autoimmunity. N. Engl. J. Med. 1999;341:2068–2074. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 78.Sherer Y., Blank M., Shoenfeld Y. Antiphospholipid syndrome (APS): Where does it come from? Best Pract. Res. Clin. Rheumatol. 2007;21:1071–1078. doi: 10.1016/j.berh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Gotoh M., Matsuda J. Induction of anticardiolipin antibody and/or lupus anticoagulant in rabbits by immunization with lipoteichoic acid, lipopolysaccharide and lipid A. Lupus. 1996;5:593–597. doi: 10.1177/096120339600500606. [DOI] [PubMed] [Google Scholar]
- 80.Subang R., Levine J.S., Janoff A.S., Davidson S.M., Taraschi T.F., Koike T., Minchey S.R., Whiteside M., Tannenbaum M., Rauch J. Phospholipid-bound β2-glycoprotein I induces the production of anti-phospholipid antibodies. J. Autoimmun. 2000;15:21–32. doi: 10.1006/jaut.2000.0382. [DOI] [PubMed] [Google Scholar]
- 81.Merrill J.T. Do antiphospholipid antibodies develop for a purpose? Curr. Rheumatol. Rep. 2006;8:109–113. doi: 10.1007/s11926-006-0050-2. [DOI] [PubMed] [Google Scholar]
- 82.Van Os G.M., Meijers J.C., Agar C., Seron M.V., Marquart J.A., Akesson P., Urbanus R.T., Derksen R.H., Herwald H., Morgelin M., et al. Induction of anti-β2-glycoprotein I autoantibodies in mice by protein H of Streptococcus pyogenes. J. Thromb. Haemost. 2011;9:2447–2456. doi: 10.1111/j.1538-7836.2011.04532.x. [DOI] [PubMed] [Google Scholar]
- 83.Biasiolo A., Rampazzo P., Brocco T., Barbero F., Rosato A., Pengo V. [Anti-β2 Glycoprotein I—β2 Glycoprotein I] immune complexes in patients with antiphospholipid syndrome and other autoimmune diseases. Lupus. 1999;8:121–126. doi: 10.1191/096120399678847506. [DOI] [PubMed] [Google Scholar]
- 84.Fischetti F., Durigutto P., Pellis V., Debeus A., Macor P., Bulla R., Bossi F., Ziller F., Sblattero D., Meroni P. Thrombus formation induced by antibodies to β2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 85.Arad A., Proulle V., Furie R.A., Furie B.C., Furie B. β2-glycoprotein-1 autoantibodies from patients with antiphospholipid syndrome are sufficient to potentiate arterial thrombus formation in a mouse model. Blood. 2011;117:3453–3459. doi: 10.1182/blood-2010-08-300715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asherson R.A. The catastrophic antiphospholipid syndrome, 1998. A review of the clinical features, possible pathogenesis and treatment. Lupus. 1998;7:S55–S62. doi: 10.1177/096120339800700214. [DOI] [PubMed] [Google Scholar]
- 87.Agarwal M.B. Antiphospholipid syndrome. East. J. Med. 2009;14:51–56. [Google Scholar]
- 88.Shoenfeld Y., Krause I., Kvapil F., Sulkes J., Lev S., Von Landenberg P., Font J., Zaech J., Cervera R., Piette J. Prevalence and clinical correlations of antibodies against six β2-glycoprotein-I-related peptides in the antiphospholipid syndrome. J. Clin. Immunol. 2003;23:377–383. doi: 10.1023/A:1025321617304. [DOI] [PubMed] [Google Scholar]
- 89.Cucnik S., Kveder T., Artenjak A., Gallova Z.U., Swadzba J., Musial J., Iwaniec T., Stojanovich L., Alessandri C., Valesini G. Avidity of anti-β2-glycoprotein I antibodies in patients with antiphospholipid syndrome. Lupus. 2012;21:764–765. doi: 10.1177/0961203312440057. [DOI] [PubMed] [Google Scholar]
- 90.Carter A.M. Complement activation: An emerging player in the pathogenesis of cardiovascular disease. Scientifica. 2012;2012:1–14. doi: 10.6064/2012/402783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jefferis R., Kumararatne D. Selective IgG subclass deficiency: Quantification and clinical relevance. Clin. Exp. Immunol. 1990;81:357. doi: 10.1111/j.1365-2249.1990.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palarasah Y., Skjodt K., Brandt J., Teisner B., Koch C., Vitved L., Skjoedt M.O. Generation of a C3c specific monoclonal antibody and assessment of C3c as a putative inflammatory marker derived from complement factor C3. J. Immunol. Methods. 2010;362:142–150. doi: 10.1016/j.jim.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 93.Banzato A., Pozzi N., Frasson R., De Filippis V., Ruffatti A., Bison E., Padayattil S., Denas G., Pengo V. Antibodies to domain I of β2 glycoprotein I are in close relation to patients risk categories in antiphospholipid syndrome (APS) Thromb. Res. 2011;128:583–586. doi: 10.1016/j.thromres.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 94.Pozzi N., Banzato A., Bettin S., Bison E., Pengo V., De Filippis V. Chemical synthesis and characterization of wild-type and biotinylated N-terminal domain 1–64 of beta2-glycoprotein I. Protein Sci. 2010;19:1065–1078. doi: 10.1002/pro.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meneghel L., Ruffatti A., Gavasso S., Tonello M., Mattia E., Spiezia L., Tormene D., Hoxha A., Fedrigo M., Simioni P. Detection of IgG anti-domain I beta2 glycoprotein I antibodies by chemiluminescence immunoassay in primary antiphospholipid syndrome. Clin. Chim. Acta. 2015;446:201–205. doi: 10.1016/j.cca.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 96.Van Lummel M., Pennings M.T., Derksen R.H., Urbanus R.T., Lutters B.C., Kaldenhoven N., De Groot P.G. The binding site in β2-glycoprotein I for ApoER2' on platelets is located in domain V. J. Biol. Chem. 2005;280:36729–36736. doi: 10.1074/jbc.M504172200. [DOI] [PubMed] [Google Scholar]
- 97.Pennings M.T., Derksen R.H., Urbanus R.T., Tekelenburg W.L., Hemrika W., De Groot P.G. Platelets express three different splice variants of ApoER2 that are all involved in signaling. J. Thromb. Haemost. 2007;5:1538–1544. doi: 10.1111/j.1538-7836.2007.02605.x. [DOI] [PubMed] [Google Scholar]
- 98.Kolyada A., Porter A., Beglova N. Inhibition of thrombotic properties of persistent autoimmune anti-β2GPI antibodies in the mouse model of antiphospholipid syndrome. Blood. 2014;123:1090–1097. doi: 10.1182/blood-2013-08-520882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palatinus A.A., Ahuja K.D., Adams M.J. Effects of antiphospholipid antibodies on in vitro platelet aggregation. Clin. Appl. Thromb. Hemost. 2012;18:59–65. doi: 10.1177/1076029611412360. [DOI] [PubMed] [Google Scholar]
- 100.Betts N.A., Ahuja K.D., Adams M.J. Anti-β2GP1 antibodies have variable effects on platelet aggregation. Pathol.-J. RCPA. 2013;45:155–161. doi: 10.1097/PAT.0b013e32835cc277. [DOI] [PubMed] [Google Scholar]
- 101.Sikara M.P., Routsias J.G., Samiotaki M., Panayotou G., Moutsopoulos H.M., Vlachoyiannopoulos P.G. β2 Glycoprotein I (β2GPI) binds platelet factor 4 (PF4): Implications for the pathogenesis of antiphospholipid syndrome. Blood. 2010;115:713–723. doi: 10.1182/blood-2009-03-206367. [DOI] [PubMed] [Google Scholar]
- 102.Ashby B., Daniel J.L., Smith J.B. Mechanisms of platelet activation and inhibition. Hematol. Oncol. Clin. North. Am. 1990;4:1–26. [PubMed] [Google Scholar]
- 103.Li Z., Delaney M.K., O’Brien K.A., Du X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pennings M., Derksen R., Van Lummel M., Adelmeijer J., Vanhoorelbeke K., Urbanus R., Lisman T., De Groot P. Platelet adhesion to dimeric β2-glycoprotein I under conditions of flow is mediated by at least two receptors: Glycoprotein Ibα and apolipoprotein E receptor 2'. J. Thromb. Haemost. 2007;5:369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 105.Lutters B.C., Derksen R.H., Tekelenburg W.L., Lenting P.J., Arnout J., De Groot P.G. Dimers of β2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2'. J. Biol. Chem. 2003;278:33831–33838. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- 106.Mesquita H.L.D., Carvalho G.R.D., Aarestrup F.M., Correa J.O.D.A., Azevedo M.R.A. Evaluation of platelet aggregation in the presence of antiphospholipid antibodies: Anti-β2GP1 and anticardiolipin. Rev. Bras. Reumatol. 2013;53:400–404. doi: 10.1016/S2255-5021(13)70110-X. [DOI] [PubMed] [Google Scholar]
- 107.Ho Y.C., Ahuja K.D., Adams M.J. Effects of anti-β2GP1 antibodies on collagen induced platelet aggregation. 2016. in preparation. [DOI] [PMC free article] [PubMed]