Abstract
Introduction
The effects of either menthol flavor cigarettes or total urinary menthol on nicotine dependence, biomarkers of addictive and carcinogenic exposure, and behavioral measures may inform differences and similarities of these two approaches.
Methods
Stratified recruitment by cigarette (menthol flavor or regular) and race (African American and white) yielded a balanced sample of 136 adult smokers in a 36-hour inpatient protocol. Exposure measures assessed during 24-hour data collection included urinary menthol, total NNAL [4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol], 10 polycyclic aromatic hydrocarbon metabolites, baseline plasma cotinine, plasma nicotine pre- and post-smoking, exhaled carbon monoxide pre- and post-smoking, and cigarette puff volumes. The latter three were measured at four specified timepoints throughout the day.
Results
There were no significant differences between menthol flavor and regular cigarette smokers in measures of nicotine dependence, biomarkers of addictive and carcinogenic exposures, or behavioral measures. Significant race × cigarette type interaction effects were found for two biomarkers: plasma nicotine and 3-hydroxyphenanthrene. Total urinary menthol was significantly associated with higher levels of nearly all dependent variables including puff volume, exhaled carbon monoxide, plasma nicotine and cotinine, NNAL, and polycyclic aromatic hydrocarbons. The significant effects of total urinary menthol were sustained after adjusting for menthol flavor and regular cigarette type and other covariates (eg, number of cigarettes per day, baseline cotinine, and baseline nicotine).
Conclusions
Urinary menthol is an independent predictive biomarker for nicotine dependence, addictive and carcinogenic exposure, and behaviors.
Implications
Comparison of the effects of menthol flavor and total urinary menthol on nicotine dependence, biomarkers of addictive and carcinogenic exposure, and behavioral measures emphasizes the important significant contribution of total urinary menthol concentrations in contrast to no significant associations by dichotomous cigarette type with these biomarkers.
Introduction
Menthol has been a characterizing flavor for many years and is an ingredient in most cigarettes.1 Menthol is added to cigarettes that are not labeled to have menthol. Although 85% of African American smokers smoke menthol flavor cigarettes, only 29% of white smokers do so.2 Nine of 14 recent studies showed that menthol flavor cigarette smokers reported increased nicotine dependence compared with regular cigarette smokers, whereas four of six studies identified shorter time to the first cigarette of the day among menthol flavor cigarette smokers in comparison to regular cigarette smokers.3 Individuals smoking menthol flavor cigarettes were significantly less likely to quit smoking than those who smoke regular cigarettes.4 On the basis of the National Survey on Drug Use and Health, menthol cigarette prevalence in recent years has increased and now exceeds nonmenthol cigarette smoking among youth and young adults.2 In the Population Assessment of Tobacco and Health study, it was noted that menthol flavor cigarette smokers tend to perceive their brand as more harmful, yet they are not more likely than regular cigarette smokers to quit smoking.5 The Tobacco Products Scientific Advisory Committee generated a comprehensive report noting that removing menthol cigarettes from the market would be in the interest of public health,6 and the 2013 Food and Drug Administration7 report concluded that “it is likely that menthol cigarettes pose a public health risk above that seen with nonmenthol cigarettes.”
African American smokers were likely to continue smoking, whereas those most likely to quit smoking were non–African American regular cigarette smokers.8 The findings from the National Health and Nutrition Examination Survey (NHANES) identified that African Americans compared with whites smoked for longer periods before quitting, with African American men smoking 2.3 years longer and women smoking 1.9 years longer before quitting.9 African American adolescents may be protected from smoking as a result of the interaction of menthol and taster status that changes with aging where a decline in one’s bitter taste facilitates smoking.4
Biomarkers in our study include polycyclic aromatic hydrocarbons (PAHs), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), and urinary menthol concentration. Cigarette smoking is a common source of exposure to carcinogenic PAHs such as phenanthrene.10 In the Multiethnic Cohort Study, African Americans and Native Hawaiians had the highest risk for lung cancer as measured by metabolites of phenanthrene.11 The Canadian Health Measures Survey implemented a nationally representative, cross-sectional survey with multiple data collection periods. Levels of total urinary NNAL were measured among tobacco users to examine changes in NNAL over time.12 Over a period of 6 years, there was a significant 64% increase in creatinine-corrected NNAL (p < .0001). Brinkman et al.13 examined mainstream smoke particulate exposure in a crossover study of smokers assigned to differing levels of menthol content. Mainstream smoke 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was 39% higher when participants smoked a menthol flavor cigarette. In the Multiethnic Cohort study, African Americans compared with whites had higher levels (per milliliter of urine) of NNAL, a potent lung carcinogen.14 Median levels of total NNAL were almost two times higher in African Americans as in Japanese Americans, and African American participants appeared to extract more nicotine and NNK per cigarette compared with whites.
To characterize menthol glucuronide (MG) exposure among smokers, Hsu et al.15 assessed biomarkers of plasma samples at two timepoints. MG levels were statistically higher in menthol flavor cigarette smokers compared with regular cigarette smokers, and these levels increased with each cigarette. Jatlow et al.16 stated that MG urinary excretion would be useful in quantifying chronic menthol exposure. Watson et al.17 compared participants’ smoking menthol study cigarettes to smoking a nonmenthol test cigarette, yielding a range of urinary menthol levels of 0.85–18.2 mg/L for menthol brand and 0.34–6.4 mg/L for the nonmenthol test cigarette, and thus significantly higher when participants smoked the menthol option.
Behaviors associated with nicotine dependence include puff volume and duration, exhaled carbon monoxide (CO), and time to first cigarette (TTFC) of the day. The study of Watson et al.17 examined differences in smoking behaviors and biomarkers of smoke exposure. When participants smoked the menthol cigarette, puff volume, puff duration, and puff depth were significantly higher. When exhaled CO was compared with number of cigarettes smoked, CO was more correlated with exposure to NNAL,18 indicating that CO more fully captures aspects of smoking behavior relevant to adverse health effects. Bloom et al.19 reported the correlation between CO and cigarettes per day (cpd) was significantly lower in African Americans than in European Americans. Findings indicate cpd was a poor proxy for exposure among African American smokers. Exhaled CO provides information beyond cpd. The National Household Survey on Drug Use yielded a large sample size over a 13-year period.20 Over time, TTFC within 30 minutes of waking had increased among those who smoke fewer cpd, whereas there was no change in TTFC for those smoking 16 or more cpd. TTFC has shown a strong prediction for nicotine dependence.20 A Multicultural Cohort from the National Longitudinal Study of Adolescent Health assessed young smokers aged 21–28 years.21 Black smokers smoked within 5 minutes of waking, which was significantly more likely than smokers who were Hispanic, white, and Asian. Number of cpd were not predictive of smoking within 5 minutes of awakening.
Literature references reflect our focus areas of menthol flavor, urinary menthol, race, nicotine dependence, addictive and carcinogenic exposures, and behavioral measures. Our study is unique and significant as it brings together these variables in an enrollment design balancing race (African American and white participants) within cigarette type (exclusive menthol flavor or regular cigarette smokers) in a controlled 36-hour inpatient environment. There are limited data regarding effect of urinary menthol on our variables of interest. We contrast the effect of menthol flavor cigarettes on dependent variables to the effect of urinary menthol on dependent variables. Use of a continuous variable such as urine menthol concentration may yield more definitive outcomes. The specific aims of our study were as follows:
1. To examine the effects of menthol flavor cigarette on nicotine dependence, biomarkers of addictive and carcinogenic exposure, and behavioral measures (Table 3).
2. To examine the effects of total urinary menthol on nicotine dependence, biomarkers of addictive and carcinogenic exposure, and behavioral measures (Table 4).
Table 3.
The Effects of Cigarette Type (Regular vs. Menthol Flavor) on Measures of Nicotine Dependence and Biomarkers of Addictive and Carcinogenic Exposure
| Adjusted mean (95% confidence interval) by cigarette typea,b | ||
|---|---|---|
| Regular (n = 65) | Menthol (n = 71) | |
| Measures of nicotine dependence | ||
| TTFC (min) | 23.24 (18.46 to 28.01) | 18.70 (14.09 to 23.30) |
| Puff volume (mL) | 62.87 (54.64 to 71.10) | 67.94 (60.34 to 75.56) |
| Exhaled carbon monoxide (ppm) | 19.55 (17.54 to 21.55) | 19.24 (17.44 to 21.05) |
| Biomarkers of addictive exposure | ||
| Plasma cotinine (ng/mL) | 218.45 (204.49 to 232.40) | 230.24 (216.78 to 243.69) |
| Biomarkers of carcinogenic exposure | ||
| Urine (24-h sample) | ||
| Total NNAL (pg/mL) | 167.70 (134.76 to 200.64) | 187.50 (155.96 to 219.05) |
| Free NNAL (pg/mL) | 56.49 (47.43 to 65.56) | 64.71 (56.03 to 73.39) |
| NNAL glucuronide (pg/mL) | 111.21 (86.21 to 136.22) | 122.79 (98.84 to 146.74) |
| Ratio of NNAL glucuronide to free NNAL | 1.88 (1.53 to 2.22) | 1.98 (1.65 to 2.31) |
| PAH (pg/mL) | ||
| 1-Hydroxynaphthalene | 7637.78 (6187.70 to 9087.86) | 8512.41 (7114.94 to 9909.88) |
| 2-Hydroxynaphthalene | 6833.00 (5872.73 to 7793.26) | 7123.81 (6198.39 to 8049.24) |
| 2-Hydroxyfluorene | 853.88 (686.88 to 1020.87) | 910.15 (749.22 to 1071.09) |
| 3-Hydroxyfluorene | 502.51 (418.07 to 586.96) | 574.57 (493.19 to 655.95) |
| 9-Hydroxyfluorene | 395.48 (312.74 to 478.21) | 392.19 (312.45 to 471.92) |
| 1-Hydroxyphenanthrene | 132.06 (104.97 to 159.15) | 153.25 (127.15 to 179.36) |
| 2-Hydroxyphenanthrene | 74.00 (59.87 to 88.12) | 80.87 (67.26 to 94.48) |
| 4-Hydroxyphenanthrene | 29.93 (0.00 to 65.42) | 73.14 (38.94 to 107.34) |
| 1-Hydroxypyrene | 182.90 (145.96 to 219.85) | 174.79 (139.19 to 210.39) |
aThe adjusted means for measures at multiple timepoints (puff volume, exhaled carbon monoxide, plasma nicotine, plasma cotinine) were estimated using mixed-effect modeling to adjust for within-subject clustering from repeated measures. The adjusted means for TTFC and biomarkers of carcinogenic exposure were estimated from multiple linear regression modeling. In all models, age, gender, race, education, and baseline cotinine were adjusted as covariates. NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; PAH = polycyclic aromatic hydrocarbon; TTFC = time to first cigarette.
bNone of the measures was significantly different between menthol flavor and regular cigarette smokers in both unadjusted (data not shown) and adjusted analyses (presented in the table).
Table 4.
The Effects of Total Menthol on Each Dependent Variable, Including Behavioral Measures of Nicotine Dependence, Biomarkers of Addictive Exposure, and Biomarkers of Carcinogenic Exposure (N = 136)
| Dependent variable | Coefficient (SE) of total menthola,b |
|---|---|
| Measures of nicotine dependence | |
| TTFC (min) | −2.42 (1.94) |
| Puff volume (mL) | 4.23 (1.94)* |
| Exhaled carbon monoxide (ppm) | 1.58 (0.42)*** |
| Biomarkers of addictive exposure | |
| Plasma nicotine (ng/mL) | 0.71 (0.36)* |
| Plasma cotinine (ng/mL) | 8.85 (3.40)** |
| Biomarkers of carcinogenic exposure | |
| Urine (24-h sample) | |
| Total NNAL (pg/mL) | 36.22 (9.53)*** |
| Free NNAL (pg/mL) | 13.09 (2.72)*** |
| NNAL glucuronide (pg/mL) | 23.13 (7.40)** |
| Ratio of NNAL glucuronide to free NNAL | −0.09 (0.14) |
| PAH (pg/mL) | |
| 1-Hydroxynaphthalene | 1241.71 (517.52)* |
| 2-Hydroxynaphthalene | 1087.92 (314.33)*** |
| 2-Hydroxyfluorene | 183.24 (49.53)*** |
| 3-Hydroxyfluorene | 119.11 (27.15)*** |
| 9-Hydroxyfluorene | 39.78 (33.39) |
| 1-Hydroxyphenanthrene | 17.51 (9.01)± |
| 2-Hydroxyphenanthrene | 9.83 (4.96)* |
| 3-Hydroxyphenanthrene | 25.64 (12.11)* |
| 4-Hydroxyphenanthrene | −23.79 (14.55) |
| 1-Hydroxypyrene | 22.05 (14.54) |
±Marginally significant (p = .055); *p < .05; **p < .01; and ***p < .001.
aThe effects on measures at multiple timepoints (puff volume, exhaled carbon monoxide, plasma nicotine, plasma cotinine) were estimated using mixed-effect modeling to adjust for within-subject clustering from repeated measures. The effects on TTFC and biomarkers of carcinogenic exposure were estimated using linear regression modeling. In each model, the dependent variable was the measure of nicotine dependence and biomarker of additive or carcinogenic exposure (listed in the first column); the independent variables included total menthol and covariates (age, gender, education, race, cigarette type [exclusive menthol flavor vs. regular cigarette], number of cigarettes per day, baseline cotinine, and baseline nicotine). Race and cigarette type interaction was also included as a covariate in models for plasma nicotine and 3-hydroxyphenanthrene. The coefficients of total menthol (shown in the second column) estimate the effect of total menthol on each dependent variable, adjusting for all the covariates in the model. For example, the coefficient of 4.23 for the adjusted effect of total menthol on puff volume is interpreted as each unit increase in total menthol was associated with 4.23 mL greater puff volume. NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; PAH = polycyclic aromatic hydrocarbon; TTFC = time to first cigarette.
bSimilar results were found in the unadjusted analyses (data not shown) and adjusted analyses (presented in the table).
Methods
To address these aims, a stratified sample of smokers (N = 136) by race (African American vs. white) and cigarette type (exclusive menthol flavor vs. regular cigarette) was recruited to examine main and interaction effects of race and cigarette type on variables identified in the specific aims. After obtaining written informed consent, 136 participants were enrolled for a 36-hour protocol in the Ohio State University Clinical Research Center (CRC) using an ad libitum cigarette smoking protocol with designated blood and urine sampling conducted by the CRC staff. The Ohio State University’s Biomedical Sciences Institutional Review Board and CRC approved this research.
Sample
Inclusion criteria were 18–50 years of age, daily cigarette smoking for at least 1 year, no prescribed medications, no acute or chronic illness, not pregnant, and women were in progesterone-confirmed mid-to-late follicular phase. Enrollment occurred over 40 months (August 2005 to December 2008) followed by 3 months of data analyses. There were no increases in federal or state of Ohio cigarette taxes during this 40-month period.22,23 The final sample (N = 136) consisted of 35 white regular cigarette smokers, 35 white menthol flavor cigarette smokers, 30 black regular cigarette smokers, and 36 black menthol flavor cigarette smokers. The sample met the original design plan of balanced representation by race and menthol flavor or regular cigarette smokers. Cigarette brand data were as follows: 49 Marlboro smokers with 28.6% smoking menthol flavor cigarettes, 42 Newport smokers with 100% smoking menthol flavor cigarettes, and 20 Camel smokers with 25% smoking menthol flavor cigarettes. The remaining 24 participants were dispersed across a number of categories.
Protocol
Stratified recruitment was conducted using previously successful strategies including advertisements in neighborhood newspapers, and informational flyers placed in work settings, clinics, and neighborhood beauty and barbershops. Participants were screened before admission to the CRC at 7 pm for the inpatient protocol. Participants were provided their usual cigarette brand that they smoked ad libitum in the reverse ventilated participant’s room during their stay. Our human laboratory study focused on each participant’s usual smoking pattern to reflect his or her typical data as measured throughout the protocol. Baseline sociodemographic information, smoking history, and physical examinations were obtained. All participants provided a 24-hour urine sample beginning with the participant’s first void after awakening the morning of day 2. Table 1 provides details of the 36-hour inpatient protocol. No menthol products beyond cigarettes were available to participants during the protocol. Participants did not use other menthol products such as lozenges, mints, or peppermint tea.
Table 1.
Data Collection of 36-h Protocol
| Day 1 | 7 pm admission. Ad libitum smoking of usual cigarettes. Baseline plasma cotinine. Self-report of usual time to first cigarette in minutes. No smoking after 11 pm. |
| Day 2 | Upon awakening 24-h urine specimen initiated with first void. Exhaled CO (eCO) measured 2 min pre- and post-smoking first cigarette of the day. Plasma blood draw 1 min pre- and post-smoking first cigarette of the day. Smoking topography puff volume during smoking bout. 10 am to noon Smoked first cigarette in this time period. Procedure as above with eCO, plasma blood draw, and puff volume. 3 pm to 5 pm Smoked first cigarette in this time period. Procedure as above with eCO, plasma blood draw, and puff volume. 8 pm to 10 pm Smoked first cigarette in this time period. Procedure as above with eCO, plasma blood draw, and puff volume. |
| Day 3 | 24-h urine collection of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, polycyclic aromatic hydrocarbon, and urinary menthol biomarkers completed at same time of day as first void of day 2. |
Measures
Nicotine Dependence
Fagerström Test for Nicotine Dependence and TTFC in minutes were implemented to assess dependence.24 Exhaled CO was measured pre- and post-smoking throughout the day using the Bedfont Mini Smokerlyzer (Innovative Marketing, Medford, NJ). Clinical Research Support System equipment was used to assess smoking topography variables of puff volume. Cigarette type was defined as exclusive menthol flavor or regular cigarette smoker.
Addictive Exposure
Nicotine and cotinine were simultaneously quantified in serum by high-performance liquid chromatography–tandem mass spectrometry with imprecision less than 10% at physiologic concentrations and limits of quantification ranging from 0.5 to 5 μg/L across five analytes by the Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, for target value of 5.0 μg/L, nicotine coefficient of variation (CV) of 12%, and cotinine CV of 9%.25
Menthol
Menthol was measured in 24-hour urine samples as free and total menthol using headspace solid-phase microextraction followed by gas chromatography/mass spectrometry.26 Quantification was based on a stable isotope labeled internal standard. Total menthol was measured following incubation with β-glucuronidase to release menthol from MG. The method detection limit for total menthol was 0.01–10 µg/mL. This analysis was conducted by the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention.
Carcinogenic Exposure
Total NNAL (free NNAL + NNAL glucuronide) measurements were made by using the liquid chromatography/atmospheric pressure ionization tandem mass spectrometry method of Xia et al.27 In these analyses, the urine samples (5 mL) were hydrolyzed with β-glucuronidase and total NNAL was measured. Briefly, the assay involved the addition of a 13C6-NNAL internal standard followed by a preliminary separation and sample cleanup using specially developed molecularly imprinted polymer columns and analysis by high-performance liquid chromatography electrospray–ionization tandem mass spectrometry. The limit of detection for these analyses using 5-mL sample aliquots was 1.6 pg/mL. For measurement of free NNAL, the preliminary hydrolysis with β-glucuronidase was omitted.
The methodology for measuring hydroxylated PAH metabolites, present in human urine as glucuronide and/or sulfate conjugates, was based on enzymatic deconjugation of the analytes to yield free OH-PAHs, followed by automatic liquid– extraction into pentene using the Gilson 215 Liquid Handler (Gilson, Inc, Middleton, WI). The sample extracts were thereafter evaporated under a chemical fume hood to remove the pentene solvent. Finally, extracts were reconstituted in toluene, derivatized to yield the trimethylsiloxane derivatives. Analytical determination of the target analytes was performed by gas chromatography isotope dilution high-resolution mass spectrometry using a MAT95XP (Thermo Finnigan MAT, Bremen, Germany) instrument.28
Statistical Analysis
Descriptive statistics were used to summarize sample characteristics, baseline plasma cotinine, TTFC, and total menthol, by race (African American and white participants) and cigarette type (menthol flavor vs. regular cigarettes). Analysis of variance was used to examine whether these measures were different by race and menthol flavor cigarette type and whether there were significant race × cigarette type interactions. Trend plots were used to visually illustrate the trajectories of exhaled CO, puff volume, plasma nicotine, and plasma cotinine throughout the day, stratified by race and menthol flavor or regular cigarette type.
Next, we conducted both unadjusted and adjusted analyses to examine the effects of cigarette type on each dependent variable of interest, including nicotine dependence and biomarkers of addictive and carcinogenic exposure. For unadjusted analyses, we used two-sample t tests for dependent variables measured at a single timepoint (TTFC and biomarkers of carcinogenic exposure) and mixed-effect modeling for those with repeated measures (puff volume, exhaled CO, plasma nicotine, and plasma cotinine), which included menthol flavor or regular cigarette type as the only predictor. The corresponding methods for adjusted analyses were multiple linear regression modeling and mixed-effect linear modeling with age, gender, race, education, and baseline cotinine adjusted as covariates in the models. Years of smoking history was not included as a covariate because of its high correlation with age (r = .91, p < .001). Significant race × cigarette type interaction effects were found for plasma nicotine and 3-hydroxyphenanthrene. Therefore, race × cigarette type interaction was also included as a predictor in models for these two biomarkers. Similar results were found in both unadjusted and adjusted analyses. We presented results from adjusted analyses.
Similarly, both unadjusted and adjusted analyses were performed to examine the effect of total menthol on each dependent variable. Linear regression modeling was used for dependent variables measured at a single timepoint, whereas mixed-effect linear regression modeling was used for those with repeated measures. In unadjusted analyses, total urinary menthol was entered into the model as a single predictor. In adjusted analyses, age, gender, education, race, cigarette type, race and cigarette type interaction (only in models for plasma nicotine and 3-hydroxyphenanthrene), number of cpd, baseline cotinine, and baseline nicotine were adjusted as covariates in the models. Again, both unadjusted and adjusted analyses yielded similar results; therefore, we presented only results from adjusted analyses. We used SAS version 9.4 (SAS Institute, Cary, NC) for all the statistical analysis. All tests were two sided with a significance level of .05.
Results
Sample
By design, the sample (N = 136) had approximately balanced distributions by race (48.5% African American) and cigarette type (52.2% smoked menthol cigarettes). Forty-five percent of the sample was male. The participants had an average age of 29.7 years (SD = 9.0, range 18–50) and average years of education of 12.6 years (SD = 1.8). Smoking characteristics were an average of 15.7 cpd (SD = 6.4), with an average TTFC of 20.8 minutes (SD = 20.8). Years of smoking history ranged from 1 to 35 years with an average of 12.6 years (SD = 9.0) (Table 2).
Table 2.
Sample Characteristics, Baseline Plasma Cotinine, Baseline Plasma Nicotine, Time to First Cigarette (TTFC), and Total Menthol by Race and Cigarette Type
| All (n = 136) | White | Black | |||
|---|---|---|---|---|---|
| Regular (n = 35) | Menthol (n = 35) | Regular (n = 30) | Menthol (n = 36) | ||
| Mean (SD) or N (%) | |||||
| Age (years)a,b | 29.7 (9.0) | 27.9 (8.4) | 24.2 (5.2) | 36.8 (9.5) | 30.8 (8.2) |
| Gender | |||||
| Male | 75 (55.1) | 17 (48.6) | 18 (51.4) | 21 (70.0) | 19 (52.8) |
| Female | 61 (44.9) | 18 (51.4) | 17 (48.6) | 9 (30.0) | 17 (47.2) |
| Education (years)c | 12.6 (1.8) | 13.9 (1.7) | 12.3 (2.0) | 12.1 (1.7) | 12.2 (1.2) |
| Cigarettes smoked per daya | 15.7 (6.4) | 16.9 (5.8) | 16.8 (6.1) | 16.0 (8.5) | 13.2 (4.1) |
| Baseline plasma cotinine (ng/mL)a | 228.9 (141.6) | 203.1 (112.8) | 178.4 (111.4) | 287.3 (170.2) | 253.1 (147.9) |
| Baseline plasma nicotine (ng/mL)c | 11.4 (9.2) | 9.2 (5.5) | 11.5 (10.6) | 15.7 (11.3) | 9.8 (7.4) |
| TTFC (min)a | 20.8 (20.8) | 32.1 (26.8) | 21.2 (21.7) | 14.8 (12.1) | 14.5 (13.7) |
| Years of smoking historya,b,d | 12.6 (9.0) | 10.9 (8.3) | 8.0 (5.9) | 19.4 (10.7) | 13.0 (7.4) |
| Total menthol (µg/mL)b | 1.6 (1.8) | 0.6 (1.4) | 2.9 (2.2) | 0.4 (0.4) | 2.3 (1.3) |
aSignificantly different by race.
bSignificantly different by cigarette type.
cSignificant race × cigarette type interaction exists.
dYears of smoking history was not included in the subsequent analyses because it was highly correlated with age (r = .91, p < .001).
Table 2 also shows the comparisons of sample characteristics, baseline cotinine, TTFC, and total urinary menthol by race and cigarette type. Compared with white participants, African American participants were significantly older, had fewer cpd, had higher baseline plasma cotinine level, had shorter TTFC, and had longer years of smoking history. Compared with participants who smoked regular cigarettes, those who smoked menthol flavor cigarettes were significantly younger, had shorter years of smoking history, and had higher levels of total menthol in the 24-hour urine sample. Significant race × cigarette type interactions were found for education and baseline nicotine. Among whites, regular cigarette smokers had significantly higher education and lower baseline nicotine than those who smoked menthol flavor cigarettes. On the other hand, among African Americans, there was no educational difference by cigarette type, and regular cigarette smokers had significantly higher baseline nicotine than those who smoked menthol flavor cigarettes.
Trajectories of Exhaled CO, Puff Volume, Plasma Nicotine, and Cotinine
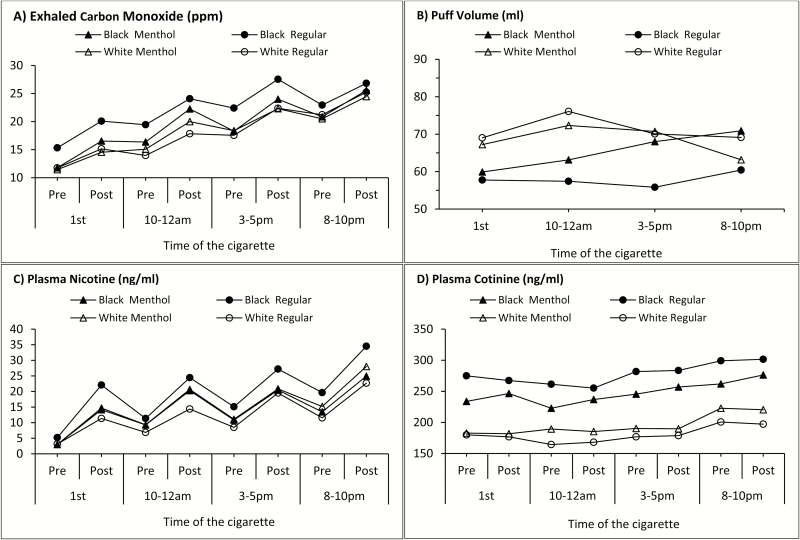
Figure 1 illustrates the trajectories of exhaled CO, puff volume, plasma nicotine, and plasma cotinine throughout the day and menthol flavor or regular cigarettes. Upward trends were observed for exhaled CO, plasma nicotine, and plasma cotinine with levels reaching the highest post-smoking at 8–10 pm. At each timepoint, levels of exhaled CO and plasma nicotine were higher post-smoking compared with pre-smoking levels, whereas no post-smoking versus pre-smoking differences were observed for plasma cotinine as would be expected with its longer half-life. At each timepoint, African American regular cigarette smokers had the lowest puff volume and highest levels of exhaled CO, plasma nicotine, and plasma cotinine. White regular cigarette smokers had the lowest levels of exhaled CO, plasma nicotine, and plasma cotinine at most timepoints.
Figure 1.
Behavioral measures of nicotine dependence (exhaled carbon monoxide [A] and puff volume [B]) and biomarkers of cigarette smoke exposure (plasma nicotine [C] and plasma cotinine [D]), by race, cigarette type (exclusive menthol flavor vs. regular cigarette), and time of cigarette (N = 136). Error bars were not shown to avoid an overbusy figure. The width of average error bars (95% confidence intervals around the means) were ±3.2 for exhaled carbon monoxide (A), ±10.9 for puff volume (B), ±3.4 for plasma nicotine (C), and ±40.4 for plasma cotinine (D).
The Effects of Menthol Flavor Cigarette
The effects of menthol flavor cigarette type on measures of nicotine dependence and biomarkers of addictive and carcinogenic exposure are summarized in Table 3 (for measures without significant cigarette type × race interaction effect) and Figure 2 (for measures with significant cigarette type × race interaction effect).
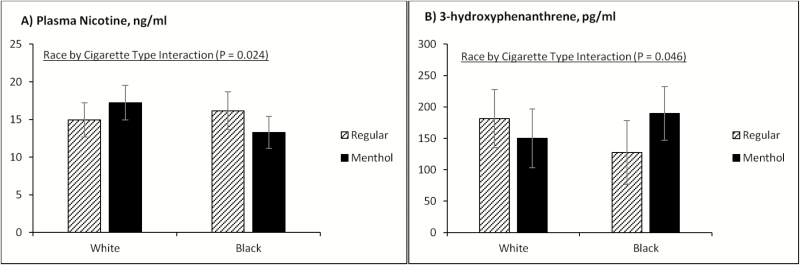
Figure 2.
The interaction effects of race and cigarette type on plasma nicotine (A) and 3-hydroxyphenanthrene (B). The adjusted means for plasma nicotine were estimated using mixed-effect modeling to adjust for within-subject clustering from repeated measures. The adjusted means for 3-hydroxyphenanthrene were estimated from multiple linear regression modeling. Age, gender, education, and baseline cotinine were adjusted as covariates in the models. Similar results were found from unadjusted (data not shown) and adjusted analyses (presented in the figure).
As shown in Table 3, menthol flavor cigarette smokers tended to have greater nicotine dependence, higher plasma cotinine level, and higher carcinogenic exposure levels, after adjusting for age, gender, race, education, and baseline cotinine in the regression models. For example, the average TTFC was 18.7 minutes among menthol flavor cigarette smokers compared with 23.2 minutes among regular cigarette smokers. However, none of the differences between menthol flavor and regular cigarette smokers reached statistical significance. Similar results were found in unadjusted analysis.
Significant race × cigarette type interaction effects were found for two biomarkers: plasma nicotine (a biomarker for addictive exposure, p = .024) and 3-hydroxyphenanthrene (a biomarker for carcinogenic exposure, p = .046). The average plasma nicotine level was lower in regular cigarette smokers (vs. menthol flavor cigarette smokers) among whites, but was higher in regular cigarette smokers (vs. menthol flavor cigarette smokers) among African Americans (Figure 2A). The average level of 3-hydroxyphenanthrene was higher in regular cigarette smokers (vs. menthol flavor cigarette smokers) among whites, but was higher in menthol flavor cigarette smokers (vs. regular cigarette smokers) among African Americans (Figure 2B).
The Effects of Total Urinary Menthol
Both unadjusted and adjusted analyses found similar results of the effects of total urinary menthol on behavioral measures, nicotine dependence, and biomarkers of addictive and carcinogenic exposure. Table 4 shows the results from the adjusted analyses. Total menthol was significantly associated with greater puff volume and exhaled CO levels. One unit increase in total menthol was associated with a 4.23-mL increase (positive difference) in puff volume and 1.58-ppm increase in exhaled CO. Higher total menthol levels were also significantly associated with higher levels of almost all the biomarkers of addictive and carcinogenic exposure, adjusting for cigarette type and other covariates (age, gender, education, number of cpd, baseline cotinine, and baseline nicotine) in the models. Repeating the analyses excluding six outliers with extremely high total menthol levels yielded similar results.
Discussion
There are limited findings in extant literature incorporating total menthol concentration, nicotine dependence, and biomarkers of addictive and carcinogen exposure. It is one of a few studies in this area on the role of total urine menthol in tobacco research. We found significant associations of 24-hour monitored urine menthol with nicotine dependence and addictive and carcinogenic biomarker exposures. In our human laboratory study, we report that urine menthol was significantly associated with higher puff volume and exhaled CO, plasma nicotine and cotinine, NNAL, and most of the PAHs. Urinary menthol is an independent predictive biomarker of nicotine dependence, addictive and carcinogenic exposure, and behavioral measures.
In contrast to our study, Benowitz et al.29 reported that when “both nicotine equivalents or plasma cotinine and urine menthol were included in the same model as predictors of NNAL or PAHs, nicotine exposure was the stronger predictor and urine menthol effect was nonsignificant.” In contrast, our findings suggested that urinary menthol was associated with greater toxicant exposure, even after adjusting for cigarette type, number of cigarette per day, baseline cotinine, and baseline nicotine. Both our study and that by Benowitz et al. recruited a nonrandom sample from a local CRC. Although complex, studies on representative samples are needed to further explore the predictive value of urinary menthol on toxicant exposures.
There were no significant differences between the dichotomous menthol flavor and regular cigarette categories in our findings and a number of studies support this. Among young adult non-daily smokers, there were no significant differences in cpd based on menthol brand preferences or state smoking policies.30 Higher levels of cotinine in African American participants were not explained by higher preference for menthol cigarettes and no differences in plasma cotinine levels for white or African American smokers were observed when using UPC-assessed menthol versus nonmenthol brands.31
Differences between menthol flavor and regular cigarettes have occurred. NHANES participants who smoked menthol cigarettes were more likely to be female and African American compared with regular cigarette smokers.32 Lewis et al.8 reported that African American menthol flavor cigarette smokers were the most likely to continue smoking, whereas those most likely to quit smoking were non–African American regular cigarette smokers. Young adult white participants had significantly higher nicotine levels, whereas there were no significant differences across Native Hawaiians and Fillipinos.33 In a study of women, it was found that menthol flavor cigarette smokers exhibited signs of greater tobacco dependence than women smoking regular cigarettes.34 Using NHANES, Rostron indicated that average NNAL concentration was lower among menthol flavor cigarette smokers compared with regular cigarette smokers overall (p = .032).30 Menthol exposures were reported by Hsu et al.15 with plasma MG levels higher among menthol versus regular cigarette smokers as would be expected. Spot urinary menthol was assessed by Watson et al.17 with some overlap of levels ranging from 0.85 to 18.2 mg/L for menthol brand and 0.34 to 6.4 mg/L for nonmenthol test cigarettes. Varied study designs and statistical analyses complicate direct comparisons across these diverse studies. Recruitment method, compensation of study participants, and a range of smaller studies to large national datasets also contribute to differences.
Significant race × cigarette type interaction effects were found for two biomarkers: plasma nicotine (p = .024) and 3-hydroxyphenanthrene (p = .046). For the interaction of race and average plasma nicotine level, white regular cigarette smokers had lower nicotine levels than menthol flavor cigarette smokers, whereas African American regular cigarette smokers had higher nicotine levels than menthol flavor cigarette smokers. Plasma nicotine post-smoking was obtained at four specific timepoints throughout the day. Although not an interaction, findings by Benowitz et al.35 identified that plasma nicotine level was significantly higher in regular cigarette smokers at 8.0 ng/mL (95% CI = 6.3 to 10.1) in comparison with menthol flavor cigarette smokers at 5.7 ng/mL (95% CI = 4.4 to 7.3) and there were no significant differences between black and white smokers.
The interaction by race and menthol flavor or regular cigarette type for 3-hydroxyphenanthrene carcinogen found that white regular cigarette smokers had a higher 3-hydroxyphenanthrene level than those smoking menthol cigarettes, and African American menthol flavor cigarette smokers had higher carcinogens than did regular cigarette smokers. Results by Patel et al.11 identified higher uptake of carcinogenic PAH in African American smokers than in whites. Benowitz et al.35 reported total PAH metabolites were significantly lower in African American compared with white smokers and higher in regular versus menthol flavor cigarette smokers. We have not located comparable data regarding the earlier interaction.
Future studies incorporating PAH metabolites may consider statistical models to estimate total concentrations of PAH metabolites in urine as developed by Jain36 using NHANES data. The model estimates total concentration of PAH metabolites in urine with knowledge of 1- and 2-hydroxynaphthalene and 9-hydroxyfluorene in addition to age, gender, and smoking status with an R2 greater than 90%.
Nicotine dependence and behavioral measures provide pertinent information. Among our African American participants at baseline, TTFC was significantly shorter in minutes consistent with Branstetter et al.21 reporting of African Americans more likely smoking within 5 minutes of waking than among Hispanic, white, and Asian smokers. Over a 13-year study period, Goodwin et al.20 observed that TTFC less than 30 minutes had increased among participants who smoked fewer cpd. In our study, urinary menthol was significantly associated with puff volume and exhaled CO, which relates to findings by Watson et al.17 that puff volumes were significantly higher with menthol cigarettes. When compared with cpd, exhaled CO was more highly correlated with NNAL exposure18 and exhaled CO was more informative than cpd.19 Baseline plasma cotinine in our study was significantly higher among African Americans compared with whites and is consistent with that reported by Mustonen et al.37 On the basis of NHANES data, African American participants demonstrated a much higher intake of nicotine per cigarette compared with white smokers.31
A strength of our study design was stratified recruitment that generated a balance of participants and a unique opportunity to discriminate effects of race (African American and white) and menthol flavor or regular cigarettes. Several previous studies illustrate less balance with 64%–74% of African American menthol flavor cigarette smokers compared with 18%–30% of white participants.29,30,38 Gender distribution was reflected with 45% enrollment of women. A 24-hour urine sample in the controlled CRC environment enhanced accuracy of NNAL, PAH, and urinary menthol measurements. Although creatinine was assessed, it was not used to adjust variables as a 24-hour urine sample was obtained. Behavioral measures were obtained four times across the day for exhaled CO, plasma nicotine, and puff volumes. There are several limitations of this study including the 36-hour inpatient protocol, which may have reduced potential participants from enrolling, and the CRC environment could have impacted smoking behaviors. Not having capabilities to measure 3-hydroxycotinine is a limitation. In spite of the relative small sample size, our study was adequately powered to identify the significant associations of total urinary menthol with nicotine dependence, behavioral measures, and biomarkers of addictive and carcinogenic exposure. However, we acknowledge that our study findings may have limited generalizability due to a nonrandomly obtained sample from a local CRC.
Conclusion
Although there were no significant differences between menthol flavor and regular cigarettes on any of our variables of interest, the effect of urine menthol was significantly associated with nearly all measures of nicotine dependence, puff volume, exhaled CO, plasma nicotine, NNAL, and PAHs. The dichotomous variable of menthol flavor or regular cigarette does not address the range of potential menthol exposure as found in a 24-hour data collection. Study findings indicate that urine menthol is predictive of exposure to nicotine and other toxicants.
Funding
This work was supported by the National Institutes of Health’s National Institute on Drug Abuse (R01DA017313 to KA) and the National Center for Research Resources (M01RR00034).
Declaration of Interests
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors wish to thank John T. Bernert, Benjamin Blount, and Andreas Sjödin for their leadership at the Centers for Disease Control and Prevention, Division of Laboratory Sciences.
References
- 1. Gordon SM, Brinkman MC, Meng RQ, et al. Effect of cigarette menthol content on mainstream smoke emissions. Chem Res Toxicol. 2011;24(10):1744–1753. [DOI] [PubMed] [Google Scholar]
- 2. Vilanti AC, Mowery PD, Delnevo CD,. et al. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004–2014. Tob Control. 2016;25(suppl 2):ii14–ii20. doi: 10.1136/tobaccocontrol-2016-053329. [DOI] [PubMed] [Google Scholar]
- 3. Villanti AC, Collins LK, Niaura RS, Gagosian SY, Abrams DB. Menthol cigarettes and the public health standard: a systematic review. BMC Public Health. 2017;17(1):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexander LA, Trinidad DR, Sakuma KL, et al. Why we must continue to investigate menthol’s role in the African American smoking paradox. Nicotine Tob Res. 2016;18(suppl 1:S91–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohn AM, Rose SW, Ilakkuvan V, et al. Harm perceptions of menthol and nonmenthol cigarettes differ by brand, race/ethnicity, and gender in US adult smokers: results from PATH Wave 1 [published online ahead of print Jan 27, 2018]. Nicotine Tob Res. 2018;27. doi: 10.1093/ntr/ntx277. [DOI] [PubMed] [Google Scholar]
- 6. Tobacco Products Scientific Advisory Committee (TPSAC). 2011. Comprehensive Report Noting That Removing Menthol Cigarettes from the Market Would Be in the Interest of Public Health. [Google Scholar]
- 7. Food and Drug Administration. 2013. Preliminary scientific evaluation of the possible public health effects of menthol versus nonmenthol cigarettes. [Google Scholar]
- 8. Lewis M, Wang Y, Berg CJ. Tobacco control environment in the United States and individual consumer characteristics in relation to continued smoking: differential responses among menthol smokers?Prev Med. 2014;65:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones MR, Joshu CE, Navas-Acien A, Platz EA. Racial/ethnic differences in duration of smoking among former smokers in the national health and nutrition examination surveys. Nicotine Tob Res. 2018;20(3):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the evaluation of carcinogenic risks to humans. 2010;92 Lyon, France: Distributed by World Health Organization. [Google Scholar]
- 11. Patel YM, Park SL, Carmella SG, et al. Metabolites of the polycyclic aromatic hydrocarbon phenanthrene in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. PLoS One. 2016;11(6):e0156203. doi: 10.1371/journal.pone.0156203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Czoli CD, Hammond D. Carcinogen exposure among Canadian tobacco users: changes in NNK exposure from 2007–2009 through 2012–2013. Cancer Epidemiol Biomarker Prev. 2018;27(3):262–267; doi: 10.1158/1055-9965.EPI-17-0715 [DOI] [PubMed] [Google Scholar]
- 13. Brinkman MC, Chuang JC, Gordon SM, et al. Exposure to and deposition of fine and ultrafine particles in smokers of menthol and nonmenthol cigarettes. Inhal Toxicol. 2012;24(5):255–269. [DOI] [PubMed] [Google Scholar]
- 14. Park SL, Carmella SG, Ming X, et al. Variation in levels of the lung carcinogen NNAL and its glucuronides in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. Cancer Epidemiol Biomarkers Prev. 2015;24(3):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu PC, Lan RS, Brasky TM, et al. Metabolomic profiles of current cigarette smokers. Mol Carcinog. 2017;56(2):594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jatlow P, Valentine G, Gueorguieva R, et al. Plasma menthol glucuronide as a biomarker of acute menthol inhalation. Tob Regul Sci. 2018;4(1):586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watson CV, Richter P, de Castro BR, et al. Smoking behavior and exposure: Results of a menthol cigarette cross-over study. Am J Health Behav. 2017;41(3):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joseph AM, Hecht SS, Murphy SE, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2963–2968. [DOI] [PubMed] [Google Scholar]
- 19. Bloom AJ, Hartz SM, Baker TB, et al. Beyond cigarettes per day. A genome-wide association study of the biomarker carbon monoxide. Ann Am Thorac Soc. 2014;11(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goodwin RD, Wall MM, Gbedemah M, et al. Trends in cigarette consumption and time to first cigarette on awakening from 2002 to 2015 in the USA: new insights into the ongoing tobacco epidemic [published online ahead of print August 10, 2017]. Tob Control. 2018;27(4):379–384. doi: 10.1136/tobaccocontrol-2016-053601. [DOI] [PubMed] [Google Scholar]
- 21. Branstetter SA, Mercincavage M, Muscat JE. Predictors of the nicotine dependence behavior time to the first cigarette in a multiracial cohort. Nicotine Tob Res. 2015;17(7):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bush T, Zbikowski S, Mahoney L, et al. The 2009 US Federal cigarette tax increase and quitline utilization in 16 states. J Environ Pub Health. 2012;2012:314740. doi: 10.1155/2012/314740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campaign for Tobacco-Free Kids. Cigarette Tax Increases by State Per Year 2000–2016 Retrieved April 26, 2016. https://www.tobaccofreekids.org/research/factsheets/pdf/0275.pdf
- 24. Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 25. Moyer TP, Charlson JR, Enger RJ, et al. Simultaneous analysis of nicotine, nicotine metabolites, and tobacco alkaloids in serum or urine by tandem mass spectrometry, with clinically relevant metabolic profiles. Clin Chem. 2002;48(9):1460–1471. [PubMed] [Google Scholar]
- 26. Huang W, Blount BC, Watson CH, et al. Quantitative analysis of menthol in human urine using solid phase microextraction and stable isotope dilution gas chromatography–mass spectrometry [published online ahead of print December 13, 2016]. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;15:1044–1045:200–205. doi: 10.1016/j.jchromb.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xia Y, McGuffey JE, Bhattacharyya S, et al. Analysis of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in urine by extraction on a molecularly imprinted polymer column and liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Anal Chem. 2005;77(23):7639–7645. [DOI] [PubMed] [Google Scholar]
- 28. Li Z, Romanoff LC, Trinidad DA, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography isotope dilution high resolution mass spectrometry. Anal Chem. 2006;78(16):5744–5751. doi: 10.1021/ac0606094 [DOI] [PubMed] [Google Scholar]
- 29. Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P III. Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3013–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rostron B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicotine Tob Res. 2013;15(10):1722–1728. [DOI] [PubMed] [Google Scholar]
- 31. Caraballo RS, Holiday DB, Stellman SD, et al. Comparison of serum cotinine concentration within and across smokers of menthol and nonmenthol cigarette brands among non-Hispanic Black and non-Hispanic White U.S. adult smokers, 2001–2006. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1329–1340. doi: 10.1158/1055-9965.EPI-10-1330 [DOI] [PubMed] [Google Scholar]
- 32. Jones MR, Tellez-Plaza M, Navas-Acien A. Smoking, menthol cigarettes and all-cause, cancer and cardiovascular mortality: evidence from the National Health and Nutrition Examination Survey (NHANES) and a meta-analysis. PLoS One. 2013;8(10):e77941. doi: 10.1371/journal.pone.0077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fagan P, Pokhrel P, Herzog TA, et al. Nicotine metabolism in young adult daily menthol and nonmenthol smokers. Nicotine Tob Res. 2016;18(4):437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenbloom J, Rees VW, Reid K, Wong J, Kinnunen T. A cross-sectional study on tobacco use and dependence among women: does menthol matter?Tob Induc Dis. 2012;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13(9):772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jain RB. Regression models to estimate the total concentration of polycyclic aromatic hydrocarbon metabolites in urine. Chemosphere. 2016;146:323–329. [DOI] [PubMed] [Google Scholar]
- 37. Mustonen TK, Spencer SM, Hoskinson RA, Sachs DP, Garvey AJ. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine Tob Res. 2005;7(4):581–590. [DOI] [PubMed] [Google Scholar]
- 38. Richie JP Jr, Carmella SG, Muscat JE, Scott DG, Akerkar SA, Hecht SS. Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiol Biomarkers Prev. 1997;6(10):783–790. [PubMed] [Google Scholar]