Abstract
Introduction
“Dual use” refers to the concurrent use of tobacco cigarettes (smoking) and electronic cigarettes (e-cigarettes; vaping). Although dual use is common among e-cigarette users, there is little evidence regarding biomarkers of exposure among dual users and how these change under different conditions of product use.
Methods
A nonblinded within-subjects crossover experiment was conducted with adult daily dual users (n = 48) in Ontario, Canada. Participants completed three consecutive 7-day periods in which the use of tobacco cigarettes and e-cigarettes was experimentally manipulated, resulting in four study conditions: Dual use, Tobacco cigarette use, E-cigarette use, and No product use. Repeated measures models were used to examine changes in product use and biomarkers of exposure.
Results
Compared to dual use, cotinine remained stable when participants exclusively smoked (p = .524), but significantly decreased when they exclusively vaped (p = .027), despite significant increases in e-cigarette consumption (p = .001). Levels of biomarkers of exposure to toxicants, including carbon monoxide (CO), 1-hydroxypyrene (1-HOP), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), were significantly lower when participants exclusively vaped than when they engaged in dual use (CO = −41%, p < .001; 1-HOP = −31%, p = .025; NNAL = −30%, p = .017). Similar findings were observed among participants abstaining from both products as compared to dual use (CO: −26%, p < .001; 1-HOP = −14% [ns]; NNAL = −35%, p = .016). In contrast, levels of biomarkers of exposure increased when participants exclusively smoked as compared to dual use (CO = +21%, p = .029; 1-HOP = +23%, p = .048; NNAL = +8% [ns]).
Conclusions
Although dual use may reduce exposure to tobacco smoke constituents to some extent, abstaining from smoking is the most effective way to reduce such exposure.
Implications
Public health authorities should clearly communicate the relative risk of e-cigarettes and tobacco cigarettes to the general public, focusing on two salient points: (1) e-cigarettes are not harmless, but they are less harmful than tobacco cigarettes; and (2) using e-cigarettes while smoking may not necessarily reduce health risks; therefore, consumers should stop smoking completely to maximize potential health benefits.
Introduction
Electronic cigarettes (e-cigarettes) are alternative nicotine delivery devices whose popularity worldwide continues to grow.1,2 E-cigarettes typically contain nicotine and are available in a wide range of flavors and product classes, including disposable products and “tank” systems.1,3 To date, evidence regarding the health effects of e-cigarettes suggests that they are likely to be harmful, but almost certainly less harmful than tobacco cigarettes.4
“Dual use”—the concurrent use of e-cigarettes and tobacco cigarettes—is the most common pattern of e-cigarette use. For instance, 63% and 70% of current vapers in Canada and the United States, respectively, also smoke tobacco cigarettes.5,6 Although the reasons for using e-cigarettes most frequently reported by dual users are to quit or reduce their smoking,7–11 it remains unclear to what extent dual users substitute tobacco cigarettes with e-cigarettes, and what, if any, impact this may have on their health.
Biomarkers of exposure are an important short-term indicator of the potential health risk of e-cigarettes. Several switching studies have prospectively examined changes in biomarker levels following a complete or partial switch from tobacco cigarettes to e-cigarettes, demonstrating that e-cigarettes expose users to nicotine but not to by-products of tobacco combustion. With respect to nicotine, evidence indicates that e-cigarettes vary greatly in their nicotine delivery potential.12 This variability is reflected in switching studies, which show that some, but not all, smokers have successfully switched from tobacco cigarettes to e-cigarettes, compensating for nicotine via a new nicotine-delivering product.10,13–19 To date, several studies indicate that e-cigarettes do not expose users to carbon monoxide13–18,20–23; however, evidence regarding other tobacco smoke constituents is limited. Comparative analyses indicate that exposure to polycyclic aromatic hydrocarbons (PAHs) was significantly lower among exclusive vapers compared to smokers.24 However, switching studies have reported mixed findings: although an industry-sponsored study reported significant decreases in PAH exposure among clinically confined subjects who completely switched to exclusive vaping, dual use, or who gave up tobacco and nicotine products entirely,18 a naturalistic study reported significant declines in some PAH biomarkers, but no change in others.17 With respect to tobacco-specific nitrosamines (TSNAs), comparative analyses have shown that exposure to the TSNA 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was significantly lower among exclusive vapers as compared to smokers and dual users.24–26 In addition, independent and industry-sponsored switching studies have shown that exposure to TSNAs declined significantly following complete smoking abstinence.16–18
Switching studies published to date have several notable limitations. First, many studies have examined early-generation devices, which provided less efficient nicotine delivery.13,14 Second, most study participants were completely or partially naive to e-cigarette use at the time of the switch,10,14,15,17 which may have implications for how these products are used, given the learning curve associated with e-cigarette adoption.27 Third, in only one study participants were allowed to select their e-cigarette flavor and nicotine concentration,15 despite evidence showing that vapers view the selection of such product characteristics as highly important with respect to satisfaction, nicotine delivery, and smoking reduction and cessation.28,29 As a result, many switching studies are limited in their external validity.
Finally, very few switching studies have examined dual use of tobacco cigarettes and e-cigarettes.14,15 This constitutes a critical evidence gap, given that in many countries, most e-cigarette users are dual users. Therefore, this study examined exposure to nicotine and tobacco smoke constituents among dual users in the context of several product switches in a naturalistic setting.
Methods
Participants
A nonblinded within-subjects experiment was conducted with adult (aged 18 years and over) dual users of tobacco cigarettes and e-cigarettes in Kitchener−Waterloo and Toronto, Ontario. Dual users were identified as current daily tobacco cigarette smokers (had smoked ≥100 cigarettes in their lifetime, and smoked ≥5 cigarettes/day) and current daily e-cigarette users (had used an e-cigarette at least once a day for each of the past 7 days).
Study eligibility also required the absence of the following: serious intentions to quit smoking in the next 6 months; use of other tobacco products in the past 7 days; use of nicotine replacement therapy in the past 7 days; use of any smoking cessation medications in the past 7 days; participation in individual or group counseling programs for smoking cessation in the past 7 days; experience of serious cardiac health issues; experience of a heart attack or stroke within the last 3 months; experience of cancer within the last year; experience of asthma, chronic obstructive pulmonary disease, a seizure disorder, or any life-threatening medical conditions with a prognosis of less than a year; and a history of psychosis, schizophrenia, bipolar disorder, or suicidal thoughts.
Participants were recruited from September 2015 to March 2016 via advertisements placed in newspapers, online, and in local vape shops, and received $295 for participating in the study. The study received clearance from the University of Waterloo’s Office of Research Ethics.
Study Design and Protocol
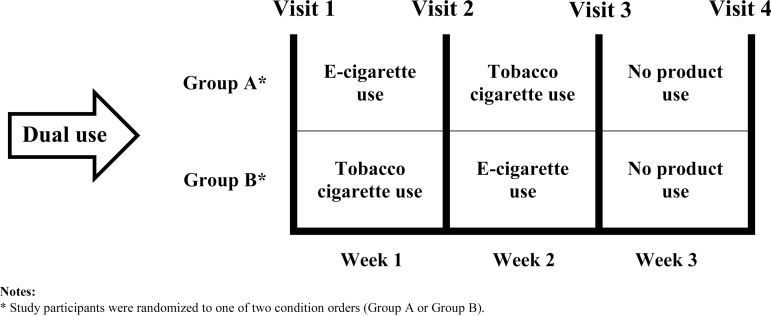
Participants completed three consecutive 7-day periods in which the use of tobacco cigarettes and e-cigarettes was experimentally manipulated, resulting in four study conditions: Dual use, Tobacco cigarette use, E-cigarette use, and No product use. To control for order effects, participants were randomly assigned to one of two condition orders, consisting of predefined sequences of product use: following the baseline condition of Dual use, Group A participants switched to E-cigarette use, then to Tobacco cigarette use, and finally to No product use; in contrast, following the baseline condition of Dual use, Group B participants switched to Tobacco cigarette use, then to E-cigarette use, and finally to No product use (Figure 1).
Figure 1.
Study design. *Study participants were randomized to one of two condition orders (Group A or Group B).
Participants were asked to attend four laboratory visits: at baseline and after each of the 7-day periods. At each visit, participants completed a 20-minute questionnaire regarding their smoking and vaping behaviors. Participants were also asked to provide a “spot” urine sample, which was frozen at −20°C immediately afterward, and two exhaled breath samples, which were measured using Bedfont Micro 4 Smokerlyzer and piCO+ Smokerlyzer machines (Bedfont Scientific Ltd). Throughout the study, participants were asked to complete a 5-minute online daily diary about their consumption of tobacco cigarettes and e-cigarettes.
Measures
Nicotine dependence for tobacco cigarettes was assessed at baseline using the Fagerström Test for Cigarette Dependence (FTCD). The measure was also adapted to assess nicotine dependence for e-cigarettes by substituting the words “smoke cigarettes” with “use e-cigarettes”. Across study conditions, patterns of use for each product were examined via self-reported questionnaire measures. Daily consumption of tobacco cigarettes and e-cigarettes was assessed using the questions “In the past 7 days, on average, how many cigarettes did you smoke per day?” and “In the past 7 days, on average, how many times did you use an e-cigarette per day?” The number of times e-cigarettes were used per day (bouts) was defined as an instance of at least one puff. Time to first use was assessed for each product using the question “In the past 7 days, on average, how soon after waking did you smoke (use) your first cigarette (e-cigarette)?,” with the following response options: “within 5 minutes,” “6–30 minutes,” “31–60 minutes,” and “after 60 minutes.” Subjective measures, such as symptoms of nicotine withdrawal and self-efficacy for quitting, were also assessed, but are not presented here.
Several biomarkers of exposure were examined in this study. Carbon monoxide was measured in exhaled breath to provide an indication of recent inhalation of tobacco smoke (half-life: 4 hours).30 Urinary concentration of cotinine, a major proximate metabolite of nicotine, was measured to assess exposure to nicotine (half-life: 16 hours).30,31 Urinary concentrations of 1-hydroxypyrene (1-HOP) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) were measured to examine carcinogen exposure: 1-HOP is the major urinary metabolite of pyrene, a noncarcinogenic component of all PAH mixtures and a by-product of tobacco combustion (half-life: 19 hours)32; and total NNAL (free unconjugated form and its glucuronides) is a metabolite of the TSNA NNK (half-life: 40–45 days).33 Validated methods were used by Roswell Park Comprehensive Cancer Center (Buffalo, NY) to analyze levels of urinary cotinine,34 urinary 1-HOP,35 and urinary total NNAL.36 All urinary biomarkers were adjusted for creatinine.
Statistical Analysis
Changes in key outcomes were examined across study conditions. Values below the limit of quantitation were substituted with constants (limit of quantitation/(√2)). Log transformations were applied to address violations from normality for several continuous outcomes (cotinine, 1-HOP, NNAL). For each key outcome, means were computed at baseline and for each study condition. Repeated measures analyses were conducted to examine mean differences for each outcome across study conditions, while accounting for correlated measurements within subjects. Covariates included the following: assigned condition order (Group A, Group B), baseline nicotine dependence (FTCD score), e-cigarette product type (tank system, other), and e-cigarette nicotine content (nicotine present, nicotine absent). Analyses were conducted using SPSS v. 24 (Chicago, IL).
Results
Sample Characteristics
Of the 293 individuals screened for study eligibility, 60 were deemed eligible. Following post hoc exclusions of those who failed to attend all study visits (n = 3), and those with very low (<5 ppm) carbon monoxide levels at baseline (n = 9), 48 participants comprised the analytic sample. Characteristics of the sample are shown in Table 1. Dual users had a mean age of 36 (SD = 11.7) years, were mostly male (71%) and white (71%), and exhibited low-to-moderate cigarette dependence (FTCD score: 4.7 [SD = 1.9]). Nicotine dependence for tobacco cigarettes was greater than that for e-cigarettes, at 4.7 (SD = 1.9) and 3.0 (SD = 2.1), respectively (t = 4.864, p < .001). Study participants had smoked and vaped daily for 17.4 (SD = 12.2) and 1.2 (SD = 0.9) years, respectively, and all reported initiating smoking before vaping. Virtually all dual users reported using tank systems (92%) and e-cigarettes with nicotine (94%). The study sample included three participants who reported exclusively vaping e-cigarettes without nicotine at baseline. Although inclusion of these participants may have impacted biomarker analyses, sensitivity analyses (data not shown) yielded nonzero levels of urinary cotinine for these subjects across all study conditions, and adjustment for e-cigarette nicotine content in the biomarker analyses yielded no significant effects; thus, these participants were retained in the analytic sample. Common e-liquid flavors included fruit (50%), tobacco (42%), and candy (42%). Among those who reported using e-cigarettes with nicotine (n = 45), nicotine concentrations less than or equal to 14 mg/mL were most commonly used (71%).
Table 1.
Sample Characteristics (n = 48)
| Characteristic | % | (n) | |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 35.9 (11.7) | ||
| 18–24 | 14.6 | (7) | |
| 25–39 | 56.3 | (27) | |
| 40–54 | 20.8 | (10) | |
| 55+ | 8.3 | (4) | |
| Sex | |||
| Male | 70.8 | (34) | |
| Female | 29.2 | (14) | |
| Ethnicity | |||
| White | 70.8 | (34) | |
| Other | 29.2 | (14) | |
| Education | |||
| High school or less | 27.1 | (13) | |
| Technical school/college | 35.4 | (17) | |
| Any university | 37.5 | (18) | |
| Nicotine dependence1 | |||
| Mean (SD) | |||
| Tobacco cigarettes | 4.7 (1.9) | ||
| E-cigarettes | 3.0 (2.1) | ||
1Nicotine dependence for tobacco cigarettes was assessed using the Fagerström Test for Cigarette Dependence (FTCD). An adapted version of the FTCD, in which the words “smoke cigarettes” were substituted with “use e-cigarettes”, was used to assess nicotine dependence for e-cigarettes. E-cigarette = electronic cigarette
To test whether randomization of participants was successful, several baseline measures were examined by assigned condition order (Group A, Group B) using independent t tests. Analyses indicated no significant differences by assigned condition order (data not shown).
Patterns of Product Use
Patterns of “permitted” (products participants were asked to use) and “non-permitted” (products participants were asked not to use) tobacco cigarette and e-cigarette use across study conditions are presented in Table 2. Throughout the study, between 54% and 58% participants smoked non-permitted tobacco cigarettes, and between 25% and 31% participants used non-permitted e-cigarettes. Participants reported smoking a significantly greater number of non-permitted tobacco cigarettes per day in the No product use condition compared to the E-cigarette use condition (t = −3.31, p = .003), although time to first use for this product did not differ across these study conditions (t = 1.27, p = .218). With respect to non-permitted e-cigarettes, daily consumption and time to first use did not differ significantly across conditions (t = 0.28, p = .790 and t = 1.16, p = .283, respectively).
Table 2.
Patterns of Use of Tobacco Cigarettes and E-cigarettes and Biomarkers of Exposure Across Study Conditions (n = 48)
| Condition | Test statistic, F (p value) | ||||
|---|---|---|---|---|---|
| Dual use | Tobacco cigarette use | E-cigarette use | No product use | ||
| Mean (95% CI) [% change from dual use] | |||||
| Use of tobacco cigarettes | |||||
| Daily consumption1 (cigarettes) | 13.71 (12.07% to 15.34%)a | 12.35 (10.54% to 14.15%)b | 1.89(n = 26) (1.05% to 2.74%) | 2.98(n = 28) (2.18% to 3.79%) | 7.89 (.008) |
| Time to first use2 | 0.88 (0.66% to 1.09%)a | 1.00 (0.77% to 1.24%)a | 2.63(n = 26) (2.37% to 2.90%) | 2.40(n = 28) (1.99% to 2.82%) | 1.60 (.213) |
| Use of e-cigarettes | |||||
| Daily consumption1 (e-cigarette bouts) | 11.11 (7.76% to 14.45%)a | 2.55(n = 12) (1.32% to 3.79%) | 17.42 (12.77% to 22.08%)b | 2.40(n = 15) (0.68% to 4.12%) | 10.11 (.003) |
| Time to first use2 | 2.00 (1.70% to 2.30%)a | 2.96(n = 12) (2.86% to 3.06%) | 1.10 (0.86% to 1.33%)b | 2.52(n = 15) (1.65% to 3.39%) | 24.00 (<.001) |
| Biomarkers of exposure | |||||
| Urinary cotinine3 (ng/mg creatinine) | 1174.44 (859.41% to 1604.72%)a [baseline] | 1282.04 (925.34% to 1776.23%)a [+9%] | 733.67 (478.41% to 1125.12%)b [−38%]* | 533.21 (326.59% to 870.56%)b [−55%]* | 5.79 (.002) |
| Exhaled CO4 (ppm) | 17.45 (14.22% to 20.68%)a [baseline] | 21.12 (17.37% to 24.87%)b [+21%]* | 10.33 (7.47% to 13.18%)c [−41%]* | 12.91 (10.18% to 15.63%)c [−26%]* | 10.12 (<.001) |
| Urinary 1-HOP3 (pg/mg creatinine) | 203.33 (153.85% to 268.66%)a [baseline] | 249.23 (197.15% to 315.14%)b [+23%]* | 141.06 (98.29% to 202.49%)c [−31%]* |
175.07 (134.28% to 228.19%)a [−14%] | 4.77 (.006) |
| Urinary NNAL3 (pg/mg creatinine) | 30.26 (21.06% to 43.48%)a [baseline] | 32.76 (23.89% to 44.91%)a [+8%] | 21.25 (14.34% to 31.47%)b [−30%]* |
19.76 (13.45% to 29.03%)b [−35%]* |
4.59 (.007) |
Gray-shaded areas indicate use of “non-permitted” products, for each study condition. Measures of patterns of use for non-permitted products were obtained through self-reported responses collected from participants’ daily diaries, whereas those for permitted products (white areas) were obtained through self-reported responses collected from scheduled laboratory visits. Summary statistics for use of non-permitted products are presented for the subset of participants who reported using a given product. CI = confidence interval; CO = carbon monoxide; e-cigarette = electronic cigarette; ppm = parts per million; 1-HOP = 1-hydroxypyrene; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
a,b,cConditions with different superscript letters were significantly different from one another, p < .05.
*Significant differences in biomarkers of exposure compared to the condition of Dual use, p < .05.
1Daily product consumption measured as: cigarettes smoked per day, in the past 7 d for tobacco cigarettes; and number of “bouts” per day, in the past 7 d (bout defined as an instance of at least one puff), for e-cigarettes.
2Mean time to first use calculated for recoded variable as a continuous measure ranging from 0 (within 5 min) to 3 (after 60 min).
3Geometric mean.
4Arithmetic mean.
Changes in patterns of permitted product use were compared across study conditions, as shown in Table 2. A repeated measures model examining daily tobacco cigarette consumption across study conditions yielded a significant effect of condition (F = 7.89, p = .008): daily tobacco cigarette consumption was significantly higher in the condition of Dual use compared to the Tobacco cigarette use condition (mean difference = 1.40, 95% CI = 0.39% to 2.40%, p = .008). There was no significant condition × condition order interaction (F = 2.99, p = .091) (Table 2).
A repeated measures model examining time to first tobacco cigarette across study conditions yielded no statistically significant differences (F = 1.60, p = .213). However, a significant interaction between assigned condition order and condition (F = 5.29, p = .027) was observed. Stratified analyses indicated that the main (null) effect of condition (described earlier) held for Group B participants (F = 0.61, p = .444). In contrast, a significant effect of condition was detected for Group A participants (F = 5.07, p = .036): time to first tobacco cigarette was significantly lower in the condition of Dual use as compared to the Tobacco cigarette use condition (mean difference = −0.33, 95% CI = −0.64% to −0.03%, p = .036) (Table 2).
As shown in Table 2, analyses examining daily e-cigarette consumption across study conditions yielded a significant effect of condition (F = 10.11, p = .003): daily e-cigarette consumption was significantly higher in the E-cigarette use condition compared to the Dual use condition (mean difference = 6.21, 95% CI = 2.27% to 10.15%, p = .003). No significant effect was detected for the interaction of condition and assigned condition order (F = 0.01, p = .921).
Further, significant differences in time to first e-cigarette use across study conditions were also observed (F = 24.00, p < .001): time to first e-cigarette was significantly lower in the E-cigarette use condition compared to the Dual use condition (mean difference = −0.88, 95% CI = −1.25% to −0.52%, p < .001). There was no significant condition × condition order interaction (F = 0.59, p = .446) (Table 2).
Exposure to Nicotine and Tobacco Smoke Constituents
Table 2 presents biomarkers of exposure across study conditions as well as changes across study conditions relative to the condition of Dual use.
Urinary levels of creatinine-corrected cotinine differed significantly by study condition (F = 5.79, p = .002): urinary cotinine was significantly higher in the Dual use condition compared to the E-cigarette use condition (mean difference = 1.60, 95% CI = 1.06% to 2.42%, p = .027) and the No product use condition (mean difference = 2.26, 95% CI = 1.31% to 3.92%, p = .004). In addition, urinary cotinine was significantly higher in the Tobacco cigarette use condition compared to the E-cigarette use condition (mean difference = 1.73, 95% CI = 1.21% to 2.46%, p = .003) and the No product use condition (mean difference = 2.44, 95% CI = 1.51% to 3.97%, p = .001). There was no significant condition × condition order interaction (F = 0.88, p = .462) (Table 2).
A repeated measures model examining exhaled carbon monoxide across study conditions yielded a significant effect of condition (F = 10.12, p < .001): exhaled carbon monoxide was significantly higher in the Tobacco cigarette use condition compared to the Dual use condition (mean difference = 3.85, 95% CI = 0.42% to 7.29%, p = .029), E-cigarette use condition (mean difference = 10.72, 95% CI = 6.43% to 15.01%, p < .001), and No product use condition (mean difference = 8.41, 95% CI = 4.78% to 12.04%, p < .001). In addition, carbon monoxide was significantly higher in the Dual use condition compared to the E-cigarette use condition (mean difference = 6.86, 95% CI = 3.79% to 9.94%, p < .001) and the No product use condition (mean difference = 4.56, 95% CI = 1.52% to 7.60%, p = .004) (Table 2).
Urinary 1-HOP also differed across study conditions (F = 4.77, p = .006): urinary 1-HOP was significantly higher in the Tobacco cigarette use condition compared to the Dual use condition (mean difference = 1.28, 95% CI = 1.00% to 1.63%, p = .048), E-cigarette use condition (mean difference = 1.78, 95% CI = 1.29% to 2.47%, p = .001), and No product use condition (mean difference = 1.41, 95% CI = 1.10% to 1.81%, p = .009). In addition, urinary 1-HOP was significantly higher in the Dual use condition compared to the E-cigarette use condition (mean difference = 1.40, 95% CI = 1.04% to 1.86%, p = .025). There was no significant condition × condition order interaction (F = 1.88, p = .148) (Table 2).
Levels of urinary NNAL across study conditions showed a similar pattern of results (F = 4.59, p = .007): urinary NNAL was significantly higher in the Tobacco cigarette use condition compared to the E-cigarette use condition (mean difference = 1.52, 95% CI = 1.17% to 1.96%, p = .002) and the No product use condition (mean difference = 1.57, 95% CI = 1.22% to 2.02%, p = .001). In addition, urinary NNAL was significantly higher in the Dual use condition compared to the E-cigarette use condition (mean difference = 1.41, 95% CI = 1.07% to 1.87%, p = .017) and the No product use condition (mean difference = 1.47, 95% CI = 1.08% to 2.00%, p = .016). There was no significant condition × condition order interaction (F = 1.26, p = .301) (Table 2).
Discussion
In this study, participants were able to effectively maintain nicotine intake when they switched from dual use to smoking. This stability of cotinine levels is consistent with published studies examining switching from exclusive smoking to dual use.15,19 In contrast, compared to dual use, participants’ cotinine levels were significantly lower when they exclusively vaped, despite significant increases in self-reported e-cigarette consumption, including increased daily use of e-cigarettes and use earlier in the day, suggesting that participants exhibited compensatory behavior with respect to e-cigarettes. However, this behavioral change appeared insufficient to maintain cotinine levels in contrast to several studies in which smokers maintained stable cotinine levels while using advanced e-cigarette products.10,15,26 Although the vast majority of dual users in this study reported using tank systems and e-liquids with nicotine, the nicotine delivery potential of these devices was not tested and may account for these results. Indeed, similar levels of cotinine among study participants across conditions of exclusive vaping and no product use support the notion that participants’ e-cigarette devices may have been limited in their ability to deliver nicotine.
Levels of several biomarkers of tobacco smoke exposure, including exhaled carbon monoxide, 1-HOP, and NNAL, were consistently lower when participants exclusively vaped compared to when they engaged in dual use. Reductions in exposure to carbon monoxide are consistent with published studies examining smokers’ switch to use of e-cigarettes.13–18,20,21,23 In addition, reductions in exposure to pyrene and the carcinogen NNK support published comparative analyses between vapers and smokers,24,25 as well as switching studies.16–18 Biomarkers of exposure were also reduced when participants abstained from both tobacco cigarettes and e-cigarettes, as compared to dual use. Significant reductions were observed for carbon monoxide and NNK when participants used neither product; although levels of 1-HOP also decreased, this difference was not statistically significant. In addition, although exposure to all examined tobacco smoke constituents decreased when participants were not permitted to smoke nor vape, exposure did not reduce to nil. This is likely due to some respondents continuing to smoke tobacco cigarettes, as well as slow clearance of some biomarkers, particularly NNAL.33 It may also reflect the presence of contaminants in e-cigarette products, or other sources of environmental exposure, particularly for PAHs.30
Exposure to carbon monoxide and PAHs was significantly greater when individuals exclusively smoked as compared to when they engaged in dual use (21% and 23%, respectively). With respect to this comparison, a nonsignificant increase in exposure to NNK was also observed (8%). These findings are generally consistent with two published switching studies. First, in a switching study with 4-week follow-up, McRobbie et al.14 reported significant reduction in exposure to carbon monoxide among smokers taking up e-cigarettes, with greater reduction observed among exclusive vapers as compared to dual users (80% vs. 52%). Further, in an industry-sponsored 1-week switching study, O’Connell et al.18 reported similar findings, with all examined biomarkers showing a decreasing trend with decreasing tobacco cigarette consumption among parallel groups of smokers. Notably, greater reduction in exposure was observed in these switching studies when compared with findings from this study. These differing results may be accounted for by the inclusion of smokers with motivations to quit14 and the clinical confinement of smokers18 in these studies, which may have contributed to greater potential substitution of tobacco cigarettes with e-cigarettes and greater compliance with forced product switching.
To date, only one other study has examined tobacco-related biomarkers of exposure in real-world settings. Shahab et al.26 examined a suite of biomarkers of exposure to TSNAs and volatile organic compounds in several groups of long-term nicotine product users. Cross-sectional comparative analyses indicated that exclusive vaping, but not dual use of tobacco cigarettes and e-cigarettes, was associated with lower levels of exposure to several tobacco smoke constituents, as compared to exclusive smoking.26 Although the authors noted that their statistical power to detect small differences (such as that between dual users and exclusive smokers) was limited, the magnitude of observed differences in exposure was similar to that in this study, at least with respect to NNK exposure. This may reflect the fact that both studies assessed experienced nicotine product users in real-world settings.
Overall, study findings regarding exposure to tobacco smoke constituents are consistent with the product design and properties of e-cigarettes, which do not contain tobacco and do not undergo combustion when used,1 and support research evidence suggesting that use of e-cigarettes is likely to be less harmful than smoking.4 Although this study is unable to discern whether dual users reduce their tobacco cigarette consumption by substitution with e-cigarettes or simply use e-cigarettes alongside their usual smoking, it appears dual users use their products to achieve a desired level of nicotine, consistent with other research.26,37
Despite slight reductions in exposure associated with dual use, the findings demonstrate that abstaining from tobacco cigarettes is the most important factor in reducing exposure to toxic smoke constituents. Research evidence indicates that smokers who quit tobacco cigarettes completely reduce their risk of premature death to levels comparable to nonsmokers.38,39 However, the potential benefits of smoking reduction, as may be the case of dual use, are less clear. To date, significant health benefits from reducing the amount of tobacco cigarettes smoked have not been demonstrated with respect to various disease outcomes.39 Although it is plausible that dual use could reduce individual risk if it results in substantial reductions in smoking, the threshold for meaningful reductions is unclear, particularly given that smokers may compensate for reductions in the number of cigarettes they smoke by smoking each cigarette more intensely.39,40 This is generally supported by the current findings, in which the differences between dual use and exclusive smoking were modest. Therefore, dual use is likely to have public health benefit to the extent that it leads to complete smoking cessation.
This study has several limitations. Participants’ patterns of product use were based on self-reported data, which are subject to recall bias. Challenges associated with measuring patterns of e-cigarette use suggest that such measures may be subject to underreporting.41 However, these limitations are tempered by the study’s use of objective biomarkers of exposure, which provide robust measures with which to examine product switching behavior. The study did not assess use of cannabis or other combusted nontobacco products, which may have influenced levels of carbon monoxide and 1-HOP.
Unlike other switching studies, this study did not confine participants to a laboratory setting, meaning participants’ adherence to the study protocol could not be verified. Biomarker data reflected this lack of compliance, particularly in the conditions in which participants were not permitted to smoke tobacco cigarettes. Sensitivity analyses showed that accounting for “cheating” adjusted the levels of biomarkers of exposure in the expected direction. Furthermore, cheating in the vaping condition would lead to reducing differences between this condition and that of dual use; that is, the potential benefits of exclusive vaping were obtained despite cheating, implying that such benefits may actually be greater than the differences observed in this study. More broadly, although cheating reduced the study’s internal validity, the study allowed for an investigation of product-switching behavior under real-world conditions, in which many smokers may not be able to achieve abstinence from smoking. Thus, a key strength of the study is its external validity, reflected in its naturalistic design and inclusion of experienced dual users using their own products.
The study findings have direct implications for public health policy. Public health authorities should acknowledge and clearly communicate differences in risk between smoking and vaping. Communicating the risk of using e-cigarettes relative to tobacco cigarettes should focus on two salient points: (1) e-cigarettes are not harmless, but they are less harmful than smoking tobacco cigarettes; and (2) using e-cigarettes while smoking may not necessarily reduce health risks; therefore, consumers should stop smoking completely to maximize potential health benefits. Although the communication of relative risk information is fraught with difficulties, public health authorities must rise to this challenge: consumers have a right to be accurately informed of product risks42,43 and e-cigarettes have become a permanent fixture of the market.44 In the absence of evidence-based communication from public health authorities, consumers will rely on less credible information from industry-sponsored marketing, media, and anecdotal evidence.45
Funding
This research was supported by an Ontario Ministry of Health and Long-Term Care Health System Research Fund grant (#06697 awarded to DH). Additional support was provided by the Canadian Institutes of Health Research (CIHR), the Vanier Canada Graduate Scholarship (CDC), a CIHR and Public Health Agency of Canada, Applied Public Health Chair (DH), and an Ontario Institute for Cancer Research Investigator Award (GTF).
Declaration of Interests
MLG reports grants from and served as an advisory board member to pharmaceutical companies that manufacture smoking cessation drugs. DH has provided paid expert testimony in tobacco litigation on behalf of governments and class-action plaintiffs on issues related to tobacco product science and regulation. The other authors have no competing interests to declare.
Acknowledgments
The authors would like to thank Ms Christine White and Ms Julia Gogoleva at the University of Waterloo for their assistance in conducting this research. The authors would also like to acknowledge Ms Mary Palumbo and Ms Taylor Vanderbush from the Nicotine and Tobacco Product Assessment Core at Roswell Park Comprehensive Cancer Center for their assistance in analyzing biomarkers of exposure.
References
- 1. Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med. 2017;52(2):e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gravely S, Fong GT, Cummings KM, et al. Awareness, trial, and current use of electronic cigarettes in 10 countries: findings from the ITC project. Int J Environ Res Public Health. 2014;11(11):11691–11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-cigarettes 2018. http://nationalacademies.org/hmd/Reports/2018/public-health-consequences-of-e-cigarettes.aspx. Accessed February 1, 2018.
- 5. Reid JL, Hammond D, Rynard VL, Madill CL, Burkhalter R. Tobacco Use in Canada: Patterns and Trends (2017 ed.) 2017. https://uwaterloo.ca/tobacco-use-canada/tobacco-use-canada-patterns-and-trends. Accessed October 18, 2017.
- 6. Coleman BN, Rostron B, Johnson SE, et al. Electronic cigarette use among US adults in the Population Assessment of Tobacco and Health (PATH) Study, 2013-2014. Tob Control. 2017;26(e2):e117–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Etter JF. Characteristics of users and usage of different types of electronic cigarettes: findings from an online survey. Addiction. 2016;111(4):724–733. [DOI] [PubMed] [Google Scholar]
- 8. Rass O, Pacek LR, Johnson PS, Johnson MW. Characterizing use patterns and perceptions of relative harm in dual users of electronic and tobacco cigarettes. Exp Clin Psychopharmacol. 2015;23(6):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rutten LJ, Blake KD, Agunwamba AA, et al. Use of E-Cigarettes among current smokers: associations among reasons for use, quit intentions, and current tobacco use. Nicotine Tob Res. 2015;17(10):1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berg CJ, Barr DB, Stratton E, Escoffery C, Kegler M. Attitudes toward E-Cigarettes, reasons for initiating E-Cigarette use, and changes in smoking behavior after initiation: a pilot longitudinal study of regular cigarette smokers. Open J Prev Med. 2014;4(10):789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel D, Davis KC, Cox S, et al. Reasons for current E-cigarette use among U.S. adults. Prev Med. 2016;93:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marsot A, Simon N. Nicotine and cotinine levels with electronic cigarette: a review. Int J Toxicol. 2016;35(2):179–185. [DOI] [PubMed] [Google Scholar]
- 13. van Staden SR, Groenewald M, Engelbrecht R, Becker PJ, Hazelhurst LT. Carboxyhaemoglobin levels, health and lifestyle perceptions in smokers converting from tobacco cigarettes to electronic cigarettes. S Afr Med J. 2013;103(11):865–868. [DOI] [PubMed] [Google Scholar]
- 14. McRobbie H, Phillips A, Goniewicz ML, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res (Phila). 2015;8(9):873–878. [DOI] [PubMed] [Google Scholar]
- 15. Pacifici R, Pichini S, Graziano S, Pellegrini M, Massaro G, Beatrice F. Successful nicotine intake in medical assisted use of e-cigarettes: a pilot study. Int J Environ Res Public Health. 2015;12(7):7638–7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cravo AS, Bush J, Sharma G, et al. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul Toxicol Pharmacol. 2016;81(suppl 1):S1–S14. [DOI] [PubMed] [Google Scholar]
- 17. Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res. 2017;19(2):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Connell G, Graff DW, D’Ruiz CD. Reductions in biomarkers of exposure (BoE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicol Mech Methods. 2016;26(6):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meier E, Wahlquist AE, Heckman BW, Cummings KM, Froeliger B, Carpenter MJ. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220–11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polosa R, Morjaria JB, Caponnetto P, et al. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med. 2014;9(5):537–546. [DOI] [PubMed] [Google Scholar]
- 23. Litt MD, Duffy V, Oncken C. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tob Control. 2016;25(suppl 2):ii67–ii72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17(6):704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 2017;26(e1):e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shahab L, Goniewicz ML, Blount BC, et al. nicotine, carcinogen, and toxin exposure in long-term E-Cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med. 2017;166(6):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McQueen A, Tower S, Sumner W. Interviews with “vapers”: Implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13(9):860–867. [DOI] [PubMed] [Google Scholar]
- 28. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10(12):7272–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farsalinos KE, Spyrou A, Stefopoulos C, et al. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers). Sci Rep. 2015;5:11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization (WHO). WHO Technical Report Series, 945. The Scientific Basis of Tobacco Product Regulation. Report of a WHO Study Group 2007. http://www.who.int/tobacco/publications/prod_regulation/trs_945/en/. Accessed April 5, 2017.
- 31. Haley NJ, Sepkovic DW, Hoffmann D. Elimination of cotinine from body fluids: disposition in smokers and nonsmokers. Am J Public Health. 1989;79(8):1046–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brandt HC, Watson WP. Monitoring human occupational and environmental exposures to polycyclic aromatic compounds. Ann Occup Hyg. 2003;47(5):349–378. [DOI] [PubMed] [Google Scholar]
- 33. Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59(3):590–596. [PubMed] [Google Scholar]
- 34. Liang SH. Rapid and Accurate LC-MS/MS Analysis of Nicotine and Related Compounds in Urine Using Raptor Biphenyl LC Columns and MS-Friendly Mobile Phases 2015. http://www.restek.com/Technical-Resources/Technical-Library/Clinical-Forensic-Toxicology/cft_CFAN2216-UNV. Accessed April 10, 2017.
- 35. Lankova D, Urbancova K, Sram RJ, Hajslova J, Pulkrabova J. A novel strategy for the determination of polycyclic aromatic hydrocarbon monohydroxylated metabolites in urine using ultra-high-performance liquid chromatography with tandem mass spectrometry. Anal Bioanal Chem. 2016;408(10):2515–2525. [DOI] [PubMed] [Google Scholar]
- 36. Jacob P 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80(21):8115–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benowitz NL. Compensatory smoking of low-yield cigarettes. In Smoking and Tobacco Control Monograph 13: Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine (Chapter 3) 2001. http://cancercontrol.cancer.gov/brp/tcrb/monographs/13/. Accessed March 15, 2017.
- 38. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. U.S. Department of Health and Human Services (USDHHS). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General 2010. http://www.cdc.gov/tobacco/data_statistics/sgr/2010/. Accessed March 10, 2017. [DOI] [PubMed]
- 40. Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1370–1375. [DOI] [PubMed] [Google Scholar]
- 41. Pearson JL, Elmasry H, Das B, et al. Comparison of ecological momentary assessment versus direct measurement of e-cigarette use with a bluetooth-enabled e-cigarette: a pilot study. JMIR Res Protoc. 2017;6(5):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kozlowski LT, Edwards BQ. “Not safe” is not enough: smokers have a right to know more than there is no safe tobacco product. Tob Control. 2005;14 (suppl 2):ii3–ii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kozlowski LT, Sweanor D. Withholding differential risk information on legal consumer nicotine/tobacco products: the public health ethics of health information quarantines. Int J Drug Policy. 2016;32:17–23. [DOI] [PubMed] [Google Scholar]
- 44. Benowitz NL, Goniewicz ML. The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–686. [DOI] [PubMed] [Google Scholar]
- 45. Zeller M, Hatsukami D; Strategic Dialogue on Tobacco Harm Reduction Group The strategic dialogue on tobacco harm reduction: a vision and blueprint for action in the US. Tob Control. 2009;18(4):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]