Abstract
Alcohol use is a well characterized risk factor for traumatic brain injury (TBI); however, emerging clinical and experimental research suggests that TBI may also be an independent risk factor for the development of alcohol use disorders. In particular, TBIs incurred early in life predict the development of problem alcohol use and increase vulnerability to neuroinflammation as a consequence of alcohol use. Critically, the neuroinflammatory response to alcohol, mediated in large part by microglia, may also function as a driver of further alcohol use. Here, we tested the hypothesis that TBI increases alcohol consumption through microglia-mediated neuroinflammation. Mice were injured as juveniles and alcohol consumption and preference were assessed in a free-choice voluntary drinking paradigm in adolescence. TBI increased alcohol consumption; however, treatment with minocycline, an inhibitor of microglial activation, reduced alcohol intake in TBI mice to sham levels. Moreover, a single injection of ethanol (2g/kg) significantly increased microglial activation in the nucleus accumbens and microglial expression of the proinflammatory cytokine IL-1β in TBI, but not sham or minocycline-treated, mice. Our data implicate TBI-induced microglial activation as a possible mechanism for the development of alcohol use disorders.
Keywords: traumatic brain injury, alcohol, neuroinflammation, microglia, minocycline
Introduction
The relationship between alcohol use and traumatic brain injury (TBI) is well documented. Clearly, there is substantial evidence that alcohol intoxication is an enormous risk factor for TBI (Bombardier et al., 2002; Opreanu et al., 2010). However, there is also mounting evidence that the inverse relationship exists, specifically that TBI may itself be a risk factor for the development of problem alcohol use (Corrigan et al., 2013; Graham and Cardon, 2008; Ilie et al., 2015; Winqvist et al., 2007). A history of TBI is a significant predictor of later alcohol abuse even among individuals that endured a mild TBI without loss of consciousness (Corrigan et al., 2013; Fishbein et al., 2016). Critically, the age of TBI is strongly correlated to alcohol use, such that alcohol use problems are highest in individuals who experienced a TBI at a younger age (Corrigan et al., 2013; Fishbein et al., 2016; McKinlay et al., 2014). Moreover, drinking after TBI is associated with poor rehabilitation outcomes, greater morbidity and a greatly increased chance of future head injuries (Corrigan, 1995; Vaaramo et al., 2014). Given that young TBI patients are less likely to have developed problem drinking prior to injury, increasing evidence suggests that the vulnerability to alcohol abuse later in life is a consequence of TBI-related pathology (Weil et al., 2016a; Weil and Karelina, 2017).
We recently reported that mild TBI produces lasting alterations in dopaminergic signaling in key brain regions associated with reward processing (Karelina et al., 2017b). Indeed there is strong evidence that the brain’s reward circuitry undergoes pathophysiological alterations following brain injury resulting in not only altered dopamine signaling, but also functional abnormalities related to inflammation and cell death in the nucleus accumbens, ventral tegmental area (VTA), amygdala, and prefrontal cortex (Sajja et al., 2013; Shah et al., 2012; Shin et al., 2013). Given the importance of these regions in establishing motivation for a rewarding substance (amygdala), dopamine release (VTA), and behavioral responses to rewarding stimuli (nucleus accumbens and prefrontal cortex), it is important to identify whether damage to these regions after TBI promotes alcohol (and other substance) abuse (Cardinal et al., 2002; Keitz et al., 2003; Kelley, 2004).
While the mechanisms by which TBI increases the risk of alcohol abuse are not well understood, the development of animal models to study alcohol consumption after TBI has led to preliminary evidence for neuroinflammation as a potential proximate mediator. Neuroinflammation is a key component of TBI pathology. Inflammation occurs in the context of impaired cerebral perfusion, oxidative damage, and ultimately, diffuse neuronal damage and cell death in the traumatized brain (Maas et al., 2008; Werner and Engelhard, 2007). The neuroinflammatory response is universal in nearly all TBI, regardless of injury severity, and is a main target of TBI treatment due to its role in exacerbating primary damage, and inflammatory contributions to the development of long-lasting functional and cognitive deficits (Das et al., 2012; Kumar and Loane, 2012; Zetterberg et al., 2013). The primary innate immune response to brain injury is mediated by microglia. Microglia are rapidly activated by brain injury and produce pro-inflammatory cytokines and chemokines that are essential for phagocytic activity but also cause damage to the surrounding healthy cells (Hernandez-Ontiveros et al., 2013; Loane and Kumar, 2016). Dysregulated microglial activation impedes TBI recovery and increases vulnerability to chronic disturbances such as affective and cognitive dysfunction which often persist years after overt symptoms of TBI have resolved (Fenn et al., 2014; Giunta et al., 2012; Walker and Tesco, 2013).
The relationship between TBI-induced microglial activation and subsequent alcohol use has not been investigated; however, alcohol is bidirectionally linked to neuroinflammation as prolonged high levels of drinking are associated with inflammation-mediated neurodegeneration and even acute intoxication can induce and modulate inflammatory processes (Crews et al., 2011; He and Crews, 2008; Mayfield et al., 2013). Recent evidence suggests that increased alcohol consumption following TBI corresponds to injury-induced microglial and astrocyte activation, and neuronal degeneration (Mayeux et al., 2015). Importantly, inflammatory signaling can drive voluntary alcohol consumption. For example, systemic treatment with lipopolysaccharide (LPS), a bacterial endotoxin that induces peripheral and central inflammatory responses, produces a long-term increase in voluntary alcohol consumption, lasting up to three months following treatment (Blednov et al., 2011). LPS-induced alcohol consumption is mediated through binding to toll-like receptor 4 (TLR4) on macrophages and microglia, as LPS treatment fails to induce alcohol consumption in mutant mice with dysfunctional TLR4 signaling (Blednov et al., 2011). In another study, TLR4 knockout mice consumed similar amounts of ethanol to wildtype controls, but exhibited attenuated behavioral and cognitive deficits typically associated with ethanol withdrawal (Pascual et al., 2011). Moreover, treating mice with minocycline, an inhibitor of microglial activation, significantly reduces spontaneous ethanol consumption (Agrawal et al., 2011). Additional evidence for a role of inflammation as a driver of alcohol consumption comes from studies of gene knockout mice: genetic deletion of chemokines Ccl2 or Ccl3, the Ccr2 chemokine receptor, interleukin 1 receptor antagonist, interleukin 6, or CD14 substantially reduces voluntary ethanol consumption (Blednov et al., 2005; Blednov et al., 2012).
There is now growing evidence that mild TBI increases voluntary alcohol consumption and preference in rats and mice without significantly affecting alcohol metabolism (Lim et al., 2015; Mayeux et al., 2015; Weil et al., 2016b). We have shown previously that juvenile TBI leads to increased alcohol consumption in adult female, but not male, mice (Weil et al., 2016b). In adulthood, female rats and mice generally consume more alcohol than males; however, alcohol consumption tends to be greater during adolescence in both sexes (Doremus et al., 2005; Vetter-O’Hagen et al., 2009; Yoneyama et al., 2008). Given that problem drinking in TBI patients occurs in both sexes, we sought to test the hypothesis that exposure to alcohol at an earlier age (i.e. during adolescence) would reveal TBI effects on drinking in male mice.
Taken together, there is compelling evidence that alcohol produces neuroinflammation and that neuroinflammation further increases alcohol preference and consumption; however, no causal relationship has been established for TBI-induced inflammation and post-injury drinking. Here, we tested this relationship using a mouse model of juvenile mild TBI in which mice were treated with minocycline and alcohol consumption was assessed in adolescence using the well characterized intermittent (every-other-day) voluntary ethanol intake test (Melendez, 2011).
Methods and materials
Animals and experimental design.
Swiss Webster mice purchased from Charles River (Wilmington, MA) were bred at OSU. Pups were weaned at 21 days of age and housed in a 14:10 light cycle with ad libitum access to food (Harlan-Teklad #8640) and filtered tap water. Mice were housed in groups of five unless otherwise stated. All procedures were approved by the OSU Institutional Animal Care and Use Committee and were conducted in accordance with NIH guidelines.
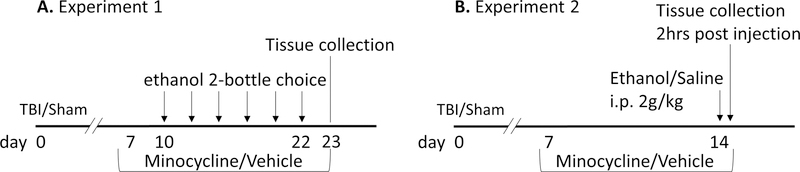
Male 21-day-old mice underwent TBI or sham injury and were returned to their home cages. Beginning one week after injury, mice were single-housed, and minocycline hydrochloride (Sigma Aldrich, 0.25mg/mL) was administered in the drinking water (and ethanol bottles when present in the two bottle choice paradigm) for half of the mice and the other half received water (and ethanol) without minocycline, producing four groups: sham/vehicle, sham/minocycline, TBI/vehicle, TBI/minocycline. The minocycline dose was selected based on previous publications (Agrawal et al., 2011; Bye et al., 2007) and was adjusted to provide a final concentration of approximately 45mg/kg/day based on total daily fluid consumption for 28–30 gram mice. Minocycline was made fresh daily. In experiment 1, mice (n = 12/group) were tested in the every-other-day ethanol drinking paradigm (see below) beginning three days after the initial administration of minocycline. Minocycline was administered continually until tissue collection at the end of the experiment (Figure 1A). In experiment 2 (n = 5–7/group), mice were administered minocycline (delivered in the drinking water) or the water vehicle for one week, and were then injected with a single bolus of ethanol (2g/kg IP of a 25% ethanol solution) or saline vehicle. Blood and brain tissue were collected two hours later for histology and assessment of cytokine expression in isolated microglia (Figure 1B).
Figure 1. Experimental timeline.
A) In experiment 1, minocycline (45mg/kg/day; or water alone) was administered beginning 7 days after TBI or sham injury and continuing until the day of tissue collection. Beginning 10 days after injury, ethanol was presented in a 2-bottle choice test every other day. B) In experiment 2, minocycline (or water control) was administered on days 7–14 after injury. On day 14, mice were treated with a single IP injection of ethanol (2g/kg) or saline control, and tissue (brain and blood) were collected 2 hours later.
Brain injury.
A closed head mild traumatic brain injury was performed on 21 day old mice as previously reported (Karelina et al., 2017a). Briefly, mice were anesthetized via inhaled isofluorane (3% in oxygen for initiation and 1.5% for maintenance) and a sagittal incision was made in the scalp to reveal the skull surface. Once the target was located (−1mm AP and −1mm ML from bregma), isofluorane was briefly (15–20 seconds) withdrawn from the mouse in order to reduce the possibility of injury-induced respiratory arrest. The injury was induced using a 2mm diameter impactor accelerated at 3mm/s to a depth of 1mm below the skull surface and isofluorane was immediately reinstated. Sham mice were exposed to the same procedure in the absence of impact. The skin was sutured and mice returned to their home cages for a one-week recovery period. This injury paradigm consistently produces axonal shearing without frank cell death. None of the animals in this study exhibited skull fracture, hemorrhage, or gross behavioral deficits.
Ethanol consumption.
Ethanol consumption was assessed using a two-bottle choice test with access to ethanol every other day as previously described (Melendez, 2011). Following a one-week recovery period, mice were single housed and water and ethanol (15% v/v) were presented in 50mL conical tubes fitted with a sipper tube. Mice remained singly housed until tissue collection at the end of the experiment. Water was available continuously and ethanol was presented every other day for a 24-hour period. The position of the bottles was switched daily to control for side bias. Body mass, water, and ethanol consumption were recorded every 48 hours for 12 days. A “ghost cage” containing similar water/ethanol bottles but without a mouse was used in order to measure the amount of liquid loss due to cage handling and dripping, consumption data were adjusted to reflect this loss. Among minocycline-treated animals, the drug was available continuously in the water and in ethanol (on the days it was presented) resulting in daily administration throughout the experiment, addition of minocycline to ethanol ensured that mice were not able to avoid drug treatment. Bottles were covered in aluminum foil to protect the light-sensitive minocycline. This minocycline treatment paradigm is preferable to daily i.p. injection or oral gavage due to the known interaction of stress (such as that induced by handling and multiple daily injections) and ethanol consumption (Albrechet-Souza et al., 2015; Hwa et al., 2016).
Sweet and bitter tastant preference were also assessed over 48 hours using the two-bottle choice paradigm in which water consumption was compared to consumption of a 3% sucrose solution or a 0.3mM quinine solution made in water. Half of the mice were also administered minocycline as described above to determine whether oral minocycline affects taste preferences.
Tissue processing and analysis.
Mice were overdosed with sodium pentobarbital (200 mg/kg) and transcardially perfused with 4% paraformaldehyde. Immunofluorescence was conducted on free-floating 40 µm thick slices by incubating tissue in a rat anti-CD68 (marker of activated microglia, BioRad #MCA1957 1:500) and a rabbit anti-Iba1 (microglial marker, Wako #019–19741 1:250) antibody overnight. Fluorescent secondary antibodies (AlexaFluor 488 #A11006 and 555 #A28180: 1:500) were used to visualize microglia. Axon damage was assessed using the NeuroSilver kit (FD Neurotechnologies #PK301) per manufacturer’s instructions.
Microscopy was conducted on a Zeiss Axioscope and images were captured using 20X and 40X objectives. Silver staining was qualitatively assessed as previously reported (Weil et al., 2014). Briefly, white matter damage was observed primarily in the corpus callosum but was also observed in the anterior commissure and the internal capsule, and was scored on a 4-point scale (0 = no axon damage, 3 = axon degeneration throughout multiple white matter tracts).
Co-expression of CD68 and Iba1 was quantified within a 0.04mm2 region of interest in the nucleus accumbens shell, core (approximately 1mm anterior of bregma) and ventral tegmental area (approximately 3mm posterior of bregma). The total number of Iba1-positive microglia were counted in both hemispheres and the percent of Iba1-positive cells that co-expressed CD68 staining were quantified.
Microglial isolation.
Microglia were extracted from the forebrain hemisphere ipsilateral to the injury (left side, between 3.56 mm and −4.16 mm from bregma) 2 hours after a single bolus ethanol injection (experimental time course depicted in Figure 1B). Isolation of microglia was conducted as previously reported via a discontinuous Percoll gradient (Karelina et al., 2017a).
qRT-PCR.
Extracted microglia were lysed and cDNA synthesized using the SuperScript III CellsDirect cDNA synthesis kit (Invitrogen # 11739010 and #18080200) according to the manufacturer’s protocol. Primer and probe sets were purchased from Applied Biosystems (CD11b: Mm00434455_m1, GFAP: Mm01253033_m1, IL1β: Mm00434228_m1) and a TaqMan 18S ribosomal RNA primer/probe set was used as an internal control (Applied Biosystems #4319413E). Amplification on an ABI 7500 Sequencing Fast System was performed using the TaqMan Fast Advanced Master mix (#4444963). Universal RT-PCR cycling conditions were used as previously reported (Karelina et al., 2017a): 50°C for 2 minutes, 95°C for 20 seconds, and 40 cycles of 95°C for 3 seconds and 60°C for 30 seconds. Gene expression is normalized to 18s rRNA and expressed as a relative quantity based on the relative standard curve method.
Statistics.
Statistical analysis was conducted using SPSS version 24 (IBM Corp.). The variables used in this analysis were “surgery” (sham or TBI), “treatment” (vehicle or minocycline), and “injection” (saline or ethanol). Ethanol consumption and preference data were initially analyzed via a 2-way repeated measures ANOVA (surgery X treatment) and followed up as a one-way ANOVA (comparing the four groups) collapsed across time. Sucrose, quinine, and total fluid intake measures were assessed using one-way ANOVA to compare fluid consumption across the groups. Immunofluorescence data were quantified using a three-way ANOVA (injury X treatment X injection). PCR data were assessed using 2-way ANOVA (surgery X treatment). Significant overall ANOVA results were followed up by a Tukey post-hoc analysis. Nonparametric silver staining data were assessed using a Kruskal-Wallis test.
Results
Minocycline reduces alcohol consumption after traumatic brain injury.
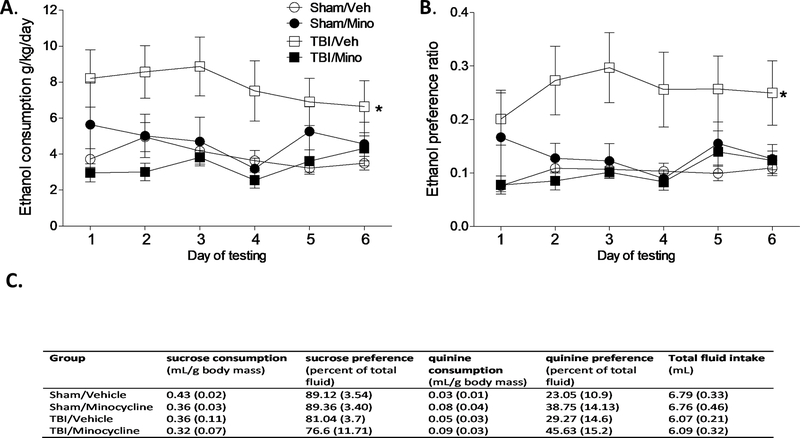
In experiment 1 (Figure 1A), alcohol consumption was assessed in adolescent males that were injured (or sham-injured) as juveniles and were treated with minocycline (or vehicle). A two-way repeated measures ANOVA revealed a significant interaction of treatment and surgery condition (F1,44 = 8.20, p = 0.006) on ethanol consumption and preference (F1,44 = 5.517, p = 0.023). A follow-up nested one-way ANOVA comparing the 4 groups revealed that consistent with previous reports (Mayeux et al., 2015; Weil et al., 2016b), injured mice exhibited both increased consumption (F3,44 = 7.252, p < 0.001), and preference (F3,44 = 5.856, p = 0.002), of ethanol compared to sham-injured mice. Minocycline treatment significantly reduced ethanol consumption and preference in brain injured mice to sham levels (Figure 2A–B; Tukey post-hoc comparisons indicate greater ethanol consumption and preference in the TBI/Veh group compared to the TBI/Mino and Sham/Veh groups, p < 0.05). Importantly, the addition of minocycline to the drinking water does not reduce water intake as mice consumed similar amounts of fluid regardless of drug condition (p> 0.05). Moreover, neither injury status nor the drug treatment significantly affected taste preference for sweet or bitter fluids as measured by sucrose and quinine consumption (Figure 2C; p > 0.05).
Figure 2. Minocycline blocks TBI-induced ethanol consumption and preference.
A) Alcohol consumption and B) preference are significantly increased in mice that underwent a TBI (TBI/Veh) but was reduced to sham levels in TBI mice that were treated with minocycline (TBI/Mino). An asterisk (*) indicates significant difference from the indicated group (p < 0.05). C) There was no effect of surgery or minocycline on sucrose and quinine consumption or total fluid intake. Data are presented as mean ± SEM.
Minocycline attenuates ethanol-induced microglial activation.
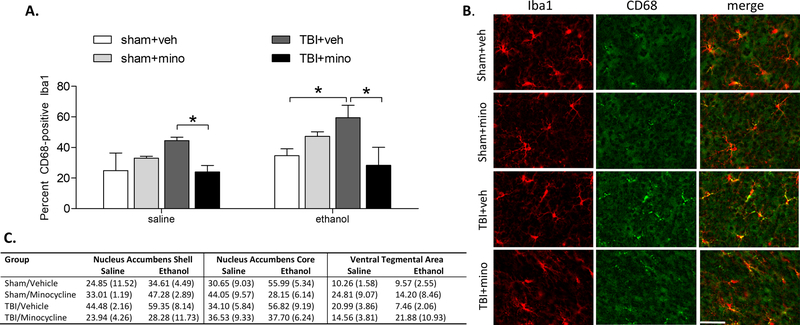
In experiment 2, mice underwent TBI or sham injury and were treated with minocycline (or vehicle control) for 1 week prior to receiving either a single IP injection of ethanol or saline, and brain tissue was collected 2 hours later for histological assessment of microglial activation. Groups did not differ in blood alcohol concentration following the injection (sham/veh 0.22 g/dL; sham/mino 0.19 g/dL; tbi/veh 0.23 g/dL; tbi/mino 0.21 g/dL), indicating that alcohol metabolism was unaltered by brain injury or minocycline treatment. Co-localization of the microglial marker Iba1 with CD68 was quantified in the nucleus accumbens and ventral tegmental area to assess microglial activation in response to ethanol. Ethanol treatment increased microglial activation in the nucleus accumbens shell compared to saline control (F1,29 = 4.151, p = 0.05). There was also a significant surgery by treatment interaction such that microglial activation was significantly attenuated by minocycline in TBI mice (F1,29 = 11.656, p = 0.003). Multiple comparisons revealed a significant reduction in microglial activation by minocycline in both the saline and ethanol injection conditions (Figure 3A–C; all p < 0.05).
Figure 3. Minocycline inhibited nucleus accumbens shell microglial activation in response to ethanol.
A) TBI significantly increased the percent of Iba1-positive microglia in the nucleus accumbens shell that co-express CD68 following a single IP injection of ethanol. Treatment with minocycline significantly reduced the percent of co-localized Iba1/CD68-positive microglia. B) Representative images of microglia in the ipsilateral nucleus accumbens shell following ethanol injection. Iba1 staining is shown in red, CD68 staining is shown in green. Scale bar = 20 µm. C) The percent of Iba1-positive microglia that co-express CD68 in the nucleus accumbens shell, core, and ventral tegmental area are represented as percent (± one standard error).
Microglial analysis in the nucleus accumbens core revealed a treatment by ethanol injection interaction (F1,32 = 8.020, p = 0.009) such that ethanol injection significantly increased microglial activation, but only in vehicle-treated animals (Figure 3C). A multiple comparisons analysis within the ethanol-injected condition did not reveal significant group differences (all p > 0.05). Microglial analysis in the ventral tegmental area did not reveal any significant group differences (all p > 0.05). Finally, it should be noted that microglial activation in the nucleus accumbens is indicative of a global pro-inflammatory response to TBI, as this region is located at a substantial distance away from the point of impact. Thus, these data may not represent a local inflammatory response due to direct mechanical damage, although there is propagation of shear waves through remote CNS tissue following focal TBI (Joldes et al., 2016).
TBI exacerbates, and minocycline attenuates, ethanol-induced cytokine expression in microglia.
An additional cohort in experiment 2 (sham/veh, sham/mino, tbi/veh, tbi/mino – all treated with a bolus injection of ethanol as described above) was used to assess microglial pro-inflammatory cytokine expression 2 hours following ethanol treatment. In order to verify that the microglial isolation process yielded an enriched microglial fraction, we performed qRT-PCR for the microglial marker CD11b (cluster of differentiation molecule 11B) and the astrocyte marker GFAP (glial fibrillary acidic protein). All of the samples expressed high relative quantities of CD11b mRNA and low to undetectable quantities of GFAP mRNA. Further, analysis of the pro-inflammatory cytokine interleukin 1-β (IL-1β) gene expression revealed increased cytokine expression following TBI (F1,19 = 6.55, p = 0.02) and a significant reduction of IL-1β expression following minocycline treatment (F1,19 = 4.52, p = 0.05). A Tukey post-hoc analysis revealed significantly higher Il-1β mRNA gene expression in the TBI-vehicle treated animals compared to sham-vehicle and sham-minocycline treated groups (Table 1).
Table 1. Cytokine gene expression.
Microglial isolation from the forebrain resulted in a population of CD11b-high and GFAP-low expressing cells. Minocycline treatment prevented ethanol-induced IL-1β production in TBI mice. Data are represented as relative gene expression using the housekeeping gene 18S as a standard (± SEM). An asterisk (*) indicates significant difference from the sham/vehicle group.
| Group | CD11b | GFAP | IL-1β |
|---|---|---|---|
| Sham/Vehicle | 37.13 (9.33) | 0.18 (0.05) | 2308.88 (559.66) |
| Sham/Minocycline | 36.39 (6.37) | 0.81 (0.44) | 984.00 (228.76) |
| TBI/Vehicle | 37.42 (9.72) | 1.87 (1.37) | 14133.61 (6476.24)* |
| TBI/Minocycline | 36.10 (9.96) | 0.58 (0.59) | 3522.89 (4769.13) |
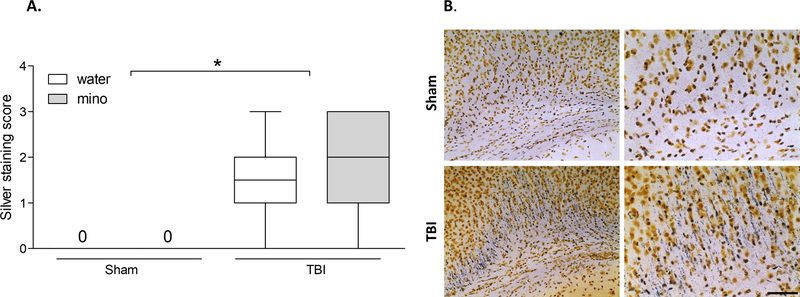
Finally, it is important to note that any significant effects of minocycline on microglial activation in response to ethanol were not mediated by reduced TBI damage. Axon degeneration was induced by TBI (F1,36 = 66.531, p < 0.001) but was not different between vehicle and minocycline treated mice (Figure 4A–B).
Figure 4. Minocycline did not reduce axon degeneration following traumatic brain injury.
A) Silver staining revealed significant axon degeneration throughout forebrain white matter following TBI which was not affected by minocycline treatment at the time point assessed. The bloxplot shows representative range (minimum and maximum), first quartile, median, and third quartile data for each group. B) Representative images of silver staining in the corpus callosum, 20X magnification images are shown on the left, and higher magnification (40X) representation of the same image is on the right; scale bar = 50 µm. An asterisk (*) indicates significant difference between the indicated groups (p < 0.05).
Discussion
A single mild juvenile TBI led to a significant increase in alcohol consumption and preference in adolescent mice. Our data suggest that alcohol preference is mediated by TBI-induced neuroinflammation in the nucleus accumbens shell, as treatment with minocycline significantly reduced ethanol consumption to sham levels. Importantly, because minocycline treatment was administered at a one week delay from injury, all injured mice experienced the acute stages of TBI pathophysiology and exhibited a similar degree of axonal degeneration regardless of drug condition. Therefore, the observed reduction in ethanol consumption was not due to reduced overall damage but appears to be related to minocycline-mediated attenuation of neuroinflammation. These data are consistent with a previous report showing minocycline-mediated reduction of microglial activation without reducing cell death in TBI (Bye et al., 2007).
To our knowledge, this is the first report to directly investigate the relationship between TBI-induced neuroinflammation and the development of increased alcohol preference after injury. However, parallel findings have reported a role for neuroinflammatory mechanisms in driving the rewarding properties of other substances of abuse. For example, opioid self-administration and conditioned place preference are suppressed following pharmacological blockade of TLR4 and in mutant mice with insufficient TLR4 signaling (Hutchinson et al., 2012). Additionally, early life treatment with the anti-inflammatory cytokine IL-10 reduces opioid self-administration in adulthood (Lacagnina et al., 2017). Further, alcohol increases liver and brain expression of pro-inflammatory cytokines (Fernandez-Lizarbe et al., 2009; Gao and Bataller, 2011) and the induction of cytokine production and release promotes voluntary alcohol intake (Blednov et al., 2011).
As a first line of defense against injury and infection, microglia are rapidly sensitized once activated and exhibit an exaggerated response to additional stimuli (Frank et al., 2011). Traumatic brain injury is a potent stimulus that primes microglia so that additional stress stimuli occurring after the TBI (even long after overt symptoms have resolved) produce an exaggerated immune response and corresponding neurobehavioral symptoms (Collins-Praino et al., 2017; Fenn et al., 2014; Witcher et al., 2015). Moreover, we recently reported that a single mild TBI potentiates the microglial response to binge-like levels of alcohol (Karelina et al., 2017a). This finding was consistent with the results of the current study, such that a single injection of ethanol increased microglial activation (CD68 expression) in response to ethanol in TBI mice. Importantly, this dose of ethanol did not induce microglial activation in sham mice. These data suggest that neuroinflammatory events following the TBI led to microglial priming such that a single dose of ethanol was sufficient to induce significant microglial activation. The time course between the TBI and ethanol injection was relatively brief (14 days) and it remains unknown how long microglia would remain primed to respond to ethanol after TBI. However, reactive microglia are a part of the chronic TBI pathology and can persist for many years after TBI (Johnson et al., 2013), thus the possibility remains that brain injured patients remain vulnerable to the neuroinflammatory effects of ethanol for many years after the initial injury.
Taken together, our data suggest that increased drinking after TBI is mediated by injury-induced neuroinflammation, and that the proinflammatory response to ethanol may be mediated by microglial production of IL-1β. Interleukin-1β is rapidly upregulated following brain injury, and although IL-1β promotes growth and trophic factor expression as a means of promoting neuronal survival following injury, it is also a potent activator of astrocytes and stimulates the production of additional pro-inflammatory cytokines as well as reactive oxygen species (For review see Allan et al., 2005). Attenuation of IL-1 activity, through administration of the IL-1 receptor antagonist or neutralizing antibodies to IL-1, significantly reduces neuronal damage and improves functional outcome in brain injury models (Clausen et al., 2009; Jones et al., 2005). Importantly, microglial upregulation of IL-1 is a key neuroimmune component that mediates alcohol dependence (Mayfield et al., 2013; Saiz et al., 2009) and chronic alcohol increases IL-1β in mice and humans (Blanco et al., 2005; Lippai et al., 2013). Thus early-life brain injuries may set up a vicious cycle such that the TBI both directly induces inflammation and exacerbates the subsequent inflammatory response to alcohol which lead to an increase in drinking behavior.
Although not directly assessed here, it is possible that TBI-induced neuroinflammation affects alcohol use by altering dopaminergic tone in the brain’s reward circuitry. Systemic injection of LPS reduces the basal firing rate of dopamine neurons in mice (Blednov et al., 2011), a pattern that is consistent with increased ethanol preference and consumption (George et al., 1995). Moreover, we previously reported that TBI induces a persistent and long-lasting state of hypodopaminergia that is associated with altered Edinger-Westphal activation in response to ethanol (Karelina et al., 2017b). Therefore, the neuroinflammatory response to TBI may affect ethanol consumption via a mechanism involving reduced dopaminergic activity. Additionally, minocycline attenuates methamphetamine and cocaine-induced behavioral sensitization through a mechanism that involves altered striatal dopamine release (Chen et al., 2009; Zhang et al., 2006). Given the reduction in ethanol consumption concurrent with minocycline treatment in the current study, it is possible that inhibiting TBI-induced inflammation also affected dopamine signaling in the reward circuitry, thus altering the rewarding properties of ethanol.
The mechanisms of action of minocycline involve modulation of microglial proliferation and activation, shifting the immune response from M1 (proinflammatory microglial state) to M2 (anti-inflammatory microglial state). Specifically, minocycline inhibits microglial production of the proinflammatory cytokines IL1β and TNFα and increases the production of the anti-inflammatory IL-10 (Kobayashi et al., 2013; Stirling et al., 2005). Minocycline further reduces microglial migration to the site of injury by altering chemoattractant protein expression (Kremlev et al., 2004). Used frequently as a neuroprotective agent, systemic treatment with minocycline improves motor function and attenuates cognitive decline in experimental TBI models (Bye et al., 2007; Sanchez Mejia et al., 2001; Siopi et al., 2012). However, although minocycline has been broadly utilized as a brain-penetrant anti-inflammatory compound and has, in the past, been considered a selective microglial activation inhibitor it is now necessary to consider several potential complications with the use of this tool (Möller et al., 2016). First, minocycline is a semi-synthetic tetracycline that has broad-spectrum antibiotic activity and thus, when administered, systemically has the potential to modulate commensal bacterial populations including the intestinal microbiota (Redin, 1966; Vaughn et al., 2017). Critically, there is mounting evidence that microbial populations can significantly alter microglial physiology, host defense, neurochemistry and behavior (Diaz Heijtz et al., 2011). Thus, it is not possible to definitively rule out that the changes in microglial physiology and alcohol self-administration are secondary to adjustments in microbiota populations. This is a critical question for future studies because both alcohol abuse and traumatic brain injuries can significantly alter intestinal permeability and the resident microbial communities (Bansal et al., 2009; Peterson et al., 2017), thus it remains possible that the neuroinflammatory and behavioral adjustments induced by minocycline are secondary to changes in the microbiota. Moreover, since there are effects of minocycline both in culture and when administered directly into CNS it is also possible that both direct and microbiota-mediated effects are at play (Erny et al., 2017; Yong et al., 2004). Additionally, there is evidence that minocycline has direct anti-inflammatory effects on other peripheral and central immune cell populations (Möller et al., 2016). Thus, we cannot say definitively that microglial modulation mediates our findings. However, we can say that minocycline both inhibited microglial activation and cytokine expression and prevented the increase in alcohol-drinking behavior.
Finally, it is worth noting that that while TBI mice increased ethanol consumption, consumption of another rewarding tastant (sucrose in experiment 1) was not significantly altered by either brain injury or treatment. Although the purpose of administering sucrose in the water was to confirm that addition of minocycline would not interfere with preference for a highly palatable substance, the observed outcome also serves to highlight that TBI may selectively increase preference for alcohol, rather than generally increase reward-seeking behavior. That minocycline reduced ethanol, but not sucrose, preference further strengthens the possibility that inflammatory events specifically drive the motivation to consume ethanol after brain injury.
Given that minocycline treatment after TBI suppressed both the voluntary consumption of, and neuroinflammatory response to, alcohol, there is strong support for the possibility that the vulnerability of pediatric TBI survivors to subsequent alcohol use disorders reflects an alteration in central inflammatory signaling and suggests microglia and related neuroimmune effectors as potential therapeutic targets in this population.
Acknowledgements
The authors are grateful to undergraduate research assistants Joseph Abraham, McKenna Guilds and Lauren Martin for their patience and hard work.
This work was supported by an OSU Neurological Institute Pilot Award, the Huron Foundation, and the National Institutes of Health (NINDS NS045758). The funding sources had no involvement in the conduct of the research or preparation of the article.
Footnotes
Declaration of Interest
The authors have no financial or personal conflicts of interest to declare.
References
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE, 2011. Minocycline reduces ethanol drinking. Brain Behav Immun 25 Suppl 1, S165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechet-Souza L, Hwa LS, Han X, Zhang EY, DeBold JF, Miczek KA, 2015. Corticotropin Releasing Factor Binding Protein and CRF2 Receptors in the Ventral Tegmental Area: Modulation of Ethanol Binge Drinking in C57BL/6J Mice. Alcohol Clin Exp Res 39, 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ, 2005. Interleukin-1 and neuronal injury. Nat Rev Immunol 5, 629–640. [DOI] [PubMed] [Google Scholar]
- Bansal V, Costantini T, Kroll L, Peterson C, Loomis W, Eliceiri B, Baird A, Wolf P, Coimbra R, 2009. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J Neurotrauma 26, 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Vallés SL, Pascual M, Guerri C, 2005. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol 175, 6893–6899. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA, 2011. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25 Suppl 1, S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA, 2005. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res 165, 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA, 2012. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol 17, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardier CH, Rimmele CT, Zintel H, 2002. The magnitude and correlates of alcohol and drug use before traumatic brain injury. Arch Phys Med Rehabil 83, 1765–1773. [DOI] [PubMed] [Google Scholar]
- Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, Morganti-Kossmann MC, 2007. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol 204, 220–233. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ, 2002. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Chen H, Uz T, Manev H, 2009. Minocycline affects cocaine sensitization in mice. Neurosci Lett 452, 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen F, Hånell A, Björk M, Hillered L, Mir AK, Gram H, Marklund N, 2009. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 30, 385–396. [DOI] [PubMed] [Google Scholar]
- Collins-Praino LE, Arulsamy A, Katharesan V, Corrigan F, 2017. The effect of an acute systemic inflammatory insult on the chronic effects of a single mild traumatic brain injury. Behav Brain Res [DOI] [PubMed]
- Corrigan JD, 1995. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil 76, 302–309. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Bogner J, Mellick D, Bushnik T, Dams-O’Connor K, Hammond FM, Hart T, Kolakowsky-Hayner S, 2013. Prior history of traumatic brain injury among persons in the Traumatic Brain Injury Model Systems National Database. Arch Phys Med Rehabil 94, 1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L, 2011. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25 Suppl 1, S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Mohapatra S, Mohapatra SS, 2012. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J Neuroinflammation 9, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S, 2011. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108, 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP, 2005. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res 29, 1796–1808. [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Prinz M, 2017. Communicating systems in the body: how microbiota and microglia cooperate. Immunology 150, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP, 2014. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry 76, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C, 2009. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol 183, 4733–4744. [DOI] [PubMed] [Google Scholar]
- Fishbein D, Dariotis JK, Ferguson PL, Pickelsimer EE, 2016. Relationships Between Traumatic Brain Injury and Illicit Drug Use and Their Association With Aggression in Inmates. Int J Offender Ther Comp Criminol 60, 575–597. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF, 2011. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun 25 Suppl 1, S21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Bataller R, 2011. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Ng GY, Jung SY, O’Dowd BF, Naranjo CA, 1995. Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: reversal by increasing synaptic dopamine. J Pharmacol Exp Ther 273, 373–379. [PubMed] [Google Scholar]
- Giunta B, Obregon D, Velisetty R, Sanberg PR, Borlongan CV, Tan J, 2012. The immunology of traumatic brain injury: a prime target for Alzheimer’s disease prevention. J Neuroinflammation 9, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DP, Cardon AL, 2008. An update on substance use and treatment following traumatic brain injury. Ann N Y Acad Sci 1141, 148–162. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT, 2008. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol 210, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV, 2013. Microglia activation as a biomarker for traumatic brain injury. Front Neurol 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR, 2012. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32, 11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA, 2016. Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology (Berl) 233, 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilie G, Mann RE, Hamilton H, Adlaf EM, Boak A, Asbridge M, Rehm J, Cusimano MD, 2015. Substance Use and Related Harms Among Adolescents With and Without Traumatic Brain Injury. J Head Trauma Rehabil 30, 293–301. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W, 2013. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joldes GR, Lanzara AL, Wittek A, Doyle B, Miller K, 2016. Traumatic brain injury: an investigation into shear waves interference effects. In: Joldes GR, Doyle B, Wittek A, Nielsen P, Miller K (Eds.), Computational biomechanics for medicine Springer International Publishing Switzerland, Switzerland, pp. 177–186. [Google Scholar]
- Jones NC, Prior MJ, Burden-Teh E, Marsden CA, Morris PG, Murphy S, 2005. Antagonism of the interleukin-1 receptor following traumatic brain injury in the mouse reduces the number of nitric oxide synthase-2-positive cells and improves anatomical and functional outcomes. Eur J Neurosci 22, 72–78. [DOI] [PubMed] [Google Scholar]
- Karelina K, Gaier KR, Prabhu M, Wenger V, Corrigan TE, Weil ZM, 2017a. Binge ethanol in adulthood exacerbates negative outcomes following juvenile traumatic brain injury. Brain Behav Immun 60, 304–311. [DOI] [PubMed] [Google Scholar]
- Karelina K, Gaier KR, Weil ZM, 2017b. Traumatic brain injuries during development disrupt dopaminergic signaling. Exp Neurol 297, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitz M, Martin-Soelch C, Leenders KL, 2003. Reward processing in the brain: a prerequisite for movement preparation? Neural Plast 10, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, 2004. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27, 765–776. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K, 2013. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4, e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremlev SG, Roberts RL, Palmer C, 2004. Differential expression of chemokines and chemokine receptors during microglial activation and inhibition. J Neuroimmunol 149, 1–9. [DOI] [PubMed] [Google Scholar]
- Kumar A, Loane DJ, 2012. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun 26, 1191–1201. [DOI] [PubMed] [Google Scholar]
- Lacagnina MJ, Kopec AM, Cox SS, Hanamsagar R, Wells C, Slade S, Grace PM, Watkins LR, Levin ED, Bilbo SD, 2017. Opioid Self-Administration is Attenuated by Early-Life Experience and Gene Therapy for Anti-Inflammatory IL-10 in the Nucleus Accumbens of Male Rats. Neuropsychopharmacology [DOI] [PMC free article] [PubMed]
- Lim YW, Meyer NP, Shah AS, Budde MD, Stemper BD, Olsen CM, 2015. Voluntary Alcohol Intake following Blast Exposure in a Rat Model of Mild Traumatic Brain Injury. PLoS One 10, e0125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G, 2013. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol 94, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A, 2016. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol 275 Pt 3, 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AI, Stocchetti N, Bullock R, 2008. Moderate and severe traumatic brain injury in adults. Lancet Neurol 7, 728–741. [DOI] [PubMed] [Google Scholar]
- Mayeux JP, Teng SX, Katz PS, Gilpin NW, Molina PE, 2015. Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behav Brain Res 279, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, Harris RA, 2013. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol 23, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A, Corrigan J, Horwood LJ, Fergusson DM, 2014. Substance abuse and criminal activities following traumatic brain injury in childhood, adolescence, and early adulthood. J Head Trauma Rehabil 29, 498–506. [DOI] [PubMed] [Google Scholar]
- Melendez RI, 2011. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res 35, 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, Eder C, Gan L, Garden GA, Hughes ZA, Pearse DD, Staal RG, Sayed FA, Wes PD, Boddeke HW, 2016. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 64, 1788–1794. [DOI] [PubMed] [Google Scholar]
- Opreanu RC, Kuhn D, Basson MD, 2010. Influence of alcohol on mortality in traumatic brain injury. J Am Coll Surg 210, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Alfonso-Loeches S, Aragón CM, Guerri C, 2011. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun 25 Suppl 1, S80–91. [DOI] [PubMed] [Google Scholar]
- Peterson VL, Jury NJ, Cabrera-Rubio R, Draper LA, Crispie F, Cotter PD, Dinan TG, Holmes A, Cryan JF, 2017. Drunk bugs: Chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav Brain Res 323, 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redin GS, 1966. Antibacterial activity in mice of minocycline, a new tetracycline. Antimicrob Agents Chemother (Bethesda) 6, 371–376. [PubMed] [Google Scholar]
- Saiz PA, Garcia-Portilla MP, Florez G, Corcoran P, Arango C, Morales B, Leza JC, Alvarez S, Díaz EM, Alvarez V, Coto E, Nogueiras L, Bobes J, 2009. Polymorphisms of the IL-1 gene complex are associated with alcohol dependence in Spanish Caucasians: data from an association study. Alcohol Clin Exp Res 33, 2147–2153. [DOI] [PubMed] [Google Scholar]
- Sajja VS, Galloway M, Ghoddoussi F, Kepsel A, VandeVord P, 2013. Effects of blast-induced neurotrauma on the nucleus accumbens. J Neurosci Res 91, 593–601. [DOI] [PubMed] [Google Scholar]
- Sanchez Mejia RO, Ona VO, Li M, Friedlander RM, 2001. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 48, 1393–1399; discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
- Shah S, Yallampalli R, Merkley TL, McCauley SR, Bigler ED, Macleod M, Chu Z, Li X, Troyanskaya M, Hunter JV, Levin HS, Wilde EA, 2012. Diffusion tensor imaging and volumetric analysis of the ventral striatum in adults with traumatic brain injury. Brain Inj 26, 201–210. [DOI] [PubMed] [Google Scholar]
- Shin SS, Bales JW, Yan HQ, Kline AE, Wagner AK, Lyons-Weiler J, Dixon CE, 2013. The effect of environmental enrichment on substantia nigra gene expression after traumatic brain injury in rats. J Neurotrauma 30, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siopi E, Llufriu-Dabén G, Fanucchi F, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M, 2012. Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: the effect of minocycline. Neurosci Lett 511, 110–115. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W, 2005. Minocycline as a neuroprotective agent. Neuroscientist 11, 308–322. [DOI] [PubMed] [Google Scholar]
- Vaaramo K, Puljula J, Tetri S, Juvela S, Hillbom M, 2014. Head trauma sustained under the influence of alcohol is a predictor for future traumatic brain injury: a long-term follow-up study. Eur J Neurol 21, 293–298. [DOI] [PubMed] [Google Scholar]
- Vaughn AC, Cooper EM, DiLorenzo PM, O’Loughlin LJ, Konkel ME, Peters JH, Hajnal A, Sen T, Lee SH, de La Serre CB, Czaja K, 2017. Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol Exp (Wars) 77, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L, 2009. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol 44, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KR, Tesco G, 2013. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci 5, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Corrigan JD, Karelina K, 2016a. Alcohol abuse after traumatic brain injury: Experimental and clinical evidence. Neurosci Biobehav Rev 62, 89–99. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Gaier KR, Karelina K, 2014. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol Dis 70, 108–116. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Karelina K, 2017. Traumatic Brain Injuries during Development: Implications for Alcohol Abuse. Front Behav Neurosci 11, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Karelina K, Gaier KR, Corrigan TE, Corrigan JD, 2016b. Juvenile Traumatic Brain Injury Increases Alcohol Consumption and Reward in Female Mice. J Neurotrauma 33, 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C, Engelhard K, 2007. Pathophysiology of traumatic brain injury. Br J Anaesth 99, 4–9. [DOI] [PubMed] [Google Scholar]
- Winqvist S, Jokelainen J, Luukinen H, Hillbom M, 2007. Parental alcohol misuse is a powerful predictor for the risk of traumatic brain injury in childhood. Brain Inj 21, 1079–1085. [DOI] [PubMed] [Google Scholar]
- Witcher KG, Eiferman DS, Godbout JP, 2015. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci 38, 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA, 2008. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM, 2004. The promise of minocycline in neurology. Lancet Neurol 3, 744–751. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Smith DH, Blennow K, 2013. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 9, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K, 2006. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry 30, 1381–1393. [DOI] [PubMed] [Google Scholar]