Abstract
Children from low income families are at greater risk of poorer health outcomes than their wealthier peers. Hospital admissions for children with gastroenteritis increase as deprivation increases. Noroviruses are responsible for 47–96% of outbreaks of acute paediatric gastroenteritis, and 5–36% of sporadic cases worldwide. However, evidence on the relationship between family income and childhood exposure to norovirus is still limited, with published studies pointing to conflicting results. This study explored the relationship between family income and early childhood exposure to norovirus in the United Kingdom using data from the Millennium Cohort Study linked to serological data. Exposure to norovirus was measured by the level of human norovirus-specific antibodies (titres) obtained from oral fluid samples collected from 5962 pre-school age UK children and tested for Norovirus-specific Immunoglobulin G (IgG). Multivariable linear and quantile regression analyses were conducted to investigate the extent to which family income was associated with child norovirus exposure, and to explore the potential mechanisms through which income might translate into norovirus exposure. Higher norovirus-specific IgG titres were associated with higher family income, but the relationship weakened after controlling for potential mediating factors, mainly increased opportunities for person-to-person contacts, such as formal childcare arrangements. This study provides novel evidence that can help inform and prioritise policy interventions (e.g. vaccination) and health promotion programmes to reduce child health inequalities in the area of gastrointestinal infections.
Keywords: UK, Child health inequalities, Income gradient, Norovirus exposure, Immunoglobulin G, Regression analysis
Highlights
-
•
There are currently conflicting results on socioeconomic patterning of child norovirus exposure.
-
•
We found a positive association between income and norovirus exposure.
-
•
The association weakens/disappears when we adjust our models with additional risk factors.
-
•
Formal childcare increases opportunities for person-to-person spread of norovirus.
-
•
Formal childcare is more prevalent in high-income families.
1. Introduction
Children born into families with low income are at a greater risk of poorer health outcomes than their wealthier peers. This positive association between health and wealth, with origins in childhood, is sometimes referred to as the ‘child health-family income gradient’ (Case, Lubotsky, & Paxson, 2002; Currie & Stabile, 2003). Gastrointestinal infections (GI) are common worldwide and affect one in four people in the UK each year (Tam et al., 2012), but published studies on whether GI is socioeconomically patterned report conflicting results (Adams et al., 2018a; Baker, Taylor, & Henderson, 1998; Beale et al., 2010; Eaton-Evans & Dugdale, 1987; Ludvigsson & Abis Study, 2006; Newman, Leon, Rebolledo, & Scallan, 2015; Phillips, Tam, Rodrigues, & Lopman, 2011), although a recent systematic review (Adams et al., 2018b) found that children from disadvantaged backgrounds have a greater risk of GI infections than their wealthier counterparts. Other recent evidence (Pockett, Adlard, Carroll, & Rajoriya, 2011) showed that paediatric hospital admissions with gastroenteritis in England increased as deprivation increased. However, evidence on the relationship between family income and childhood exposure to norovirus (NoV) is still limited. Norovirus is a major cause of sporadic cases and outbreaks across all age groups (Ahmed et al., 2014), with adverse consequences in the community in both high- and low-income regions. In particular, NoVs are responsible for 47–96% of outbreaks of acute paediatric gastroenteritis, and 5–36% of sporadic cases worldwide (Esposito, Ascolese, Senatore, & Codecà, 2014). In the UK, the median estimated norovirus costs to patients and the health service at 2008–09 prices were £81 million (95% CI: £63 m-£106 m), indicating that norovirus caused greater economic burden than Campylobacter (£50 m) and rotavirus (£25 m) combined (Tam & O'Brien, 2016). Approximately 15% of UK children <5 years of age experience NoV-associated gastroenteritis in the community each year (O'Brien, Donaldson, Iturriza-Gomara, & Tam, 2016). Similarly, a recent US-based study found that 20.7% children <5 years of age sought medical care in the community for NoV-associated gastroenteritis in the year under analysis (September 2012–September 2013) (Grytdal et al., 2016). The primary route of transmission of NoV is faecal-oral through direct contact with infected individuals (De Wit, Koopmans, & Van Duynhoven, 2003) or contaminated surfaces and food, although airborne transmission may also occur (Esposito et al., 2014). NoV infection often causes a self-limiting disease, as it usually lasts for up to two or three days and recovery is typically the rule. NoV infection is however highly contagious, is associated with high rates of morbidity, and is a frequent cause of hospitalisation. It can also be a cause of death among immunocompromised patients (Esposito et al., 2014). Understanding the socioeconomic patterning of NoV exposure in early childhood is timely and crucial to better inform NoV prevention and control measures currently under development (e.g., vaccines) (Lopman, Steele, Kirkwood, & Parashar, 2016), so that they can specifically target the most-at-risk populations who could benefit the most from such prevention measures, while reducing the overall burden of this gastrointestinal infection. This study investigates whether family income is associated with NoV exposure in UK children three years of age and identifies some mechanisms through which income may translate into exposure to NoV.
2. Materials and methods
2.1. Data sources
We used data from the Millennium Cohort Study (MCS), a nationally representative longitudinal birth cohort study collecting information on health, wealth, education and other family circumstances from parents of almost 19,000 children born in the UK in 2000–2001 (Hansen, 2012). The stratified clustered sample design allowed oversampling of families living in areas of child poverty and those with high proportions of ethnic minority groups. This study only used data collected on pre-school age children. It therefore included data collected when children were aged 9 months (henceforth called survey 1) and 3 years (henceforth called survey 2), which were then linked to the ‘biomedical data enhancement study of infections and later allergies’ (Bartington, Peckham, Brown, Joshi, & Dezateux, 2009; Townsend et al., 2012). The latter study was conducted alongside survey 2. Further details on these data sources can be found elsewhere (Bartington et al., 2009; Hansen, 2012; Townsend et al., 2012). As this study falls within the remit of the original parent ethics approval (Hansen, 2012) no additional ethics clearance was required.
2.2. Theoretical framework and empirical specification
To investigate the relationship between family income and NoV exposure and the likely mechanisms that may mediate this relationship, we modelled our empirical analyses within the economic framework of the Grossman model (Grossman, 1972; Jacobson, 2000). In this model, child health is considered the result of parental characteristics (e.g., genetics, education, income) and other family-related ‘inputs’ (e.g., maternal child-health-related behaviours such as smoking during pregnancy and breastfeeding). This is known as the ‘child health production function’. The choice of the type of variables to include in our empirical specifications, as predictors of NoV exposure and/or potential mediators of the ‘child NoV exposure-family income’ relationship, was informed by theoretical frameworks previously used in epidemiology and in the economic empirical literature investigating the ‘child health-family income’ gradient (Barker, 1990, 1993, 1995; Khanam, Nghiem, & Connelly, 2009; Noonan, Burns, & Violato, 2018; Propper, Rigg, & Burgess, 2007; Violato, Petrou, Gray, & Redshaw, 2011). The specific variables used to operationalise these frameworks (which we describe in the following paragraphs) had to be relevant to our child outcome of interest, namely exposure to NoV. They were, therefore, identified from the literature on GI/NoV risk factors (see Table 1).
Table 1.
Model specifications and covariates.
| Model specifications | Covariates |
|---|---|
| Model 0 (Unadjusted) | Family income (Case et al., 2002; Currie & Stabile, 2003) |
| Model 1 (Baseline) | Family income (Case et al., 2002; Currie & Stabile, 2003) + Group 1 (G1) baseline variables, namely: child gender (s1a), age (s2b) and ethnicity (s1); month when oral fluid sample was collected (s2 - proxy for seasonality of NoV). (Phillips et al., 2011; O'Brien et al., 2016; Phillips, Tam, Rodrigues, & Lopman, 2010) |
| Model 2 | Same variables as in Model 1 + Group 2 (G2) - Child characteristics at birth and early years variables: Gestational age (Barker, 1990; Bentley et al., 2016; Quigley, Kelly, & Sacker, 2007) (s1); parity (firstborn- s1) (Beale et al., 2010; Mucci et al., 2004; Vianna & Polan, 1978); delivery mode (s1) (Bentley et al., 2016); antibiotics use when child older than one year (s2) (Dennehy et al., 2006) |
| Model 3 | Same variables as in Model 2 + Group 3 (G3) - Maternal characteristics and child-health-related behaviours variables, namely: Maternal education (s1) (Ludvigsson & Abis Study, 2006; Newman et al., 2015); maternal smoking during pregnancy (s1) (Ludvigsson & Abis Study, 2006); breastfeeding duration (s1) (Baker et al., 1998; Bentley et al., 2016; Eaton-Evans & Dugdale, 1987; Ludvigsson & Abis Study, 2006; Quigley et al., 2007) |
| Model 4 | Same variables as in Model 3 + Group 4 (G4) – Home environment variables, namely: siblings living in the household (s2) (Baker et al., 1998; Ludvigsson & Abis Study, 2006; Phillips et al., 2011); house tenure (s2c) (Baker et al., 1998; Ludvigsson & Abis Study, 2006); whether anyone smokes in the same room as the child (i.e. exposure to passive smoking) (s2) (Baker et al., 1998; Ludvigsson & Abis Study, 2006) |
| Model 5 | Same variables as in Model 4 + Group 5 (G5) – Environment outside the home variables, namely: childcare arrangements (s2) (Phillips et al., 2011; De Wit et al., 2003; Menon et al., 2013); local pollution, grime or other environmental problems (s1c) (Dai et al., 2004; Gray, Jiang, Morgan-Capner, Desselberger, & Estes, 1993; Peasey et al., 2004); UK country of residence (s2) (Dai et al., 2004; Gray et al., 1993; Peasey et al., 2004) |
| Model 6 (Fully adjusted) | Same variables as in Model 5 + Group 6 (G6) – Other family characteristics variables, namely: maternal depression (s2) (Silverstein et al., 2010); language spoken at home (s2) (Bartington et al., 2009) |
Measured at survey 1 (age 9 months).
Measured at survey 2 (age 3).
A variable indicating whether the family had changed address since last survey was controlled for.
The first set of variables that we included in our empirical models referred to events during fetal life and early childhood, and we called them ‘the importance of early years’ variables. It is in fact widely recognized that early life experiences are important determinants of child health throughout childhood and, subsequently, through adulthood. Even before conception and throughout pregnancy, a multiplicity of social, biological and genetic factors combine to influence the health of the baby with far-reaching consequences (Barker, 1990; Case, Fertig, & Paxson, 2005), and some of these may be socio-economic patterned. Furthermore, given that money by itself is neither an antidote for illness nor a direct cause of it, our attempt to identify the channels through which income may affect NoV exposure was informed not only by previous literature on childhood NoV risk factors but also informed by and framed within the ‘parental investment’ (Becker & Tomes, 1986) and ‘parental stress’ (Violato et al., 2011; Mayer, 1997) theoretical frameworks. The ‘parental investment’ framework, originating within the economics literature (Becker & Tomes, 1986), refers to the effects of income transmitted through the parents' capacity to invest resources (both monetary and non-monetary) in their children's health. In the context of our NoV exposure model, this refers mainly to ‘investments’ in the home environment (e.g. house type) and in environments outside the home (e.g. type of childcare arrangements). Throughout this study we refer to these variables as the ‘the parental investment’ variables. The ‘parental stress’ framework, which had its origins in the psychological and developmental literature but counts applications also in the empirical economic literature (Khanam et al., 2009; Noonan et al., 2018; Propper et al., 2007, Violato et al., 2011), focuses on the effects of income on child outcomes through parental mental health. It suggests that low income negatively affects child outcomes by adversely impacting on parents' emotional wellbeing (e.g. psychological distress/depression) and parenting practices (e.g. interactions with their children). While this framework has mainly been applied in studies investigating the relationship between child development and family income, we extended its application to the child NoV exposure context. We hypothesised that low income, and the potentially associated maternal psychological distress, may have implications for child exposure to NoV insofar as it induces maternal behaviours which reduce opportunities for the child to be in contact with other children (Silverstein, Feinberg, Young, & Sauder, 2010), given that person-to-person transmission is prominent in NoV infections. We therefore included maternal depression in our empirical specifications. This variable, together with another cultural characteristic of the family, language(s) spoken at home (Bartington et al., 2009), are henceforth referred to as the ‘other family characteristics’ variables. The resulting empirical specification of the child health production function can therefore be written as follows:
| (1) |
were CO represents the child outcome of interest for child i, i.e. NoV exposure; α0 is the constant; hi0 is the child's initial health endowment, such as gestational age; Xi is a set of variables including family sociodemographic controls and some child characteristics; Y is family income taken in its logarithmic form; PIi and OFCi refer to sets of variables that operationalise the ‘parental investment’ and ‘other family characteristics’ frameworks; εi is the random-error term.
2.3. Variables definitions
2.3.1. Norovirus exposure
Norovirus-specific Immunoglobulin G (IgG), a marker of past NoV infection, was measured from oral fluid samples collected from pre-school children aged 3 years, using enzyme-linked immunoassay (ELISA). The ELISA used non-replicating virus like particles generated by cloning and expressing the VP1 encoding gene of a GII-4 strain as a capture antigen; a standard plasma control was used for normalization and semi quantitation across tests (Menon et al., 2013; Townsend et al., 2012). In addition, total IgG was measured against a commercially available IgG standard in all oral fluid samples to obtain a measure of the quality of the samples. Samples with total IgG values ≥ 2 mg/L were considered of good quality. Norovirus antibody levels (good quality samples only) were used as a measure of child exposure to NoV, with higher titres being interpreted as greater frequency of exposure. Given skewness of data (Supplementary Fig. S1), NoV titres were expressed in logarithmic form.
2.3.2. Family income
As is common in the economic literature on the ‘child health-family income gradient’ (Case et al., 2002; Currie & Stabile, 2003), the main covariate of interest was ‘permanent’ family income. This measure of income was originally developed in the context of the ‘permanent income hypothesis’, which is an economic theory (Friedman, 1957) explaining how individuals usually spread their consumption over their lifetime and often spend money according to their long-term average income (the ‘permanent’ income) rather than their current income (also called ‘transitory’). In our study, we measured permanent income by averaging family income over surveys 1 and 2, adjusting for family composition and inflation (at 2004 prices, when survey 2 occurred), and expressed in logarithmic form (Case et al., 2002, 2005).
2.3.3. Covariates
Covariates that operationalised the theoretical frameworks explained in section 2.2 and reflected known GI/NoV risk factors identified in the literature are reported in Table 1, together with the relevant references and the model specifications (M0 to M6) in which they were included. In order to better illustrate the stepwise approach in model building (see section 2.4) and more clearly report results, we have grouped covariates into six groups (G1 to G6), as described in Table 1. Each group of variables was added sequentially to the unadjusted model M0 to form the fully adjusted model M6. Succinctly, the ‘standard sociodemographic’ variables (G1) were included in all model specifications. The ‘importance of early years’ variables (G2 + G3), referred to events during fetal life and early childhood characteristics, which may combine to impact health over the life course (Barker, 1990; Case et al., 2005). The ‘parental investment’ (G4 + G5) and the ‘other family characteristics’ (G6) variables, operationalised potential mechanisms by which family income may impact on child exposure to NoV within the theoretical frameworks outlined in section 2.2.
2.4. Statistical analysis
In primary analyses, we first explored the univariate association between NoV exposure and all risk factors, and between family income and the other risk factors. Stepwise Ordinary Least Squares (OLS) multivariable regression models, as outlined in equation (1) and Table 1, were then estimated. Empirical selection was conducted by assessing conceptually similar variables for collinearity and statistical significance. We followed microeconometrics practice when including regressors of theoretical importance (e.g. gender, age) even if not statistically significant (Cameron & Trivedi, 2009), and kept other covariates in the models if p-value < 0.10 (although this rule was relaxed in sensitivity analyses/robustness checks). Groups of variables were added sequentially to the baseline model (M1) using a life-course approach to model building (order indicated in Table 1) to identify the overall key factors in the fully adjusted model. Given that the life course rationale was not compelling for the ‘parental investment’ and the ‘other family characteristics’ variables, we also explored how results varied by interchanging the order in which these variables were adjusted for. Any observed variation in the coefficient of family income on the addition of groups of risk factors was taken to suggest “potential” mediation (Baron & Kenny, 1986), as differently from traditional mediation analysis, which relies on causal models, our models are only associational (Baron & Kenny, 1986; Kenny, 2018). Mediators are variables that fall along the causal explanatory pathway between an exposure (family income) and an outcome (NoV titres) of interest. Our model hypothesised (and showed) that family income had a direct relationship with NoV exposure, and we attempted to identify a chain of impacts where family income influences (some of the) covariates, and those, in turn influence NoV exposure. We therefore reported also on the association between family income and the covariates, and between the covariates and NoV exposure. Survey weights were included in all analyses to account for the stratified cluster sample design of the study and attrition bias. In secondary analyses, quantile regression (QR) multivariable models (Koenker, 2005) were also estimated to investigate whether the effects of the covariates varied by the level of NoV exposure, and all covariates were centred (Koenker, 2005). The conventional OLS approach used in our primary analyses, in fact, estimated the covariates effects on the conditional mean of the NoV exposure. By doing this, it neglected to consider other locations throughout the NoV exposure distribution. This is, instead, relevant given the way in which we measured NoV exposure, where higher antibody titres represent greater frequency of exposure. Comprehensive sensitivity analyses/robustness checks were conducted as detailed in Supplementary Table S1. In the first sensitivity analysis (SA1), variables not statistically significant at the 5-percent level were dropped from the fully adjusted model, as it is typical of some biostatistics disciplines. In the second sensitivity analysis (SA2), we re-run primary analyses including also oral fluid samples of poor quality (i.e. total IgG values < 2 mg/L). In the third sensitivity analysis (SA3), family income was replaced with an alternative measure of socioeconomic status based on occupational status; while in the fourth sensitivity analysis (SA4), ‘permanent’ income was replaced with ‘transitory’ lagged income, as measured at survey 1. Finally, given that listwise deletion was adopted in the primary analyses, a further robustness check was performed using multiple imputation (SA5) (White, Royston, & Wood, 2011). All analyses were conducted using Stata 13 (StataCorp LP; College Station, TX).
3. Results
Of the 11,034 oral fluid samples from singleton children aged 3, 5962 (54.13%) were of good quality (total IgG ≥ 2 mg/L (Townsend et al., 2012)). Supplementary Table S2 summarises the characteristics of the participants with IgG concentration <2 mg/L and ≥2 mg/L, respectively. The two sub-samples were very similar. The percentage of missing values for each variable is reported in Supplementary Table S3.
3.1. Association of covariates with NoV and family income
Higher family income was positively associated with NoV exposure (Table 2). All covariates of interest, some of which could also potentially act as mediators, were associated with income, except for gestation and antibiotics exposure, and varied by income quintiles (Supplementary Table S4). In univariate regression (Table 3) being firstborn, antibiotic exposure, a mother with overseas qualification only, speaking only a foreign language at home, attending formal childcare, and living in pollution- and grime-free areas were significantly associated with higher NoV titres. Characteristics associated with lower NoV titers included: being male, born through a planned caesarian section, having siblings aged 5 or older, living in rented accommodation, being exposed to passive smoking at home, having a mother diagnosed with depression, and living in Scotland or Northern Ireland. There was no significant association between NoV titres and child age, gestation, or maternal smoking during pregnancy.
Table 2.
Association between family income and norovirus exposure – Ordinary Least Squares models.
| Log of family permanent income coefficients |
||
|---|---|---|
| Model specification |
Groups of variables (added sequentially to the baseline model) |
|
| Coefficients | 95% CIa | |
| M0b | 0.08*** | (0.02, 0.14) |
| M1c | 0.09*** | (0.03, 0.14) |
| M2d | 0.09*** | (0.03, 0.15) |
| M3d | 0.09** | (0.02, 0.16) |
| M4d | 0.05 | (-0.03, 0.013) |
| M5d | 0.01 | (-0.07, 0.10) |
| M6d/Fully adjusted model) | 0.002 | (-0.08, 0.09) |
| Fully adjusted specification (only significant covariates) | 0.004 | (-0.08, 0.09) |
Table 3.
Association of covariates with norovirus exposure.
| Variablesa | Coefficients | 95% CIb |
|---|---|---|
| Baseline model variables | ||
| Male | −0.07* | (-0.13, 0.001) |
| Child age (months) | ||
| <36 | --c | --c |
| 36–39 | 0.02 | (-0.13, 0.17) |
| >39 | 0.11 | (-0.08, 0.29) |
| Child ethnicity | ||
| White | – | – |
| Mixed | 0.15 | (-0.05, 0.35) |
| Indian | 0.02 | (-0.21, 0.25) |
| Pakistani and Bangladeshi | 0.06 | (-0.07,0.18) |
| Black or black British | −0.06 | (-0.25, 0.13) |
| Other | 0.17 | (-0.07, 0.40) |
| ‘Importance of early years’ variables | ||
| Gestation < 37 weeks | −0.08 | (-0.24,0.08) |
| Antibiotic at > 1 year | 0.45** | (0.05, 085) |
| Child firstborn | 0.13*** | (0.05, 0.21) |
| Delivery mode | ||
| Normal/instrumental- spontaneous onset | – | – |
| Normal/instrumental- induced labour | −0.03 | (-0.12, 0.06) |
| Planned section- spontaneous onset of labour | −0.18** | (-0.33, −0.04) |
| Planned section- induced labour | −0.23** | (-0.45, −0.01) |
| Emergency section- spontaneous onset of labour | 0.02 | (-0.12, 0.17) |
| Emergency section- induced labour | 0.01 | (-0.16, 0.19) |
| Maternal educationd | ||
| Nvq level 1 | – | – |
| Nvq level 2 | −0.001 | (-0.16, 0.16) |
| Nvq level 3 | −0.02 | (-0.19, 0.15) |
| Nvq level 4 & 5 | 0.07 | (-0.09, 0.45) |
| Overseas qualification only | 0.23** | (0.004, 0.45) |
| None | −0.02 | (-0.19, 0.15) |
| Maternal smoking during pregnancy | ||
| Never smoked | – | – |
| Stopped smoking | 0.01 | (-0.11, 0.13) |
| Smoked throughout | 0.02 | (-0.07, 0.12) |
| Breastfeeding | ||
| Never | – | – |
| Less than 7 days | −0.01 | (-0.14, 0.12) |
| 1 week to 3 months | 0.09 | (-0.02, 0.20) |
| 3–6 months | 0.05 | (-0.06, 0.17) |
| More than 6 months | 0.06 | (-0.05, 0.17) |
| ‘Parental investment’ variables | ||
| Siblings at home | ||
| No siblings | – | – |
| 1 or more aged <5 years | −0.07 | (-0.19, 0.05) |
| 1 or more aged ≥ 5 years | −0.12*** | (-0.21, −0.03) |
| House tenure | ||
| Mortgaged/owned | – | – |
| Council rented | −0.07 | (-0.18, 0.04) |
| Rented (housing association or private) | −0.13*** | (-0.23, −0.03) |
| Other | 0.09 | (-0.10, 0.28) |
| Exposure to passive smoking | −0.12** | (-0.22, −0.01) |
| Childcare arrangements | ||
| No childcare | – | – |
| Formal care | 0.15*** | (0.05, 0.26) |
| Informal care | 0.04 | (-0.05, 0.13) |
| Exposure to local pollution and grime | ||
| Very/Fairly common | – | – |
| Not very common | 0.07 | (-0.03, 0.18) |
| Not at all common | 0.11** | (0.01, 0.21) |
| Country of the UK | ||
| England | – | – |
| Wales | 0.004 | (-0.11, 0.12) |
| Scotland | −0.15*** | (-0.26, −0.05) |
| Northern Ireland | −0.28*** | (-0.40, −0.16) |
| ‘Other family characteristics’ variables | ||
| Maternal GP-diagnosed depression | −0.10** | (-0.19,-0.01) |
| Language spoken at home | ||
| English only | – | – |
| English and other language(s) | 0.02 | (-0.09, 0.13) |
| Other language(s) only | 0.18** | (-0.002, 0.36) |
∗ Significant at the 10-percent level.
∗∗ Significant at the 5-percent level.
∗∗∗ Significant at the 1-percent level.
Missing data for each variable are reported in Table S3.
CI: Confidence Interval.
Reference category.
Nvq: national vocational qualifications (UK system); NVQ level 1: GCSE grades D-G, NVQ/SVQ/GSVQ level 1; NVQ level 2: O level/GCSE grades A-C, trade apprenticeships, NVQ/SVQ/GSVQ level 2; NVQ level 3: A/AS/S levels, NVQ/SVQ/GSVQ level 3; NVQ level 4 & 5: first degree, diplomas in higher education, professional qualification at degree level, nursing/other medical qualification, higher degree.
3.2. Association between family income and NoV adjusted for covariates
Family income was significantly associated with higher NoV titres. Table 2 summarises the OLS estimates of the association between family income and NoV exposure for the various stepwise model specifications. When blocks of variables were added sequentially to the baseline model M1 (Table 2, columns 2 and 3; and Supplementary Table S5), the positive relationship between income and NoV exposure was not only attenuated from 0.09 to 0.002, but also rendered statistically insignificant when variables representing the ‘parental investment’ framework were included in the model. As both NoV titres and family income were expressed in their logarithmic form in all model specifications, an income coefficient of 0.09 can be better understood by saying that, under the assumption that our models were causal, the effect of a 10% increase in the average annual family income was associated with almost a 1% increase in NoV titres.
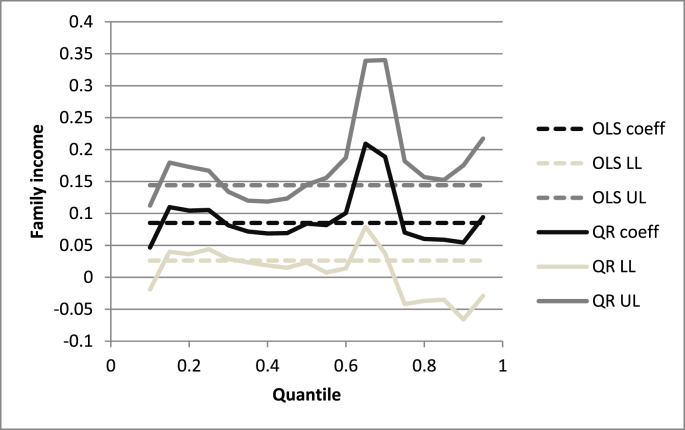
QR results from secondary analyses are reported graphically (Fig. 1 and Supplementary Fig. S2) and show, at any chosen quantile of NoV titre, how the association with income varies. Focusing, for instance, on the baseline model (M1), the QR income effect was considerably larger than the OLS coefficient of 0.09 in the upper half of the NoV distribution (Fig. 1). At the 0.65 quantile, the income effect was more than double (coeff: 0.21; 95% CI; 0.08, 0.34; p = 0.002). Similar results held across all model specifications (Supplementary Fig. S2), with the effect of income varying by the levels of NoV exposure, and a tendency to have larger effects in the upper half of the NoV distribution.
Fig. 1.
Family income quantile regression estimates for norovirus exposure model M1.
*Fig. 1 legend: Fig. 1 has a horizontal quantile scale and the vertical scale in norovirus antibody level (log) indicates the family income effect. The solid black line (‘QR coeff’) is the quantile regression estimate of income at each quantile; the dark grey (‘QR UL’) and the light grey (‘QR LL’) solid lines are the upper limit and lower limit, respectively, of the 95% confidence intervals of the quantile estimates. Ordinary least squares estimates - black dashed line ‘OLS coeff’ - and their 95% confidence intervals (dark and light grey dashed lines ‘OLS UL’ and ‘OLS LL’) are reported as a way of comparison.
3.3. Association between NoV and other covariates
Table 4 reports results from the OLS estimates of the fully adjusted model and shows that the main significant associations between NoV exposure and risk factors identified in the univariate analysis (Table 3), some of which may be considered potential mediators in the relationship NoV exposure-family income, still held in the multivariable context. In particular, the following variables were significantly associated with higher NoV titres: being firstborn; antibiotic exposure; having a mother with overseas qualifications only, or a mother who smoked throughout pregnancy; living in a family where only a language other than English was spoken at home; attending formal childcare; and living in an area where local pollution and grime was not at all common. Being born through a planned caesarian section rather than through normal vaginal delivery, living in a rented accommodation as opposed to a owned/mortgaged one, being exposed to passive smoke at home, having a mother diagnosed with depression, and living in Scotland or Northern Ireland rather than in England were instead characteristics significantly associated with lower NoV titres.
Table 4.
Fully adjusted model – Ordinary Least Squares estimates.
| Fully adjusted modela |
||
|---|---|---|
| Variablesb | Coefficients | 95% CIc |
| Family income | 0.002 | (-0.08, 0.09) |
| ‘Importance of early years’ variables | ||
| Gestation < 37 weeks | −0.05 | (-0.21, 0.11) |
| Antibiotic at > 1 year | 0.53*** | (0.15, 0.92) |
| Child firstborn | 0.18*** | (0.05, 0.32) |
| Delivery mode | ||
| Normal/instrumental- spontaneous onset | --d | --d |
| Normal/instrumental- induced labour | −0.02 | (-0.10, 0.07) |
| Planned section- spontaneous onset of labour | −0.21*** | (-0.35,-0.07) |
| Planned section- induced labour | −0.25*** | (-0.43, −0.06) |
| Emergency section- spontaneous onset of labour | 0.02 | (-0.13, 0.17) |
| Emergency section- induced labour | −0.10 | (-0.26, 0.06) |
| Maternal educatione | ||
| Nvq level 1 | – | – |
| Nvq level 2 | −0.04 | (-0.19, 0.12) |
| Nvq level 3 | −0.05 | (-0.21, 0.11) |
| Nvq level 4 & 5 | 0.03 | (-0.13, 0.18) |
| Overseas qualifications only | 0.26** | (0.03, 0.50) |
| None | 0.01 | (-0.16, 0.19) |
| Maternal smoking during pregnancy | ||
| Never smoked | – | – |
| Stopped smoking | 0.05 | −0.06 |
| Smoked throughout | 0.20*** | 0.09 |
| Breastfeeding | ||
| Never | – | – |
| Less than 7 days | −0.01 | (-0.14, 0.13) |
| 1 week to 3 months | 0.05 | (-0.06, 0.16) |
| 3–6 months | −0.03 | (-0.15, 0.08) |
| More than 6 months | 0.003 | (-0.11, 0.12) |
| ‘Parental investment’ variables | ||
| Siblings at home | ||
| No siblings | – | – |
| 1 or more aged <5 years | 0.001 | (-0.12, 0.13) |
| 1 or more aged ≥ 5 years | 0.08 | (-0.08, 0.24) |
| House tenure | ||
| Mortgaged/owned | – | – |
| Council rented | −0.06 | (-0.20, 0.07) |
| Rented (housing association or private) | −0.16*** | (-0.29, −0.04) |
| Other | 0.02 | (-0.18, 0.22) |
| Exposure to passive smoking | −0.14** | (-0.25, −0.03) |
| Childcare arrangements | ||
| No childcare | – | – |
| Formal care | 0.13** | (0.03, 0.24) |
| Informal care | 0.04 | (-0.05, 0.12) |
| Country of the UK | ||
| England | – | – |
| Wales | −0.01 | (-0.12, 0.11) |
| Scotland | −0.19*** | (-0.29, −0.09) |
| Northern Ireland | −0.28*** | (-0.41, −0.15) |
| ‘Other family characteristics’ variables | ||
| Maternal GP-diagnosed depression | −0.12*** | (-0.21, −0.04) |
| Language spoken at home | ||
| English only | – | – |
| English and other language(s) | −0.004 | (-0.17, 0.16) |
| Other language(s) only | 0.24** | (0.01, 0.039) |
∗∗ Significant at the 5-percent level.
∗∗∗ Significant at the 1-percent level.
Controlled for child age (months), gender and ethnicity, month in which the oral fluid sample was collected, whether the family changed address between surveys 1 and 2.
Sample size: 5063.
CI: Confidence Interval.
Reference category.
Nvq: national vocational qualifications (UK system); NVQ level 1: GCSE grades D-G, NVQ/SVQ/GSVQ level 1; NVQ level 2: O level/GCSE grades A-C, trade apprenticeships, NVQ/SVQ/GSVQ level 2; NVQ level 3: A/AS/S levels, NVQ/SVQ/GSVQ level 3; NVQ level 4 & 5: first degree, diplomas in higher education, professional qualification at degree level, nursing/other medical qualification, higher degree.
3.4. QR regressions and sensitivity analyses/robustness checks
QR regression estimates generally corroborated the OLS results, but uncovered a varying impact of some covariates– both in magnitude and statistical significance - by the level of NoV exposure, especially at the upper tail of the NoV exposure distribution. Key QR results are reported in Supplementary Fig. S3. For example, larger positive impacts (i.e. higher frequency of NoV exposure) of being firstborn (Fig. S3, Panel C) and attending formal childcare on NoV exposure (Fig. S3, Panel I) were observed in the upper half of the NoV distribution. Larger negative impacts (i.e. lower frequency of NoV exposure) of planned c-section compared with normal delivery (Fig. S3, Panels D and E) and maternal depression (Fig. S3, Panel O) were more apparent in the in the right tail of the NoV exposure distribution.
The main OLS findings were maintained in the extensive sensitivity analyses/robustness checks (Supplementary Tables S6, S7, S8, S9, S10).
4. Discussion
To our knowledge, this study is the first to extend the investigation of the ‘child health-family income gradient’ to the context of GI infections within the theoretical framework of the Grossman model (Grossman, 1972; Jacobson, 2000). Current (epidemiological and public health) evidence on the association between socioeconomic status (SES) and GI is still inconclusive, with some studies indicating higher rates of GI among disadvantaged populations, and others suggesting the opposite. We found that children living in higher income households had higher NoV titres at age 3 years, with higher effects in the upper tail of the NoV distribution. However, the relationship was attenuated and rendered statistically insignificant when variables from the ‘parental investment’ framework were included in the model. These results were confirmed by multiple sensitivity analyses/robustness checks.
A plausible explanation for a positive univariate association between NoV exposure and family income may be that higher income influences the prevalence of known high-risk factors for NoV infection (Whitney et al., 2015). In other words, income matters for ‘what it can buy’, which may differentially expose children to the infection, indicating in this way the potential mechanisms through which income may translate into differential child NoV exposure. In particular, a key result was that child attendance to formal childcare (e.g., nursery, crèches) compared with no childcare arrangements was associated with higher NoV antibody titres; and formal childcare arrangements were more prevalent in the highest quintiles of the income distribution. This finding is in line with, and further corroborates, the knowledge that childcare centres are common settings in which the primary infection in households with children is usually acquired (De Wit et al., 2003).
Significantly lower levels of antibody titres were found in children living in rented accommodations – arrangements more prevalent in lower income families - as opposed to children living in own/mortgaged accommodations. Whilst living in rented accommodation may sometimes be associated with residential crowding and increased rate of person-to-person transmission of NoV (My et al., 2013), our results seem to mainly capture what income may afford in term of housing type and, therefore, may simply represent a proxy for socioeconomic status. Alternatively, it may be a proxy of or be associated with other observed (no formal childcare) and unobserved behaviours more prevalent in people of low socioeconomic status, which may in turn reduce opportunities of exposure to NoV in children. A similar interpretation may apply to the results that significantly lower titres were found in children exposed to passive smoking, and significantly higher titres in children living in better off neighbourhoods. Both circumstances were inversely socioeconomically patterned.
The results that significantly lower NoV antibody titres were found in children residing either in Scotland or Northern Ireland, compared with those living in England, needs to be further explored. However, a potential explanation may be ascribed to the larger prevalence of rural areas in those regions of the UK (in our sample about 46% and 49% for Scotland and Northern Ireland, respectively) compared with England (about 15%). More rural areas are generally less densely populated, which, in conjunction with the fact that in our sample the percentage of children attending formal childcare in Scotland and Northern Ireland was lower than in England, may cautiously explain the reduced occasions for children to be exposed to NoV infection through contact with other children.
Statistically significant associations of NoV exposure with variables other than those operationalising the ‘parental investment’ framework appear to be mostly conducible to the predominant person-to-person spread of the NoV infection. In this light, the significantly lower NoV antibody titres found in children of mothers suffering from depression is consistent with existing literature (Silverstein et al., 2010) showing that children of mentally distressed mothers are less likely to participate in age-appropriate preschool activities, reducing opportunities of contact with other children and occasions for the person-to-person transmission of the infection.
The significantly higher NoV titres in children whose mothers were exclusively educated abroad and/or growing in families where only a foreign language was spoken at home is difficult to interpret due to the absence of specific confirmatory evidence. However, under the assumption that those children may have extended family abroad, it could be suggested that both associations might be explained by the likely larger amount of foreign travel (Al-Abri, Beeching, & Nye, 2005; Phillips et al., 2011) to visit family abroad, which in turn implies increased contact with people and different exposures to infections than for children who travel less. We acknowledge that this hypothesis would need to be validated when suitable data become available.
Children born through planned caesarian section rather than normal vaginal delivery had significantly lower NoV antibody titres. Clinical studies suggest that caesarean section alters normal gut microbiota development, immune system response and homeostasis in early life (Bentley et al., 2016). This may make the child more vulnerable and may trigger a more protective behaviour in parents, with fewer occasions for the child to acquire infections transmitted person-to-person. While this hypothesis requires further investigation, our data show that the percentage of children born through caesarian section who attended formal childcare (20%) was lower than for children born through vaginal delivery (28%), which may have driven our results.
Being firstborn was significantly associated with higher NoV exposure, and the percentage of children attending formal childcare was higher for firstborn than for late-born children (33% versus 25%). Childcare is expensive in the UK and, for families with more than one pre-school age child, economic reasons may contribute to choices between return to work and formal childcare (Harding, Wheaton, & Butler, 2017). Child's use of antibiotics was significantly associated with higher NoV titres, as highlighted in previous studies (Dennehy et al., 2006). Antibiotic use early in life has been associated with an increase in rates and frequency of diarrheal episodes in some populations (Rogawski et al., 2015). It may in fact disturb gut homeostasis and increase susceptibility to diarrheal disease. If antibiotic exposure is more common in children attending childcare - and therefore more exposed to a multiplicity of other infections – it might also be a confounder, but our data do not indicate a differential distribution of antibiotic use by childcare arrangements. The prevalence of antibiotic use was small in our sample.
Higher NoV antibody titres were found in children whose mother reported to continue smoking throughout pregnancy compared to children whose mothers never smoked. This appears counterintuitive, as smoking during pregnancy is more prevalent in low income mothers, but a similar result was found in a large Swedish study of gastrointestinal infections in young children (Ludvigsson & Abis Study, 2006). The association of higher NoV titers and antibiotic use or mother reporting smoking during pregnancy may have a biological explanation that requires further investigation.
A recent systematic review and meta-analysis of the relationship between SES and GI in developed countries (Adams et al., 2018b) found a significant inverse association between SES and risk of GI infections for children. While those results (Adams et al., 2018b) may seem at odds with ours, their evidence relied on hospitalised cases. There is a substantial body of empirical evidence showing that hospital admissions for children with gastroenteritis increase as area-level (Pockett et al., 2011) and individual-level (Biering-Sorensen, Sondergaard, Vitting Andersen, Andersen, & Mortensen, 2012) SES decreases. Our results, rather than contradicting those findings, may offer a plausible explanation as to why this may be observed in relation to paediatric NoV gastroenteritis. Infants from disadvantaged families may be less exposed to the virus early in life, and therefore may become more vulnerable to it as they age, with potentially more severe consequences requiring hospitalisation. Further UK evidence confirms this potential explanation, as researchers (Adams et al., 2018a) found that lower SES was associated with lower GI disease risk in a large community cohort, but with higher odds of severe illness (Phillips et al., 2011; Rose et al., 2017).
Our study increases the understanding of the relationship between family income and NoV exposure in pre-school age children, and may help inform and prioritise policy and health promotion programmes to reduce inequalities in NoV infections in childhood. Firstly, differential NoV exposure by income in pre-school years may lead to differential risk of disease at school age, with potential adverse effects on educational attainment. Exposure to NoV infection is not a synonym for disease. Infections can also be asymptomatic. However, both symptomatic and asymptomatic infections strengthen the immune response to subsequent exposure to the virus. So low income children, who are less exposed in the early years, become more vulnerable to the virus as they age. Vaccination in early years may therefore create a more level playing field across the income distribution, so that children enter education with a more equitable risk of NoV infection. Promising results in this direction have recently been reported following the introduction of childhood rotavirus vaccination in the UK, with the study finding that vaccination could help reduce GI related health inequalities in infants (Hungerford et al., 2018). Secondly, further educational efforts should be made to increase awareness of childcare staff and families with young children of young children's relatively higher risk of NoV exposure and the actions that can be taken to reduce such risk. Finally, our results suggest that broadening the research horizon to consider the broader wellbeing of the whole family (e.g., maternal psychological distress) may give a better understanding of the heterogeneity of behaviours that can impact on differential child exposure to NoV infections.
Our study contributes novel empirical evidence to the wider literature on the relationship between SES and GI by focusing - as called for by recent systematic reviews (Adams et al., 2018b; Newman et al., 2015) – on a specific age (children) and type of pathogen (person-to-person spread). The novel application of a well-established economic framework (Grossman, 1972; Jacobson, 2000) may open the way to valuable synergies between health economics and epidemiology to enhance understanding of socioeconomic inequalities in GI and other infections in childhood. Another strength of our study was the use of general population survey data linked to laboratory data, which provided a unique opportunity to adjust our empirical models for a variety of covariates not usually available in most serological studies. Furthermore, the value of our results was enhanced by our secondary analyses using QR regression. This is a novel application of an econometric method to serological data. Given that high NoV antibodies were interpreted as greater frequency of exposure, we considered it informative to explore which variables had a significant impact in the upper tail of the NoV exposure distribution. The conventional linear regression estimates of the covariates effects on the conditional mean of NoV exposure is not in fact necessarily indicative of size and significance of these effects on the upper tail of the NoV exposure distribution. QR allowed us investigating the impact of each covariate on the full distribution of NoV exposure.
Our study also has some limitations. Measuring IgG in children aged 3 years alone cannot recapitulate the natural history of exposure to NoV in infancy. This could only be done in a cohort study design in which samples are collected at regular intervals from birth, given that first norovirus infections occur very early in life, and should include the measurement of IgA vs IgG to assess first exposure to NoV in young babies. However, IgG titre increases with number of exposures to NoV and, as such, it represents a marker of frequency of NoV exposure, with higher IgG titres indicating greater frequency of NoV exposure. Also, the multipurpose nature of the MCS surveys meant that we were unable to adjust for some common NoV-specific risk factors, such as contact with other people with gastroenteritis (in and outside the household), details of recent consumption of at-risk foods, hygienic food-handling practices and common hand-washing patterns. The interpretation of some of our results requires further confirmatory analyses, which could not be conducted with the available data. Therefore, some of our findings are best interpreted with caution. We acknowledge that interpreting the impact of individual covariates in our model specifications may be complicated by conceptual overlap between some measures, although collinearity was not detected through formal testing. Finally, the estimated relationship between each analysed variable and seroresponse is associational only and causality remains to be established.
5. Conclusions
Our study contributes to an existing, albeit limited, body of empirical health economics literature on the ‘child health-family income gradient’ as well as to the general social epidemiological and public health evidence on the wider association between socioeconomic status and gastrointestinal infections. Using a large UK birth cohort study of pre-school age children and their families linked to serological data, we have shown that there exists a positive univariate association between family income and exposure to NoV. However, when we adjusted our models for relevant covariates/potential mediating mechanisms, the relationship disappeared. Our results suggest that income mainly matters to the extent to which it may influence the prevalence of risk factors for NoV infection, such as increased opportunities for the person-to-person transmission of the virus (e.g. formal childcare). Further research is warranted to assess the validity of the associations identified in this study to improve our understanding of children at increased risk of NoV infection and the mechanisms driving socio-economic inequalities in risk, in order to more effectively address these. Our secondary analyses suggested that relationships between NoV exposure and variables of interest may also differ by quantile, highlighting the usefulness of focusing on more than one part of the NoV exposure distribution to understand more completely the relative importance of risk factors for NoV exposure in young children.
Declaration of interest statement
The authors report no conflict of interests.
Financial disclosure statement
The authors have nothing to disclose.
Acknowledgements
The research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections at University of Liverpool in partnership with Public Health England (PHE), in collaboration with University of East Anglia, University of Oxford and the Quadram Institute. M. Violato and A. Gray are based at the University of Oxford; D. Taylor-Robinson, D. Hungerford, S. O'Brien, and M. Iturriza-Gomara are based at the University of Liverpool. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care or Public Health England. A. Gray was supported by the NIHR Biomedical Research Centre, Oxford. We would also like to thank Joel Smith, University of Oxford, and Joy Wang, StataCorp Technical Support, for their advice on the STATA software syntax for quantile regression with complex survey data; all participants to the Millennium Cohort Study and the Biomedical data enhancement study of infections and later allergies, without whom this work would have not been possible.
Footnotes
This study was a secondary analysis of the Millennium Cohort Study, using de-identified data available in the public domain. As the project falls within the remit of the original parent ethics approval (Hansen K (editor). Millennium Cohort Study: A Guide to the Datasets (Eighth Edition) First, Second, Third, Fourth and Fifth Surveys. London: Centre for Longitudinal Studies, 2014) no additional ethics clearance was required.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2019.100445.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adams N.L., Rose T.C., Hawker J., Violato M., O'Brien S.J., Whitehead M. Socioeconomic status and infectious intestinal disease in the community: A longitudinal study (IID2 study) The European Journal of Public Health. 2018;28(1):134–138. doi: 10.1093/eurpub/ckx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams N.L., Rose T.C., Hawker J., Violato M., O'Brien S.J., Barr B. Relationship between socioeconomic status and gastrointestinal infections in developed countries: A systematic review and meta-analysis. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0191633. e0191633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.M., Hall A.J., Robinson A.E., Verhoef L., Premkumar P., Parashar U.D. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. The Lancet Infectious Diseases. 2014;14(8):725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Abri S.S., Beeching N.J., Nye F.J. Traveller's diarrhoea. The Lancet Infectious Diseases. 2005;5(6):349–360. doi: 10.1016/S1473-3099(05)70139-0. [DOI] [PubMed] [Google Scholar]
- Baker D., Taylor H., Henderson J. Inequality in infant morbidity: Causes and consequences in England in the 1990s. ALSPAC study team. Avon longitudinal study of pregnancy and childhood. Journal of Epidemiology & Community Health. 1998;52(7):451–458. doi: 10.1136/jech.52.7.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J. Fetal origins of coronary heart disease. British Heart Journal. 1993;69(3):195–196. doi: 10.1136/hrt.69.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bartington S.E., Peckham C., Brown D., Joshi H., Dezateux C. Feasibility of collecting oral fluid samples in the home setting to determine seroprevalence of infections in a large-scale cohort of preschool-aged children. Epidemiology and Infection. 2009;137(2):211–218. doi: 10.1017/S0950268808000927. [DOI] [PubMed] [Google Scholar]
- Beale N., Peart C., Kay H., Taylor G., Boyd A., Herrick D. ALSPAC' infant morbidity and council tax band: Doctor consultations are higher in lower bands. The European Journal of Public Health. 2010;20(4):403–408. doi: 10.1093/eurpub/ckp211. [DOI] [PubMed] [Google Scholar]
- Becker G.S., Tomes N. Human capital and the rise and fall of families. Journal of Labor Economics. 1986;4(3):S1–S39. doi: 10.1086/298118. [DOI] [PubMed] [Google Scholar]
- Bentley J.P., Simpson J.M., Bowen J.R., Morris J.M., Roberts C.L., Nassar N. Gestational age, mode of birth and breastmilk feeding all influence acute early childhood gastroenteritis: A record-linkage cohort study. BMC Pediatrics. 2016;16:55. doi: 10.1186/s12887-016-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biering-Sorensen S., Sondergaard G., Vitting Andersen K., Andersen A.M., Mortensen L.H. Time trends in socio-economic factors and risk of hospitalisation with infectious diseases in pre-school children 1985-2004: A Danish register-based study. Paediatric & Perinatal Epidemiology. 2012;26(3):226–235. doi: 10.1111/j.1365-3016.2011.01255.x. [DOI] [PubMed] [Google Scholar]
- Cameron A.C., Trivedi P.K. Stata Press; Texas: 2009. Microeconometrics using Stata. [Google Scholar]
- Case A., Fertig A., Paxson C. The lasting impact of childhood health and circumstance. Journal of Health Economics. 2005;24(2):365–389. doi: 10.1016/j.jhealeco.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Case A., Lubotsky D., Paxson C. Economic status and health in childhood: The origins of the gradient. The American Economic Review. 2002;92(5):1308–1334. doi: 10.1257/000282802762024520. [DOI] [PubMed] [Google Scholar]
- Currie J., Stabile M. Socioeconomic status and child health: Why is the relationship stronger for older children? The American Economic Review. 2003;93(5):1813–1823. doi: 10.1257/000282803322655563. [DOI] [PubMed] [Google Scholar]
- Dai Y.C., Nie J., Zhang X.F., Li Z.F., Bai Y., Zeng Z.R. Seroprevalence of antibodies against noroviruses among students in a Chinese military medical university. Journal of Clinical Microbiology. 2004;42(10):4615–4619. doi: 10.1128/JCM.42.10.4615-4619.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit M.A.S., Koopmans M.P.G., Van Duynhoven Y.T.H.P. Risk factors for norovirus, sapporo-like virus, and group A rotavirus gastroenteritis. Emerging Infectious Diseases. 2003;9(12):1563–1570. doi: 10.3201/eid0912.020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy P.H., Cortese M.M., Begue R.E., Jaeger J.L., Roberts N.E., Zhang R. A case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in U.S. children. The Pediatric Infectious Disease Journal. 2006;25(12):1123–1131. doi: 10.1097/01.inf.0000243777.01375.5b. [DOI] [PubMed] [Google Scholar]
- Eaton-Evans J., Dugdale A.E. Effects of feeding and social factors on diarrhoea and vomiting in infants. Archives of Disease in Childhood. 1987;62(5):445–448. doi: 10.1136/adc.62.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S., Ascolese B., Senatore L., Codecà C. Pediatric norovirus infection. European Journal of Clinical Microbiology & Infectious Diseases. 2014;33(3):285–290. doi: 10.1007/s10096-013-1967-9. [DOI] [PubMed] [Google Scholar]
- Friedman M. Princeton University Press; Princeton: 1957. The permanent income hypthesis. A theory of the consumption function. [Google Scholar]
- Gray J.J., Jiang X., Morgan-Capner P., Desselberger U., Estes M.K. Prevalence of antibodies to norwalk virus in England: Detection by enzyme-linked immunosorbent assay using baculovirus-expressed norwalk virus capsid antigen. Journal of Clinical Microbiology. 1993;31(4):1022–1025. doi: 10.1128/jcm.31.4.1022-1025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. On the concept of health capital and the demand for health. Journal of Political Economy. 1972;80(2):223–255. [Google Scholar]
- Grytdal S.P., DeBess E., Lee L.E., Blythe D., Ryan P., Biggs C. Incidence of norovirus and other viral pathogens that cause acute gastroenteritis (AGE) among kaiser permanente member populations in the United States, 2012-2013. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0148395. e0148395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K. 7th ed. Centre for Longitudinal Studies; London: 2012. Millennium cohort study: A guide to the datasets. [Google Scholar]
- Harding C., Wheaton B., Butler A. Childcare survey 2017: Family and childcare trust 2017. https://www.familyandchildcaretrust.org/childcare-survey-2017 Available from:
- Hungerford D., Vivancos R., Read J.M., Iturriza-Gomara M., French N., Cunliffe N.A. Rotavirus vaccine impact and socioeconomic deprivation: An interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Medicine. 2018;16(1):10. doi: 10.1186/s12916-017-0989-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. The family as producer of health--an extended grossman model. Journal of Health Economics. 2000;19(5):611–637. doi: 10.1016/s0167-6296(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Kenny D.R. Mediation 2018. http://davidakenny.net/cm/mediate.htm Available from:
- Khanam R., Nghiem H.S., Connelly L.B. Child health and the income gradient: Evidence from Australia. Journal of Health Economics. 2009;28(4):805–817. doi: 10.1016/j.jhealeco.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Koenker R. Cambridge University Press; Cambridge and New York: 2005. Quantile regression: Econometric society monographs, no. 38. 2005. [Google Scholar]
- Lopman B.A., Steele D., Kirkwood C.D., Parashar U.D. The vast and varied global burden of norovirus: Prospects for prevention and control. PLoS Medicine. 2016;13(4) doi: 10.1371/journal.pmed.1001999. e1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J.F., Abis Study G. Epidemiological study of constipation and other gastrointestinal symptoms in 8000 children. Acta Paediatrica. 2006;95(5):573–580. doi: 10.1080/08035250500452621. [DOI] [PubMed] [Google Scholar]
- Mayer S.E. Harvard University Press; Cambridge and London: 1997. What money can't buy: Family income and children's life chances. 1997. [Google Scholar]
- Menon V.K., George S., Aladin F., Nawaz S., Sarkar R., Lopman B. Comparison of age-stratified seroprevalence of antibodies against norovirus GII in India and the United Kingdom. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056239. e56239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci L.A., Hsieh C.C., Williams P.L., Dickman P.W., Bjorkman L., Pedersen N.L. Birth order, sibship size, and housing density in relation to tooth loss and periodontal disease: A cohort study among Swedish twins. American Journal of Epidemiology. 2004;159(5):499–506. doi: 10.1093/aje/kwh063. [DOI] [PubMed] [Google Scholar]
- My P.V., Thompson C., Phuc H.L., Tuyet P.T., Vinh H., Hoang N.V. Endemic norovirus infections in children, Ho chi minh city, vietnam, 2009-2010. Emerging Infectious Diseases. 2013;19(6):977–980. doi: 10.3201/eid1906.111862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman K.L., Leon J.S., Rebolledo P.A., Scallan E. The impact of socioeconomic status on foodborne illness in high-income countries: A systematic review. Epidemiology and Infection. 2015;143(12):2473–2485. doi: 10.1017/S0950268814003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K., Burns R., Violato M. Family income, maternal psychological distress and child socio-emotional behaviour: Longitudinal findings from the UK Millennium Cohort Study. SSM Population Health. 2018;4:280–290. doi: 10.1016/j.ssmph.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien S.J., Donaldson A.L., Iturriza-Gomara M., Tam C.C. Age-specific incidence rates for norovirus in the community and presenting to primary healthcare facilities in the United Kingdom. The Journal of Infectious Diseases. 2016;213(Suppl 1):S15–S18. doi: 10.1093/infdis/jiv411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peasey A.E., Ruiz-Palacios G.M., Quigley M., Newsholme W., Martinez J., Rosales G. Seroepidemiology and risk factors for sporadic norovirus/Mexico strain. The Journal of Infectious Diseases. 2004;189(11):2027–2036. doi: 10.1086/386310. [DOI] [PubMed] [Google Scholar]
- Phillips G., Tam C.C., Rodrigues L.C., Lopman B. Prevalence and characteristics of asymptomatic norovirus infection in the community in England. Epidemiology and Infection. 2010;138(10):1454–1458. doi: 10.1017/S0950268810000439. [DOI] [PubMed] [Google Scholar]
- Phillips G., Tam C.C., Rodrigues L.C., Lopman B. Risk factors for symptomatic and asymptomatic norovirus infection in the community. Epidemiology and Infection. 2011;139(11):1676–1686. doi: 10.1017/S0950268810002839. [DOI] [PubMed] [Google Scholar]
- Pockett R.D., Adlard N., Carroll S., Rajoriya F. Paediatric hospital admissions for rotavirus gastroenteritis and infectious gastroenteritis of all causes in England: An analysis of correlation with deprivation. Current Medical Research and Opinion. 2011;27(4):777–784. doi: 10.1185/03007995.2011.555757. [DOI] [PubMed] [Google Scholar]
- Propper C., Rigg J., Burgess S. Child health: Evidence on the roles of family income and maternal mental health from a UK birth cohort. Health Economics. 2007;16(11):1245–1269. doi: 10.1002/hec.1221. [DOI] [PubMed] [Google Scholar]
- Quigley M.A., Kelly Y.J., Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119(4):e837–e842. doi: 10.1542/peds.2006-2256. [DOI] [PubMed] [Google Scholar]
- Rogawski E.T., Westreich D.J., Becker-Dreps S., Adair L.S., Sandler R.S., Sarkar R. Antibiotic treatment of diarrhoea is associated with decreased time to the next diarrhoea episode among young children in Vellore, India. International Journal of Epidemiology. 2015;44(3):978–987. doi: 10.1093/ije/dyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T.C., Adams N.L., Barr B., Hawker J., O'Brien S.J., Violato M. Socioeconomic status is associated with symptom severity and sickness absence in people with infectious intestinal disease in the UK. BMC Infectious Diseases. 2017;17(1):447. doi: 10.1186/s12879-017-2551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein M., Feinberg E., Young R., Sauder S. Maternal depression, perceptions of children's social aptitude, and reported activity restriction among former very low birth weight infants. Archives of Disease in Childhood. 2010;95(7):521–525. doi: 10.1136/adc.2009.181735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C.C., O'Brien S.J. Economic cost of Campylobacter, norovirus and rotavirus disease in the United Kingdom. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0138526. e0138526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C.C., Rodrigues L.C., Viviani L., Dodds J.P., Evans M.R., Hunter P.R. Longitudinal study of infectious intestinal disease in the UK (IID2 study): Incidence in the community and presenting to general practice. Gut. 2012;61(1):69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend C., Cortina-Borja M., Peckham C., Brown D., Johnson J., Joshi H. 2012. Technical Report on the Millennium Cohort Study biomedical data enhancement study of infections and later allergies. London. [Google Scholar]
- Vianna N.J., Polan A.K. Immunity in hodgkin's disease: Importance of age at exposure. Annals of Internal Medicine. 1978;89(4):550–556. doi: 10.7326/0003-4819-89-4-550. [DOI] [PubMed] [Google Scholar]
- Violato M., Petrou S., Gray R., Redshaw M. Family income and child cognitive and behavioural development in the United Kingdom: does money matter? Health Economics. 2011;20(10):1201–1225. doi: 10.1002/hec.1665. [DOI] [PubMed] [Google Scholar]
- White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Whitney B.M., Mainero C., Humes E., Hurd S., Niccolai L., Hadler J.L. Socioeconomic status and foodborne pathogens in Connecticut, USA, 2000-2011(1) Emerging Infectious Diseases. 2015;21(9):1617–1624. doi: 10.3201/eid2109.150277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.