Abstract
Background:
The application of three-dimensional (3D) visualization techniques to evaluate the earliest visible onset of abnormal retinal vascular development in preterm infants with retinopathy of prematurity (ROP), using bedside non-contact optical coherence tomography (OCT) imaging to characterize morphology and sequential structural changes of abnormal extraretinal neovascularization.
Methods:
Thirty-one preterm infants undergoing routine ROP screening with written informed consent for research imaging were enrolled in this prospective observational study. We imaged the macula and temporal periphery of preterm infants using a handheld OCT system (Envisu 2300 or handheld swept-source research system). The scans obtained were segmented and using enhanced ray casting were converted to 3D volumes to which color filter were applied.
Results:
Using colorized 3D visualization, we defined extraretinal neovascular structures as buds, bridging networks and placoid lesions. We could longitudinally follow progression and regression of extraretinal neovascularization in stage 3 ROP after treatment in one infant over 12 weeks and document the appearance of early buds, and formation of florid neovascularization. From stage 2 to 3 ROP, we observed progression from sessile buds to a complex plaque that corresponded to stage 3 ROP on clinical examination. We demonstrated regression of neovascular complexes to small pre-retinal tufts after treatment with anti-VEGF.
Conclusions:
The extension of OCT processing to include surface flattening and colorization that further improved structural analysis rendered better understanding of extraretinal tissue. Our ability to image similar areas in the same infant over multiple visits enabled us to study the evolution of these structural components and follow pathological vascular events longitudinally in development and regression after treatment. These methods can be applied to further study which are likely contribute to our understanding of the pathophysiology of neovascularization in ROP.
Keywords: oct, optical coherence tomography, 3D, visualization, ROP, retinopathy of prematurity
Introduction:
Retinopathy of prematurity (ROP) is a leading cause of blindness in preterm infants.[1] The vision loss associated with ROP is often a result of anomalous extraretinal angiogenesis, or neovascularization, in stage 3 ROP that leads to progressive vitreoretinal fibrovascular traction forces culminating in tractional retinal detachment.[2] A better understanding of the sequential structural retinal changes that lead to abnormal retinal and extraretinal neovascular development may provide opportunities for therapeutic interventions and identify surrogate early markers of severe ROP.
The course of human retinal vascular development has been studied for about a century.[3] Most studies focused on retinal vascular development are in animal models,[4–7] although there have also been histological studies in post-mortem infant eyes with and without ROP.[8–11] The advent of optical coherence tomography (OCT) has allowed for visualization of the retinal micro-anatomical cross-section in vivo in real time.[12] The use of bedside, non-contact, handheld, spectral domain (SD) OCT to image infants has increased our understanding of retinal development and maturation in infants.[13] Prior studies have used SDOCT to assess disease severity and the presence of plus disease in ROP;[14] however, SDOCT studies have rarely considered three-dimensional (3D) visualization of normal and pathologic tissue relationships at sites of extraretinal neovascular development.[15]
We propose that 3D structural analysis of neovascular processes will provide valuable information regarding complex relationships among the onset, development and regression of neovascularization. Our group has previously described enhanced 3D rendering of OCT data with high performance volumetric filtering, edge and feature enhancement, depth-based shading, and lighting.[16] While this tool was designed to improve 3D visualization of microanatomy in ocular surgery, it is also useful in viewing OCT volumes captured at the bedside, such as from an infant with ROP.[16] With this image processing, we are able to change our viewpoint (i.e., tilt and rotate the volume) and appreciate 3D extraretinal angiogenic structures relative to the retinal surface and surrounding tissues.[16] In this study we apply these 3D visualization techniques to evaluate the earliest visible onset of abnormal retinal vascular development leading to extraretinal neovascularization or later retinal detachment in preterm infants with ROP, using bedside non-contact OCT imaging. From this analysis, we characterize the morphology and sequential structural changes of abnormal extraretinal neovascularization in premature infants by 3D OCT.
Methods:
In this study, we describe extraretinal neovascularization in preterm infant retinas within a prospective OCT retinal imaging cohort of preterm infants. This study was conducted under two study protocols and was approved by the Duke University Medical Center institutional review board and adhered to the tenets of the Declaration of Helsinki. Infants enrolled in these study protocols had written informed consent from a parent or legal guardian to participate in research imaging and allow access to medical records. We imaged the infants during routine weekly screening examinations for ROP, which included dilated fundus examination using indirect ophthalmoscopy. We gathered demographic and clinical information such as gender, race, birth weight, gestational age and maximum ROP severity from the medical records.
We performed research imaging on the same day as the clinical examination using the hand-held SDOCT imaging system (Envisu 2300, Leica Microsystems, Research Triangle Park, North Carolina, USA) optimized for preterm infant use in the Intensive Care Nursery (ICN) and as per an age-specific protocol published by Maldonado et al.[13] After July, 2016, the research protocol (NCT02887157, clincaltrials.gov) allowed the use of an investigational swept source (SS)OCT system with a MEMS-scanner-based ultracompact hand piece with imaging centered at 1047nm wavelength. The hand piece was lighter and smaller than the commercially available system. Bioptigen (SDOCT) imaging was typically performed with 630–730 A-scans per B-scan and 63–73 B-scans per volume at 2 seconds per volume while the investigational device (SSOCT) allowed 512 A-scans per B-scan and 112 B-scans per volume at <1 second per volume.
We reviewed SDOCT images from all preterm infants with a clinical diagnosis of ROP from stage 0 to stage 5 with or without plus disease. Of the 106 infants in the prospective retinal imaging study, 91 infants were enrolled for ROP screening and we began imaging of the vascular–avascular junction at the 60th infant. Of the 91 infants, 31 eyes had stage 0 ROP, 21 eyes had stage 1, 61 eyes had stage 2, 51 eyes had stage 3 and 18 eyes had retinal detachment (stage 4, 4B, 5) as the highest stage of ROP assessed by an expert clinical examiner. We reviewed macular volumes in all 91 infants and the peripheral volumes in 14/41 infants in whom we captured acceptable quality volumes from the temporal periphery at multiple visits. 30/91 infants had adequate macular and/or peripheral scan quality to be studied in 3D. We also included one infant with stage 3 ROP imaged on the SSOCT system, so that a total of 31 infants with macular and/or peripheral scans are presented in this study. We excluded visits in which we were unable to capture adequate quality images in infants with advanced stages of ROP (4A, 4B and 5) requiring surgical interventions.
Image Processing and Analysis
We chose OCT volumes with good B-scan quality and minimal motion artifact between B scans and applied enhanced ray casting and volumetric filtering to generate 3D visualization of the scan. [ 16] The 3D volume could be manipulated by the viewer to allow for multiple viewing angles. We used these 3D volumes with corresponding B-scans and the en face view to examine and analyze the ROP findings. The location of the volume was determined using the en face retinal view as well as the B-scan images. (Supplementary Figure 1).
Each volume was automatically segmented using Duke OCT Retinal Analysis Program (DOCTRAP, Duke University). We isolated and manually corrected the retinal pigment epithelium (RPE) segmentation for each B-scan. The corrected RPE position for each A-scan was used to shift the A-scan linearly to achieve a common baseline. This technique flattens the RPE across the volume to allow for improved visualization of retinal surface features while preserving measurements along the A-scan dimension. The 3D volumes were re-rendered using the flattened data. Color gradients were applied; red superiorly, yellow medially and blue inferiorly with positions of these colors adjusted to differentiate and emphasize surface features.[17]
Longitudinal analyses:
In 31 infants, we had multiple visits available for the fovea and optic nerve regions. In 13/31 infants, we had at least one follow-up visit with imaging of the temporal periphery and, of these, 3 infants were re-imaged at a similar location as the previous visit. Because the handheld OCT system could not automatically locate the same area of imaging during follow-up, we manually used retinal vascular patterns to match retinal regions.
Results:
Fifty-five eyes from 31 preterm infants, imaged between 31 weeks and 48 weeks post menstrual age (PMA), were included in this study. All infants had a wince to light in each eye at the time of each examination. Some of these infants had more than one visit from which the OCT volumes were analyzed. From indirect ophthalmoscopy, these infants had clinically determined ROP of Stages 0, 1, 2, 3 or Stage 3 regressed on the day of the OCT imaging sessions. Macular edema of prematurity was also present on macular OCT imaging in 21 of the 31 infants (42 eyes) and often on multiple exam days: in 21 eyes (24 visits) before treatment and 14 eyes (40 visits) after treatment. Macular edema was found in eyes with all ROP Stages 0, 1, 2, 3 and regressed 3.
We observed multiple morphologies of neovascular elevations, and various stages of neovascularization both around the posterior pole and the vascular-avascular (V-AV) junction in 20 eyes of 11 patients. These findings were present before and/or after ROP treatment on multiple exam days. The clinical ROP stages for these 20 eyes ranged from Stage 2 to Stage 3 in zone I and II.
Extraretinal Neovascularization
Extraretinal neovascularization was defined as neovascular tissue, perfused, and regressing vessels extending above the inner retinal surface on 3D OCT. We categorized these pathologies as buds, bridging networks, placoid neovascularization, regressed neovascularization and retinal vessel elevation. These terms were defined by their characteristic appearances and with attempts to align OCT features with descriptions from Foos[8] (Table 1). Extraretinal neovascular features were found in eyes clinically determined to be stage 2 ROP or worse.
Table 1:
Histopathological, optical coherence tomography and clinical appearance definitions of terms used to describe extraretinal neovascularization in retinopathy of prematurity.
| Term | Histopathology Foos description | SDOCT Definition | Clinical Appearance |
|---|---|---|---|
| Extraretinal Neovascularization | Extraretinal vascularization (Foos 1987) | Cells, vessels, perfused and regressing vessels in ROP extending above the inner retinal surface in ROP. | |
| Sessile buds | Uncommon and occur as isolated sessile mounds on the retinal surface behind, but usually in the same quadrant as the ridge. Also called polypoidal. (Foos 1987) | Dome shaped, knob-like growth, extending above the surface of the vascularized retina and into the vitreous cavity. These may be isolated or in clusters, visible as posterior as at the margin of the optic nerve head and as peripheral as just posterior to the ridge. | No distinguishing features from non neovascular ROP |
| Pedunculated buds | Less common and arise on thin delicate stalks from the retina behind the ridge*. | These buds appear to arise on a thin delicate stalk or are constricted at the lower end close to the retinal surface (pinching in). They arise from the retinal surface and may extend high into the vitreous. These commonly occurred posterior to a bridging network. | Popcorn |
| Bridging networks | Pedunculated forms which resemble “frond, palm tree or sea fan” and “congeries [jumble] of delicate vessels”. | Bridging of pre-retinal tissue with a network-like appearance. between two buds or large networks with multiple “stems” from the retina. These appear to arise from closely packed pedunculated and/or polypoid buds. There may be individual buds posterior to the network. | NV Stage 3 |
| Placoid | Occurs in the region of the ridge* and is by far the most common and, by virtue of its relationship to the early stages of retinal detachment is the most important type. May also include “nonvascular proliferative extraretinopathy” (Foos 1987) | These arise around the ridge, and have a more circumferential orientation due to multiple buds coalescing to form an elevated neovascular complex which may be a thickened broad sheet. | NV Stage 3 |
| Regressed Extraretinal Neovascularization | Not described in detail | Vessels and buds are thin, irregular and attenuated. With regression, the tissue may shift to be closer to the retinal surface, or alternately may extend high into the vitreous and out of range of OCT imaging the retina. | Regression |
| Vessel elevation | Not described | Retinal vessel(s) posterior to extraretinal neovascularization may appear to partially bulge upward across the retinal surface (yet remain intra-retinal) on 3D producing linear and web-like patterns of surface elevation. This can occur across any part of the retinal vasculature from the optic nerve head to the V- AV junction. On cross-sectional scans these give a spiked pointy appearance as opposed to a normal smooth retina surface. | Stage 3, or Plus, or Pre-plus** |
Foos described sessile (polypoidal), pedunculated and placoid types of extraretinal vascularization and their relationship to the thickened Stage 2 ridge at the vascular-avascular j unction.
when the vascular changes appeared in posterior vessels, the clinicians described plus or preplus, however the more peripheral changes were not necessarily associated with preplus or plus disease.
Buds
Based on appearance, we described buds as either sessile or pedunculated (Figure 1). Sessile buds were visible as isolated, dome-shaped, smooth, and rounded elevations that appeared to directly arise from the retinal surface and extend into the vitreous. These buds were not immediately adjacent to one another and were greater than 100–200μm apart. The buds were located over moderate-sized retinal vessels on the retinal surface posterior to the V-AV junction. They could be found from the optic nerve head to the V-AV junction, although they were often found closer to the fovea than to the vascular-avascular junction.
Figure 1:

Colorized three-dimensional (3D) and B-scan visualization of different types of buds (red arrows) found in stage 2 and above retinopathy of prematurity. a, b show dome-shaped, rounded sessile buds arising directly from the retinal surface and extending into the vitreous cavity. c, d show pedunculated buds that appear to arise on a thin delicate stalk at the lower end close to the retinal surface.
Pedunculated buds were visible as extraretinal tissue that appeared to arise on a thin delicate stalk or to be constricted (pinched in) at the lower end of the structure close to the retinal surface. These buds arose from the retinal surface and extended about 20–400μm into the vitreous. They were found more peripherally in the region posterior to the V-AV junction, and could be seen isolated or grouped directly over the vessel. These were visualized posterior to stage 3 neovascular networks both before and after treatment. We visualized sessile or pedunculated buds in: 4 eyes with stage 2 ROP, 5 eyes with stage 3 ROP before treatment and in 20 eyes after treatment (includes multiple exam visits from infants) for stage 3 ROP.
Bridging network
Bridging networks were classified as connections between two or more buds with multiple stems connected to the retina (Figure 2), creating a network-like appearance of pre-retinal tissue. The OCT cross sectional views of the retina surrounding these sites showed no distinct changes in choroid, inner or outer retina adjacent to these extraretinal neovascular sites except for the grossly increased elevation of retinal vessels causing a slight upward surface bulge in the retina. The bridging networks were observed in five eyes with stage 3 ROP prior to treatment and regressed as described below. These structures appeared consistent with early neovascular networks.
Figure 2:

En face view (a), three-dimensional (3D) volume (b), B-scan (c) and colorized 3D volume (d): of the neovascularization and avascular retina at the vascular-avascular junction. The 3D volume shows vascular elevations along dilated retinal blood vessels (red arrows) and bridging into extraretinal neovascular networks. The B-scans demonstrate avascular retina (dotted red line) and the vascular elevations (blue line) indicated on the en face and 3D view. Colorized 3D volume demonstrates the elevation of extraretinal tissue from the retinal surface with red indicating maximum elevation and blue indicating the retinal surface.
Placoid lesions
Placoid lesions were visible as elevated webs of extraretinal neovascular tissue arising in a ridge and comprised of coalescing buds and bridging networks (Figure 3 a,b,). These lesions had a circumferential orientation at the retinal periphery and had multiple focal or broad attachments to the underlying retina. We observed intravitreal traction on these lesions resulting in upward deformation of the neovascular tissue and tenting of the retinal surface. The OCT cross-section demonstrated inner retinal hypo-reflective slits extending both anterior and posterior to the junction in the region abutting to the ganglion cell layer. Placoid lesions were observed in six eyes with advanced stage 3 ROP.
Figure 3:
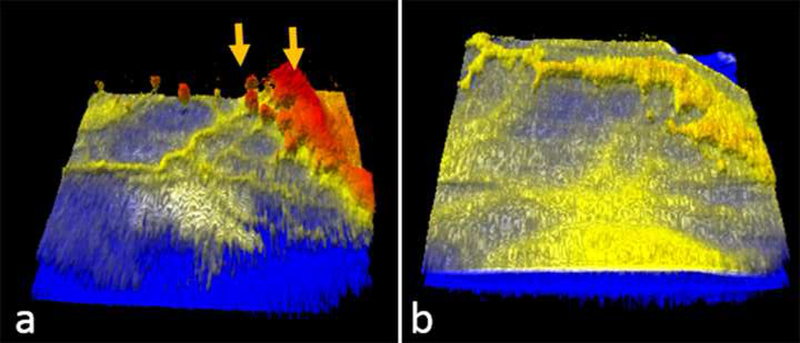
Representative optical coherence tomography colorized three-dimensional (3D) volumes of a preterm infant with zone I stage 3 retinopathy of prematurity, prior to (a) and three weeks after (b) intravitreal bevacizumab injection. The yellow arrows in a indicating extraretinal neovascular tissue is seen regressing three weeks later.
Regressed neovascularization
In eyes imaged after laser or anti-vascular endothelial growth factor (VEGF) injection, vessels and buds were found to be thin, irregular, and attenuated. They were often closer to the retinal surface and did not extend high into the vitreous (Figure 3). On 3D OCT, regression of 3D structures and reduced elevation were evident. Of the 6 eyes with treatment and regression of stage 3 ROP, we were able to image similar locations pre-and post-treatment in 3 eyes. In one eye, the thick extraretinal neovascular complex diminished to a thin extraretinal lesion without evidence of tractional elevation or distortion of the retinal surface, three weeks after a single anti-VEGF injection. In another eye, a similar finding was observed one week after anti-VEGF treatment. In the third eye, there was progressive traction that developed into stage 4B ROP with vitreous hemorrhage over the macula after two bevacizumab injections and laser treatment. Pronounced traction radiating into the vitreous from the neovascularization was visible in that eye a week prior to progression to stage 4B ROP with vitreous hemorrhage.
Vessel elevation
Retinal vessels appeared to bulge upward across the retinal surface (yet remain intra-retinal), producing curvilinear and web-like patterns of surface elevations visible in 3D. This was observed both at the posterior pole and in the periphery. On cross-sectional scans, these vessels appear to be spiked in contrast to a normal smooth retina surface. In some eyes with stage 3 ROP, retinal vessels in the posterior pole and in the region of extraretinal neovascularization were prominent and bulged vitread across the retinal surface in a branching pattern. This pattern was never definitively identified in eyes with stage 2 ROP. In two eyes with stage 2 ROP and pre-plus or plus disease, we observed mild elevation of the arcade vessels without neovascular budding in the regions captured on OCT.
Recurrent Neovascularization
We had one infant with a good example of recurrent neovascularization at 45 weeks PMA and 12 weeks after the administration of anti-VEGF treatment. Recurrent neovascularization (Figure 4c) appeared as a dramatic increase in the number of buds, both sessile and pedunculated, and a large macular neovascular lesion with a placoid formation. Buds appeared along both large and small blood vessels and arose from prior lesions (Figures 4a,b) and also de novo. The macular lesion appears to arise from an area of mildly elevated tufting, which may have been a regressed lesion after initial anti-VEGF treatment. A prominent, raised blood vessel appears to feed this lesion. Vascular elevation and engorgement is apparent and appears to precede the development of neovascular buds (Figure 4b). We followed the course of recurrent neovascularization over the course of 7 weeks. On clinical examinations, this infant was found to have zone 1 stage 1 with plus disease at 33 weeks PMA and was treated with bevacizumab. The first OCT scan of this infant at 41 weeks PMA (Figure 4a) shows a region of mild elevation and tufting in the nasal macula with sessile buds along the superior arcade. At 43 weeks PMA (Figure 4b), vascular elevation and engorgement is visible. The buds are slightly increased in size with minimal change in the area of tufting compared to findings at 41 weeks. No new lesions are clearly visible. Imaging at 45 weeks PMA (Figure 4c) was previously described but shows placoid development of the previously tufted region, de novo lesion formation, significant increase in number and size of sessile and pedunculated buds, and vascular elevations. Buds appear to coalesce in some areas, especially along the superior arcade. The infant was treated with another intravitreal injection of bevacizumab at this time. Follow-up imaging at 48 weeks PMA (Figure 4d) shows interval regression of the placoid region, significant reduction in neovascular budding and no evidence of vascular elevation or engorgement. The infant underwent laser photocoagulation of the avascular periphery 2 months later and no further progression. This case shows a form of regression of recurrent extraretinal neovascularization following subsequent anti-VEGF treatment.
Figure 4:
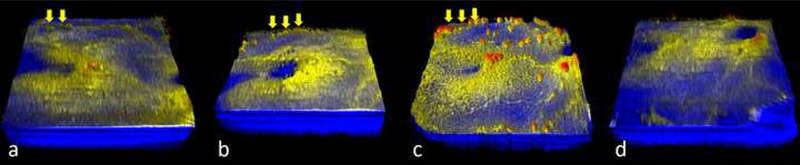
Colorized, three-dimensional, swept-source optical coherence tomography volumes of the posterior retina of a preterm infant with recurrent neovascularization over time. (a) 8 weeks after anti-VEGF at 41 weeks PMA residual tufts of neovascular tissue are visualized at the macula and along the superior arcade (yellow arrows) (b) at 43 weeks PMA shows increased vascular elevation, minimal change in the tufted lesion, and a mild increase in existing buds (yellow arrows) (c) at 45 weeks shows recurrent neovascularization. The number of buds increases dramatically (yellow arrows) and they are present over large and small blood vessels, appearing coalesced in parts of the superior arcade. The previously tufted area adjacent to the fovea developed into a placoid lesion with a prominent feeding vessel. The infant was treated with bevacizumab and follow-up imaging (d) was performed at 48 weeks showing interval regression in buds, placoid lesion and vascular elevation. All volumes are imaged with 100 b-scans per volume, except (c) which has 1,000 b-scans per volume.
Longitudinal progression from normal surface to earliest buds and neovascularization
We followed the 3D extraretinal neovascular development in one eye of one infant over 11 weeks (Figure 5). On clinical examinations, this eye progressed from stage 0 (32 weeks PMA) to stage 2 (35 weeks PMA) to stage 3 (39 weeks PMA). Weekly OCTs were obtained until 43 weeks PMA, allowing documentation of the onset and development of neovascular buds. During the first four weeks (32–36 weeks PMA), the retina appeared as a bland inner surface without focal elevation of vasculature. We first documented buds and vessel elevation at 37 weeks in stage 2 ROP. One week later, we documented both sessile and pedunculated buds found isolated and in clusters. Pedunculated buds were found closer to the ridge. When the eye progressed to stage 3 ROP at 39 weeks PMA, we were able to document bridging networks that progressed to a placoid lesion a week later. At the surface level, we observed that multiple buds coalesced to form bridging networks, then a placoid lesion. We were able to trace the outline of the buds in this placoid complex. As we maneuvered the visualization of the 3D volumes to observe the under-surface of this lesion, we noted that the placoid lesion had numerous vessels feeding into it at 39 and 40 weeks PMA (Figure 6). We were able to image similar regions (Supplementary figure 1) in the temporal retina at 37, 39 and 40 weeks PMA. We documented progression of buds as well as progressive vessel elevation. Two isolated sessile buds with minimal elevation from the retinal surface were documented at 37 weeks PMA. At 39 weeks, these buds, appeared to be pedunculated, clustered together and were moderately elevated from the retinal surface. At 40 weeks, we could demonstrate coalescing of these buds, which nearly merged into the placoid complex. The buds were at maximum elevation when compared to the previous two weeks (Figure 6). As seen in Figure 6, some buds at 40 weeks appeared to cast shadows on the retinal surface. A number of buds that were present at 39 weeks merged into the placoid complex at 40 weeks PMA.
Figure 5:

Colorized three-dimensional swept-source optical coherence tomography volumes of the temporal retina of a preterm infant followed over time. (a) at 33 weeks postmenstrual age (PMA) and stage 0 retinopathy of prematurity (ROP), the retina appears as a bland surface with no apparent extraretinal tissue or vessel elevation; (b) at 35 weeks PMA and stage 2 ROP, the retina appears similar to (a); (c) at 37 weeks PMA and stage 2 ROP shows the appearance of a single sessile bud; (d) represents another area of the temporal retina at 37 weeks, showing numerous small and large buds, both sessile and pedunculated type either found isolated or in clusters; ( e) at 39 weeks PMA and stage 3 ROP with pre-plus demonstrates vessel elevation and multiple buds coalescing to form a network and a placoid lesion; (f) at 40 weeks PMA and stage 3 ROP with pre-plus disease, demonstrates further increase in the elevation of the placoid lesion as indicated by the red color on the colorized volume, when compared to the previous week, along with vessel elevation and presence of multiple buds.
Figure 6:

Colorized three-dimensional (3D) swept-source optical coherence tomography volumes and B-scans of the temporal retina at a similar location (Supplementary figure 1) imaged at 37, 39 and 40 weeks PMA of a preterm infant. The white arrows in a,c,e, show buds followed over the three imaging timepoints. The buds are seen progressing from two small isolated sessile buds at 37 weeks to two pedunculated buds, clustered together, to nearly a bridging network at 40 weeks that appear to be more elevated when compared to the previous weeks. The yellow hollow arrows in b,d,f indicate the position of the buds on the 3D volume and their corresponding B-scan. The white line across the B-scan in (d) is an artifact from imaging. Imaging at 37 weeks (a, b) show the presence of early buds and extraretinal tissue at the ridge. The buds are mildly elevated at 39 weeks (c, d) with the presence of multiple sessile and pedunculated buds forming a network and moderately elevated early placoid lesion. Retinal vessel elevation is clearly evident and the retinal vessels are seen feeding into the extraretinal neovascular lesions in (d). Imaging at 40 weeks (e, f) shows further elevation of the extraretinal neovascular placoid lesion, with retinal vessels feeding into the placoid lesion and new posterior pedunculated buds when compared to 39 weeks.
Discussion:
Using colorized 3D visualization of retinal OCT images, we were able to examine the extraretinal vascular structures by 3D OCT imaging for the first time. We delineated and defined extraretinal neovascular structures as buds, bridging networks and placoid lesions. While bridging networks and placoid lesions matched with clinical findings of Stage 3 ROP, we cannot evaluate the strength of a possible correlation from this small first-in human series. We were able to longitudinally follow progression and regression of extraretinal neovascularization in stage 3 ROP after treatment in one infant over 12 weeks and document the appearance of early buds, retinal vessel elevation at the time of bud formation and florid neovascularization. From stage 2 to 3 ROP, we observed progression from sessile buds to a complex plaque that corresponded to stage 3 ROP on clinical examination. In another infant, we demonstrated regression of neovascular complexes to small pre-retinal tufts after treatment with anti-VEGF. The current study identifies buds as possible early markers of dysregulated angiogenesis.
Historical histopathologic human and animal studies have led to various theories for physiological and pathological retinal vasculature development. As early as 1928, Ida Mann, in her human histology drawings, described the emergence of retinal vessels from the optic disc at about the fourth month of gestation extending to the ora serrata by the eighth month.[3] Normal retinal vascular development is hypothesized to be driven initially by vasculogenesis, de novo formation of vessels by differentiation of endothelial precursor cells, through about 22 weeks gestation, transitioning to angiogenesis, budding from pre-existing vessels during the third trimester.[18–22] Investigators proposed abnormal neovascularization to be from vascular or mesenchymal proliferation, budding, or elongation of capillary loops.[23] However, confirmation of these theories has been limited by difficulty in visualization of the infant retina necessitating the use of histochemical studies.
In this study, we demonstrated that buds may be the earliest form of extraretinal neovascular tissue seen in ROP stage 2 or worse. Both sessile and pedunculated buds stem from existing retinal vasculature near the V-AV junction. This is consistent with the current studies using experimental models showing that endothelial stalk cells, located posterior to the endothelial tip cells at the V-AV junction,[24] are responsible for proliferation into extraretinal neovascularization. The premise that neovascularization occurs from budding and early endothelial proliferation from existing blood vessels,[18] early human histopathology by Kushner et al[25], in 1977 who described these buds as “peculiar dilatations or tufts” arising from the capillary networks and found to have a lumen.[25] We found both sessile and pedunculated buds in eyes with stage 3 ROP and mainly sessile buds in stage 2 ROP. Wallace et al studied the significance of isolated buds in stage 2 ROP.[26] They hypothesized that these isolated neovascular tufts, described clinically as “popcorn”, can occur either early or late during active disease and may be associated with progression or regression of disease.[26] Similar to our observation, Wallace et al found single or multiple of these tufts in one or more quadrants either immediately posterior to the V-AV junction in stage 3 ROP but at a significant distance from the V-AV junction in stage 2 eyes. From their study, they concluded that there may be two types of popcorn lesions; one associated with increased risk of disease progression, and one occurring later as the advancing ridge leaves behind benign tufts. The buds found in stage 3 ROP may fall into the former category and will be examined in the ongoing longitudinal study. The neovascular complex seen in the OCT images was consistent with Foos’s findings in his human histopathology reports.[8, 9] Foos attributed the progressive change in morphology to a multifactorial process resulting in intraretinal cellular changes in the vanguard and rearguard eventually leading to retinal detachment.[8] We believe that the morphologic heterogeneity in the neovascular elevations we imaged may be the result of different forms of vitreoretinal traction.[27] Further studies of the vitreoretinal interactions will yield more insights into the etiology of these morphologically distinct neovascularization patterns.
Our finding of neovascular buds arising and recurring from pre-existing vessels appeared to concur with what Lapore et al described as the “rosary-bead-like” hyperfluorescent lesions inside the vessels on fluorescein angiography.[28] Although fluorescein angiography allows appreciation of a wider angle of the retina, our 3D imaging provides superior spatial resolution when compared with the en face view of fluorescein angiography, and shows that these are indeed small elevations from the retinal surface. Another benefit of our approach over fluorescein angiography for future studies is that the visualization of these neovascular structures is not confounded by late leakage of fluorescein.
Color imaging has been used to characterize and classify the pathophysiologic stages of ROP. However, color images offer a less detailed appearance than do images obtained with SDOCT and viewed with 3D visualization. The similarity in appearances between 3D SDOCT and flat mounted retinas from representative models of ROP has been valuable in translating molecular mechanisms of pathologic features possible only through experimental study to what occurs in the premature infant with ROP. An example is the rat oxygen-induced retinopathy (OIR) model, which exposes newborn full-term rats to fluctuations in oxygen levels that cause similar arterial oxygen extremes as in human infants with severe ROP.[4, 29, 30] The rat OIR model recreates two sequential phases of ROP. The first involves delayed physiologic vascular development causing peripheral avascular retina at the V-AV junction with vascular tortuosity and dilation similar to plus disease and compromise of physiologic vascularity within the existing vasculature. Subsequently, intravitreal or extraretinal neovascularization develops at the V-AV junction similar to stage 3 ROP in type 1 ROP that occurs in zone II. [4] Flat mounts labeled for vascularization show two dimensional appearances of bridging networks of vessels posterior to the V-AV junction (Supplementary Figure 2) similar in appearance to OCT images of stage 3 ROP in preterm infants (Figure 6).[2] To-date, 3D visualization in these animals are lacking. As suggested in the animal model studies, signaling pathways triggered by variable oxygen levels and oxidative stress lead to VEGF receptor 2-induced disordered angiogenesis[31],[32] and intravitreal neovascularization.[5, 29] [31] OCT imaging with 3D visualization provides a more distinct appearance of the inter-connections between vascular structures at V-AV junction in human infants compared to indirect ophthalmoscopy or imaging with a contact camera. Longitudinal studies show similar findings by 3D SDOCT as that seen in experimental models in which VEGF signaling is inhibited.[31, 33, 34] Similarities in features between the experimental rat OIR model and human disease lend greater ability to translate findings experimentally to the human disease.
Despite these advances, our study has several limitations. Our data was limited by the small sample size due to which quantitative analysis was not possible. Our patients were a mix of cross-sectional and short longitudinal follow up. We had limited views of the periphery. Additionally, we did not have fluorescein angiography or OCT angiography as adjuncts to structural 3D OCT volumes; the former was not performed so as to avoid exposing vulnerable infants to intravenous dye for research imaging, and the latter was not possible with the handheld systems that were used for bedside OCT imaging. These studies would be useful in the future to gain further insight on blood flow patterns associated with the identified formations. We do not have the data to determine whether these methods would be reproducible or useful for ROP screening or will contribute to the choice of future therapeutic interventions, as this would depend on results from clinical studies larger than this small pilot group. We believe that this visualization is likely to contribute to our understanding of the pathophysiology of neovascularization in ROP and thus will be useful in future research into the evolution and regression of neovascularization in ROP. Continued study of neovascular development in ROP with larger samples and a wider set of diagnostic tools will further elucidate the pathophysiology of this disease.
In summary, we extended OCT processing to include surface flattening and colorization that further improved structural analysis and better understanding of the extraretinal tissue. We compared our 3D findings with early histopathology reports, the experimental rat OIR model, and with published clinical studies. The use of handheld OCT with colorized 3D visualization of OCT volumes in infants extends our understanding of human infant retinal vascular pathology through a bedside, portable, non-invasive technique that provides superior ability to visualize the V-AV junction and discriminate the patterns and stages of vascular pathology. Our ability to image similar areas in the same infant over multiple visits enabled us to study the evolution of these structural components and follow pathological vascular events longitudinally in development and regression after treatment. These methods can be applied to further larger studies with 3D OCT visualization and are likely to contribute to our understanding of the pathophysiology of neovascularization in ROP.
Supplementary Material
Acknowledgements:
The authors would like to thank Du Tran-Viet and Alexandria Dandridge (Duke University Eye Center), for data acquisition, Dr. Neeru Sarin, MBBS (Duke University Eye Center) and Dr. C Michael Cotten (Department of Neonatology, Duke University Medical Center) for recruiting infants for the study. We also thank Colin A. Bretz, PhD (Moran Eye Center, Utah) for preparing, staining and imaging the retinal flat-mount of the rat OIR model.
Funding: Bayer global ophthalmology awards program (GOAP) fellowship project award, K23EY028227 and RPB career development award (XC); The Hartwell Foundation (CAT); The Andrew Family Charitable Foundation (CAT); Grants P30 EY001583, RO1 EY025009 and R01 EY015130 and R01 EY017011 from the National Eye Institute (NEI); Rockefeller Writing Residency (CAT); and an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah, Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NEI, or NIH. The sponsors or funding organizations had no role in the design or conduct of this research.
Financial Support: Bayer global ophthalmology awards program (GOAP) fellowship project award K23EY028227 and RPB career development award (XC); The Hartwell Foundation (CAT); The Andrew Family Charitable Foundation (CAT); Grants P30 EY001583, RO1 EY025009 and R01 EY015130 and R01 EY017011 from the National Eye Institute (NEI); Rockefeller Writing Residency (CAT); and an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah, Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NEI, or NIH. The sponsors or funding organizations had no role in the design or conduct of this research.
Meeting Presentation: Part of this manuscript was presented by Dr. Cynthia A Toth for the Hermann Wacker Prize of the Club Jules Gonin at the XXXIst Annual Meeting of Club Jules Gonin held July 11-14, 2018, at Association for Research in Vision and Ophthalmology (ARVO) 2016 meetings.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: Dr. Toth receives royalties through her university from Alcon. Dr. Izatt has a patent and receives royalties from Leica Microsystems. No other authors have financial disclosures. No authors have a proprietary interest in the current study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Written informed consent was obtained from all individual participants (parent/guardian) included in the study.
References:
- 1.Gilbert C, Foster A (2001) Childhood blindness in the context of VISION 2020--the right to sight. Bull World Health Organ 79: 227–232 [PMC free article] [PubMed] [Google Scholar]
- 2.Hartnett ME (2010) The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol 120: 25–39 DOI 10.1007/s10633-009-9181-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann IC (1928) The develpoment of the human eye. Cambridge University Press, London [Google Scholar]
- 4.Hartnett ME (2015) Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 122: 200–210 DOI 10.1016/j.ophtha.2014.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartnett ME (2017) Advances in understanding and management of retinopathy of prematurity. Surv Ophthalmol 62: 257–276 DOI 10.1016/j.survophthal.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutty GA, McLeod DS, Bhutto I, Wiegand SJ (2011) Effect of VEGF trap on normal retinal vascular development and oxygen-induced retinopathy in the dog. Invest Ophthalmol Vis Sci 52: 4039–4047 DOI 10.1167/iovs.10-6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutty GA, Chan-Ling T, Phelps DL, Adamis AP, Berns KI, Chan CK, Cole CH, D’Amore PA, Das A, Deng WT, Dobson V, Flynn JT, Friedlander M, Fulton A, Good WV, Grant MB, Hansen R, Hauswirth WW, Hardy RJ, Hinton DR, Hughes S, McLeod DS, Palmer EA, Patz A, Penn JS, Raisler BJ, Repka MX, Saint-Geniez M, Shaw LC, Shima DT, Smith BT, Smith LE, Tahija SG, Tasman W, Trese MT (2006) Proceedings of the Third International Symposium on Retinopathy of Prematurity: an update on ROP from the lab to the nursery (November 2003, Anaheim, California). Mol Vis 12: 532–580 [PubMed] [Google Scholar]
- 8.Foos RY (1987) Retinopathy of prematurity. Pathologic correlation of clinical stages. Retina 7: 260–276 [PubMed] [Google Scholar]
- 9.Foos RY, Kopelow SM (1973) Development of retinal vasculature in paranatal infants. Survery of Ophthalmology 18: 117–127 [Google Scholar]
- 10.Ashton N, Cook C (1954) Direct observation of the effect of oxygen on developing vessels: preliminary report. Br J Ophthalmol 38: 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashton N (1954) Pathological basis of retrolental fibroplasia. Br J Ophthalmol 38: 385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt-Erfurth U, Leitgeb RA, Michels S, Povazay B, Sacu S, Hermann B, Ahlers C, Sattmann H, Scholda C, Fercher AF, Drexler W (2005) Three-dimensional ultrahigh-resolution optical coherence tomography of macular diseases. Invest Ophthalmol Vis Sci 46: 3393–3402 DOI 10.1167/iovs.05-0370 [DOI] [PubMed] [Google Scholar]
- 13.Maldonado RS, Izatt JA, Sarin N, Wallace DK, Freedman S, Cotten CM, Toth CA (2010) Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci 51: 2678–2685 DOI 10.1167/iovs.09-4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado RS, Yuan E, Tran-Viet D, Rothman AL, Tong AY, Wallace DK, Freedman SF, Toth CA (2014) Three-dimensional assessment of vascular and perivascular characteristics in subjects with retinopathy of prematurity. Ophthalmology 121: 1289–1296 DOI 10.1016/j.ophtha.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA (2009) Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology 116: 2448–2456 DOI 10.1016/j.ophtha.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viehland C, Keller B, Carrasco-Zevallos OM, Nankivil D, Shen L, Mangalesh S, Viet du T, Kuo AN, Toth CA, Izatt JA (2016) Enhanced volumetric visualization for real time 4D intraoperative ophthalmic swept-source OCT. Biomed Opt Express 7: 1815–1829 DOI 10.1364/boe.7.001815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleicher ID, Jackson-Atogi M, Viehland C, Gabr H, Izatt JA, Toth CA (2018) Depth-Based, Motion-Stabilized Colorization of Microscope-Integrated Optical Coherence Tomography Volumes for Microscope-Independent Microsurgery. Translational Vision Science and Technology In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes S, Yang H, Chan-Ling T (2000) Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci 41: 1217–1228 [PubMed] [Google Scholar]
- 19.Smith LE, Hard AL, Hellström A (2013) The Biology of Retinopathy of Prematurity: How Knowledge of Pathogenesis Guides Treatment. Clin Perinatol 40: 201–214 DOI 10.1016/j.clp.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartnett ME, Penn JS (2012) Mechanisms and management of retinopathy of prematurity. N Engl J Med 367: 2515–2526 DOI 10.1056/NEJMra1208129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, Purcaro V, Giannantonio C, Papacci P, Romagnoli C (2014) Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology 121: 2212–2219 DOI 10.1016/j.ophtha.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 22.Purcaro V, Baldascino A, Papacci P, Giannantonio C, Molisso A, Molle F, Lepore D, Romagnoli C (2012) Fluorescein angiography and retinal vascular development in premature infants. J Matern Fetal Neonatal Med 25 Suppl 3: 53–56 DOI 10.3109/14767058.2012.712313 [DOI] [PubMed] [Google Scholar]
- 23.Garoon I, Epstein G, Segall M, Rabb MF, LaFranco F, Quirk TC 3rd, (1980) Vascular tufts in retrolental fibroplasia. Ophthalmology 87: 1128–1132 [DOI] [PubMed] [Google Scholar]
- 24.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177 DOI 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushner BJ, Essner D, Cohen IJ, Flynn JT (1977) Retrolental Fibroplasia. II. Pathologic correlation. Arch Ophthalmol 95: 29–38 [DOI] [PubMed] [Google Scholar]
- 26.Wallace DK, Kylstra JA, Greenman DB, Freedman SF (1998) Significance of isolated neovascular tufts (“popcorn”) in retinopathy of prematurity. J aapos 2: 52–56 [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Mangalesh S, Dandridge A, Tran-Viet D, Wallace DK, Freedman SF, Toth CA (2018) Spectral-Domain OCT Findings of Retinal Vascular-Avascular Junction in Infants with Retinopathy of Prematurity. Ophthalmology Retina DOI 10.1016/j.oret.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepore D, Molle F, Pagliara MM, Baldascino A, Angora C, Sammartino M, Quinn GE (2011) Atlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurity. Ophthalmology 118: 168–175 DOI 10.1016/j.ophtha.2010.04.021 [DOI] [PubMed] [Google Scholar]
- 29.Cunningham S, Fleck BW, Elton RA, McIntosh N (1995) Transcutaneous oxygen levels in retinopathy of prematurity. Lancet 346: 1464–1465 [DOI] [PubMed] [Google Scholar]
- 30.Penn JS, Tolman BL, Lowery LA, Koutz CA (1992) Oxygen-induced retinopathy in the rat: hemorrhages and dysplasias may lead to retinal detachment. Curr Eye Res 11: 939–953 [DOI] [PubMed] [Google Scholar]
- 31.Simmons AB, Bretz CA, Wang H, Kunz E, Hajj K, Kennedy C, Yang Z, Suwanmanee T, Kafri T, Hartnett ME (2018) Gene therapy knockdown of VEGFR2 in retinal endothelial cells to treat retinopathy. Angiogenesis DOI 10.1007/s10456-018-9618-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker S, Wang H, Simmons AB, Suwanmanee T, Stoddard GJ, Kafri T, Hartnett ME (2018) Targeted Knockdown of Overexpressed VEGFA or VEGF164 in Muller cells maintains retinal function by triggering different signaling mechanisms. Sci Rep 8: 2003 DOI 10.1038/s41598-018-20278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME (2013) Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 54: 2020–2026 DOI 10.1167/iovs.13-11625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Yang Z, Jiang Y, Flannery J, Hammond S, Kafri T, Vemuri SK, Jones B, Hartnett ME (2014) Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 55: 737–744 DOI 10.1167/iovs.13-13429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


