Abstract
PURPOSE
The aim of the current study was to increase the uptake of screening mammography among high-risk women who were treated for a childhood cancer with chest radiotherapy.
PATIENTS AND METHODS
Two hundred four female survivors in the Childhood Cancer Survivor Study who were treated with chest radiotherapy with 20 Gy or greater, age 25 to 50 years, and without breast imaging in the past 24 months were randomly assigned 2:1 to receive a mailed informational packet followed by a tailored telephone-delivered brief motivational interview (intervention) versus an attention control. Primary outcome was the difference in the proportion of participants who completed a screening mammogram by 12 months as evaluated in an intent-to-treat analysis. Stratum-adjusted relative risk (RR) and 95% CI were estimated using the Cochran-Mantel-Haenszel method. Secondary outcomes included the completion of screening breast magnetic resonance imaging (MRI) and barriers to screening and moderating factors.
RESULTS
Women in the intervention group were significantly more likely than those in the control group to report a mammogram (45 [33.1%] of 136 v 12 [17.6%] of 68; RR, 1.9; 95% CI, 1.1 to 3.3). The intervention was more successful among women age 25 to 39 years (RR, 2.2; 95% CI, 1.1 to 4.7) than among those age 40 to 50 years (RR, 1.4; 95% CI, 0.6 to 3.2). The proportion of women who reported a breast MRI at 12 months was similar between the two groups: 16.2% (intervention) compared with 13.2% (control; RR, 1.2; 95% CI, 0.6 to 2.5). Primary barriers to completing a screening mammogram and/or breast MRI included lack of physician recommendation, deferred action by survivor, cost, and absence of symptoms.
CONCLUSION
Use of mailed materials followed by telephone-delivered counseling increased mammography screening rates in survivors at high risk for breast cancer; however, this approach did not increase the rate of breast MRI. Cost of imaging and physician recommendation were important barriers that should be addressed in future studies.
INTRODUCTION
By age 50 years, one in three women who were treated for a childhood cancer with chest radiotherapy will be diagnosed with breast cancer, a risk equivalent to that of BRCA1 carriers.1 Because early detection of breast cancer is strongly associated with survival in the general population, breast cancer surveillance with annual screening mammography and breast magnetic resonance imaging (MRI) is recommended for female survivors of childhood cancer who were treated with chest radiotherapy, starting at age 25 or 8 years after chest radiation, whichever occurs last.2-4 As we have previously documented, the majority of women in this risk group is not adherent to these recommendations.5,6 Magnifying this problem, most survivors of childhood cancer are unaware of their risks,5,7 are no longer observed at a cancer center,8,9 and are instead observed by primary care providers (PCPs) who rarely receive a survivorship care plan for these survivors and, as a result, are poorly informed about recommended follow-up care.10-12
Our preliminary studies suggested that an educational intervention with a recommendation for annual mammography may lead to increased surveillance rates.13 Furthermore, our data suggested that inclusion of a behaviorally based method to address screening self-efficacy and other related individual factors would enhance the intervention and facilitate the initiation and maintenance of screening.5,14,15 This approach, which is especially relevant for women who require a more intensive and personalized health-related, behavior-based intervention, can optimally be delivered by telephone via a brief motivational interview.16-20
Thus, we conducted the two-arm, unblinded, randomized controlled EMPOWER study (ClinicalTrials.gov identifier: NCT01579552) among high-risk female survivors age 25 to 50 years to test the efficacy of mailed educational materials, followed by a telephone-delivered brief motivational interview, on completing breast cancer screening—primarily with mammography and secondarily with breast MRI—compared with an attention control group.
PATIENTS AND METHODS
Study Design and Participants
Participants were recruited for this institutional review board–approved study from the Childhood Cancer Survivor Study, a 31-institution retrospective cohort that consists of 24,363 long-term childhood cancer survivors who were diagnosed before age 21 years, between 1970 and 1999, surviving at least 5 years from diagnosis and living in the United States or Canada. The Childhood Cancer Survivor Study cohort methodology and study design have been previously described in detail.21,22 Survivors were eligible to participate in the EMPOWER study if they were female, treated with chest radiotherapy with 20 Gy or greater, at least 8 years from chest radiation, age 25 to 50 years at time of enrollment, English speaking, did not have a personal history of breast cancer or myocardial infarction, and had not undergone a mammogram or other breast imaging in the previous 24 months.
Random Assignment and Study Interventions
After the receipt of informed consent, participants were enrolled from October 7, 2010, through April 1, 2014, and were randomly assigned 2:1 via a computer algorithm using a permuted block randomization scheme with a block size of four to the targeted intervention or attention control group. On the basis of our previous findings of lower screening mammography rates among women younger than age 40 years, we stratified randomization by age at enrollment (25 to 39 and 40 to 50 years).5 We also stratified on the basis of race and ethnicity. The baseline survey completed by participants is available at https://ccss.stjude.org/tools-and-documents/questionnaires.html.
The intervention consisted of mailed informational print materials followed by a tailored telephone-delivered brief motivational interview. The intervention was guided by two health behavior theories, the Health Belief Model23-25 and the Transtheoretical Model.26-30 The marriage of the Health Belief Model and Transtheoretical Model models has been particularly successful with mammography interventions.31-37 The mailed packet (Data Supplement) included a cover letter that provided information in lay terms about the risks associated with the woman’s previous chest radiotherapy and the clinical guideline recommendation for annual screening mammography and breast MRI. Four other components included were a one-page description of the potential benefits and other considerations of breast cancer screening for women with a similar cancer history, two laminated cards—one for the participant and one to give to her PCP—highlighting the recommendations, a list of low-cost options for mammography, and a letter template that could be used to obtain approval for coverage of breast MRI from an insurance company, if needed. Two to four weeks later, women were contacted by telephone and a brief motivational interview16,17,38-41 with computer-assisted telephone interviewing was conducted by a trained counselor. The 30- to 45-minute brief motivational interview was tailored to the stage of readiness. Overarching goals of the session were to answer questions about the mailed materials, elicit intrinsic motivation for breast cancer surveillance, create and resolve ambivalence, develop an action plan, and strengthen client commitment to initiate and maintain regular screening. The attention control group received the same number of contacts, but mailed information and telephone calls focused on cardiac health rather than breast cancer risk (Data Supplement). After the completion of the study, women in the control group were provided with the breast cancer–related informational materials sent to the intervention group.
Assessment of Study Outcomes
Primary outcome in the protocol was screening mammography by 12 months after random assignment. As a result of difficulties in obtaining medical record confirmation (described below), we analyzed self-reported completion of a mammogram by 12 months. Secondary aims included moderating factors that predict mammogram completion, the proportion of women who completed a breast MRI, and perceived barriers to completing surveillance imaging. For this latter variable, we asked the question, “How important were each of the following reasons for not having a mammogram?” After this question was a list of items, based on our previous studies,5 using a five-point Likert scale (not at all, a little bit, moderately, quite a bit, extremely). A similar question was used for breast MRI.
Statistical Analysis
The trial was powered to detect a difference of 15% between the intervention and attention control arms, assuming that the proportion of women in the control arm having a mammogram by 12 months would be 10% to 15%.5 We planned to enroll 360 women (intervention, n = 240; control, n = 120) to have at least 85% power and a Type I error rate of 0.05 using a two-sided test. However, the accrual rate was less than anticipated and the study closed with a total of 204 participants enrolled. Sociodemographic characteristics were compared between participants and nonparticipants using Fisher’s exact test and the Wilcoxon rank-sum test for categorical and continuous covariates, respectively.
Primary analysis was an intent-to-treat analysis that included all women who were randomly assigned to the study. Participants who did not complete the 12-month assessment were considered to not have had a mammogram. Intervention and control groups were compared using the Cochran-Mantel-Haenszel test stratifying by the randomization strata. In secondary analyses, we restricted our analyses to the subsets of participants who completed the 12-month assessment.
We examined factors that potentially moderated the efficacy of the intervention using Poisson regression models with a log link function and robust SEs to estimate relative risks (RRs). Fitting separate models for each moderating factor, we modeled the probability of obtaining a mammogram within 12 months as a function of the intervention, factor, interaction between the intervention and the factor, and randomization strata. Similar analyses were performed for receipt of breast MRI. Potential moderating factors, informed by our previous work,5,13-15 included age, race/ethnicity, education level, health insurance status, household income, presence of chronic health conditions, having a cancer treatment summary, and knowledge that chest radiation increases the risk of breast cancer.
Among women who completed the 12-month assessment who did not report a mammogram, the proportions of women who listed key barriers to obtaining a mammogram were compared between arms by modeling the barrier as a function of group and randomization strata also using Poisson models.
All statistical analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.4, 1990; SAS Institute, Cary, NC) or StataSE 15.0 for Windows (STATA, College Station, TX; Computing Resource Center, Santa Monica, CA) using two-sided tests and a significance level of P ≤ .05.
RESULTS
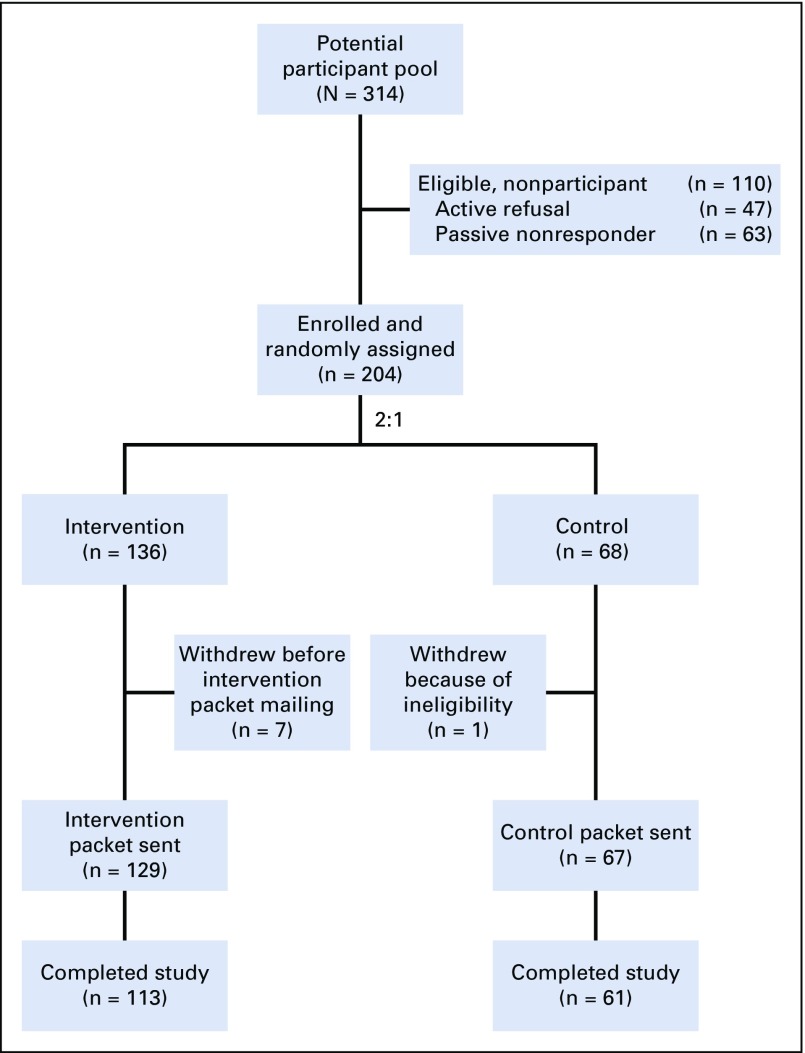
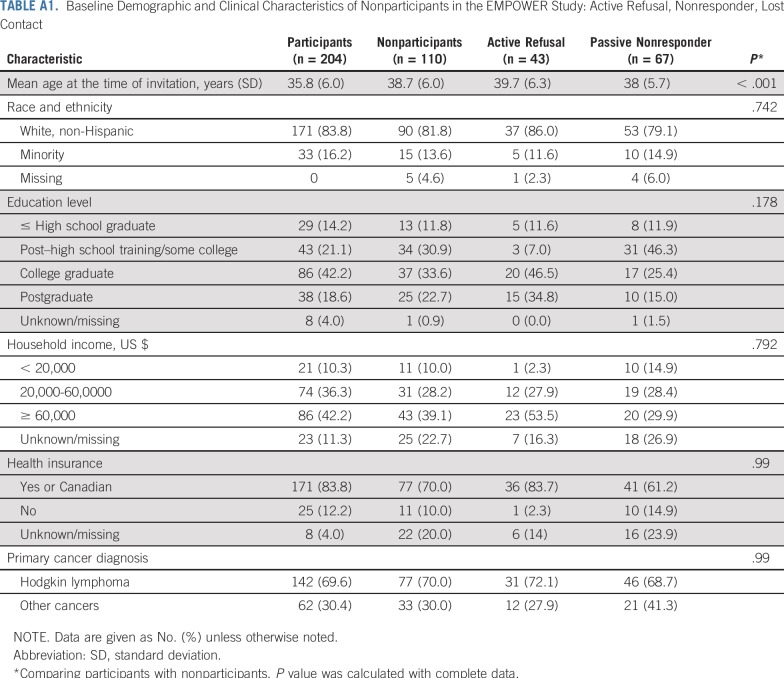
Of the 314 women living in the United States or Canada who were eligible and successfully contacted with the introductory study packets, 204 (65.0%) consented and were randomly assigned—68 to the attention control group and 136 to the intervention group (Fig 1). Forty-three women (13.7%) were successfully contacted but did not consent to participate (active nonparticipants) and another 67 women (21.3%) did not respond to the study invitation (passive nonparticipants). Nonparticipants were modestly older than participants (38.7 years v 35.8 years, respectively; P < .001). There were no significant differences on the basis of race/ethnicity, attained educational level, household income, or health insurance status, according to status at last contact (Appendix Table A1, online only).
FIG 1.
EMPOWER study CONSORT diagram (ClinicalTrials.gov identifier: NCT01579552).
Of the 136 women who were randomly assigned to the intervention group, 80% (109 of 136) received all components of the intervention. Of the 68 women in the control group, 90% (61 of 68) received all components. In total, 174 women (85%) completed the 12-month measurements (intervention group, n = 113 of 136; control group, n = 61 of 68). Intention-to-treat analysis included all randomly assigned participants.
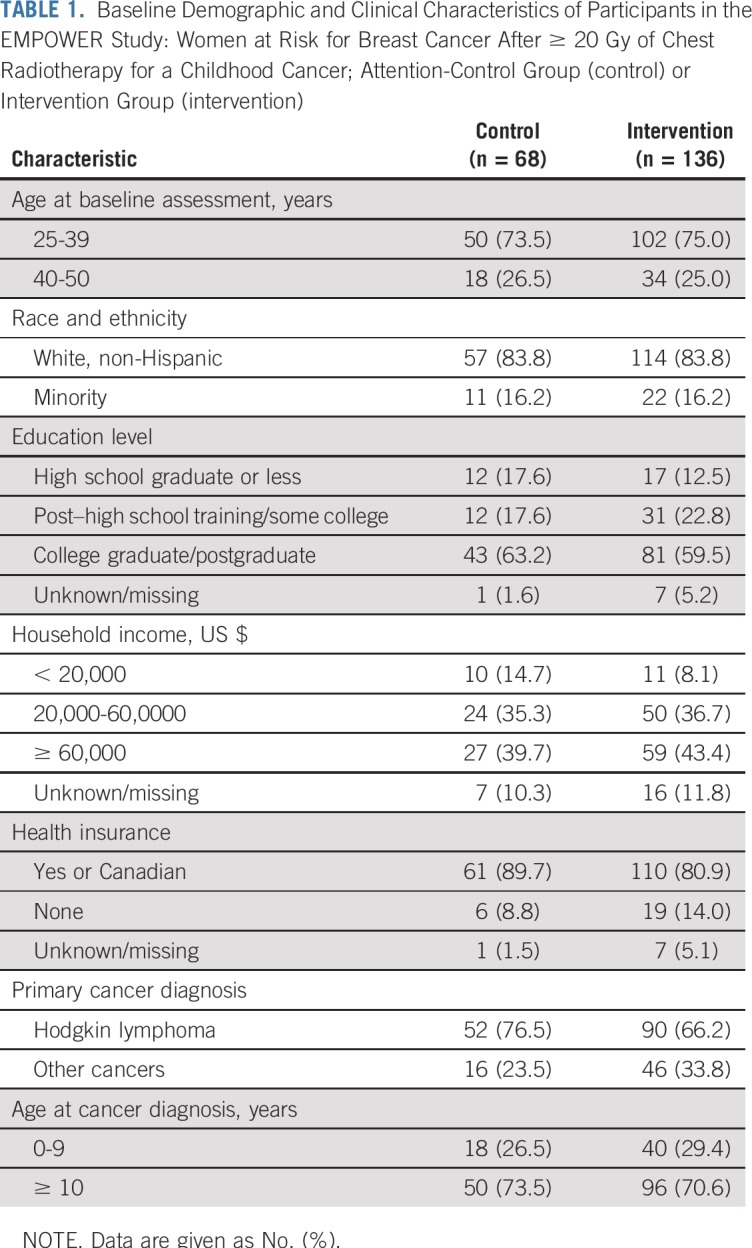
Of the 204 women who were randomly assigned, mean age was 35.8 years, 16.2% were a racial/ethnic minority, and 10.3% had a household income of less than $20,000 (Table 1). Most had some form of insurance (83.8%), although 32% reported a large deductible.
TABLE 1.
Baseline Demographic and Clinical Characteristics of Participants in the EMPOWER Study: Women at Risk for Breast Cancer After ≥ 20 Gy of Chest Radiotherapy for a Childhood Cancer; Attention-Control Group (control) or Intervention Group (intervention)
Primary and Secondary Outcomes
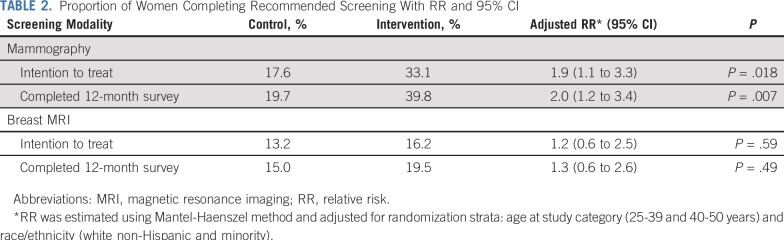
Women in the intervention group were more likely than those in the attention control group to report a mammogram by 12-months (intent-to-treat analysis; 33.1% [45 of 136] v 17.6% [12 of 68]; RR, 1.9; 95% CI, 1.1 to 3.3; Table 2). When restricting the analysis to those who completed the 12-month measurements, the difference between the two groups was slightly greater. Women in the intervention group (45 [39.8%] of 113) were more likely to complete the mammogram than women in the control group (12 [19.7%] of 61; RR, 2.0; 95% CI, 1.2 to 3.4).
TABLE 2.
Proportion of Women Completing Recommended Screening With RR and 95% CI
Although the intervention was associated with increased mammography rates, the proportion of women who reported a breast MRI was similar between the two groups (intervention, 16.2%; control, 13.2%; intention-to treat RR, 1.2; 95% CI, 0.6 to 2.5).
Consistent with the low breast MRI completion rate, only 13.2% of women who were randomly assigned to the intervention group and 10.3% of women in the control group completed both a screening mammogram and breast MRI (intention-to-treat: RR, 1.3; 95% CI, 0.6 to 2.9). When comparing the completion of at least one breast imaging study (mammogram or breast MRI), women who were randomly assigned to the intervention group were 75% more likely to report any screening test than women in the control group (intention-to-treat: RR, 1.7; 95% CI, 1.1 to 2.9).
Of 63 women who reported a screening test, we obtained medical confirmation of breast imaging for 54 of them (85.7%; 12 of 14 in the control group and 42 of 49 in the intervention group). Reasons for the inability to confirm the imaging study included a lack of information regarding the imaging facility, the facility being unable to locate records, and the facility sending only partial information. When we repeated the analysis including only women with a medically confirmed imaging study, results were not substantively different.
Factors Moderating the Efficacy of the Intervention
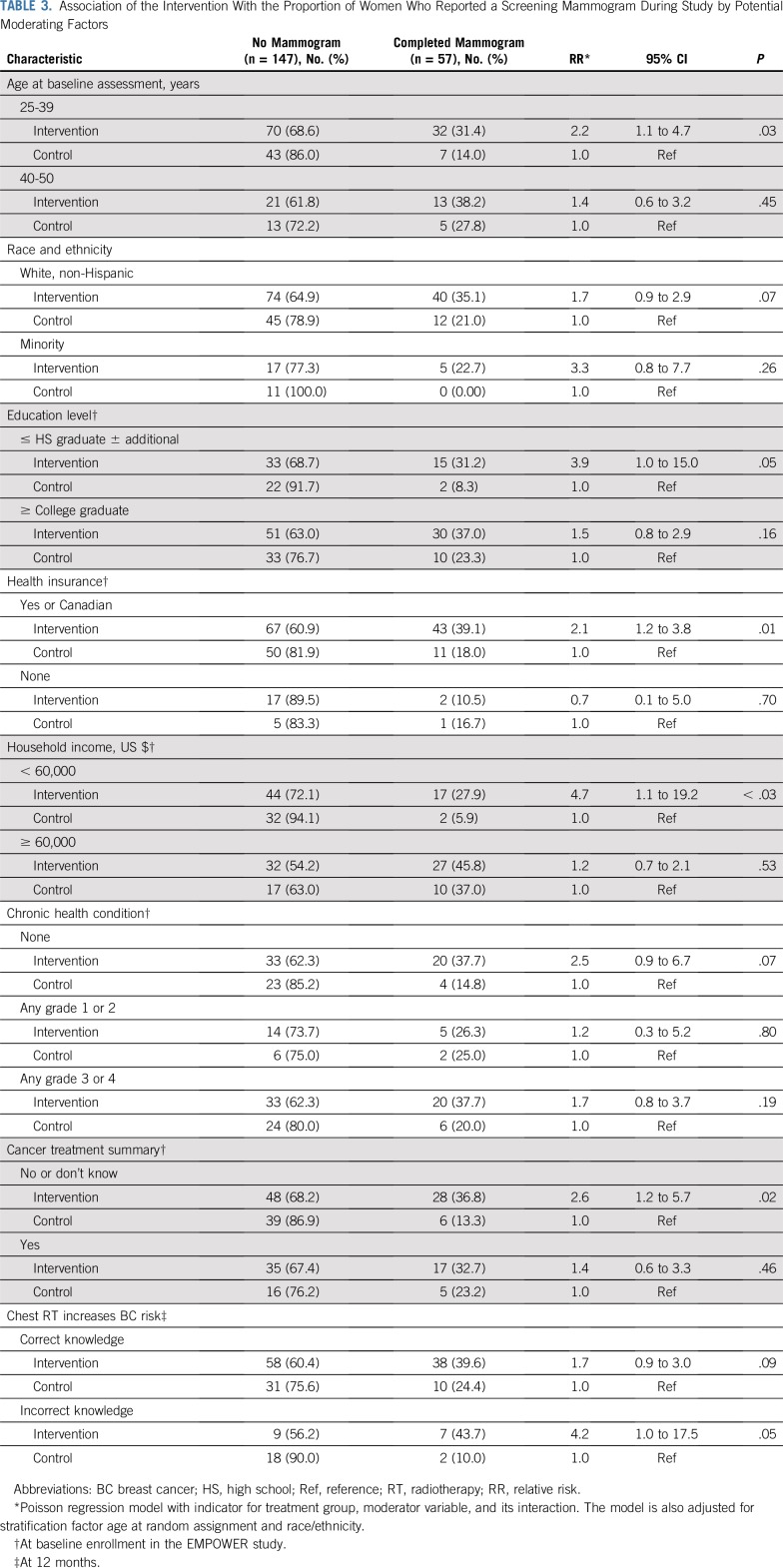
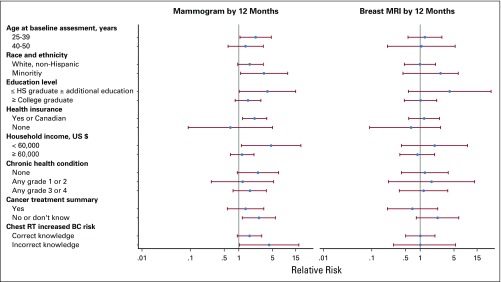
Several factors moderated the efficacy of the intervention (Table 3 and Fig 2). The intervention was more successful among women age 25 to 39 years (RR, 2.2; 95% CI, 1.1 to 4.7) than among those age 40 to 50 years (RR, 1.4; 95% CI, 0.6 to 3.2). Similarly, the intervention seemed to be more efficacious among women with a lower household income, a lower educational attainment, those without a cancer treatment summary, or a lack of awareness of their breast cancer risk before the study. Indeed, less than 10% of women in the control group who had a lower level of education or who were unaware of their breast cancer risk completed a mammogram, whereas more than 35% in the intervention group completed one. Thus, it seems that the intervention was particularly efficacious among the more vulnerable women.
TABLE 3.
Association of the Intervention With the Proportion of Women Who Reported a Screening Mammogram During Study by Potential Moderating Factors
FIG 2.
Association of the intervention with the proportion of women who reported a screening mammogram during study by potential moderating factors. BC, breast cancer; HS, High School; MRI, magnetic resonance imaging; RT, radiotherapy.
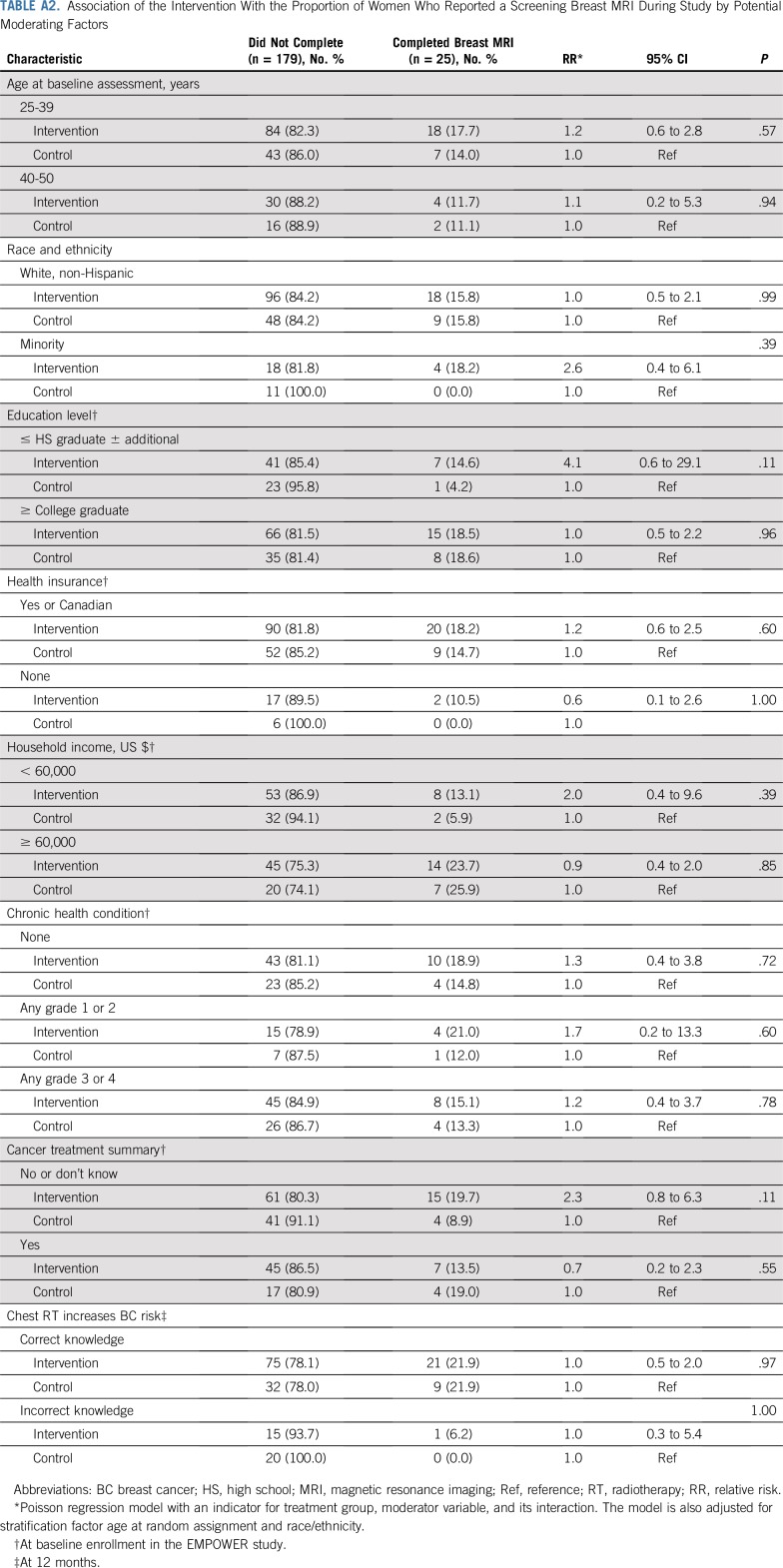
In contrast, none of these factors moderated the efficacy of the intervention on completing a breast MRI (Fig 2 and Appendix Table A2, online only).
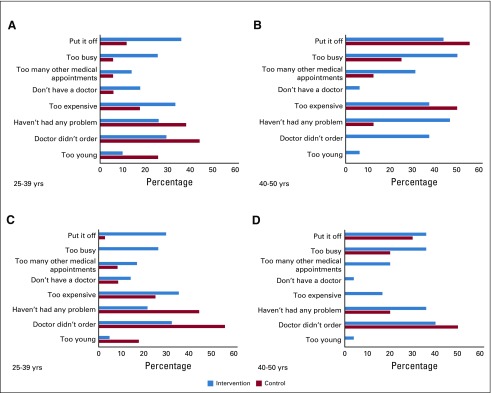
Barriers to Breast Cancer Surveillance
Because primary barriers differed by age group, results are presented separately in Figure 3. Among women in the intervention group age 25 to 39 years, primary barriers to completing a mammogram were “put it off” (36.0%), “too expensive” (34.3%), and “doctor didn’t order it” (29.4%). Among women age 40 to 50 years, primary barriers were “too busy” (50.0%), “haven’t had any problems” (46.7%), “put it off” (43.8%), “doctor didn’t order it” (37.5%), and “too expensive” (37.5%). Barriers to completing a breast MRI were similar (Fig 3).
FIG 3.
Barriers (quite a bit/extremely) to obtaining (A and B) a screening mammogram or (C and D) breast magnetic resonance imaging (MRI)among women who did not complete the recommended surveillance. (NOTE. Participants could have had more than one reason for not completing the breast imaging study.)
DISCUSSION
Recognizing that the cumulative incidence of breast cancer in this population is 30% by age 50 and that mortality after second breast cancers is substantially elevated,1 breast cancer surveillance is an essential component of risk-based health care among women who were treated for a pediatric cancer with chest radiotherapy.2,3 To our knowledge, this is the largest multicenter randomized controlled trial to date aimed at increasing breast cancer surveillance rates in a high-risk population. We determined that women who received a mailed informational packet followed by a telephone-delivered brief motivational interview were twice as likely to report completing a mammogram within 12 months. Furthermore, the intervention may have been more efficacious among younger women and among more vulnerable women (eg, lower level of education or household income or lack of knowledge of risk); however, this subgroup analysis was limited by sample size. Despite the efficacy of the intervention, the overall proportion of high-risk survivors in the intervention group who completed a mammogram remained relatively low at less than 40%. In addition, the intervention did not substantively increase the rate of breast MRI. Key barriers to completing these breast imaging studies included patients’ doctors not ordering the test(s), putting testing off, cost, and the absence of symptoms.
We are aware of only one other randomized trial aimed at increasing breast cancer surveillance among this high-risk population. Bloom et al42 previously conducted a single-institution randomized trial among 157 survivors of Hodgkin lymphoma who were treated with chest radiotherapy before age 35 years and compared mammography rates after a risk notification letter (control) with the letter plus telephone counseling (intervention). Breast MRI rates were not evaluated. Nearly one half of women (47%) reported a mammogram in the 14 months before study enrollment. Among the 133 women who completed the 6-month study, telephone counseling, compared with control, was associated with a 3.6-fold increased likelihood of being in mammography maintenance. Notable differences from our study were the inclusion of women who were already undergoing mammography before enrollment and not using an intent-to-treat analysis. Nevertheless, these two randomized trials found that an informative letter followed by telephone counseling is an effective strategy by which to increase the likelihood of beginning or continuing mammography in this high-risk population.
A novel contribution of this study is exploring factors that moderate the efficacy of the intervention. Although caution is recommended when interpreting these findings on the basis of relatively small subgroup analyses, there seemed to be some important moderators. The intervention may have been more efficacious among women who might be considered less likely to begin screening—younger women, lower household income, or lower level of education—or who were unaware of their risk. Indeed, as our control group illustrates, having these factors of vulnerability was associated with a low likelihood of completing a mammogram without the intervention.
Despite the efficacy of the intervention on mammography rates, this approach led to only a modest increase in screening breast MRI rates. Breast MRI with mammography has been shown to be superior to either test alone among women with a hereditary risk for breast cancer,43-47 or among survivors of Hodgkin lymphoma who were treated with chest radiotherapy.48,49 Recognizing that radiotherapy may be associated with an increased mammographic breast density,2,3 thereby rendering mammography somewhat less sensitive, breast MRI is an essential tool for early breast cancer detection in this young, high-risk population. Indeed, Hodgson et al50 simulated the benefit of breast MRI among women who were treated with chest radiotherapy and observed that approximately 80 women would need to be invited to MRI-based screening to prevent one breast cancer death.
Thus, to build upon the efficacy of our intervention that increases screening mammography and to identity new ways with which to increase the rate of breast MRI use among this high-risk group, it is important to consider the barriers to breast MRI that women face in our trial. Although cost is an obvious barrier, there are several strategies that may help lessen its impact. First, most insurance companies cover the cost of a screening breast MRI among women with a lifetime risk of breast cancer that exceeds 20%.4 In the informational packet, we provided women with a template letter to be sent to insurance companies, if needed; we do not know how often this letter was used by their PCPs. In addition, the Right Action for Women/Christina Applegate Foundation provides low- to no-cost breast MRI for young women with an elevated risk of breast cancer. This avenue should be explored in future studies.51
In addition to cost, three other barriers must be addressed to improve breast cancer surveillance rates among these women: “put it off,” “too busy,” and “doctor didn’t order it.” Studies of different methods of patient and physician activation are warranted. Recognizing that our intervention seemed to be more successful among younger women completing a mammogram, additional efforts may be needed for women older than age 40.
This study has several strengths, including being the largest randomized controlled trial completed to date; using a conceptually based approach; and enrolling a racially, socioeconomically, and geographically diverse group of women with an elevated risk of breast cancer. There were also limitations that should be considered when interpreting the results. Women who participated in this study are part of a cohort study and may be more informed about their risks than other women with similar histories; however, in a population with lower awareness, the intervention may be more effective. Second, we relied on self-reported imaging for our primary analyses. Using self-reported mammography is associated with an overestimation of completion rate.52-54 As noted above, restricting the analysis to include only medical record–confirmed imaging studies did not change the findings. Lastly, 14% of women in the intervention group did not complete the telephone-based brief motivational interview. These limitations are important not only for interpreting the findings of the study, but also for planning future studies.
In summary, an intervention that consisted of a mailed informational packet followed by a telephone-delivered brief motivational interview was associated with a doubling of the screening mammography rate while having minimal impact on breast MRI rate. Recognizing the high risk of breast cancer and breast cancer–specific mortality among women who are treated for a childhood cancer with chest radiotherapy, testing of other approaches aimed at enhancing breast cancer surveillance are urgently needed.
APPENDIX
TABLE A1.
Baseline Demographic and Clinical Characteristics of Nonparticipants in the EMPOWER Study: Active Refusal, Nonresponder, Lost Contact
TABLE A2.
Association of the Intervention With the Proportion of Women Who Reported a Screening Breast MRI During Study by Potential Moderating Factors
Footnotes
Presented at the 2016 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2016.
Supported by National Cancer Institute Grants No. U24-CA55727 (G.T.A.), R01-CA134722 (K.C.O.), K05-CA160724 (K.C.O.) R01-CA136783 (C.S.M.), K07-CA134935 (T.O.H.), and P30-CA008748, and the Meg Berté Owen Foundation. Support to St Jude Children’s Research Hospital was also provided by Cancer Center Support Grant No. CA21765 and the American Lebanese-Syrian Associated Charities.
Clinical trial information: NCT01579552.
AUTHOR CONTRIBUTIONS
Conception and design: Kevin C. Oeffinger, Jennifer S. Ford, Chaya S. Moskowitz, Joanne F. Chou, Tara O. Henderson, Melissa M. Hudson, Lisa Diller, Elena B. Elkin, Kathleen Garrett, Margaret Rebull, Wendy Leisenring, Leslie L. Robison, Gregory T. Armstrong
Financial support: Kevin C. Oeffinger, Leslie L. Robison, Gregory T. Armstrong
Administrative support: Nidha Z. Mubdi, Dayton Rinehart, Christopher Vukadinovich, Gregory T. Armstrong
Provision of study materials or patients: Melissa M. Hudson, Leslie L. Robison, Gregory T. Armstrong
Collection and assembly of data: Kevin C. Oeffinger, Jennifer S. Ford, Joanne F. Chou, Melissa M. Hudson, Aaron McDonald, James Ford, Nidha Z. Mubdi, Dayton Rinehart, Christopher Vukadinovich, Todd M. Gibson, Nassim Anderson, Margaret Rebull, Wendy Leisenring, Leslie L. Robison, Gregory T. Armstrong
Data analysis and interpretation: Kevin C. Oeffinger, Jennifer S. Ford, Chaya S. Moskowitz, Joanne F. Chou, Tara O. Henderson, Melissa M. Hudson, Lisa Diller, Aaron McDonald, Todd M. Gibson, Leslie L. Robison, Gregory T. Armstrong
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Promoting Breast Cancer Surveillance: The EMPOWER Study, a Randomized Clinical Trial in the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Chaya S. Moskowitz
Consulting or Advisory Role: BioClinica
Tara O. Henderson
Research Funding: Seattle Genetics
Other Relationship: Seattle Genetics
Melissa M. Hudson
Consulting or Advisory Role: Coleman Supportive Oncology Initiative for Children with Cancer, Oncology Research Information Exchange Network, Princess Máxima Center
Lisa Diller
Stock and Other Ownership Interests: Novartis (I), Amgen (I), Roche (I), CRISPR Therapeutics (I), Baxter (I), Spark Therapeutics (I), Regenxbio (I), LabCorp (I), Portola Pharmaceuticals (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217–2223. doi: 10.1200/JCO.2013.54.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: Surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152:444–455. W144–W154. doi: 10.1059/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulder RL, Kremer LC, Hudson MM, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14:e621–e629. doi: 10.1016/S1470-2045(13)70303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301:404–414. doi: 10.1001/jama.2008.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2010;153:442–451. doi: 10.1059/0003-4819-153-7-201010050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2363–2373. doi: 10.1200/JCO.2008.21.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan PC, Daugherty CK, Wroblewski KE, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv. 2013;7:275–282. doi: 10.1007/s11764-013-0271-0. [DOI] [PubMed] [Google Scholar]
- 11.Oeffinger KC, Robison LL. Childhood cancer survivors, late effects, and a new model for understanding survivorship. JAMA. 2007;297:2762–2764. doi: 10.1001/jama.297.24.2762. [DOI] [PubMed] [Google Scholar]
- 12.Suh E, Daugherty CK, Wroblewski K, et al. General internists’ preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med. 2014;160:11–17. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Hudson MM, Mertens AC, et al. Increasing rates of breast cancer and cardiac surveillance among high-risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatr Blood Cancer. 2011;56:818–824. doi: 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SM, Ford JS, Rakowski W, et al. Inconsistent mammography perceptions and practices among women at risk of breast cancer following a pediatric malignancy: A report from the Childhood Cancer Survivor Study. Cancer Causes Control. 2010;21:1585–1595. doi: 10.1007/s10552-010-9587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SM, Moskowitz CS, Ford JS, et al. Health care utilization, lifestyle, and emotional factors and mammography practices in the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2015;24:1699–1706. doi: 10.1158/1055-9965.EPI-14-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: The Guilford Press; 1991. [Google Scholar]
- 17.Miller WR, Sanchez VC. Motivating young adults for treatment and lifestyle change. In: Howard G, editor. Alcohol Use and Misuse by Young Adults. Notre Dame, IN: University of Notre Dame Press; 1994. pp. 55–82. [Google Scholar]
- 18.West DS, DiLillo V, Bursac Z, et al. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30:1081–1087. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- 19.Ludman EJ, Curry SJ, Meyer D, et al. Implementation of outreach telephone counseling to promote mammography participation. Health Educ Behav. 1999;26:689–702. doi: 10.1177/109019819902600509. [DOI] [PubMed] [Google Scholar]
- 20.Lando HA, Hellerstedt WL, Pirie PL, et al. Brief supportive telephone outreach as a recruitment and intervention strategy for smoking cessation. Am J Public Health. 1992;82:41–46. doi: 10.2105/ajph.82.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker MH, Drachman RH, Kirscht JP. A new approach to explaining sick-role behavior in low-income populations. Am J Public Health. 1974;64:205–216. doi: 10.2105/ajph.64.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15:175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 25.Cummings KM, Jette AM, Rosenstock IM. Construct validation of the Health Belief Model. Health Educ Monogr. 1978;6:394–405. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- 26.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 27.Prochaska JO, DiClemente CC. Self change processes, self efficacy and decisional balance across five stages of smoking cessation. Prog Clin Biol Res. 1984;156:131–140. [PubMed] [Google Scholar]
- 28.Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. Prog Behav Modif. 1992;28:183–218. [PubMed] [Google Scholar]
- 29.Prochaska JO, DiClemente CC, Velicer WF, et al. Standardized, individualized, interactive, and personalized self-help programs for smoking cessation. Health Psychol. 1993;12:399–405. doi: 10.1037//0278-6133.12.5.399. [DOI] [PubMed] [Google Scholar]
- 30.Velicer WF, DiClemente CC, Prochaska JO, et al. Decisional balance measure for assessing and predicting smoking status. J Pers Soc Psychol. 1985;48:1279–1289. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- 31.Champion V, Maraj M, Hui S, et al. Comparison of tailored interventions to increase mammography screening in nonadherent older women. Prev Med. 2003;36:150–158. doi: 10.1016/s0091-7435(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 32.Champion VL, Skinner CS. Differences in perceptions of risk, benefits, and barriers by stage of mammography adoption. J Womens Health (Larchmt) 2003;12:277–286. doi: 10.1089/154099903321667618. [DOI] [PubMed] [Google Scholar]
- 33.Champion VL, Springston JK, Zollinger TW, et al. Comparison of three interventions to increase mammography screening in low income African American women. Cancer Detect Prev. 2006;30:535–544. doi: 10.1016/j.cdp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Rakowski W, Ehrich B, Goldstein MG, et al. Increasing mammography among women aged 40-74 by use of a stage-matched, tailored intervention. Prev Med. 1998;27:748–756. doi: 10.1006/pmed.1998.0354. [DOI] [PubMed] [Google Scholar]
- 35.Rimer BK, Halabi S, Sugg Skinner C, et al. The short-term impact of tailored mammography decision-making interventions. Patient Educ Couns. 2001;43:269–285. doi: 10.1016/s0738-3991(00)00172-5. [DOI] [PubMed] [Google Scholar]
- 36.Rimer BK, Halabi S, Sugg Skinner C, et al. Effects of a mammography decision-making intervention at 12 and 24 months. Am J Prev Med. 2002;22:247–257. doi: 10.1016/s0749-3797(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 37.Crane LA, Leakey TA, Rimer BK, et al. Effectiveness of a telephone outcall intervention to promote screening mammography among low-income women. Prev Med. 1998;27:S39–S49. doi: 10.1006/pmed.1998.0395. [DOI] [PubMed] [Google Scholar]
- 38.Miller WR, Rollnick S.(eds)Motivational interviewing and the stages of changeinMotivational Interviewing: Preparing People for Change ed 2New York, NY: The Guilford Press; 2002. pp201–216. [Google Scholar]
- 39.Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med. 2001;20:68–74. doi: 10.1016/s0749-3797(00)00254-3. [DOI] [PubMed] [Google Scholar]
- 40.Knight KM, McGowan L, Dickens C, et al. A systematic review of motivational interviewing in physical health care settings. Br J Health Psychol. 2006;11:319–332. doi: 10.1348/135910705X52516. [DOI] [PubMed] [Google Scholar]
- 41.Wilson GT, Schlam TR. The transtheoretical model and motivational interviewing in the treatment of eating and weight disorders. Clin Psychol Rev. 2004;24:361–378. doi: 10.1016/j.cpr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Bloom JR, Stewart SL, Hancock SL. Breast cancer screening in women surviving Hodgkin disease. Am J Clin Oncol. 2006;29:258–266. doi: 10.1097/01.coc.0000209447.63640.5a. [DOI] [PubMed] [Google Scholar]
- 43.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 44.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–1778. doi: 10.1016/S0140-6736(05)66481-1. Erratum: Lancet 365:1848, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: The EVA trial. J Clin Oncol. 2010;28:1450–1457. doi: 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- 46.Chiarelli AM, Prummel MV, Muradali D, et al. Effectiveness of screening with annual magnetic resonance imaging and mammography: Results of the initial screen from the Ontario high risk breast screening program. J Clin Oncol. 2014;32:2224–2230. doi: 10.1200/JCO.2013.52.8331. [DOI] [PubMed] [Google Scholar]
- 47.Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29:1664–1669. doi: 10.1200/JCO.2009.27.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tieu MT, Cigsar C, Ahmed S, et al. Breast cancer detection among young survivors of pediatric Hodgkin lymphoma with screening magnetic resonance imaging. Cancer. 2014;120:2507–2513. doi: 10.1002/cncr.28747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng AK, Garber JE, Diller LR, et al. Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol. 2013;31:2282–2288. doi: 10.1200/JCO.2012.46.5732. [DOI] [PubMed] [Google Scholar]
- 50.Hodgson DC, Cotton C, Crystal P, et al. Impact of early breast cancer screening on mortality among young survivors of childhood Hodgkin’s lymphoma. J Natl Cancer Inst. 2016;108:djw010. doi: 10.1093/jnci/djw010. [DOI] [PubMed] [Google Scholar]
- 51.Right Action for Women Assistance. https://www.rightactionforwomen.org/assistance
- 52.Tiro JA, Sanders JM, Shay LA, et al. Validation of self-reported post-treatment mammography surveillance among breast cancer survivors by electronic medical record extraction method. Breast Cancer Res Treat. 2015;151:427–434. doi: 10.1007/s10549-015-3387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cronin KA, Miglioretti DL, Krapcho M, et al. Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomarkers Prev. 2009;18:1699–1705. doi: 10.1158/1055-9965.EPI-09-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King ES, Rimer BK, Trock B, et al. How valid are mammography self-reports? Am J Public Health. 1990;80:1386–1388. doi: 10.2105/ajph.80.11.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]