Abstract
PURPOSE
Female survivors of childhood cancer have a high risk of subsequent breast cancer. We describe the ensuing risk for mortality and additional breast cancers.
PATIENTS AND METHODS
Female participants in the Childhood Cancer Survivor Study, a cohort of 5-year survivors of cancer diagnosed between 1970 and 1986 before age 21 years, and subsequently diagnosed with breast cancer (n = 274; median age at breast cancer diagnosis, 38 years; range, 20 to 58 years) were matched to a control group (n = 1,095) with de novo breast cancer. Hazard ratios (HRs) and 95% CIs were estimated from cause-specific proportional hazards models.
RESULTS
Ninety-two childhood cancer survivors died, 49 as a result of breast cancer. Overall survival after breast cancer was 73% by 10 years. Subsequent risk of death as a result of any cause was higher among childhood cancer survivors than among controls (HR, 2.2; 95% CI, 1.7 to 3.0) and remained elevated after adjusting for breast cancer treatment (HR, 2.4; 95% CI, 1.7 to 3.2). Although breast cancer–specific mortality was modestly elevated among childhood cancer survivors (HR, 1.3; 95% CI, 0.9 to 2.0), survivors were five times more likely to die as a result of other health-related causes, including other subsequent malignant neoplasms and cardiovascular or pulmonary disease (HR, 5.5; 95% CI, 3.4 to 9.0). The cumulative incidence of a second asynchronous breast cancer also was elevated significantly compared with controls (P < .001).
CONCLUSION
Mortality after breast cancer was higher in childhood cancer survivors than in women with de novo breast cancer. This increased mortality reflects the burden of comorbidity and highlights the need for risk-reducing interventions.
INTRODUCTION
Breast cancer (BC) is one of the most frequently occurring subsequent malignancies in adult survivors of childhood or adolescent cancer.1-3 The highest risk of BC among these survivors is observed in women treated with wide chest radiation fields, who have a risk comparable to women with BRCA mutations.4-7 Risk also is elevated to a lesser degree among women not exposed to chest radiotherapy. The elevated risk may be conferred by treatment with chemotherapy, including anthracyclines and alkylating agents; by genetic mutations; or by gene-chemotherapy interactions.2,8,9 Of note, although considerable work has been done to characterize risk, less is known about the pathology of these cancers and survival after a BC diagnosis in this population. Using data from the Childhood Cancer Survivor Study (CCSS), a large North American cohort of childhood cancer survivors, we sought to define mortality after BC in adult survivors of childhood cancer and to compare these outcomes with those of women with de novo BC.
PATIENTS AND METHODS
Study Population
The CCSS is a retrospective cohort study with longitudinal follow-up of 5-year survivors of cancer diagnosed before age 21 years. Participants were diagnosed and treated between 1970 and 1986 for leukemia, CNS tumor, Hodgkin lymphoma (HL), non-HL, Wilms tumor, neuroblastoma, soft tissue sarcoma, or a bone tumor at one of 26 institutions in North America. The CCSS was approved by institutional review boards at participating centers. Participants, or proxies if younger than 18 years of age, provided informed consent. Information on diagnosis and treatment of childhood cancer was ascertained through abstraction of medical records using standardized protocols. Descriptions of the cohort design and methodology have been previously detailed.10,11
Ascertainment of BC and BC Therapy Information
Participants with BC, subsequent invasive BC, or ductal carcinoma in situ (DCIS) were identified through self- or proxy report or through the National Death Index (NDI) and confirmed by pathology reports or other medical records. BC characteristics, including histology, size, location, grade, hormone receptor status, and human epidermal growth factor receptor 2 (HER2) status were abstracted. Radiation therapy (yes/no) and chemotherapy (yes/no) for treatment of BC were self-reported.
Ascertainment of Cause of Death
NDI death records were searched for cause of death through 2013. Underlying causes of death for deceased participants (using the International Classification of Diseases, Ninth and Tenth Revisions) were provided and identified the initiating cause of death using standardized rules. For deaths that predated the NDI (ie, those in 1975 to 1978; n = 139), death certificates were requested from states where the deaths occurred. For Canadian participants (n = 7, one death), cause of death was unavailable; sensitivity analyses that excluded them yielded no substantive differences.
Comparison With Women With De Novo BC in the SEER Program
Using SEER*Stat version 8.3.4, we extracted case listings from the 18-registry database of women diagnosed between 1983 and 2012 with DCIS or invasive BC as their first cancer. We selected five women for every female CCSS participant with BC, matching on race (white non-Hispanic, black non-Hispanic, Latino-Hispanic, other), age (within 5 years), stage (0, I, II, III, IV), and calendar year of BC diagnosis. Forty-six CCSS participants were missing the stage at BC diagnosis and were excluded from analyses that compared the two groups. A description of the matched populations is presented in the Data Supplement.
Statistical Analysis
Overall survival (all-cause mortality) was defined as the time from BC diagnosis until death as a result of any cause and was estimated using the Kaplan-Meier method. Women lost to follow-up or alive at last contact were censored. Cumulative incidence of cause-specific (BC or other cause) mortality was obtained from a nonparametric estimate12 that treated the competing cause of death as a competing risk. Women lost to follow-up or alive at last contact were censored. Years since BC diagnosis was the time scale.
Overall survival was compared between groups using proportional hazards frailty models, where the correlation induced by matching was modeled with a shared gamma frailty. To explore whether differences in survival persisted after accounting for BC treatment (because treatment dates were unavailable), a landmark analysis that counted survival starting at 1 year after the first BC was performed. SEER combines no with unknown in its therapy databases; consequently, this analysis compared yes with no/unknown for each treatment. Cox models stratified on BC stage evaluated the association of potential risk factors with mortality among childhood cancer survivors.
Breast tumor characteristics were compared with controls and within childhood cancer survivors by age at BC diagnosis using generalized estimating equations with an independent working correlation matrix and robust variance estimates. Using a 1-year landmark analysis, the cumulative incidence of a second asynchronous BC was estimated among women alive without a second BC diagnosis within 1 year of a first BC. Women with less than 1 year of follow-up or with a bilateral mastectomy (98 childhood cancer survivors) after the first BC were excluded. Analyses were conducted using Stata/SE 15.1 for Windows (StataCorp, College Station, TX) and SAS 9.4 for Windows (SAS Institute, Cary, NC) statistical software.
RESULTS
Childhood Cancer Survivor Population
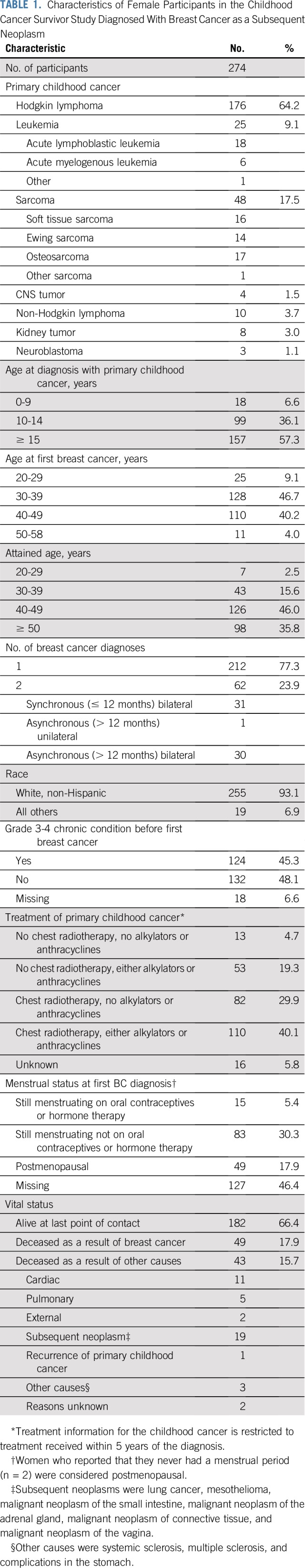
There were 277 childhood cancer survivors subsequently diagnosed with BC. Three were male, are described in the Data Supplement, and are not included in the analyses. Among 274 females, 64% (n = 176; Table 1) were survivors of HL, 71% (n = 195; Data Supplement) had been treated with chest-directed radiation, and 61% (n = 165) had been treated with chemotherapy (n = 88; 32% with anthracyclines). Median age at first BC diagnosis was 38 years (range, 20 to 58 years), median time between childhood cancer and first BC diagnosis was 23 years (range, 7 to 40 years), and median follow-up after the first BC diagnosis among those still alive at last contact was 8 years (range, less than 1 year to 26 years).
TABLE 1.
Characteristics of Female Participants in the Childhood Cancer Survivor Study Diagnosed With Breast Cancer as a Subsequent Neoplasm
Tumor Characteristics
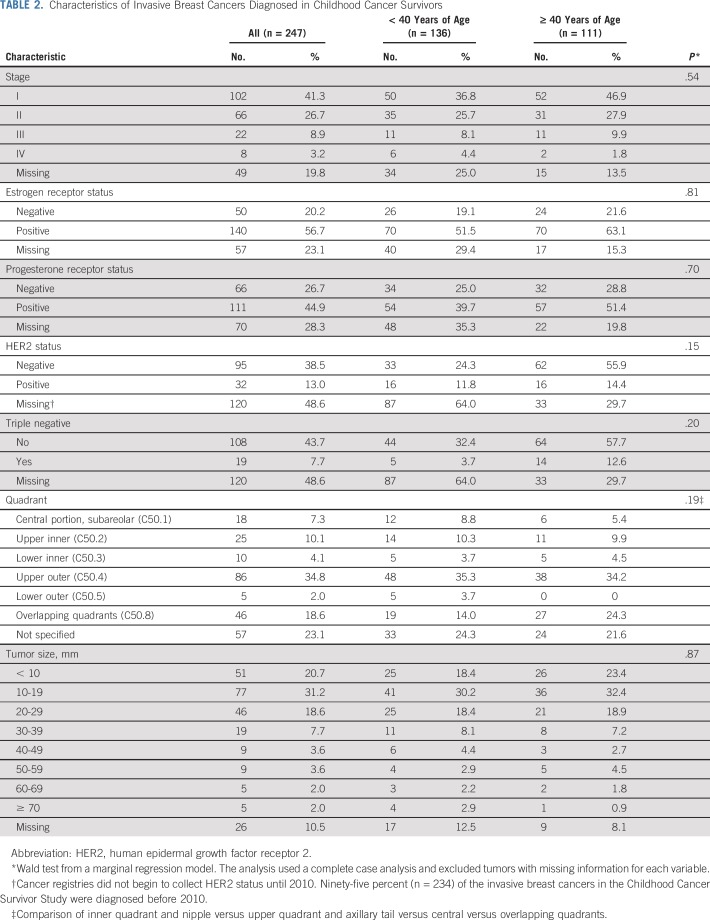
There were 336 BC diagnoses among 274 childhood cancer survivors; 74% (n = 247) were invasive, and 24% (n = 81) were DCIS. Sixty-two survivors had two BCs diagnosed. In 33 instances, both BCs were invasive, and in 24, one was invasive and one was DCIS. Most invasive BCs were diagnosed at an early stage (stage I/II, n = 168; 68%; Table 2). Histopathology and receptor status characteristics were not significantly different from de novo tumors diagnosed in controls (Data Supplement) and did not differ substantively by age at diagnosis.
TABLE 2.
Characteristics of Invasive Breast Cancers Diagnosed in Childhood Cancer Survivors
Overall Survival
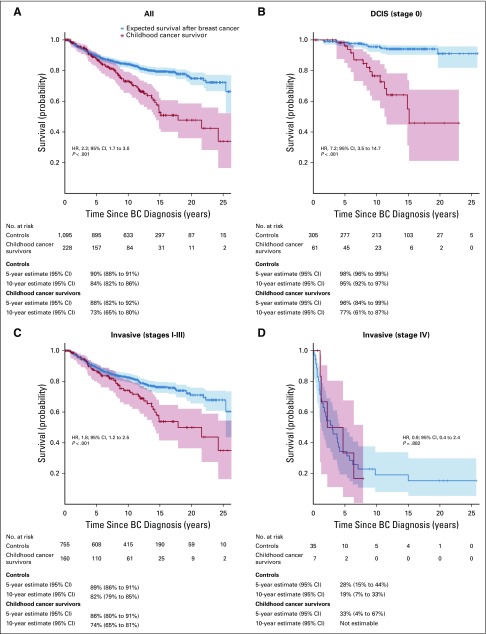
Ninety-two childhood cancer survivors died after a subsequent BC diagnosis (Table 1). Risk of death after BC was more than twofold higher among childhood cancer survivors than among controls (hazard ratio [HR], 2.2; 95% CI, 1.7 to 3.0; Fig 1). Risk remained elevated after adjusting for BC treatment with radiotherapy (HR, 2.2; 95% CI, 1.7 to 3.1), chemotherapy (HR, 2.3; 95% CI, 1.8 to 3.2), or both (HR, 2.4; 95% CI, 1.7 to 3.2). Risk of death was significantly elevated in childhood cancer survivors compared with controls after early-stage BC (DCIS: n = 61 [HR, 7.2; 95% CI, 3.5 to 14.7]; stages I to III: n = 160 [HR, 1.8; 95% CI, 1.2 to 2.5]) but was not elevated after late-stage BC (stage IV: n = 7 [HR, 0.9; 95% CI, 0.4 to 2.4]), although the sample size was small. Risk was significantly elevated among childhood cancer survivors regardless of age at BC (diagnosed before age 40 years: HR, 1.7 [95% CI, 1.2 to 2.5; P = .002]; diagnosed at age 40 years or older: HR, 3.0 [95% CI, 1.8 to 4.9; P < .001]).
FIG 1.
Overall survival after breast cancer (BC) by stage at BC diagnosis. (A) All stages. (B) Ductal in situ carcinoma (DCIS; stage 0). (C) Invasive BC (stages I to III). (D) Invasive BC (stage IV).
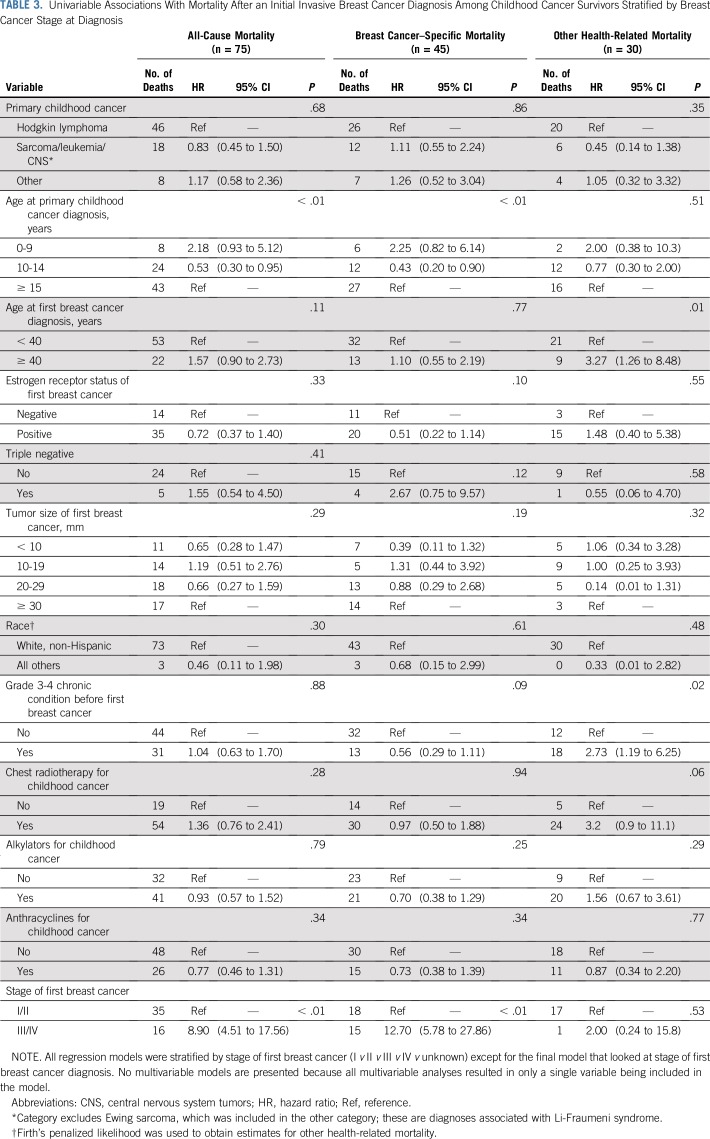
Among childhood cancer survivors, young age at childhood cancer diagnosis was associated with a higher risk of all-cause mortality after invasive BC (Table 3). The data indicate that an older age (40 years or older) at BC diagnosis may be associated with a higher risk of all-cause mortality, but the association was not statistically significant.
TABLE 3.
Univariable Associations With Mortality After an Initial Invasive Breast Cancer Diagnosis Among Childhood Cancer Survivors Stratified by Breast Cancer Stage at Diagnosis
BC-Specific Mortality
Among childhood cancer survivors, 49 women died as a result of BC. Five- and 10-year cumulative incidence rates of BC-specific mortality were 12% (95% CI, 8% to 17%) and 20% (95% CI, 15% to 25%), respectively, among childhood cancer survivors compared with 9% (95% CI, 7% to 11%) and 13% (95% CI, 11% to 16%) among women with de novo BC.
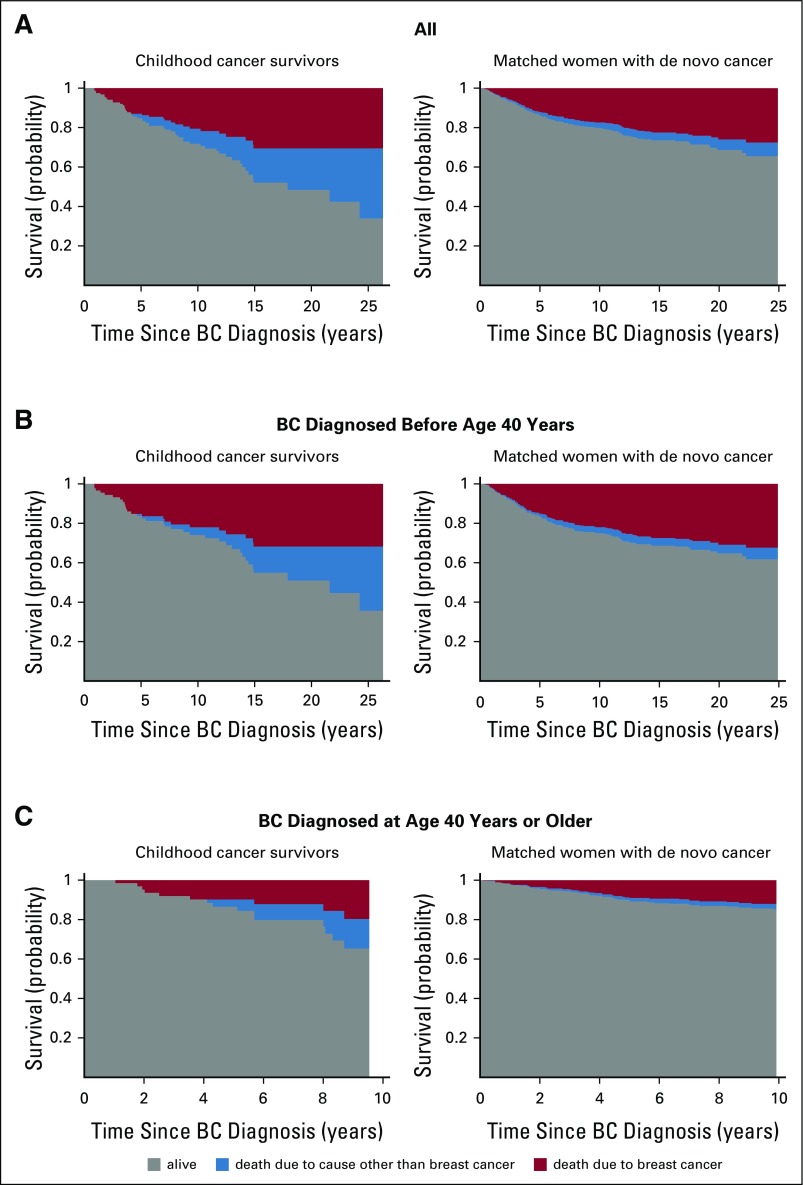
Figure 2 depicts the probability of survival partitioned by cause of death. The risk of childhood cancer survivors dying as a result of BC was slightly elevated but not statistically significantly compared with controls (HR, 1.3; 95% CI, 0.9 to 2.0) and was less than the increased risk observed for all-cause mortality. Results are similar when restricted to women diagnosed with invasive BC (data not shown; HR, 1.2; 95% CI, 0.84 to 1.84). Childhood cancer survivors diagnosed with BC at younger than 40 years of age had a risk of BC-specific mortality similar to controls (HR, 1.1; 95% CI, 0.7 to 1.8), but those diagnosed with BC at age 40 years and older had a somewhat higher risk (HR, 1.8; 95% CI, 0.9 to 3.5). BC-specific mortality by stage at diagnosis is provided in the Data Supplement. Three childhood cancer survivors died as a result of BC after DCIS; two had a subsequent invasive BC at 1 and 6 years after the initial BC.
FIG 2.
Survival outcomes after invasive breast cancer (BC). The gray areas represent the probability of surviving after a BC diagnosis. The other two areas partition death into death as a result of BC (red) and death as a result of other causes (blue). For other, non-BC causes of death among childhood cancer survivors, 42% (n = 18) were attributed to other subsequent neoplasms, 33% (n = 14) to cardiac causes, and 16% (n = 7) to pulmonary complications. In contrast, among the matched general population, only 11% (n = 4) were attributed to other neoplasms, 14% (n = 5) to cardiac causes, and 14% (n = 5) to pulmonary conditions, whereas 28% were attributed to other health-related conditions (eg, pregnancy complications, septicemia, congenital conditions) and 20% to non–health-related causes (eg, accidents). (A) All BC diagnoses. (B) BCs diagnosed before 40 years of age. (C) BCs diagnosed at age 40 years or older.
Among childhood cancer survivors, a young age at childhood cancer diagnosis was associated with a higher risk of BC-specific mortality (Table 3), but this should be interpreted cautiously given the small number of events. A decreased risk of BC-specific mortality was associated with estrogen receptor–positive BC and an increased risk associated with triple-negative cancers, although sample sizes were relatively small among women diagnosed with invasive BC, and associations were not statistically significant (Table 3). The same trends were evident when also looking at women diagnosed with DCIS (Data Supplement). We did not find evidence that other factors, including chest radiotherapy, were significantly associated with BC-specific mortality.
Other Causes of Mortality
All other ascertained causes of death among childhood cancer survivors were health related (Table 1). Of 43 deaths, 42% (n = 18) were attributed to other subsequent neoplasms, 33% (n = 14) to cardiac causes, and 16% (n = 7) to pulmonary complications. In contrast, among women with de novo BC, of deaths not attributed to BC, 20% (n = 10) were unrelated to heath conditions (Fig 2). The 5- and 10-year cumulative incidence rates of other-cause mortality were 3% (95% CI, 1% to 6%) and 13% (95% CI, 9% to 19%), respectively, among childhood cancer survivors compared with 1% (95% CI, 0.8% to 2%) and 3% (95% CI, 2% to 4%) among women with de novo BC.
Other-cause mortality after BC was significantly higher among all childhood cancer survivors (HR, 5.5; 95% CI, 3.4 to 9.0; P < .001), among those diagnosed with early-stage BC (DCIS: HR, 12.3 [95% CI, 5.0 to 30.0; P < .001]; stages I to III: HR, 4.4 [95% CI, 2.3 to 8.2; P < .001]), among those diagnosed with BC at a young age (less than 40 years: HR, 5.1 [95% CI, 2.7 to 10.0; P < .001]), and among those diagnosed with BC at age 40 years or older (HR, 7.0; 95% CI, 3.1 to 15.3; P < .001).
In addition to a young age at childhood cancer diagnosis, having a grade 3 or 4 chronic condition before diagnosis of invasive BC was significantly associated with other-cause mortality in childhood cancer survivors (HR, 2.7; 95% CI, 1.19 to 6.25; Table 3). The data also suggest an association between chest radiotherapy for the childhood cancer and other-cause mortality (HR, 3.2; 95% CI, 0.9 to 11.1). Relative to other childhood cancer survivors, we did not observe an elevated risk of other-cause mortality among survivors with primary cancers typically associated with Li-Fraumeni syndrome (sarcoma, leukemia, and CNS tumors) who are known to have poor survival as a result of other malignancies.
Incidence of Second BCs
Among 176 childhood cancer survivors alive and without a second BC within 1 year of an initial BC, 31 were diagnosed with a second BC 1 or more years later. The cumulative incidence rate of a second asynchronous BC was significantly elevated among childhood cancer survivors relative to controls (P < .001). At 5 years, it was 8.0% (95% CI, 4.4% to 12.7%) among childhood cancer survivors and 2.7% (95% CI, 1.9% to 3.9%) among controls.
DISCUSSION
Our study is the first, to our knowledge, to analyze a large number of childhood cancer survivors with subsequent BC and to address, in a single population, multiple aspects related to subsequent BC characteristics and outcomes. We found that mortality is significantly elevated among survivors of childhood cancer, even in the setting of early-stage BC, despite apparent similarities between BCs as subsequent neoplasms in childhood cancer survivors and de novo BCs in the general population. Although BC-specific mortality was modestly higher in childhood cancer survivors, deaths attributable to health conditions other than BC seem to be the driving force in the elevated all-cause mortality. To change the dismal outcomes of these women, our results suggest that it is imperative that at the time of a secondary BC diagnosis, they have a comprehensive evaluation that extends beyond a singular focus of the BC. This should include an assessment of existing cardiopulmonary disease and a plan for future cancer screening to optimize the management of comorbidities and cardiopulmonary disease and prolong the lifespan of these survivors.
Although much has been written about the risks childhood cancer survivors have for other subsequent cancers and cardiopulmonary disease,3,13-15 the prevalence of these conditions at the time that a subsequent BC is diagnosed has not been well described. Because childhood cancer survivors tend to have high rates of multiple chronic conditions and frailty, even at young ages,14,16,17 we expect that at the time of BC diagnosis, relative to women with de novo BC, they are more likely to be frail and have multiple chronic health conditions, and these conditions contribute to differences in mortality (Data Supplement). Of note, the leading non-BC causes of death in our study (cardiovascular disease, other subsequent neoplasms, and lung disease) are conditions known to be associated with premature mortality in childhood cancer survivors.18 Differences in overall survival between the groups were particularly striking in women diagnosed with early-stage disease and substantially less pronounced in women diagnosed with late-stage disease, likely because of the severity of the disease in the latter case.
Studies that focused exclusively on HL survivors diagnosed at a wide range of ages found similar trends.19-21 However, although age at a de novo BC diagnosis is an important prognostic factor used for guiding treatment decisions,22 these prior studies did not describe outcomes separately by age at BC diagnosis. Our data are the first to suggest that differences in BC-specific mortality between childhood cancer survivors and women with de novo BC may be smaller for women diagnosed at younger than 40 years of age than differences observed for women diagnosed at age 40 years or older. This observation may reflect the tendency for women diagnosed with de novo BC who are younger than age 40 years to have more aggressive disease than those diagnosed at age 40 years or older.23 With insufficient data to evaluate this comparison formally, these findings need to be explored in future work. Differences in other-cause mortality were markedly elevated regardless of age at BC diagnosis.
Among women with de novo BC, all-cause and BC-specific mortality as well as incidence of second BCs vary by patient and disease-related factors, including race,24-28 age at BC diagnosis,22,25,27-30 stage at diagnosis,25,26,28,30,31 tumor size,27 and hormone receptor status of the tumor.25,30,32 Whether these factors are similarly associated with prognosis after BC subsequent to a childhood cancer is unknown. Although not key contributors to all-cause mortality, our data suggest that many of these same factors may be associated with BC-specific mortality. Studies of de novo BCs also have suggested that tumor characteristics differ by menopausal status after controlling for age at BC.33 Our ability to assess formally differences by menopausal status among childhood cancer survivors was limited by missing data, but we show descriptive data on tumor characteristics by menopausal status in the Data Supplement.
Strengths of our study include the use of a relatively large cohort that is geographically, racially, and socioeconomically diverse, with detailed information on childhood cancer treatment exposures available, as well as the use of SEER data. Several limitations also are important to consider. First, analyses from SEER among women with de novo BC at a young age likely included women with hereditary cancers. Mortality rates may be elevated after hereditary BCs, which would lead to an underestimation of the association between a primary childhood cancer and mortality after BC. One analysis, however, suggested no difference in overall survival after BC diagnosed at a young age when comparing carriers of mutations in BRCA1/2 versus women who are BRCA mutation negative.34 Second, others have pointed to difficulties in ascertaining cause of death from death certificates.35 We have likely misclassified cause of death to some extent but expect misclassification to be similar in CCSS and SEER. Of note, we lacked precise information on BC treatments in CCSS and SEER; a substantial amount of these data are missing in both data sets, and neither data set contains information on HER2-directed therapy or anthracyclines, which limits our ability to account for the affect that BC treatment has on survival and the additional anthracycline exposure has on other-cause mortality through increased cardiac morbidity.36 We were also missing information on hormone receptor status as well as HER2 status because of the era in which many BCs were diagnosed. SEER began collecting information on hormone receptor status in 1990 and HER2 status in 2010 and infrequently collected hormone receptor status for DCIS in the early years. In our data, 6% of the childhood cancer survivors’ BCs were diagnosed before 1990 and 95% before 2010. Although a limitation, we do not expect that these missing data had a large impact on the comparison between the childhood cancer survivors and women with de novo BC because the reason the data were missing is not related to the BCs or the women and likely affects both CCSS and SEER similarly. Of note, among women with invasive BC, a smaller proportion were missing hormone receptor status.
In summary, childhood cancer survivors with BC have elevated mortality rates. Primary drivers of this elevated mortality are chronic morbidities associated with previous therapies. Although guidelines recommend related follow-up care, many primary care physicians are unaware of these guidelines, and many childhood cancer survivors are not receiving the recommended surveillance.37,38 Advocacy and research efforts focused on increasing awareness and education around the specialized long-term follow-up care and available guidelines for survivors of childhood, adolescent, and young adult cancer are critical. Moreover, at the time of a BC diagnosis, we strongly recommend that women have a comprehensive evaluation for late effects related to their prior childhood cancer therapy. Finally, we observed a modestly elevated risk of BC-specific mortality. It has been suggested that therapeutic options may be limited because of prior treatment (eg, anthracyclines, radiotherapy); however, supporting data are limited, and additional work is needed to assess this hypothesis better. Regardless, our results emphasize the need for this population with a high risk of mortality to be followed by clinicians familiar with the health conditions faced by childhood cancer survivors.
Footnotes
Presented at the 2018 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2018.
Supported by grants from the National Cancer Institute (R01CA136783, C.S.M., principal investigator [PI]; U24CA55727, G.T.A., PI; K05CA160724 and R01CA134722, K.C.O., PI; K07CA134935, T.O.H., PI; P30CA21765, C. Roberts, PI), Memorial Sloan Kettering Cancer Center Core grant P30 CA008748, and the Meg Berté Owen Foundation. Support to St Jude Children’s Research Hospital was provided by the American Lebanese Syrian Associated Charities.
AUTHOR CONTRIBUTIONS
Conception and design: Chaya S. Moskowitz, Wendy M. Leisenring, Gregory T. Armstrong, Kevin C. Oeffinger, Tara O. Henderson
Administrative support: Chaya S. Moskowitz, Gregory T. Armstrong, Leslie L. Robison
Financial support: Chaya S. Moskowitz, Gregory T. Armstrong, Kevin Oeffinger, Tara Henderson
Provision of study material or patients: Joseph P. Neglia, Lisa R. Diller, Gregory T. Armstrong, Leslie L. Robison
Collection and assembly of data: Chaya S. Moskowitz, Joanne F. Chou, Joseph P. Neglia, Rebecca M. Howell, Danielle Novetsky Friedman, Lucie M. Turcotte, Michael A. Arnold, Gregory T. Armstrong, Leslie L. Robison, Tara O. Henderson
Data analysis and interpretation: Chaya S. Moskowitz, Joanne F. Chou, Ann H. Partridge, Dana Barnea, Lindsay M. Morton, Wendy M. Leisenring, Gregory T. Armstrong, Kevin C. Oeffinger, Tara O. Henderson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Mortality After Breast Cancer Among Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Chaya S. Moskowitz
Consulting or Advisory Role: Bioclinica
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: Small royalty payments for co-authoring the breast cancer survivorship section of UpToDate
Lisa R. Diller
Stock and Other Ownership Interests: Novartis (I), Amgen (I), Roche (I), CRISPR Therapeutics (I), Baxter (I), Spark Therapeutics (I), REGENXBIO (I), LabCorp (I), Portola Pharmaceuticals (I)
Tara O. Henderson
Research Funding: Seattle Genetics
Other Relationship: Seattle Genetics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373:2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 2.Teepen JC, van Leeuwen FE, Tissing WJ, et al. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER study cohort: Role of chemotherapy. J Clin Oncol. 2017;35:2288–2298. doi: 10.1200/JCO.2016.71.6902. [DOI] [PubMed] [Google Scholar]
- 3.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA. 2017;317:814–824. doi: 10.1001/jama.2017.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: Lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239–4246. doi: 10.1200/JCO.2008.19.9174. [DOI] [PubMed] [Google Scholar]
- 5.Lange JM, Takashima JR, Peterson SM, et al. Breast cancer in female survivors of Wilms tumor: A report from the National Wilms Tumor Late Effects Study. Cancer. 2014;120:3722–3730. doi: 10.1002/cncr.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217–2223. doi: 10.1200/JCO.2013.54.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swerdlow AJ, Cooke R, Bates A, et al. Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: A national cohort study. J Clin Oncol. 2012;30:2745–2752. doi: 10.1200/JCO.2011.38.8835. [DOI] [PubMed] [Google Scholar]
- 8.Henderson TO, Moskowitz CS, Chou JF, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34:910–918. doi: 10.1200/JCO.2015.62.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Wilson CL, Easton J, et al. Genetic risk for subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2018;36:2078–2087. doi: 10.1200/JCO.2018.77.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103–112. [Google Scholar]
- 13.Armstrong GT, Yasui Y, Robison LL. Reduction in late mortality after childhood cancer. N Engl J Med. 2016;375:290–292. doi: 10.1056/NEJMc1604184. [DOI] [PubMed] [Google Scholar]
- 14.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 15.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE) Lancet. 2017;390:2569–2582. doi: 10.1016/S0140-6736(17)31610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkin EB, Klem ML, Gonzales AM, et al. Characteristics and outcomes of breast cancer in women with and without a history of radiation for Hodgkin’s lymphoma: A multi-institutional, matched cohort study. J Clin Oncol. 2011;29:2466–2473. doi: 10.1200/JCO.2010.32.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milano MT, Li H, Gail MH, et al. Long-term survival among patients with Hodgkin’s lymphoma who developed breast cancer: A population-based study. J Clin Oncol. 2010;28:5088–5096. doi: 10.1200/JCO.2010.29.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veit-Rubin N, Rapiti E, Usel M, et al. Risk, characteristics, and prognosis of breast cancer after Hodgkin’s lymphoma. Oncologist. 2012;17:783–791. doi: 10.1634/theoncologist.2011-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredholm H, Magnusson K, Lindström LS, et al. Long-term outcome in young women with breast cancer: A population-based study. Breast Cancer Res Treat. 2016;160:131–143. doi: 10.1007/s10549-016-3983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narod SA. Breast cancer in young women. Nat Rev Clin Oncol. 2012;9:460–470. doi: 10.1038/nrclinonc.2012.102. [DOI] [PubMed] [Google Scholar]
- 24. Howlader N, Noone AM, Krapcho M, et al: SEER Cancer Statistics Review, 1975-2014. https://seer.cancer.gov/csr/1975_2014.
- 25.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 26. American Cancer Society: Breast Cancer Facts & Figures 2017-2018. Atlanta, GA, American Cancer Society, 2017. [Google Scholar]
- 27.Rosenberg J, Chia YL, Plevritis S. The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Breast Cancer Res Treat. 2005;89:47–54. doi: 10.1007/s10549-004-1470-1. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein JL, Lapinski RH, Thakore SS, et al. The descriptive epidemiology of second primary breast cancer. Epidemiology. 2003;14:552–558. doi: 10.1097/01.ede.0000072105.39021.6d. [DOI] [PubMed] [Google Scholar]
- 29.Adami HO, Malker B, Holmberg L, et al. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. 1986;315:559–563. doi: 10.1056/NEJM198608283150906. [DOI] [PubMed] [Google Scholar]
- 30.Buist DS, Abraham LA, Barlow WE, et al. Diagnosis of second breast cancer events after initial diagnosis of early stage breast cancer. Breast Cancer Res Treat. 2010;124:863–873. doi: 10.1007/s10549-010-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters S, Maringe C, Butler J, et al. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: A population-based study. Br J Cancer. 2013;108:1195–1208. doi: 10.1038/bjc.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurian AW, McClure LA, John EM, et al. Second primary breast cancer occurrence according to hormone receptor status. J Natl Cancer Inst. 2009;101:1058–1065. doi: 10.1093/jnci/djp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collaborative Group on Hormonal Factors in Breast Cancer Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018;19:169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begg CB, Schrag D. Attribution of deaths following cancer treatment. J Natl Cancer Inst. 2002;94:1044–1045. doi: 10.1093/jnci/94.14.1044. [DOI] [PubMed] [Google Scholar]
- 36.Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with Medicare claims. Med Care. 2016;54:e55–e64. doi: 10.1097/MLR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suh E, Daugherty CK, Wroblewski K, et al. General internists’ preferences and knowledge about the care of adult survivors of childhood cancer: A cross-sectional survey. Ann Intern Med. 2014;160:11–17. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]