Abstract
Fragile X syndrome (FXS) is characterized by both social approach and social avoidance. However, the age of emergence and developmental trajectory of social avoidance has not been examined. This study investigates the longitudinal developmental trajectory and dynamic nature of social avoidance in males with FXS from infancy through young adulthood (n=191). Multiple facets of social avoidance were collected using the Social Avoidance Scale (Roberts et al., 2007, 2009). Overall, 81% of males with FXS displayed social avoidance, which emerged during infancy, increased in severity across childhood, and stabilized through adolescence and early adulthood. An exaggerated “warm up” effect was also observed in FXS. This study delineates the complex profile of social avoidance, a common and impairing behavioral feature of FXS.
Keywords: Fragile X, autism spectrum disorder, social approach, social anxiety, infant
Introduction
Social avoidance is characterized by a range of behaviors including failure to initiate interactions, reduced time spent interacting with others, and social interaction restricted to a subset of preferred individuals (Kaldewaij, Cock, Volman, Toni, & Roelofs, 2017; Kampman, Viikki, Jarventausta, & Leinonen, 2014). Avoidance of social interactions can be driven by a lack of interest or desire to interact with others, or by elevated levels of discomfort or stress related to social interactions (Torvik et al., 2016; White et al., 2014). Elevated social avoidance is associated with negative outcomes, including social anxiety, reduced relationship quality, and educational and vocational difficulties (Clauss & Blackford, 2012; McLaughlin, Hatzenbuehler, Mennin, & Nolen-Hoeksema, 2011; Schneier et al., 1994). Examining social avoidance in young children is complex, however, given rapid developmental shifts and the fact that social avoidance can represent a signal of normative and “healthy’ development (Bretherton & Ainsworth, 1974; Brooker et al., 2013). As such, longitudinal studies identifying trajectories of social avoidance across time yield critical data to inform the nature and impact that avoidance of social stimuli may have on outcomes. In fact, evidence suggests that trajectories of avoidance, but not mean levels at a given age, predict ASD (Zwaigenbaum et al., 2005) and ADHD outcomes (Miller et al. 2013). Given that interventions have been shown to reduce social avoidance (see Reichow & Volkmar, 2010 for a review), increased understanding of the trajectory and mechanisms associated with social avoidance is critical for early detection and optimizing outcomes (Beadle-Brown, Murphy, & Wing, 2005; Landa, Holman & Garret-Mayer, 2007).
Although social avoidance is represented across a continuum in neurotypical individuals, increased levels and atypical profiles of social avoidance are present in specific neurodevelopmental disorders. Fragile X syndrome (FXS) is a single-gene disorder caused by an expansion of cytosine-guanine-guanine (CGG) trinucleotide repeats on the FMR1 gene and a subsequent reduction of fragile X mental retardation protein (FMRP). FXS affects approximately 1 in 4,000 males (Crawford, Acuna, & Sherman, 2001), with females being affected less often (i.e., 1 in 8,000) and less severely (Turner, Webb, Wake, & Robinson, 1996). In males, the phenotype associated with FXS encompasses mild to severe intellectual disability, with social impairment as one of the core features.
The social impairment in FXS represents a unique and complex profile of both social approach and avoidance. Interactions with unfamiliar people or when in environments that are novel can result in acute elevations of social avoidance in FXS (Cohen et al., 1988; Kau, Meyer, & Kaufmann, 2002; Kau, Reider, Payne, Meyer, & Freund, 2000). Reduced eye contact is one of the most salient and pervasive features of social impairment and is reported to characterize 83% of children with FXS and up to 98% of adults with FXS (Lachiewicz, Dawson, & Spiridigliozzi, 2000; Merenstein et al., 1996; Roberts, Weisenfeld, Hatton, Heath, & Kaufmann, 2007). Despite the pervasiveness of social avoidance in FXS, there are numerous reports that show behaviors suggestive of a willingness or desire to interact with others (Cohen et al., 1988). The term ‘fragile X handshake’ has been coined to describe how individuals with FXS display a wish to initiate social interaction by offering a handshake and moving towards a social partner whilst simultaneously avoiding eye contact and twisting away upon greeting (Cornish, Turk, & Hagerman, 2008). The ‘fragile X handshake’ exemplifies the paradox of the social interaction profile in FXS in that features of both social avoidance and social approach present simultaneously.
Documenting the developmental trajectory of social avoidance is crucial for promoting both early detection and further understanding regarding the most optimal time to deliver interventions. Existing work points to the possibility of a change in social avoidance across the lifespan in FXS. Specifically, our previous work documented a unique profile of social avoidance in males with FXS, characterized by social avoidance to initial interactions and a significant reduction in social avoidance after time, suggesting a “warm up” effect. This “warm up” effect was more striking in children aged younger than 5 years compared to those 5 years and above (Roberts et al., 2007), indicating more severe social avoidance in older males that is less influenced by the familiarity of the assessor. Other longitudinal research has indicated that males with FXS demonstrate a significant decrease in socialization skills between the ages of 2–6 years, 6–10 years, and 10–14 years, although this stabilizes between 14–18 years (Klaiman et al., 2014). Taken together, this research points to key developmental changes in social impairment across early childhood; yet, no studies have conducted a longitudinal examination from infancy.
Due to the overlap of behavioral features, social avoidance in FXS is often attributed to comorbid social anxiety and/or autism spectrum disorder (ASD), both of which occur at elevated rates in FXS (Williams, Porter, & Langdon, 2014; Roberts et al., 2007, 2018, 2019). There is increased interest in examining these complex relationships among social avoidance, ASD and anxiety in FXS and other neurodevelopmental disorders, such as ASD, given the importance of differential diagnoses and targeted treatment planning. However, this work is challenging given measurement limitations including a lack of instruments normed on persons with intellectual impairment and the potential lack of validity for many existing measures given the cognitive and communication impairments inherent in many clinical groups that can affect performance on these measures. Also, most measures do not capture important dynamics of social avoidance including distinguishing reactions to initial interactions with novel people versus interactions with familiar people. Instead, most published work uses rating scales that require a qualitative choice of yes/no or present/absent which can obscure important nuances of social avoidance. In addition, many studies rely on parental ratings of social avoidance which can be biased towards an over or under-endorsement based on the restricted familial context or level of understanding and inference that varies across parents. Direct observations of behaviors by trained individuals and inclusion of multiple measures are recommended as best practice (Mian, Carter, Pine, Wakschlag, & Briggs-Gowan, 2015). As such, examining the relationship of social avoidance to features of ASD and anxiety, and the trajectory of social avoidance across a wide developmental span, will provide crucial information to refine the phenotype of FXS and its shared symptomatology with ASD.
To summarize, social avoidance in FXS appears to be an important, but complex, feature that is implicated as a core phenotypic feature but also as a symptom of co-morbid ASD and anxiety disorders. Evidence suggests that there is a developmental effect with social avoidance increasing over time in young children; however, the age at which social avoidance emerges and the trajectory of social avoidance over childhood into early adulthood has not been reported. Moreover, many previous studies have involved small samples of participants and age trends have often been examined cross-sectionally rather than longitudinally. In addition, most attempts to characterize social avoidance in FXS have not employed multidimensional coding schemes or examined changes over the course of an interaction, thereby potentially obscuring clinical and mechanistic meaningful differences across disorders and individuals. Tracing developmental trajectories of social avoidance in FXS through a multidimensional, dynamic characterization will provide critical insight to guide early identification and treatment efforts (Karmiloff-Smith & Farran, 2011).
The Present Study
The present study addressed two primary research aims. First, we sought to characterize the developmental trajectory of multiple dimensions of social avoidance in males with FXS from infancy through early adulthood. We hypothesized that social avoidance becomes more pronounced as individuals with FXS age. Our second aim was to examine the dynamic aspect of social avoidance in males with FXS. We addressed this aim by quantifying change in social avoidance across the course of the assessment visit, focusing on a “warm up” effect (i.e., initial rating at onset of interaction contrasted with the rating at the end of a prolonged social interaction). We hypothesized that individuals with FXS will display a greater “warm up” effect than typically developing (TD) controls, representing a high degree of initial avoidance followed by a marked reduction in avoidance by the end of the interaction, a pattern that will be most pronounced in younger participants. Even after warm up, however, we anticipate higher levels of social avoidance by individuals with FXS.
Methods
Participants
A total of 263 male participants were included in this longitudinal study, 191 with FXS and 72 TD control subjects representing 648 observations (see Table 1 for details). These participants were drawn from three sites (the University of South Carolina [USC], the University of North Carolina at Chapel Hill [UNC], and the University of California, Davis MIND Institute [UC-Davis]) that conducted longitudinal studies and included the Social Avoidance Scale (SAS) (Roberts et al., 2007, 2009, 2019) as one of the measures (R01MH090194, R01MH107573, PI: Roberts; R01HD024356, PI: Abbeduto; P30HD003110, PI: Bailey). Thus, this is a convenience sample representing the opportunity to analyze the developmental trajectory of social avoidance from a large sample with a wide age distribution. The first author, who developed the SAS, was part of these studies across the sites. The data collection, coding and analyses for the SAS were identical across the studies as were the recruitment strategies and participant characterization. Recruitment sources for participants with FXS included past studies, national parent listservs, social media, colleagues, postings by the National Fragile X Foundation, and support from the Research Participant Registry Core of the Carolina Institute for Developmental Disabilities at the University of North Carolina at Chapel Hill and the Volunteer Registry at the University of South Carolina. Participants with FXS had the full mutation of the FMR1 gene (>200 CGG repeats), confirmed through genetic testing. TD controls were recruited through flyers posted in local daycares and electronic posts on playgroup sites. The controls had no family history of ASD or FXS and demonstrated typical development through parental report (no documented delay), which was confirmed by developmental testing as part of the study protocols.
Table 1.
Participant Demographic Information
| All Participants | Infant/Preschool (Age-Matched) | Adolescent/Adult | |||
|---|---|---|---|---|---|
| FXS (n = 191) | TD (n = 72) | FXS (n = 66) | TD (n = 59) | FXS (n = 101) | |
| Number of Observations | 459 | 189 | 153 | 137 | 212 |
| Observations per Participant | |||||
| M | 2.40 | 3.63 | 2.32 | 2.32 | 2.10 |
| (SD) | (0.86) | (1.33) | (0.75) | (0.66) | (0.73) |
| Range | 1 – 5 | 1 – 6 | 1 – 4 | 1 – 4 | 1 – 3 |
| CA (months) | |||||
| M | 121.51 | 29.32 | 32.55 | 30.64 | 207.49 |
| (SD) | (88.17) | (19.94) | (18.26) | (15.99) | (46.14) |
| Range | 4.57– 304.43 | 5.66– 100.18 | 4.57– 71.98 | 6.07– 71.94 | 120.04– 304.43 |
| Mullen AE (months) | |||||
| M (SD) | -- | -- | 17.27 (10.12) | 30.75 (16.30) | -- |
| Range | 3.50 – 61.75 | 7.25 – 67.25 | |||
| n | 147 | 132 | |||
| Leiter-R AE (years) | |||||
| M (SD) | -- | -- | -- | -- | 5.17 (1.13) |
| Range | 2.21 – 8.15 | ||||
| n | 141 | ||||
Notes: FXS is participants with fragile X syndrome, TD is typically-developing participants; M is mean; SD is standard deviation; CA is chronological age; AE is age equivalent
For the psychometric and descriptive analyses, all observations were utilized. For the inferential analyses, participants were divided into two cohorts: 1) An infant/preschool cohort, ranging in age from 4 to 72 months and including both FXS and TD participants, and 2) an adolescent/young adult cohort, ranging from 10 to 25 years, including only FXS participants. The maximum age of 25 years is due to the focus and inclusionary criteria of the larger longitudinal grants from which these data were drawn for the present study. Due to the small number of observations available from participants 6 to 10 years of age, observations from this span were not included in inferential models but are depicted descriptively.
Measures
Social Avoidance.
The Social Avoidance Scale (SAS) is an in-vivo experimental direct observation scale that is completed by trained research specialists as part of the larger assessment battery. Examiners are trained to ≥ 80% agreement on the SAS with periodic inter-rater reliability calculated to avoid drift. The intra-class correlation coefficients (ICCs) reflect a moderate to high degree of reliability between raters on all of the SAS scales, with a range from .82 to .90. For the initial rating (rating 1=R1), the research team observes and records the participant’s response to their initial interactions for the first minute of greeting and interaction. For the second rating (rating 2=R2), the research team observes and records the participant’s response to their interactions across the final hour of interaction once that child has become familiar with them. As such, the SAS ratings are based on behavioral observations of the participant during “naturalistic” interactions with the research team rather than videotaped sessions of discrete tasks.
The SAS measures multiple components of social avoidance at the beginning and end of a social interaction to document changes in social avoidance as the participant becomes more familiar with the context and social partner(s). Social avoidance is measured with three scales: physical movement, facial expression, and eye contact. Higher ratings on each scale indicate more avoidant behavior. Scale 1, Physical Movement, measures an individual’s avoidance through physical movement ranging from 0 “child clearly moves toward you” to 4 “clearly moves away.” Scale 2 measures an individual’s social shyness and avoidance through facial expressions. The Facial Expression scale ranges from 0 “no sign of vocal, facial, or postural wariness at all” to 4 “frank and clear-cut shyness.” Scale 3, Eye Contact, measures an individual’s use of eye contact during the interaction. The eye contact scale ranges from 0 “age appropriate eye contact” to 5 “no eye contact at all.”
To assess change in scores from R1 to R2, four categories of change were computed: 1) “Consistent Low Avoidance” (0 or 1 at both R1 and R2); 2) “Increasing Avoidance” (0 or 1 at R1 and > 1 at R2); 3) “Warms Up” (> R1 at R2); and 4) “Consistent High Avoidance” (> 1 at R1 and ≥ R1 at R2). The SAS also documents the location of the assessment (e.g., home, school, lab), study site (e.g., USC, UNC, UC-Davis) and the duration of the assessment visit to examine their effect on SAS ratings. In the infant/preschool cohort, SAS ratings were not significantly different across home or lab locations for any ratings, X2s(2) < 6.00, ps > .05, with the exception of Eye Contact R1, X2(2) = 6.84, p < .05. In the adolescent/adult cohort, SAS ratings varied by home or lab location for Physical Movement R1, and Facial Expression R1/R2, X2s(2) > 6.82, ps < .05. Differences in ratings across location are not unexpected given the logistical and physical differences inherent in laboratory v. home-based assessments. In the infant/preschool cohort, site effects (USC v. UNC) were significant for Physical Movement R1/R2, Facial Expression R1, and Eye Contact R1, Us < 8121.50, ps < .01. In the adolescent/adult cohort, site effects (USC v. UNC v. UC-Davis) were significant only for Physical Movement R1 and Eye Contact R2, X2s(2) > 6.29. Site was included as a predictor in all inferential models. As expected, the duration of the assessment visit was correlated with R2 for all three SAS scales across groups, ρs >.27, ps < .001. Thus, duration of assessment was included as a predictor in all R2 inferential models.
Other measures.
Developmental ability or non-verbal cognitive level was measured via the Mullen Scales of Early Learning (Mullen, 1995) (if chronological age <= 68 months) or the Leiter International Performance Scale-Revised (Leiter-R; Roid & Miller, 1997) (if chronological age >68 months). The Anxiety, Depression, and Mood Scale (ADAMS; Esbensen, Rojahn, Aman, & Ruedrich, 2003), Childhood Autism Rating Scale – 2 (Schopler, Van Bourgondien, Wellman, & Love, 2010) and the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000) were used in construct validity analyses.
Procedure
The assessment protocol involved two consecutive days of evaluation across all 3 sites (see Baker et al., 2011; Roberts et al., 2016; Thurman, et al., 2014; and Warren et al., 2010, for more details). Given the primary aims of each study, a standard battery of measures was administered by trained personnel across all three sites including direct child assessments in addition to parent/caregiver rating scales that spanned two days. To control for potential study effects associated with variation in the nature of the assessments and our primary interest on initial social avoidance, this study focused on SAS ratings for the initial day of the assessment only and site was also included in the analyses.
To investigate convergent validity evidence for the SAS, relationships with theoretically-related subscales (CARS Childhood Autism Rating Scale, CBCL Withdrawn subscale and ADAMS Social Avoidance subscale) were investigated with Spearman’s correlations (see Table 2). The SAS is strongly associated with features of ASD, with moderate to strong correlations between the Eye Contact scale and elements of social anxiety and social withdrawal. A moderate relationship between Physical Movement at post-assessment and social anxiety was also observed. Divergent validity evidence was examined using measures hypothesized not to be associated with social avoidance (ADAMS Manic/Hyperactive and CBCL Externalizing). Evidence suggests that the SAS scales are generally not related to either of these measures. However, a moderate relationship was observed between the Eye Contact scale for post-assessment and the ADAMS manic/hyperactive scale.
Table 2.
Convergent and Discriminant Validity of SAS
| Convergent | Discriminant | ||||
|---|---|---|---|---|---|
| ADAMS Social Avoidance (n=77) | CBCL Withdrawn (n=215) | CARS Total (n=257) | ADAMS Manic/Hyperactive (n=77) | CBCL Externalizing (n=215) | |
| Physical Movement R1 | .21 | .10 | .22** | .08 | .04 |
| Physical Movement R2 | .29* | −.07 | .39** | .15 | −.08 |
| Facial Expression R1 | .14 | .13 | .43** | .10 | −.04 |
| Facial Expression R2 | .21 | .08 | .54** | .19 | −.09 |
| Eye Contact R1 | .2 | .20** | .63** | .22 | −.08 |
| Eye Contact R2 | .35** | .19** | .71** | .27* | −.06 |
Notes:
p < .05
p < .01
Values indicate Spearman coefficient ρ.
Analytic Plan
Analyses were run using SAS 9.4 PROC GLIMMIX and SPSS 24.0. To address the first aim of characterizing the trajectory of multiple dimensions of social avoidance in males with FXS from infancy through early adulthood, we conducted a series of analyses representing both descriptive and inferential approaches. We employed a simple descriptive approach to characterize the entire cohort (participants from 4 months to 25 years old). These data were restricted to descriptive analyses given the uneven distribution of participants across the entire age cohort preventing an inferential approach.
Inferential models were run in the two age cohorts that contained sufficient data to do so: 1) an infant/preschool cohort of TD males and males with FXS (aged 4–72 months), and 2) an adolescent/young adult cohort of males with FXS only (aged 10–25 years). In the infant/preschool cohort, males with FXS were matched on chronological age to TD males to examine group and age effects. A pseudo-randomized matching process was employed in which observations were a) assigned a random number; b) split into quartiles; c) sorted by random number and number of observations available for each participant; and d) systematically trimmed to remove cases until the mean chronological age was statistically equivalent (p > .50) and the distributions of chronological age were similar between groups.
Due to the ordinal nature of the data, hierarchical generalized linear models (HGLMs) were employed to examine initial (R1) and familiar (R2) scores on all three scales, with chronological age nested within participants. This is a robust statistical approach for examining behavior longitudinally, because models account for nesting of observations within individuals and also allow for inter-individual differences in the timing and number of observations. A model-building approach was utilized to determine the most parsimonious, best-fitting model (Ene, Leighton, Blue, and Bell, 2015). As is commonly done in this approach, an unconditional model with no predictors (Model 1) was first run to calculate the intra-class correlation coefficient (ICC). In Model 2, the Level-2 fixed effects of chronological age (grand-mean centered at 31.65 months), group (TD, FXS), site and duration of visit (for R2 only) were included. Model 3 added the Level-2 fixed effect of the chronological age by group interaction. Model fit was assessed via a X2 deviance test of the −2 log likelihood (−2LL) ratio. All models were run using the Laplace estimation method for a multinomial distribution, and a cumulative logit link was used to compute the cumulative odds for being at or below each rating. Because developmental level (i.e., Mullen age equivalent scores) was redundant with group and highly correlated with chronological age, it was excluded from the inferential models. All described inferential models were repeated for the older cohort, though only chronological age was included in Model 2 due to the lack of a TD control group. Age was grand-mean centered at 17.29 years.
To address the second aim (i.e., to identify the dynamic aspects of change in scores from R1 to R2 using the four categories of change described earlier), we computed the percentage of observations in each change category for each group on each SAS scale in each cohort. This approach was used given the ordinal nature of the data. Chi-square models were run to investigate whether the proportion of participants in the “Warms Up” category differed between TD and FXS participants in the infant/preschool cohort and between younger and older FXS participants (Infant/Preschool v. Adolescent/Adult cohorts) for each of the SAS scales. We ran analyses for the “Warms Up” category only given previous evidence that this dynamic change was a particularly salient trait for the FXS group (Roberts et al., 2007), and because we wanted to be mindful of the number of analytical models computed. The other change categories are characterized descriptively.
Results
Aim 1 – Characterize the longitudinal trajectory of social avoidance in males with FXS from infancy through early adulthood
Descriptive Analyses: Entire Sample (4 months to 25 years).
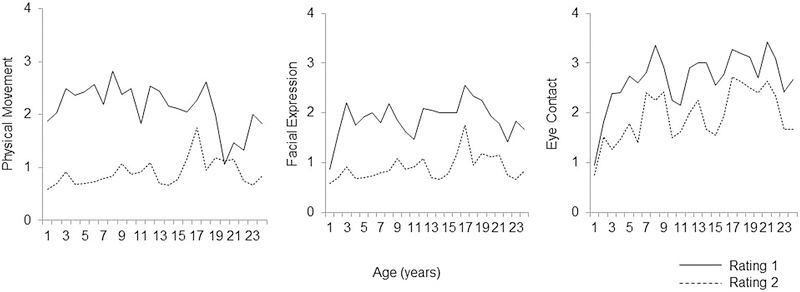
Results using the summary variable (≥1 on any of the three SAS scales at either the R1 or R2 ratings) indicated that of the entire sample of males with FXS (4 months to 25 years), 81% displayed social avoidance at R1, and 49% at R2. Within the younger cohort, 77% of males with FXS displayed social avoidance at R1 and 34% at R2. In contrast, 44% and 10% of TD males displayed social avoidance at R1 and R2, respectively. In the adolescent/adult cohort of males with FXS, 82% at R1 and 64% at R2 exhibited social avoidance. Mean SAS scores for males with FXS are depicted in Figure 1.
Figure 1.
Mean SAS scores for males with FXS.
Inferential Analyses: Infant/Preschool Cohort (4 to 72 months).
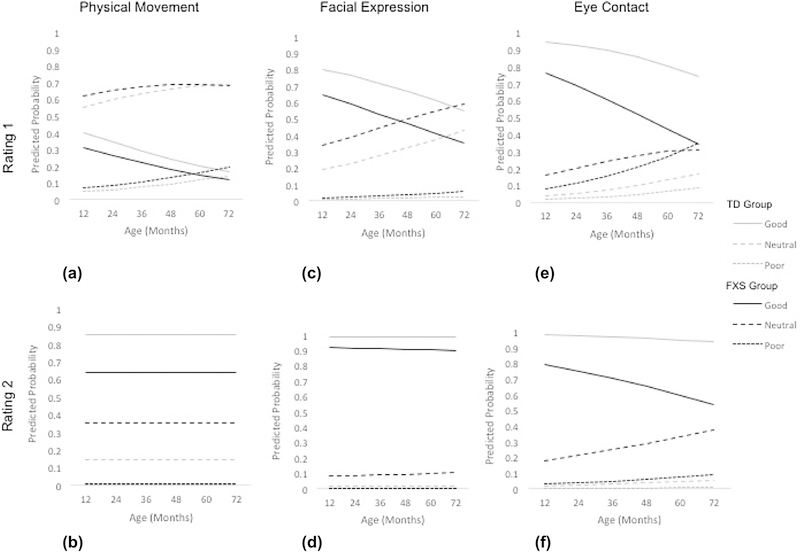
For the infant/preschool cohort, fixed-effects models were determined to be the best-fitting. Fixed-effects models including age, group, site, and duration (R2 only) with random intercepts were computed for all these analyses (Table 3; Figure 2) with the ICC’s included to reflect the model variance. The interactions of age by group did not contribute to the model so were not included.
Table 3.
Estimates for Generalized Linear Models in Infant/Preschool Cohort
| Physical Movement | Facial Expression | Eye Contact | |
|---|---|---|---|
| Rating 1 (n = 290) | Rating 1 (n = 290) | Rating 1 (n = 280) | |
| Fixed Effects | |||
| Intercept (0) | −2.32*** (0.30) | −1.03*** (0.24) | 0.76* (0.29) |
| Intercept (1) | −0.80*** (0.23) | 1.00*** (0.24) | 2.26*** (0.34) |
| Intercept (2) | 1.22*** (0.24) | 2.21*** (0.28) | 3.22*** (0.39) |
| Intercept (3) | 2.60*** (0.31) | 4.39*** (0.44) | 3.54*** (0.41) |
| Intercept (4) | -- | -- | 6.16*** (0.63) |
| Age | −0.02* (0.01) | −0.02** (0.01) | −0.03** (0.01) |
| Group (FXS) | −0.38 (0.24) | −0.80** (0.25) | −1.70*** (0.34) |
| Site (USC) | −0.30 (0.27) | −0.33 (0.27) | −0.97** (0.35) |
| Error Variance | |||
| Intercept | 0.24 (0.29) | 0.35 (0.28) | 1.09* (0.50) |
| Model Fit | |||
| −2LL | 816.60‡ | 791.30‡ | 742.17‡ |
| ICC (from Model 1) | .11 | .16 | .42 |
| Rating 2 (n = 279) | Rating 2 (n = 279) | Rating 2 (n = 279) | |
| Fixed Effects | |||
| Intercept (0) | 0.33 (0.47) | 2.44*** (0.57) | 2.11*** (0.49) |
| Intercept (1) | 1.74*** (0.49) | 4.30*** (0.67) | 3.48*** (0.54) |
| Intercept (2) | 4.02*** (0.61) | 6.83*** (0.89) | 4.64*** (0.59) |
| Intercept (3) | 6.05*** (0.92) | 8.27*** (1.25) | 5.63*** (0.65) |
| Intercept (4) | -- | -- | 7.98*** (1.03) |
| Age | 0.00 (0.01) | 0.00 (0.01) | −0.02† (0.01) |
| Group (FXS) | −1.18** (0.39) | −1.99*** (0.48) | −2.53*** (0.44) |
| Site (USC) | 1.91*** (0.45) | −0.01 (0.45) | 0.53 (0.41) |
| Duration | −0.05 (0.11) | −0.15 (0.12) | −0.19† (0.11) |
| Error Variance | |||
| Intercept | 1.49* (0.69) | 1.91* (0.97) | 1.19* (0.59) |
| Model Fit | |||
| −2LL | 547.04‡ | 463.61‡ | 590.92‡ |
| ICC (from Model 1) | .45 | .45 | .44 |
Notes:
p < .10
p < .05
p < .01
p < .001
= Likelihood ratio test significant; Entries show parameter estimates with standard errors in parentheses; Estimation Method = Laplace.
Figure 2.
Predicted Probabilities of SAS Ratings in Infant/Preschool cohort.
For the R1 ratings for the Physical Movement scale, results indicated that increased chronological age over time was associated with decreased likelihood of scoring a “0” (i.e., “child clearly moves toward you”; b = −0.02, p < .05). While the effect of group was not significant at R1 (b = −0.38, p = .12), the Odds Ratio estimates indicated that children with FXS were 1.46 times less likely than TD children to receive a score of “0”, thus corresponding to a small effect size (Cohen et al., 1988). For the R2 ratings for the Physical Movement scale, there was an effect of group indicating that children with FXS were less likely to score a “0” (b = −1.18, p < .01). In fact, children with FXS were 3.26 times less likely than TD children to receive a score of “0” for R2 Physical Movement, corresponding to just under a medium effect size (Cohen, 1988).
For the R1 ratings for the Facial Expression scale, results indicated that increased chronological age over time (b = −0.02, p < .01) and group (b = −0.80, p < .01) were associated with decreased likelihood of scoring a “0” (i.e., “no sign of vocal, facial, or postural wariness”). The Odds Ratios indicated that children with FXS were 2.16 times less likely than TD children to receive a score of “0” for R1 Facial Expression. For the R2 ratings for the Facial Expression scale, chronological age over time did not affect the likelihood of scoring a “0” on Facial Expression (b = 0.00, p = 0.73). However, the FXS group was significantly less likely than the TD group to score a “0” (b = −1.99, p < .001). In fact, the children with FXS were 7.33 times less likely than TD children to receive a score of “0” for Facial Expression R2, almost approaching a large effect size (i.e., OR=9; Cohen et al., 1988).
For the Eye Contact scale, results indicated main effects of chronological age over time and group for R1 with only group associated with R2. Specifically, as chronological age increased, the likelihood of scoring a “0” on Eye Contact R1 decreased (b = −0.03, p < .01). The FXS group was less likely than the TD group to score a “0” (i.e., “age appropriate eye contact”) on Eye Contact R1 (b = - 1.70, p < .001). Children with FXS were 5.50 times less likely than TD children to receive a score of “0” for Eye Contact at R1, indicating the effect was between medium and large size (Cohen et al., 1988). Chronological age over time had a marginally significant effect on the likelihood of being scored a “0” on Eye Contact at R2 (b = −0.02, p = 0.08). Group did predict the likelihood of scoring a “0” on the scale, with the FXS group significantly less likely to score a “0” (b = −2.53, p < .001). Odds Ratios revealed that children with FXS were 12.56 times more likely than TD children to receive a score of “0” for Eye Contact at R2, indicating that the effect exceeded a large magnitude as defined by Cohen et al. (1988).
Inferential Analyses: Adolescent/Young Adult Cohort (10 to 25 years).
For the adolescent/adult cohort, unconditional models were determined to be the best-fitting as inclusion of the predictors did not add appreciably to the model. This indicates that, within this cohort, neither chronological age over time, site, nor duration of assessment influenced either R1 or R2 ratings. A control group was not available for this cohort and so interaction effects were not tested. Results, including ICCs, are depicted in Table 4.
Table 4.
Estimates for Generalized Linear Models in Adolescent/Adult Cohort
| Physical Approach | Facial Expression | Eye Contact | |
|---|---|---|---|
| Rating 1 (n = 211) | Rating 1 (n = 212) | Rating 1 (n = 212) | |
| Fixed Effects | |||
| Intercept (0) | −2.05*** (0.30) | −3.20*** (0.39) | −5.32*** (0.65) |
| Intercept (1) | −0.86*** (0.24) | −0.65** (0.25) | −2.59*** (0.37) |
| Intercept (2) | 0.64** (0.23) | 1.17*** (0.26) | −0.47† (0.28) |
| Intercept (3) | 2.19*** (0.30) | 2.29*** (0.32) | 0.68* (0.28) |
| Intercept (4) | -- | -- | 3.15*** (0.41) |
| Error Variance | |||
| Intercept | 2.26** (0.85) | 2.65** (0.97) | 4.05** (1.37) |
| Model Fit | |||
| −2LL | 650.58 | 620.19 | 656.49 |
| ICC | .41 | .45 | .55 |
| Rating 2 (n = 210) | Rating 2 (n = 212) | Rating 2 (n = 212) | |
| Fixed Effects | |||
| Intercept (0) | −1.23*** (0.25) | −1.04** (0.36) | −3.84*** (0.47) |
| Intercept (1) | 0.51* (0.23) | 2.11*** (0.44) | −1.08*** (0.29) |
| Intercept (2) | 2.39*** (0.33) | 4.29*** (0.60) | 0.73* (0.29) |
| Intercept (3) | 4.00*** (0.47) | 5.53*** (0.72) | 2.25*** (0.36) |
| Intercept (4) | -- | -- | 5.61*** (0.72) |
| Error Variance | |||
| Intercept | 2.18** (0.85) | 7.40** (2.53) | 4.23** (1.42) |
| Model Fit | |||
| −2LL | 587.34 | 511.94 | 645.73 |
| ICC | .40 | .69 | .56 |
Notes:
p < .10
p < .05
p < .01
p < .001
Entries show parameter estimates with standard errors in parentheses; Estimation Method = Laplace.
Aim 1 Results Summary:
In summary, 81% of males with FXS aged 4 months to 25 years displayed evidence of social avoidance. Trajectories of increasing social avoidance over time was evident for all three scales, albeit at a trend level for eye contact at R1. In contrast, social avoidance at R2 was not associated with increases across age over time on any scale. However, this pattern was observed only in the infant/preschool cohort for the inferential models. SAS ratings were not associated with trajectories across time for any scale at R1 and R2 for the adolescent/young adult cohort. Contrasted to TD controls, the infant/preschool-aged males with FXS displayed elevated social avoidance across the majority of scales and at both R1 and R2 and the elevation was evident within the first year of life. However, the discrimination between the FXS and TD groups was greater for the R2 ratings, when the participants had increased familiarity, with Odds Ratios more than doubled for the R2 ratings compared to the R1 ratings for all three scales.
Aim 2 – Identify the dynamic aspect of social avoidance in males with FXS.
Comparison to TD Controls: Infant/Preschool Cohort.
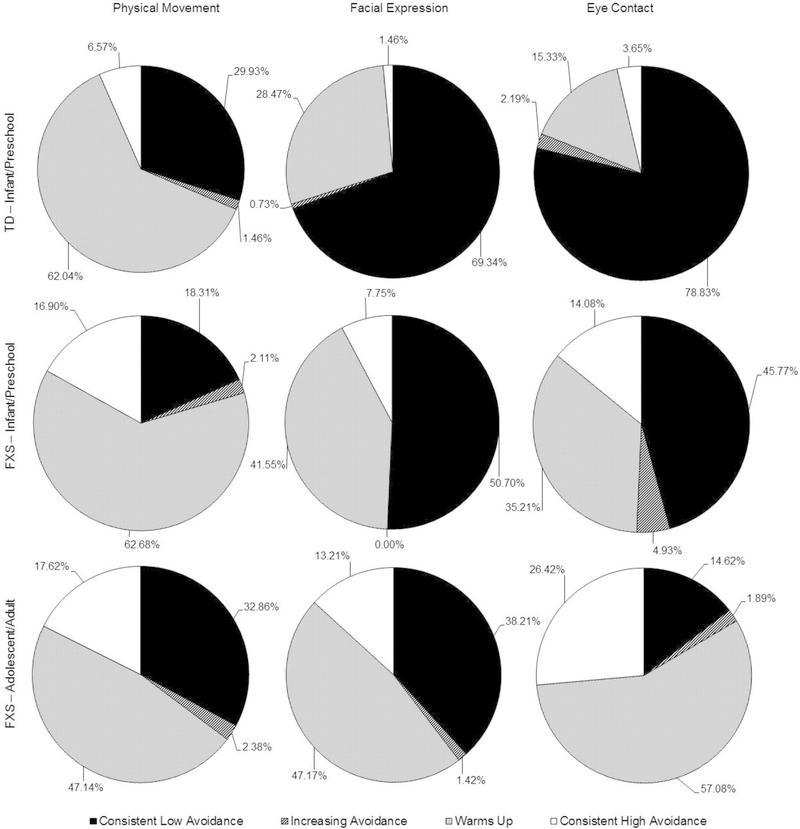
For Physical Movement, similar proportions of participants in the young FXS and TD groups (63% versus 62%) exhibited a “warm up” effect (Χ2(1) = 0.01, p = .91). For the Facial Expression scale, the FXS group had a larger proportion of participants who warmed up (42% versus 29%; Χ2(1) = 5.22, p < .05), suggesting that a “warm-up” effect is more common in the FXS group than the TD group. Likewise, for the Eye Contact scale, a warm-up effect was more common in the FXS group than the TD group (35% versus 15%; Χ2(1) = 14.47, p < .001). See Figure 3 for the proportion of participants for each of the change categorizations.
Figure 3.
Proportion of participants in each SAS change category.
Comparison by Age within FXS: Infant/Preschool Cohort v. Adolescent/Adult Cohort.
For Physical Movement, the warm-up effect was more common in the younger cohort than the older cohort (63% versus 47%; Χ2(1) = 8.20, p < .01). In contrast, for Facial Expression, the warm-up effect occurred at similar rates in the younger cohort and the older cohort (42% versus 47%; Χ2(1) = 1.08, p = .30). For Eye Contact, however, the warm-up effect was more common in the older cohort than the younger cohort (57% versus 35%; Χ2(1) = 16.24, p < .001). See Figure 3 for the proportion of participants for each of the change categorizations.
Aim 2 Results Summary.
In summary, the infant/preschool group showed increasing “warm up” in both the Facial Expression and Eye Contact scales but not for the Physical Movement scale compared to the TD children. Across the two FXS age groups, the infant/preschool group showed increased “warm up” during the Physical Movement scale, the same degree of “warm up” for the Facial Expression and less “warm up” for the Eye Contact scale compared to the adolescent/young adult group. As a descriptive variable to describe the consistency of elevated social avoidance, we also report the proportion that displayed “consistently high social avoidance”.
Discussion
Social avoidance is a core phenotypic feature of FXS, however, social avoidance in FXS is unique in that elements of social interest and approach are often coupled with features of social avoidance (Cornish et al., 2008; Roberts et al., 2007; Roberts et al., 2007, 2018, 2019). Elevated social avoidance is associated with poor outcomes and increased impairment in FXS including reduced independence, elevated social isolation and increased severity of social anxiety and ASD symptoms (Ouyang, Grosse, Raspa, & Bailey et al., 2010; Roberts et al., 2009; Williams et al., 2014). Despite the pervasive and impairing presence of social avoidance in FXS, little research has examined the longitudinal course or predictors of social avoidance. In this study, we investigate social avoidance by using a multi-dimensional direct observation scale that includes physical movement, facial expression, and eye contact with 191 males with FXS aged 4 months to 25 years. To our knowledge, this is the first study to document the nature of social avoidance in young infants (from 4 months of age) with FXS. In contrast to previous “static” snapshots of avoidance, such as those elicited via rating scales, our approach was to examine dynamic responses to both an initial social encounter and in response to social interactions with a familiar partner. This is a novel approach but one we believe is critical given the limitations of many scales that require a forced choice (i.e., often a binary designation of presence or absence) and thus, do not adequately address the complexity of social avoidance in FXS.
Trajectory of Social Avoidance in FXS.
The overarching findings from our study indicate that 81% of males with FXS (77% of the younger cohort and 82% of the older cohort) display socially avoidant behavior that emerges during infancy and increases in severity across early childhood. Our findings also indicate that the trajectory of social avoidance across time in FXS is complex and multi-faceted with non-linear age-related patterns across development. Specifically, social avoidance increased across longitudinal assessments over time in the infant/preschool cohort while there was relative stability in the adolescent/young adult cohort. This trajectory is particularly interesting given recent research indicating no effect of chronological age on eye contact avoidance in males aged 8–16 years with FXS (Hall & Venema, 2017). Given our study representing repeated assessments of the same individuals over time across a very wide developmental span from infancy through young adulthood, we extend the literature in important ways by documenting the variation in developmental trajectories across key developmental periods. These results highlight the merit of the approach adopted here in tracing developmental trajectories from infancy through to adulthood. The increase in social avoidance across age during the infant/preschool developmental period appears to be nearly universal across both initial social encounters and for social interactions with familiar persons for all three scales. The only exception is that physical movement during initial social encounters does not appear to be associated with advancing chronological age over time.
The severity of social avoidance clearly distinguishes children with FXS from chronological age- matched TD male controls. Specifically, infants and preschool-aged males with FXS showed elevated social avoidance that was pervasive across nearly all three SAS scales for both initial and familiar contexts. The only dimension in which the social avoidance of the young FXS group was not elevated from the TD group was in their physical movement for the initial interactions. Earlier work reported similar differences to those reported here between TD and FXS males on measures of eye contact avoidance and facial expressions (Roberts et al., 2009). In this previous work, however, there were no differences between these two participant groups on physical movement for the familiar interaction rather than the initial interaction as reported here. As such, future work is needed to clarify the role of physical movement in contributing to the overall social avoidance profile observed in FXS.
The role of the familiarity of the social partner is often not considered in studies of social avoidance in FXS. Here, it is reported that the increasing familiarity of the social partner is a critical facet to understanding the nature of social avoidance in FXS, as social avoidance during interactions with a familiar person discriminated the group with FXS from TD controls to a greater degree than interactions with unfamiliar people (e.g., the Odds Ratios more than doubled for the familiar versus initial ratings). Specifically, elevated social avoidance in response to initial interactions discriminated young infants and preschool males with FXS from TD controls. However, the persistence of social avoidance even at the end of the interaction, after becoming more familiar with the context and social partners, most discriminated the FXS from the TD participants. Earlier work demonstrated a decrease in social avoidance as a function of increased familiarity with the assessor (Roberts et al., 2007). Existing literature has, therefore, indicated that individuals with FXS demonstrate elevated social avoidance with unfamiliar people and in novel situations (Cohen et al., 1988; Hessl et al., 2006; Hall et al., 2006; Kau et al., 2000, 2002; Roberts et al., 2007). The current study extends these findings by highlighting that social avoidance, even when with a familiar assessor, is atypical in males with FXS. In support of this notion, Hall and Venema (2017) reported that parent ratings reflected less avoidance of eye contact during interactions with caregivers and those most familiar to the individual with FXS than with people less familiar. At the same time, however, the high rates of social avoidance at the end of the social encounter suggests a resistance of social anxiety to increased familiarity in individuals with FXS or their slower accrual of the social cues that lead a situation to be perceived as familiar and thus, less anxiety provoking.
Several important differences across the three social avoidance scales were evident. Not surprisingly, avoidant eye contact appeared to have particular salience in characterizing social avoidance profiles in FXS contrasted to TD controls. Despite all three dimensions of social avoidance showing group distinctions across both initial and familiar contexts, the magnitude of the distinction was the greatest for the Eye Contact scale (R1 OR = 5.50; R2 OR = 12.56), which complements research documenting the almost universal eye contact avoidance reported in this population (Lachiewicz et al., 2000; Merenstein et al., 1996). Infants and preschool-aged males with FXS were nearly twice as likely to have at least mild avoidant eye contact contrasted to the TD group at both the initial and familiar ratings than for the physical movement and facial expression scales.
The distinctions between participants with FXS and TD controls were evident across the entire age range for the infant/preschool cohort suggesting that elevated social avoidance in males with FXS emerged within the first year of life. This finding suggests a strong heritable influence presumed to be linked to FMR1 gene function. It has been hypothesized that social avoidance occurs downstream of a dysregulated arousal system (Hall, Lightbody, Huffman, Lazzeroni, & Reiss, 2009; Roberts et al., 2009). The finding reported here, that social avoidance emerges within the first year of life, corroborates with previous studies indicating atypicalities in physiological arousal also emerging within the first year of life (Roberts, Tonnsen, Robinson, & Shinkareva, 2012) to lend support to this hypothesis. We were not able to determine if the social avoidance of males with FXS differed from TD age-matched controls beyond 6 years-of-age given the lack of adolescent/adult TD comparison data available in our convenience sample.
Dynamic Aspects of Social Avoidance.
A larger proportion of participants in the FXS infant/preschool than the TD group warmed up for the Facial Expression (42% versus 29%) and Eye Contact scales (35% versus 15%) with the two groups nearly identical for the Physical Movement scale (63% versus 62%). The greater proportion of “warm up” in the young FXS group was largely driven by the TD group having a larger proportion of participants that demonstrated little to no social avoidance at the initial ratings which constrained the potential to “warm up” (e.g., 79% of the TD group had consistently good eye contact versus 46% from the young FXS group). Although not statistically analyzed, the FXS figures indicate that a higher proportion of participants with FXS demonstrated a “warm up” effect in the Facial Expression and Physical Movement scales compared to the Eye Contact scale. This supports previous work indicating that, compared to the other two scales, eye contact is less likely to improve with the amount of time spent with the examiner. This adds to a body of work highlighting the nature of the eye contact impairment in FXS (Roberts et al., 2007; Hall & Venema, 2017).
Across the two FXS age cohorts, the infant/preschool cohort demonstrated more “warm up” than the adolescent/adult cohort (63% versus 47%) for the Physical Movement scale, whereas there were no differences in “warm up” for the Facial Expression scale between the two cohorts (42% versus 47%). In contrast, the adolescent/adult cohort “warmed up” more than the infant/preschool cohort (57% versus 35%) on the Eye Contact scale. This extends previous work, which documented a flatter “warm-up” profile of social avoidance in FXS males over the age of 5 years compared to younger males (Roberts et al., 2007). Notably, 14% of the young group with FXS had “consistently high” eye contact avoidance contrasted to 4% from the TD group. The prevalence of consistently high eye contact avoidance increased from 14% in the young FXS group to 26% in the adolescent FXS group. Thus, despite evidence of a “warm up” effect across some SAS scales, the overall pattern clearly demonstrates that males with FXS show elevated and persistent social avoidance throughout social encounters.
Developmental Findings.
There are a number of important developmental considerations evident through this study. First, the predictors of social avoidance differed across the age cohorts. Increased chronological age and a shorter duration of interaction were associated with increased social avoidance, but only for the infant/preschool cohort. Second, modulation of social avoidance, depicted as increased “warm up” was more common in the infant/preschool cohort for the Physical Movement scale but more common in the adolescent/young adult cohort for the Eye Contact scale. Third, consistently high eye contact avoidance nearly doubled across age with 26% of adolescent and young adult males demonstrating impaired eye contact at both initial social interactions and during social interactions with familiar persons. This work suggests that diagnostic efforts should employ a developmental framework as features of social avoidance may change over time and could serve as precursors to later-emerging disorders (e.g., social avoidance could lead to social anxiety disorder or signal elevated risk for ASD). Likewise, our findings have clear treatment implications. Initial intervention efforts have revealed a potential utility for intranasal administration of oxytocin in reducing eye contact avoidance and hyperarousal, as measured through salivary cortisol, in adolescent/adult males with FXS (Hall, Lightbody, McCarthy, Parker, & Reiss, 2012). A recent review also highlighted the utility of behavioral interventions to improve target behaviors in individuals with FXS, although interventions targeting social pragmatic skills and eye contact were mixed (Moscowitz & Jones, 2015). Importantly, this review stated the clear need for such interventions to be guided more by phenotypic characteristics of FXS. The current study delineates the phenotype of social avoidance from a developmental perspective and suggests a need for initiating treatment at a very young age and employing multiple methods of treatment (e.g., behavioral, psychopharmacological) aimed to reduce social avoidance.
Limitations and Future Directions.
While this is the largest study to date documenting social avoidance in males with FXS using a direct observation rating scale, and is the first to employ a scale that reflects dynamic aspects of social avoidance, there are a number of limitations and important future directions. We did not include females in our sample given the complexity of the analyses and our smaller sample of females. As such, it will be important to characterize the trajectory of social avoidance in females with FXS in the future. Second, our convenience sample pulled from multiple related longitudinal studies limited our maximum age to 25 years. Thus, we can not make conclusions about social avoidance profiles later in adulthood in males with FXS. There is a critical paucity of research on older adults with FXS, and our understanding about developmental changes in brain function and resultant behaviors across the lifespan of individuals with FXS would greatly benefit from inclusion of older adults. Additionally, the SAS scale showed adequate reliability and validity and most strongly associated with ASD symptom severity which is similar to findings in a related study (Roberts et al., 2019). However, future work should examine the relationship of the SAS as a predictor to relevant outcomes including ASD features, social anxiety and ADHD which are three highly prevalent co-morbid conditions. We have studies under way to examine these relationships which is beyond the scope of this paper. Finally, this study is strictly behavioral so the inclusion of biomarkers such as heart activity or salivary cortisol as potential mechanistic factors is an important future line of research.
Summary and Implications.
Our results show that 81% of males with FXS displayed evidence of social withdrawal that emerged during infancy with a significant increase across the infant, toddler and preschool years. We also found that social avoidance is strongly related to features of ASD and, albeit to a lesser degree, to symptoms of social anxiety and withdrawal. Thus, this study contributes to the question in the FXS field regarding the relationship of social anxiety and ASD symptom severity as social withdrawal appears to be common across both of these conditions that are often co-morbid in FXS. Given evidence that social skills can be improved and social avoidance reduced (Hall, 2015; Moskowitz & Jones, 2015). We reported that social avoidance during interactions with familiar individuals and avoidant eye contact were highly salient features that characterize the FXS phenotype. As such, this study highlights the importance of examining social avoidance using a dynamic and multi-dimensional scale.
Acknowledgments
This study was funded by 2R01MH090194 PI: Roberts (NIMH); 1R01MH107573 PI: Roberts (NIMH); 5R01HD024356 PI: Abbeduto (NICHD); P30-HD003110–35 PI: Bailey (NICHD). We want to thank the families who gave their time and support as participants of these studies.
Footnotes
No change in author affiliations at this time
References
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms & profiles: an integrated system of multi-informant assessment Burlington: University of Vermont, Research Center for Children, Youth & Families. [Google Scholar]
- Baker S, Hooper S, Skinner M, Hatton D, Schaaf J, Ornstein P, et al. (2011). Working memory subsystems and task complexity in young boys with Fragile X syndrome. Journal of Intellectual Disability Research, 55(1), 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle-Brown J, Murphy G, & Wing L (2005). Long-term outcome for people with severe intellectual disabilities: impact of social impairment. American Journal on Intellectual and Developmental Disabilities, 110(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Bretherton I, & Ainsworth M (1974). Responses of one-year-olds to a stranger in a strange situation. In Lewis M & Rosenblum L (Eds.), The origins of fear: The origins of behavior (Vol. 2, pp. 131–164). New York: Wiley. [Google Scholar]
- Brooker RJ, Buss KA, Lemery‐Chalfant K, Aksan N, Davidson RJ and Goldsmith HH (2013), The development of stranger fear in infancy and toddlerhood: normative development, individual differences, antecedents, and outcomes. Developmental Science, 16, 864–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J, & Blackford J (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry, 51(10), 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, … Brown WT (1988). Social gaze, social avoidance, and repetitive behavior in fragile X males: A controlled study. American Journal on Mental Retardation, 92(5), 436–446. [PubMed] [Google Scholar]
- Cornish K, Turk J, & Hagerman R (2008). The fragile X continuum: New advances and perspectives. Journal of Intellectual Disability Research, 52(6), 469–482. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, & Sherman SL (2001). FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine, 3(5), 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene M, Leighton EA, Blue GL, & Bell BA (2015). Proceedings from the SAS Global Forum 2015: Multilevel models for categorical data using SAS PROC GLIMMIX: The basics, paper no. 3430–2015 Cary, NC: SAS Institute Inc. [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, & Ruedrich S (2003). Reliability and Validity of an Assessment Instrument for Anxiety, Depression, and Mood Among Individuals with Mental Retardation. Journal Of Autism & Developmental Disorders, 33(6), 617–629. [DOI] [PubMed] [Google Scholar]
- Hall S, DeBernardis M, & Reiss AL (2006). Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 36(7), 935–947. [DOI] [PubMed] [Google Scholar]
- Hall S, Lightbody AA, Huffman LC, Lazzeroni LC, & Reiss AL (2009). Physiological correlates of social avoidance behavior in children and adolescents with fragile X syndrome. Journal of the American Academy of Child & Adolescent Psychiatry, 48(3), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, McCarthy BE, Parker KJ, & Reiss AL (2012). Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome. Psychoneuroendocrinology, 37(4), 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS (2015). Treatments for fragile X syndrome: A closer look at the data. Journal of Developmental Disabilities Research Reviews, 15(4), 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, & Venema K (2017). A Screening Tool to Measure Eye Contact Avoidance in Boys with Fragile X Syndrome. Journal Of Autism & Developmental Disorders, 47(7), 2254–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, & Reiss AL (2006). Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines, 47(6), 602–610. [DOI] [PubMed] [Google Scholar]
- Kaldewaij R, Koch SB, Volman I, Toni I, & Roelofs K (2017). On the control of social approach-avoidance behavior: Neural and endocrine mechanisms. Current Topics in Behavioral Neuroscience, 30 275–293. [DOI] [PubMed] [Google Scholar]
- Kampman O, Viikki M, Jarventausta K, & Leinonen E (2014). Meta-analysis of anxiety disorders and temperament. Neuropsychobiology, 69(3), 175–186. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A, & Farran EK (2011). Williams syndrome: A model for the neuroconstructivist approach. In Farran EK & K.-S. A (Eds.), Neurodevelopmental disorders across the lifespan: A neuroconstructivist approach: OUP Oxford. [Google Scholar]
- Kau ASM, Meyer WA, & Kaufmann WE (2002). Early development in males with Fragile X syndrome: A review of the literature. Microscopy Research and Technique, 57(3), 174–178. [DOI] [PubMed] [Google Scholar]
- Kau ASM, Reider EE, Payne L, Meyer WA, & Freund LS (2000). Early behavior signs of psychiatric phenotypes in fragile X syndrome. American Journal on Mental Retardation, 105(4), 286–299. [DOI] [PubMed] [Google Scholar]
- Klaiman C, Quintin EM, Jo B, Lightbody AA, Hazlett HC, Piven J, Hall SS, & Reiss AL (2014). Longitudinal Profiles of Adaptive Behavior in Fragile X Syndrome. Pediatrics, 134(2), 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachiewicz AM, Dawson DV, & Spiridigliozzi GA (2000). Physical characteristics of young boys with fragile X syndrome: Reasons for difficulties in making a diagnosis in young males. American Journal of Medical Genetics, 92(4), 229–236. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E (2007) Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch. Gen. Psychiatry 64, 853–864. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, Mennin DS, Nolen-Hoeksema S (2011). Emotion dysregulation and adolescent psychopathology: A prospective study. Behaviour Research and Therapy, 49(9), 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein SA, Sobesky WE, Taylor AK, Riddle JE, Tran HX, & Hagerman R (1996). Molecular-clinical correlations in males with an expanded FMR1 mutation. American Journal of Medical Genetics, 64(2), 388–394. [DOI] [PubMed] [Google Scholar]
- Mian ND, Carter AS, Pine DS, Wakschlag LS, & Briggs-Gowan MJ (2015). Development of a novel observational measure for anxiety in young children: The Anxiety Dimensional Observation Scale. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 56(9), 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AC, Keenan JM, Betjemann RS, Willcutt E, Pennington BF, & Olson RK (2013). “Reading Comprehension in Children with ADHD: Cognitive Underpinnings of the Centrality Deficit.” Journal of Abnormal Child Psychology 41, no. 3: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskoqitz LJ, & Jones EA (2015). Uncovering the evidence for behavioral interventions with individuals with fragile X syndrome: A systematic review. Research in Developmental Disabilities, 38, 223–241. [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995) Mullen Scales of Early Learning: AGS Edition, American Guidance Service, Circle Pines, MN. [Google Scholar]
- Ouyang L, Grosse S, Raspa M, & Bailey D (2010). Employment impact and financial burden for families of children with fragile X syndrome: Findings from the National Fragile X Survey. Journal of Intellectual Disability Research, 54(10), 918–928. [DOI] [PubMed] [Google Scholar]
- Reichow B, & Volkmar F (2010). Social skills interventions for individuals with autism: Evaluation for evidence-based practices within a best evidence synthesis framework. Journal of Autism and Developmental Disorders, 40(2), 149–166. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Clarke MA, Alcorn K, Carter JC, Long AC, & Kaufmann WE (2009). Autistic behavior in boys with fragile X syndrome: social approach and HPA-axis dysfunction. Journal of Neurodevelopmental Disorders, 1(4), 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Crawford H, Will EA, Hogan AL, McQuillin S, Tonnsen BL, et al. (2019). Infant social avoidance predicts autism but not anxiety in fragile X syndrome. Frontiers in Psychiatry, 10, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Ezell JE, Fairchild AJ, Klusek J, Thurman AJ, McDuffie A et al. (2018). Biobehavioral composite of social aspects of anxiety in young adults with fragile X syndrome contrasted to autism spectrum disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 177(7), 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, McCary LM, Caravella KE, & Shinkareva SV (2016). Brief report: Autism symptoms in infants with fragile X syndrome. Journal of Autism and Developmental Disorders, 46(12), 3830–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen B, Robinson A, & Shinkareva SV (2012). Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 117(2), 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LAH, Hatton DD, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 37(9), 1748–1760. [DOI] [PubMed] [Google Scholar]
- Roid GH, & Miller LJ (1997). Leiter International Performance Scale-Revised Wood Dale, IL: Stoelting. [Google Scholar]
- Schneier FR, Heckelman LR, Garfinkel R, Campeas R, Fallon BA, Gitow A, … Liebowitz MR (1994). Functional impairment in social phobia. Journal of Clinical Psychiatry, 55(8), 322–331. [PubMed] [Google Scholar]
- Schopler E, Van Bourgondien ME, Wellman GJ, & Love SR (2010). Childhood Autism Rating Scale, Second Edition (CARS-2): Manual Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Thurman AJ, McDufe A, Hagerman R, & Abbeduto L (2014). Psychiatric symptoms in boys with fragile X syndrome: A comparison with nonsyndromic autism spectrum disorder. Research in Developmental Disabilities, 35(5), 1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torvik FA, Welander-Vatn A, Ystrom E, Knudsen GP, Czajkowski N, Kendler KS, & Reichborn-Kjennerud T (2016). Longitudinal associations between social anxiety disorder and avoidant personality disorder: A twin study. Journal of Abnormal Psychology, 125(1), 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, & Robinson H (1996). Prevalence of fragile X syndrome. American Journal of Medical Genetics, 64(1), 196–197. [DOI] [PubMed] [Google Scholar]
- White SW, Mazefsky CA, Dichter GS, Chiu PH, Richey JA, & Ollendick TH (2014). Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: understanding anxiety in autism spectrum disorder. International Journal of Developmental Neuroscience, 39, 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TA, Porter MA, & Langdon R (2014). Social approach and emotion recognition in fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 119(2), 133–150. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, & Szatmari P (2005). “Behavioral Manifestations of Autism in the First Year of Life.” International Journal of Developmental Neuroscience 23, no.2–3: 143–152. [DOI] [PubMed] [Google Scholar]