Abstract
PURPOSE
To compare the survival outcomes of neoadjuvant three-dimensional conformal radiotherapy (RT) followed by hepatectomy with hepatectomy alone in patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT).
PATIENTS AND METHODS
A randomized, multicenter controlled study was conducted from January 2016 to December 2017 in patients with resectable HCC and PVTT. Patients were randomly assigned to receive neoadjuvant RT followed by hepatectomy (n = 82) or hepatectomy alone (n = 82). The modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines were used to evaluate the therapeutic effects of RT. The primary end point was overall survival. The expression of interleukin-6 (IL-6) in patients’ serum before RT and in surgical specimens was correlated with response to RT.
RESULTS
In the neoadjuvant RT group, 17 patients (20.7%) had partial remission. The overall survival rates for the neoadjuvant RT group at 6, 12, 18, and 24 months were 89.0%, 75.2%, 43.9%, and 27.4%, respectively, compared with 81.7%, 43.1%, 16.7%, and 9.4% in the surgery-alone group (P < .001). The corresponding disease-free survival rates were 56.9%, 33.0%, 20.3%, and 13.3% versus 42.1%, 14.9%, 5.0%, and 3.3% (P < .001). On multivariable Cox regression analyses, neoadjuvant RT significantly reduced HCC-related mortality and HCC recurrence rates compared with surgery alone (hazard ratios, 0.35 [95% CI, 0.23 to 0.54; P < .001] and 0.45 [95% CI, 0.31 to 0.64; P < .001]). Increased expressions of IL-6 in pre-RT serum and tumor tissues were significantly associated with resistance to RT.
CONCLUSION
For patients with resectable HCC and PVTT, neoadjuvant RT provided significantly better postoperative survival outcomes than surgery alone. IL-6 may predict response to RT in these patients.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related death worldwide.1 HCC tends to invade the portal vein system and form a portal vein tumor thrombus (PVTT). PVTT is one of the most important predictors of poor survival in HCC. The Barcelona Clinic Liver Cancer (BCLC) staging classification2 recommends sorafenib as the only treatment for these patients. However, survival probabilities differ significantly according to the extent and severity of PVTT.3,4 In selected patients with resectable HCC and PVTT, surgery may offer better survival rates than nonsurgical treatments.5-8 However, once PVTT invades the right-side, left-side, or main trunk of the portal vein, postoperative prognosis becomes dismal.6,9 The combined use of surgery and multimodal therapies may improve long-term survival in these patients.
Radiotherapy (RT) is increasingly used in advanced HCC and has been reported to confer survival benefits.10-12 In a previous retrospective study by us, neoadjuvant RT significantly reduced the extent of PVTT in a proportion of patients and improved postoperative survival.13 However, the level of evidence of this study was low because of its retrospective design and relatively small sample size. Reports have demonstrated that interleukin-6 (IL-6), a multifunctional cytokine, is positively linked to radiation resistance in various malignancies, including HCC.14-16 The current study was conducted to determine the roles of neoadjuvant RT in patients with HCC and PVTT and IL-6 expression in the prediction of radiation response in these patients.
PATIENTS AND METHODS
Study Design
This randomized, open-label, multicenter controlled clinical study was approved by the ethics committees of the participating centers, and its protocol conformed to the 1975 Declaration of Helsinki. All patients provided written informed consent to a trialist and noninvestigator before participation. The trial conformed to the CONSORT statement and was registered in the Chinese Clinical Trial Registry. The research data were used only for publication or shared with other researchers. The identity and medical information of each participant remain strictly confidential.
Patient Eligibility
The key inclusion criteria were age 18 to 70 years, HCC diagnosed by biopsy or by the noninvasive criteria of the European Association for the Study of Liver guidelines,2 the primary HCC being resectable, and Cheng’s type II/III PVTT (ie, PVTT that involved the right- or left-side branch or main trunk of the portal vein)9 (Appendix Table A1, online only). The assessment of resectability is described in the Appendix (online only). The key exclusion criteria were a history of other malignancy in the past 5 years, any previous antitumor treatment of HCC within 1 year, and hepatitis C virus (HCV) or HIV infection.
Sample Size Estimation
The sample size was estimated on the basis of a 1-year overall survival (OS) rate of 69% in the neoadjuvant RT group and 35.6% in the surgery-alone group, which were obtained from the data of our retrospective cohort of patients treated from January 2010 to December 2013.13 The minimum sample size was 75 for each group (two-sided α = .05; β = .10; power, 90%), and 10% of patients were added to compensate for any loss to follow-up. Finally, 82 patients were included in each of the two groups.
Randomization and Masking
The patients were randomly assigned 1:1 to the neoadjuvant RT or control group according to a sequestered, computer-generated randomization code that used permuted blocks of treatment group allocations without stratification. Allocation concealment was conducted using sequentially numbered opaque sealed envelopes.
Neoadjuvant RT and Evaluation of Response/Toxicity
The imaging evaluation is described in the Appendix. Three-dimensional conformal RT (3DCRT) was delivered to the neoadjuvant RT group within 5 days of random assignment. The gross tumor volume was defined as the tumor volume that was enhanced in the arterial phase combined with the PVTT volume, which was shown as a filling defect in the portal venous phase of the computed tomography (CT) scan. The clinical tumor volume (CTV) was generated by adding 5 to 10 mm to the gross tumor volume. The planning target volume was expanded to include a 5- to 10-mm margin from the CTV to compensate for internal physiologic movements and variations in size, shape, and position of the CTV. The planned total dose to the planning target volume was 18 Gy, with a fractional size of 3.0 Gy, using 6-MV x-rays with a linear accelerator (Elekta Synergy; Elekta, Stockholm, Sweden) at five fractions per week. Before each treatment session, patients underwent a megavoltage cone beam CT scan and were positioned with automated image registration by application of the image-guided RT system (Elekta Synergy). Respiratory gating technique was used to reduce dose delivered to healthy tissues and surrounding organs. The RT regimen was in accordance with that used in our previous studies.13,17
The modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines18 were used to evaluate the therapeutic effects of RT on primary liver tumor. For the evaluation of PVTT, any downstaging in the Cheng’s PVTT classification or any conspicuous restoration of blood flow in the portal vein were regarded as partial remission (PR) and any upstaging in the PVTT classification as progressive disease (PD). Otherwise, the effects were defined as stable disease (SD). The overall RT response was defined as PD if either the primary tumor or the PVTT was classified as PD; it was defined as PR if either the primary tumor or the PVTT was classified as PR while the other was SD. Acute RT toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (version 4.0).19
Surgical Procedure
For patients who were randomly assigned to the surgery-alone group, surgery was planned immediately and carried out within 5 days after assignment. Patients assigned to the neoadjuvant RT group were re-evaluated in 4 weeks after complement of RT, and surgery was planned immediately and carried out within 5 days if the patients did not develop a contraindication to surgery. The procedure of hepatectomy is described in the Appendix. Morbidity was defined as occurrence of any postoperative complications and graded by the Clavien-Dindo classification.20
Assessment of Outcomes and Follow-Up
The primary end point was OS, which was defined as the time from random assignment to tumor-related death. One of the secondary outcomes was disease-free survival (DFS), which was defined as the time from random assignment to the time when a recurrent tumor was first diagnosed. The other secondary end points were surgery-related complications and in-hospital mortality. The data were analyzed on an intention-to-treat basis. The follow-up protocol is described in the Appendix. This study was censored on October 1, 2018.
Immunohistochemistry and Enzyme-Linked Immunosorbent Assay for IL-6
HCC tissues were collected from the 73 patients in the neoadjuvant RT group after RT and surgical treatment. Immunohistochemistry (IHC) staining for IL-6 was performed according to the method reported in a previous report.21 For the 82 patients in the neoadjuvant RT group, the levels of IL-6 and several other cytokines with possible associations with radioresistance were analyzed in the blood samples by enzyme-linked immunosorbent assay. The relationship between the various responses to RT and expressions of these cytokines and the patients’ clinical characteristics were analyzed.
Statistical Analysis
Variables were compared using the χ2 test, Fisher’s exact test, independent t test, Mann-Whitney U test, or Wilcoxon rank sum test, as appropriate. Survival analysis was estimated by the Kaplan-Meier method. Factors that were significantly (P < .1) associated with survival in the univariable analysis were entered into the Cox proportional hazards regression model. P < .05 was considered statistically significant. All statistical analyses were performed with Stata 12.0 software (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
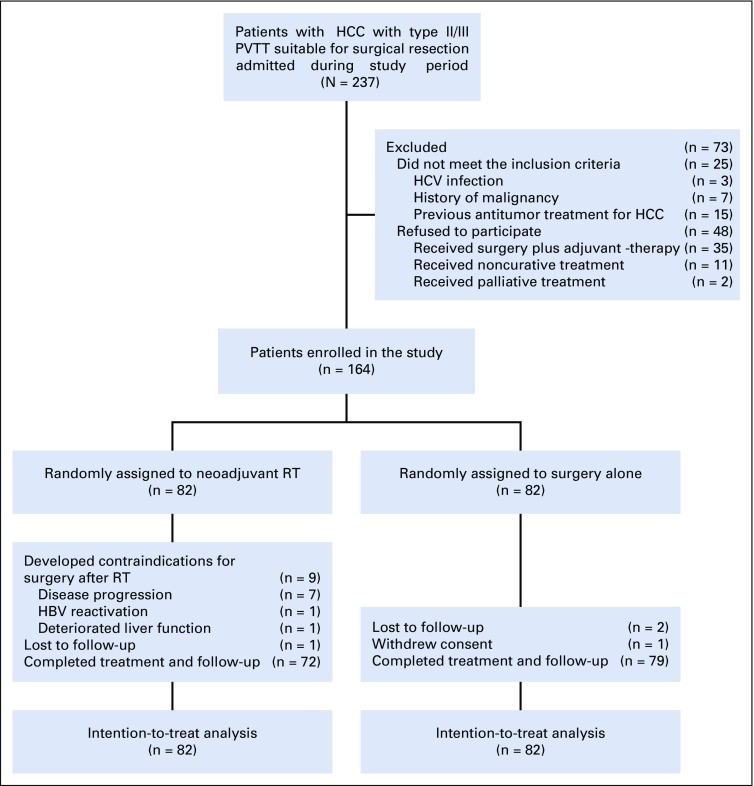
From January 2016 to December 2017, of 237 patients who met the inclusion criteria, 73 were excluded. Finally, 164 patients were randomly assigned to the neoadjuvant RT group (n = 82) and the surgery-alone group (n = 82; Fig 1). These patients came from the Eastern Hepatobiliary Surgery Hospital (n = 157), the Fujian Provincial Cancer Hospital (n = 5), and the Zhongshan Hospital (n = 2).
FIG 1.
CONSORT diagram of the randomized clinical trial. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; PVTT, portal vein tumor thrombus; RT, radiotherapy.
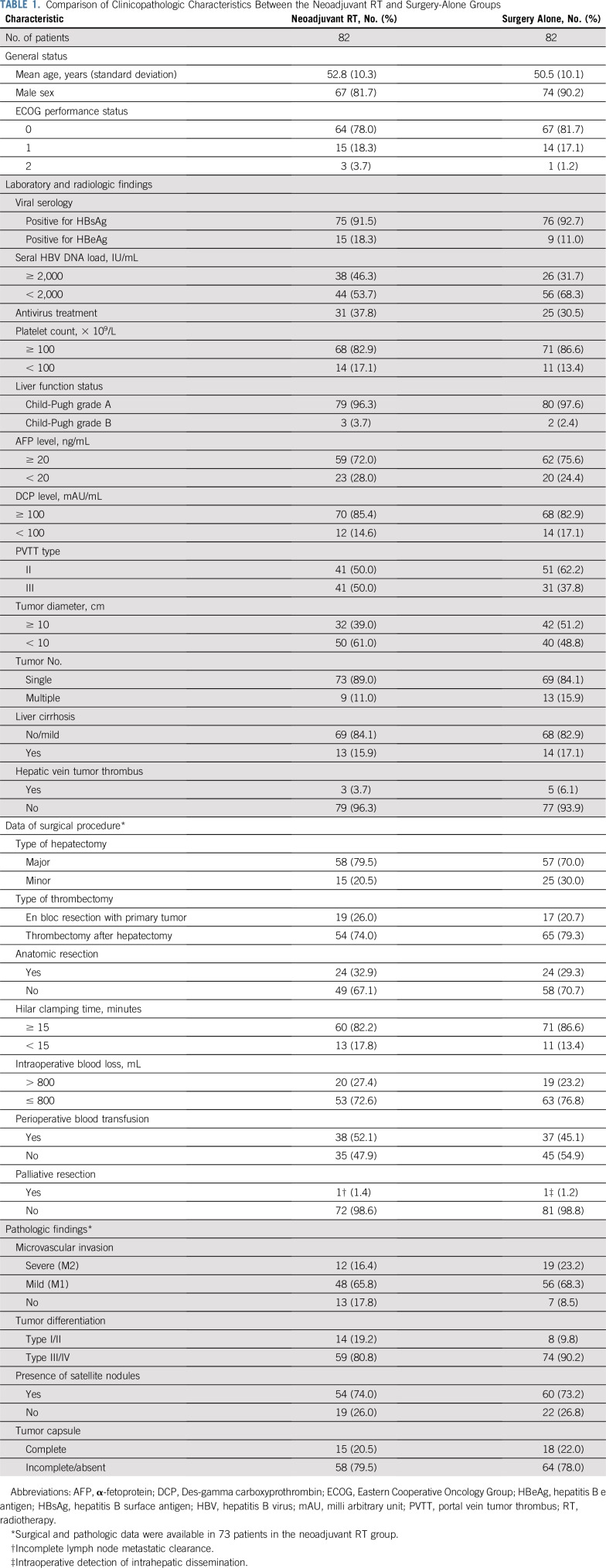
In the neoadjuvant RT group, nine patients developed contraindications to surgery after RT, and they underwent nonsurgical therapy (transarterial chemoembolization [TACE], n = 7; sorafenib, n = 2). In the surgery-alone group, patients received upfront surgery after random assignment; thus, there was no patient with contraindication to surgery. One patient in the surgery-alone group withdrew from the study after random assignment and underwent surgical resection in another institution. The baseline characteristics were well matched between groups (Table 1).
TABLE 1.
Comparison of Clinicopathologic Characteristics Between the Neoadjuvant RT and Surgery-Alone Groups
Response Rate and RT Toxicity
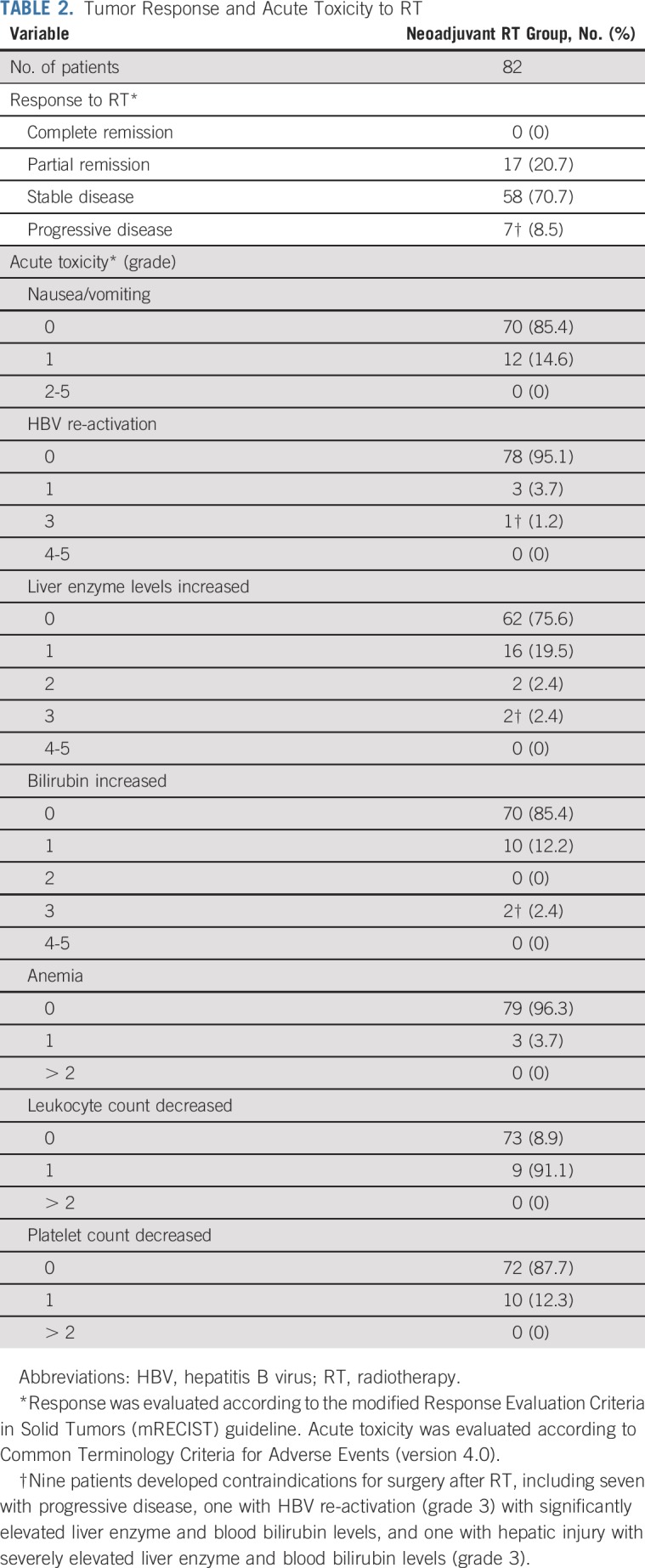
The overall response rate of RT was 20.7% (17 of 82 patients), with no complete response; 17 (20.7%) patients had PR, 58 (70.7%) SD, and seven (8.5%) PD. Of the 17 patients with PR, the PVTT was downstaged from Cheng’s type III to type II (representative patient image shown in Appendix Fig A1, online only) or from type II to type I in 12 patients. After RT, two patients had grade 3 liver toxicity and were considered to be unsuitable for surgery because of insufficient liver function. Another two patients with grade 2 liver enzyme elevations had their enzyme levels return to normal after 7 days of oral administration of glutathione and bicyclol (Table 2).
TABLE 2.
Tumor Response and Acute Toxicity to RT
Surgical Procedure and Postoperative Complications
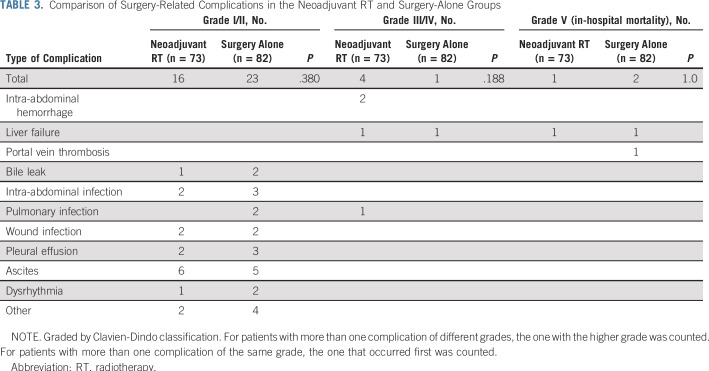
For the two patients who had grade 2 liver enzyme elevations, surgery was delayed for 7 days. Nine patients developed contraindications to surgery, including seven with PD and two with grade 3 liver toxicity; the remaining 73 in the neoadjuvant RT group underwent surgical treatment. All patients were confirmed to have PVTT on intraoperative exploration and pathologic examination. Major postoperative complications (Clavien-Dindo classification of 3 or greater) were observed in eight patients. The surgery-related morbidity and mortality rates were comparable between groups (Table 3).
TABLE 3.
Comparison of Surgery-Related Complications in the Neoadjuvant RT and Surgery-Alone Groups
Follow-Up Data
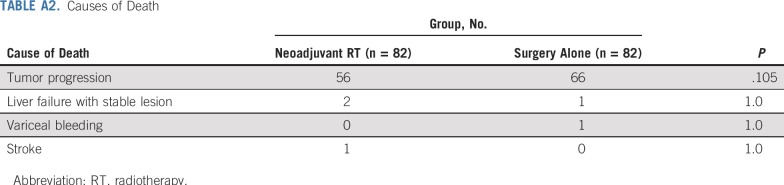
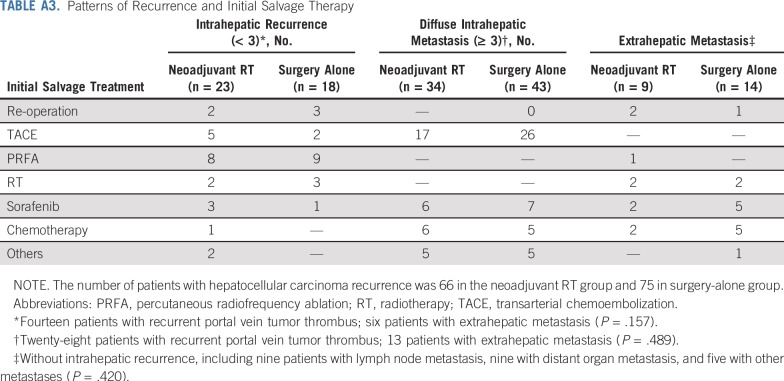
The median follow-up was 15.2 months (interquartile range, 10.5-21.5 months) in the neoadjuvant RT group and 10.8 months (interquartile range, 6.8-15.6 months) in the surgery-alone group. In the neoadjuvant RT group, 66 patients developed HCC recurrence, and 56 patients died as a result of HCC. In the surgery-alone group, 75 patients developed HCC recurrence, and 66 patients died as a result of HCC. The causes of death are listed in Appendix Table A2 (online only). The patterns of recurrence and salvage therapies are listed in Appendix Table A3 (online only).
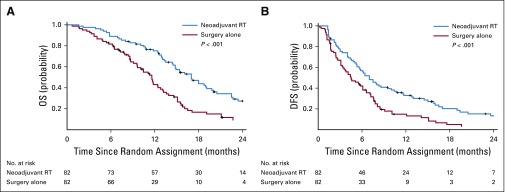
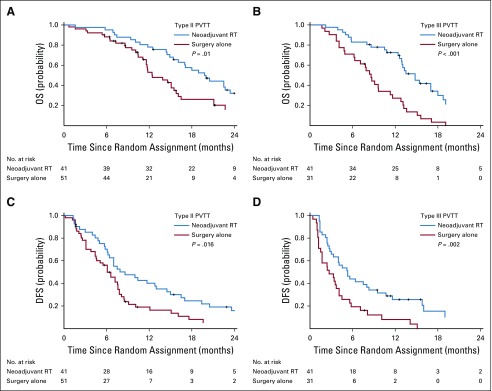
The 6-, 12-, 18-, and 24-month OS rates were 89.0%, 75.2%, 43.9%, and 27.4%, respectively, in the neoadjuvant RT group versus 81.7%, 43.1%, 16.7%, and 9.4% in the surgery-alone group (P < .001; Fig 2A). The 6-, 12-, 18-, and 24-month DFS rates were 56.9%, 33.0%, 20.3%, and 13.3%, respectively, in the neoadjuvant RT group versus 42.1%, 14.9%, 5.0%, and 3.3% in the surgery-alone group (P = .009; Fig 2B). RT significantly increased both the OS and the DFS rates in patients with Cheng’s type II PVTT (P = .01 and P = .016, respectively) as well as in those with type III PVTT (P < .001 and P = .002, respectively; Appendix Fig A2, online only).
FIG 2.
(A) Overall survival (OS) and (B) disease-free survival (DFS) curves for the neoadjuvant three-dimensional conformal radiotherapy (RT) plus surgery and surgery-alone groups.
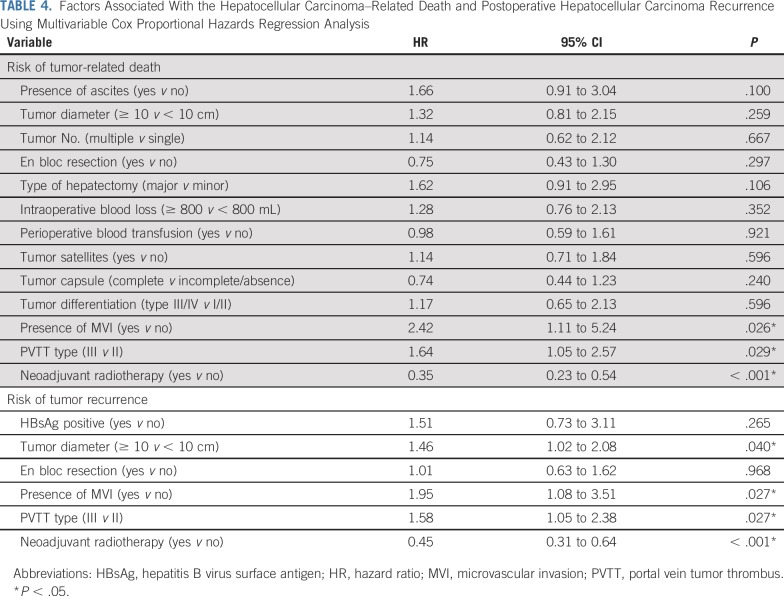
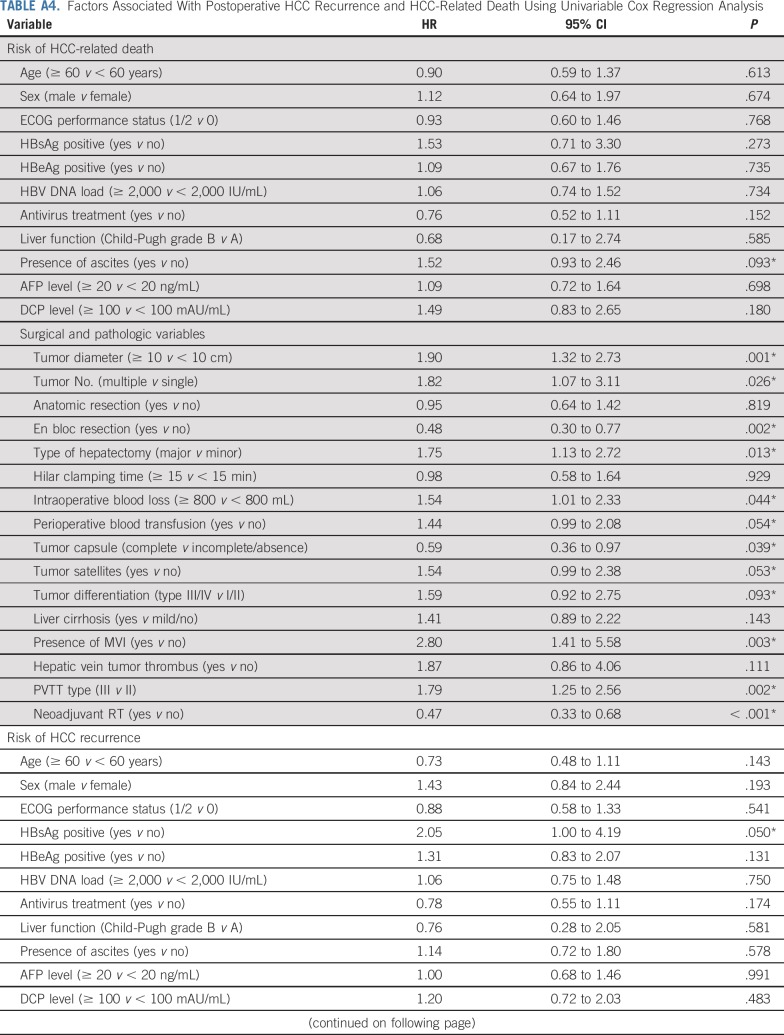
The factors that were significantly (P < .1) associated with HCC recurrence and HCC-related death on univariable Cox regression analysis (Appendix Table A4, online only) were analyzed using the multivariable Cox proportional hazards regression model. The results show that neoadjuvant RT significantly decreased both the HCC-related mortality and the HCC recurrence rates compared with surgery alone (hazard ratios, 0.35 [95% CI, 0.23 to 0.54; P < .001] and 0.45 [95% CI, 0.31 to 0.64; P < .001]; Table 4).
TABLE 4.
Factors Associated With the Hepatocellular Carcinoma–Related Death and Postoperative Hepatocellular Carcinoma Recurrence Using Multivariable Cox Proportional Hazards Regression Analysis
Association of IL-6 Expression With RT Response
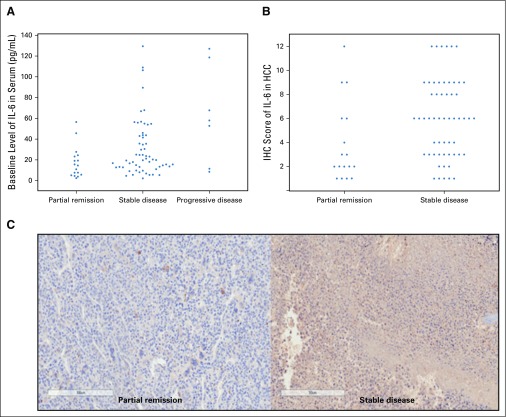
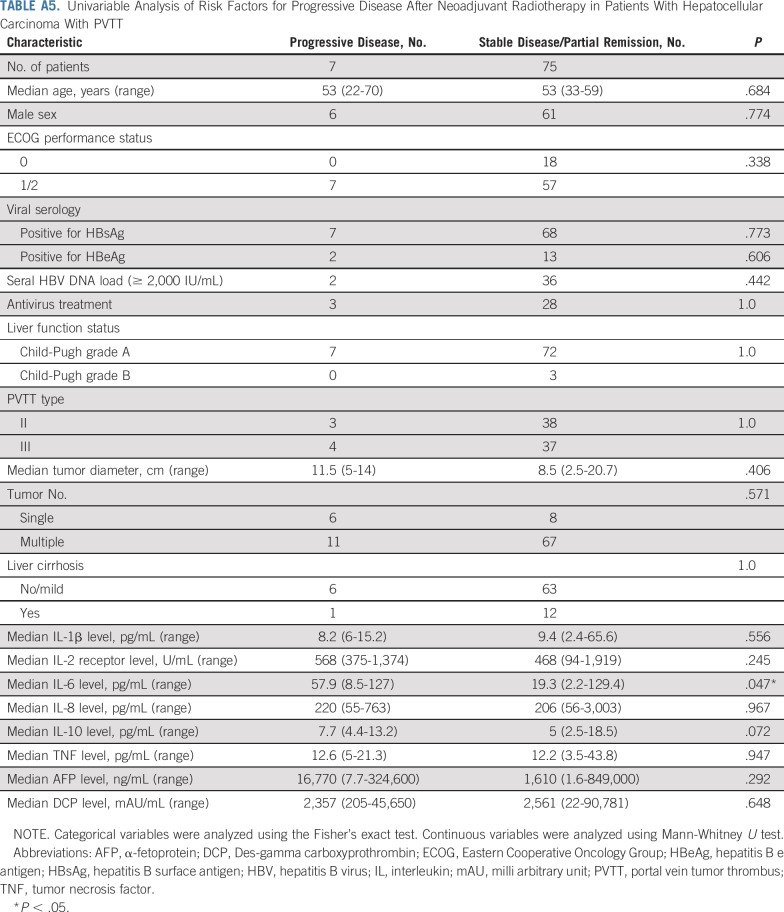
The baseline serum IL-6 levels were significantly higher in patients with PD after 3DCRT, than in those with PR and SD (P = .047; Appendix Fig A3, online only; Appendix Table A5, online only). The IL-6 expression levels in the HCC tissues were significantly higher in patients with SD than in those with PR on the basis of IHC scoring (P = .018; Appendix Fig A3).
DISCUSSION
Controversy exists among experts from the West and the East on the treatment of patients with HCC and PVTT. Western guidelines, which are based on the BCLC classification, consider HCC with PVTT to be at the advanced BCLC stage C, and sorafenib is the only recommended therapy.2,22 In China/Southeast Asia, where the common etiology of HCC is hepatitis B virus, patients usually have better liver function reserves and long-term survival outcomes after hepatectomy compared with patients in Europe, North America, and Japan, where HCV-related HCC is predominant.23,24 Furthermore, hepatitis B virus–related HCC usually progresses faster with worse survival outcomes from sorafenib treatment compared with HCV-related HCC.23 As a consequence, surgery is more frequently adopted for treatment of selected patients with HCC and PVTT in China and Southeast Asia.25-27
Although randomized comparative trials are still lacking, many retrospective studies have shown surgical treatment to provide survival benefit in selected patients with HCC and PVTT compared with nonsurgical treatments.7,28,29 In a multicenter, nationwide study of 6,474 patients, Kokudo et al28 showed that surgical treatment is associated with better survival outcomes than nonsurgical treatment of PVTT limited to the first-order branch of the main portal vein (Cheng’s type I/II). The location and severity of PVTT are significant factors that affect postoperative prognoses. When the PVTT has invaded into the left- or right-side branch or main trunk of the portal vein (Cheng’s type II/III), it is difficult to perform en bloc resection of the tumor together with the PVTT, which is widely believed to have a better curative effect than thrombectomy after hepatectomy.6,9 Adjuvant and neoadjuvant therapy have been advocated to improve the postoperative prognoses of these patients. Lau and colleagues30,31 reported that systemic or local treatment could downstage initially unresectable HCC to become resectable, which thus allows for salvage surgery in a proportion of patients to result in better long-term survival. Our multicenter randomized study was conducted on the basis of the hypothesis that by downstaging the extent of PVTT and/or HCC before surgery using 3DCRT, the chance of tumor spread or residual disease could be lowered and thus result in better postoperative survival.
RT is important in the treatment of patients with HCC and PVTT probably because of radiosensitivity of PVTT cells to irradiation.17 When combined with TACE or hepatic arterial infusion chemotherapy, 3DCRT has been shown to achieve adequate thrombus shrinkage and portal vein flow restoration in patients with HCC and PVTT.10,32-34 A randomized clinical trial by Yoon et al10 using a combination of RT and TACE (n = 45) as a first-line treatment of HCC with PVTT resulted in significantly better progression-free survival, objective response rate, and time to progression compared with sorafenib (n = 45). The OS in the TACE plus RT group was significantly improved (55.0 v 43.0 weeks), even after 48.9% of patients with PD within 12 weeks in the sorafenib group were switched to receive TACE plus RT.
However, only a few reports exist on the use of RT as a neoadjuvant therapy for HCC. A retrospective study found that neoadjuvant RT is associated with improved long-term survival in patients with HCC compared with adjuvant RT.35 Nevertheless, the dose of neoadjuvant RT was not described in this research. Surgical treatment of HCC with PVTT is complex, and a major hepatectomy with or without thrombectomy is required. Thus, to ensure safety of the subsequent hepatectomy, it is necessary to use a low dose of irradiation to minimize radiation injury to the nontumorous liver and to shorten the time interval between the neoadjuvant treatment and surgery. Our previous clinical experience and basic research have indicated that a total radiation dose of 18 Gy in six fractions can be used as a bridge to liver resection for HCC with PVTT.13,17 In our previous study in 95 patients, 12 of 45 in the neoadjuvant RT group had significant reduction in the extent of PVTT after RT, whereas four had PD. Survival analyses showed that neoadjuvant RT resulted in significantly better OS and DFS than hepatectomy alone.13 The current prospective randomized study also confirmed the benefit of neoadjuvant RT in patients with HCC with PVTT, and RT resulted in a low rate of adverse effects. RT contributed to the improved survival outcomes mainly by decreasing the tumor volume in PVTT, which is a known poor prognostic indicator of postoperative survival. The reduction in PVTT volume, especially by downstaging the PVTT type, facilitated surgical treatment and reduced the possibility of residual tumor or spread in the portal vein during surgery.
Despite the favorable local control rates with radiation, treatment failure in our study was still a major problem, which negates the chance for curative surgery in a small proportion of patients with initially resectable lesions. The ability to identify biomarkers to predict the responses to RT is of critical importance to select patients for neoadjuvant RT. IL-6 is a major activator of signal transducer and activator of transcription 3 signaling pathway. The mechanisms that underlie IL-6 in conferring radioresistance, in addition to promoting tumor cell proliferation and survival, also may come from promoting DNA repair after radiation-induced double-strand breaks.36,37 The overexpression of IL-6 was reported to associate with increased radioresistance in several cancers.14-16,37,38 One study demonstrated that IL-6 overexpression in serum is associated with in-field and extrahepatic failure after RT in patients with HCC.14 Our results indicate that IL-6 overexpression in serum or tumor tissues is related to radioresistance. More fundamental and clinical studies are needed to verify this hypothesis.
This study has potential limitations. First, blinding was not possible because of the nature of the intervention. Thus, this study has the inherent biases of an open-label trial. Second, salvage treatments, including local or systemic therapies that may affect the result of survival, were used to treat HCC recurrence for ethical reasons. Finally, the generalizability of our results may be limited because patients with HCV-related HCC were excluded from this study and the indications for surgery differed among centers.
In conclusion, despite PD and adverse events in a small proportion of patients, neoadjuvant RT is effective in improving OS and DFS rates in patients with HCC and PVTT. IL-6 may act as a biomarker in predicting the response to radiation in these patients.
Appendix
Resectability of Tumor
Experienced surgeons resected tumors in patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT). Patients were not considered for surgery if they had one or more of the following clinical features: Eastern Cooperative Oncology Group performance status greater than 2, multiple disseminated nodules (more than three) on imaging, distant metastasis, Child-Pugh class C liver function, Child-Pugh class B liver function for a tumor that required major hepatectomy defined as resection of three or more Couinaud liver segments, Cheng’s type IV PVTT, inferior vena cava tumor thrombus, and severe coexisting systemic diseases.
Imaging
All patients underwent a routine three-phase intravenous contrast dynamic computed tomography (CT) scan. For patients in the neoadjuvant radiotherapy (RT) group, another CT scan was performed 4 weeks after radiation. The CT data were reconstructed into three-dimensional (3D) images using 3D image processing software (3D Plus Body Visible System; Yorktal Medical, Shenzhen, People’s Republic of China). The 3D reconstructed images were used to evaluate the extent of HCC and PVTT, simulate surgery by determining the liver parenchymal transection plane, estimate the residual and remnant liver volumes, and evaluate tumor response to RT.
Surgical Procedure
Partial hepatectomy was performed through a right-side subcostal incision with a midline extension. After mobilization of the liver, intraoperative ultrasonography was performed to assess tumor number and size, the relationship of the tumor with major vascular structures, and the extent of PVTT. Liver parenchymal transection was conducted using the clamp crushing method. Pringle’s maneuver, if necessary, was applied to occlude the blood inflow of the liver using a clamp and unclamp cycle time of 15 and 5 minutes, respectively.
For patients with PVTT confined to the ipsilateral branch of the portal vein (Cheng’s type I/II), anatomic en bloc liver resection together with the PVTT and primary tumor was preferred, provided that an adequate volume of residual liver would remain. When the thrombus protruded beyond the transection plane or into the main trunk of the portal vein (type III), the tumor thrombus was extracted from the opened stump of the portal vein after removal of the primary tumor (thrombectomy). After flushing with normal saline and confirming that no residual thrombus remained, the stump was closed with a continuous suture.
Follow-Up Protocol
The patients were followed up in the outpatient clinic once a month in the first year and subsequently at longer intervals. Serum α-fetoprotein measurements and abdominal ultrasounds were performed once every month. Contrast CT scan or magnetic resonance imaging was performed once every 3 months for surveillance of recurrence or if HCC recurrence was suspected clinically. Transarterial chemoembolization, local ablative therapy, or systemic therapy was used for the treatment of HCC recurrence, which depended on the location of the recurrent lesion, size and number of recurrences, liver function status, and presence/absence of extrahepatic disease. Palliative treatment was provided to patients with end-stage disease, poor general status, or poor liver function.
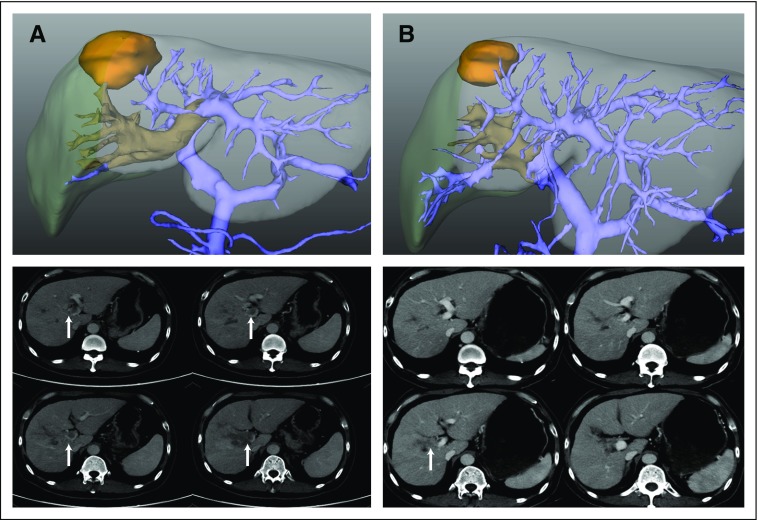
FIG A1.
Tumor and extent of portal vein tumor thrombus (PVTT) before and after neoadjuvant three-dimensional conformal radiotherapy (RT) on computed tomography scan and three-dimensional reconstructed images (with hiding of irrelevant anatomic structures) in a representative patient with partial remission to neoadjuvant RT. (A) A 55-year-old male with an original 4 × 3.5-cm hepatocellular carcinoma with PVTT (arrow), which had extensively invaded the right-side branch, bifurcation, and left-side branch of the portal vein (Cheng’s type III) before RT. (B) Four weeks after RT, tumor size shrunk to 2.5 × 2.5 cm, and the PVTT (arrow) was confined to the right side ipsilateral of the portal vein (type II).
FIG A2.
Kaplan-Meier analysis of patient survival with various types of portal vein tumor thrombus (PVTT) in the neoadjuvant radiotherapy (RT) plus surgery and surgery-alone groups. (A) Overall survival (OS) and (C) disease-free survival (DFS) among patients with Cheng’s type II PVTT in the neoadjuvant RT and surgery-alone groups. (B) OS and (D) DFS among the patients with type III PVTT in the neoadjuvant RT and surgery-alone groups.
FIG A3.
Interleukin-6 (IL-6) expression in patients with different responses to radiotherapy (RT). (A) Comparison of baseline IL-6 levels in serum evaluated by enzyme-linked immunosorbent assay (progressive disease v partial remission/stable disease; P = .047). (B) Comparison of IL-6 expression in hepatocellular carcinoma (HCC) tissues by immunohistochemistry scoring (P = .018). (C) Representative slides of immunohistochemistry (magnification, ×200).
TABLE A1.
Cheng’s Classification of Portal Vein Tumor Thrombus
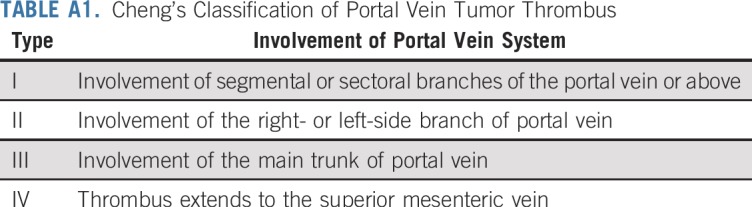
TABLE A2.
Causes of Death
TABLE A3.
Patterns of Recurrence and Initial Salvage Therapy
TABLE A4.
Factors Associated With Postoperative HCC Recurrence and HCC-Related Death Using Univariable Cox Regression Analysis
TABLE A5.
Univariable Analysis of Risk Factors for Progressive Disease After Neoadjuvant Radiotherapy in Patients With Hepatocellular Carcinoma With PVTT
Footnotes
Supported by the National Key Basic Research Program 973 project (No. 2015CB554000), the Project of Shanghai Shenkang Hospital Development Center (No. SHDC12015106), the Key Project of the Natural Science Foundation of China (No. 81730097), the grants of the Science Fund for Creative Research Groups (No. 81521091), and the Chang Jiang Scholars Program (2013) of the Ministry of Education of the People’s Republic of China.
Clinical trial information: ChiCTR-INR-17012510.
AUTHOR CONTRIBUTIONS
Conception and design: Xubiao Wei, Yabo Jiang, Xiuping Zhang, Wan Yee Lau, Shuqun Cheng
Financial support: Shuqun Cheng
Administrative support: Yan Meng, Wenming Cong, Wan Yee Lau, Shuqun Cheng
Provision of study material or patients: Jie Shi, Weixing Guo, Dong Zhou, Hui Zhang, Huichuan Sun, Cheng Huang, Congde Lu, Yaxin Zheng, Shuqun Cheng
Collection and assembly of data: Xubiao Wei, Yabo Jiang, Xiuping Zhang, Shuang Feng, Bin Zhou, Xiaofei Ye, Hui Xing, Ying Xu, Jie Shi, Weixing Guo, Dong Zhou, Hui Zhang, Huichuan Sun, Cheng Huang, Congde Lu, Yaxin Zheng, Yan Meng
Data analysis and interpretation: Xubiao Wei, Yabo Jiang, Xiuping Zhang, Xiaofei Ye, Bin Huang, Wenming Cong
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
No potential conflicts of interest were reported.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2. doi: 10.1016/j.jhep.2018.03.019. European Association for the Study of the Liver: EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182-236, 2018 [Erratum: J Hepatol 70:817, 2019] [DOI] [PubMed] [Google Scholar]
- 3.Shuqun C, Mengchao W, Han C, et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54:499–502. [PubMed] [Google Scholar]
- 4.Sakata H, Konishi M, Ryu M, et al. Prognostic factors for hepatocellular carcinoma presenting with macroscopic portal vein tumor thrombus. Hepatogastroenterology. 2004;51:1575–1580. [PubMed] [Google Scholar]
- 5.Le Treut YP, Hardwigsen J, Ananian P, et al. Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature. A European case-control series. J Gastrointest Surg. 2006;10:855–862. doi: 10.1016/j.gassur.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Chen XP, Qiu FZ, Wu ZD, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:940–946. doi: 10.1245/ASO.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Peng ZW, Guo RP, Zhang YJ, et al. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118:4725–4736. doi: 10.1002/cncr.26561. [DOI] [PubMed] [Google Scholar]
- 8.Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West Study Group. Ann Surg. 2013;257:929–937. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073–2080. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 10.Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: A randomized clinical trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su F, Chen KH, Liang ZG, et al. Comparison of three-dimensional conformal radiotherapy and hepatic resection in hepatocellular carcinoma with portal vein tumor thrombus. Cancer Med. 2018;7:4387–4395. doi: 10.1002/cam4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano R, Ohira M, Kobayashi T, et al. Hepatectomy versus stereotactic body radiotherapy for primary early hepatocellular carcinoma: A propensity-matched analysis in a single institution. Surgery. 2018;164:219–226. doi: 10.1016/j.surg.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Feng S, Xue J, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: A comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB. 2016;18:549–556. doi: 10.1016/j.hpb.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cha H, Lee EJ, Seong J. Multi-analyte analysis of cytokines that predict outcomes in patients with hepatocellular carcinoma treated with radiotherapy. World J Gastroenterol. 2017;23:2077–2085. doi: 10.3748/wjg.v23.i11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu CT, Chen MF, Chen WC, et al. The role of IL-6 in the radiation response of prostate cancer. Radiat Oncol. 2013;8:159. doi: 10.1186/1748-717X-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen MF, Hsieh CC, Chen WC, et al. Role of interleukin-6 in the radiation response of liver tumors. Int J Radiat Oncol Biol Phys. 2012;84:e621–e630. doi: 10.1016/j.ijrobp.2012.07.2360. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Li N, Feng S, et al. Portal vein tumor thrombus is more sensitive to irradiation. Int J Clin Exp Med. 2017;10:4486–4496. [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19. National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0, 2009. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng YZ, Cai Z, Shi S, et al. Cilia loss sensitizes cells to transformation by activating the mevalonate pathway. J Exp Med. 2018;215:177–195. doi: 10.1084/jem.20170399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng J, Wu J. Hepatitis B- and hepatitis C-related hepatocellular carcinomas in the United States: Similarities and differences. Hepat Mon. 2012;12:e7635. doi: 10.5812/hepatmon.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Utsunomiya T, Shimada M, Kudo M, et al. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: A nationwide study of 11,950 patients. Ann Surg. 2015;261:513–520. doi: 10.1097/SLA.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 25.Cheng S, Chen M, Cai J. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget. 2017;8:8867–8876. doi: 10.18632/oncotarget.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang T, Lin C, Zhai J, et al. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol. 2012;138:1121–1129. doi: 10.1007/s00432-012-1188-0. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa K, Shiba H, Horiuchi T, et al. Survival benefit of hepatic resection for hepatocellular carcinoma beyond the Barcelona Clinic Liver Cancer classification. J Hepatobiliary Pancreat Sci. 2017;24:199–205. doi: 10.1002/jhbp.436. [DOI] [PubMed] [Google Scholar]
- 28.Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938–943. doi: 10.1016/j.jhep.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 29.Liang L, Chen TH, Li C, et al. A systematic review comparing outcomes of surgical resection and non-surgical treatments for patients with hepatocellular carcinoma and portal vein tumor thrombus. HPB. 2018;20:1119–1129. doi: 10.1016/j.hpb.2018.06.1804. [DOI] [PubMed] [Google Scholar]
- 30.Lau WY, Ho SK, Yu SC, et al. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299–305. doi: 10.1097/01.sla.0000133123.11932.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol. 2007;14:3301–3309. doi: 10.1245/s10434-007-9549-7. [DOI] [PubMed] [Google Scholar]
- 32.Shui Y, Yu W, Ren X, et al. Stereotactic body radiotherapy based treatment for hepatocellular carcinoma with extensive portal vein tumor thrombosis. Radiat Oncol. 2018;13:188. doi: 10.1186/s13014-018-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. doi: 10.1111/hepr.12392. Fujino H, Kimura T, Aikata H, et al: Role of 3-D conformal radiotherapy for major portal vein tumor thrombosis combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res, 45:607-617, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Tang QH, Li AJ, Yang GM, et al. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: A comparative study. World J Surg. 2013;37:1362–1370. doi: 10.1007/s00268-013-1969-x. [DOI] [PubMed] [Google Scholar]
- 35.Lin H, Li X, Liu Y, et al. Neoadjuvant radiotherapy provided survival benefit compared to adjuvant radiotherapy for hepatocellular carcinoma. ANZ J Surg. 2018;88:E718–E724. doi: 10.1111/ans.14387. [DOI] [PubMed] [Google Scholar]
- 36.Centurione L, Aiello FB. DNA repair and cytokines: TGF-β, IL-6, and thrombopoietin as different biomarkers of radioresistance. Front Oncol. 2016;6:175. doi: 10.3389/fonc.2016.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Zhang F, Tsai Y, et al. IL-6 signaling promotes DNA repair and prevents apoptosis in CD133+ stem-like cells of lung cancer after radiation. Radiat Oncol. 2015;10:227. doi: 10.1186/s13014-015-0534-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Guo Y, Xu F, Lu T, et al. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]