Abstract
Background:
The objective of this study was to evaluate whether autologous stem-cell-based therapy may mitigate the symptoms of interstitial cystitis.
Methods:
Stromal vascular fraction (SVF) rich in stem cells and derived from autologous adipose tissue was deployed into 109 men and women with interstitial cystitis/painful bladder syndrome as a surgical procedure. This stem-cell-rich biologic product was injected both systemically and regionally into pelvic floor targets. Patients were queried about quality of life and symptom and bother subjective outcomes tests every 3 months for 2 years.
Results:
A total of 78 patients reported a positive response at 1 year. Symptom and bother metrics were statistically improved at 1 year. There were minimal adverse events associated with the harvesting, procurement, and clinical deployment of SVF.
Conclusion:
Interstitial cystitis is a complex clinical problem that is known for its resistance to conventional therapies. SVF as an autologous personalized regenerative strategy shows good safety and efficacy and may potentially have a role in the mitigation of interstitial cystitis.
Keywords: autologous stem cells, chronic pelvic pain, interstitial cystitis, stromal vascular fraction
Introduction
Surgically procured stromal vascular fraction (SVF) derived at the point of care is an autologous biologic product derived from the enzymatic digestion of lipo-aspirate and is widely being investigated for its regenerative, immunomodulatory, antinociceptive, and anti-inflammatory properties. As interstitial cystitis(IC)/painful bladder syndrome (PBS) has degenerative aspects, autoimmune features, and is clinically associated with significant pain and inflammation, an investigation is indicated to determine whether autologous SVF as a form of cell therapy can mitigate IC symptoms. This prospective study consists of a pilot series of 109 IC patients who underwent treatment with combined regional and systemic deployment of autologous SVF and were assessed with self-reported subjective outcomes testing to evaluate safety and clinical efficacy.
Methods
A total of 109 patients (aged 52–70, mean 61) consisting of 91 women and 18 men, all of whom were diagnosed by their primary urologist with IC were enrolled in this study. The study was approved by an IRB (International Cell Surgical Society IRB) and patient funded. Patients underwent basic urologic evaluation with records review and signed IRB approved consents for the invetigational deployment of autologous SVF. Most patients in the study had a history of receiving multiple various medications and procedural interventions with generally limited clinical responses. All patients were maintained on their usual and customary medications and no new medications for IC were initiated during the first 180 days of the study. Predeployment cystoscopy was not performed to stratify patients based on the presence or absence of Hunner’s ulcers and no specific measures of disease severity other than baseline pain, O’Leary-Sant, and Pelvic Pain and Urgency/Frequency Patient Symptom Scale (PUF) scores were obtained.
The CSN Time Machine® system and method (Cell Surgical Network, USA) was used to harvest, centrifuge, incubate, and isolate the autologous SVF cellular product. Local anesthesia (lidocaine 0.5% with epinephrine 1:400,000 and sodium bicarbonate 8.4%) was injected into patient’s abdomen or flank regions and a #11 blade was used to puncture the skin. Lipoharvesting was performed under operating room sterility conditions with a negative pressure syringe technique using a 3 mm cannula. Lipo-aspirate (50 cc) was collected in a specially designed closed harvesting syringe (TP-101 Syringe by Medikhan, Los Angeles, USA) and condensed to 25 cc by centrifugation at 2800 rpm for 3 min. Next, 12.5 Wunsch units of T-MAX® Good Manufacturing Practices (GMP) grade collagenase (private label name for Liberase by Roche, Indianapolis, IN, USA) was added to the condensed lipo-aspirate and incubated at 38°C for 30 min to digest the collagen matrix in order to allow separation of the ‘fat fraction’ from the remaining ‘stromal fraction’ and ‘vascular fraction’ in a second closed processing syringe (TP-102 syringe by Medikhan, Los Angeles, CA, USA). The remaining ‘stromal and vascular fraction’ inside the TP-102 syringe is then washed with D5LR Lactated Ringer’s solution sequentially three times, concentrated down to 10 cc and then filtered to isolate the final SVF product. The entire SVF separation process is performed in closed syringes in the operating room at the bedside, requiring less than 80 min. Photomicrography using the Invitrogen by Countess (Invitrogen, Waltham, Ma) was used to count cells, and measure cell viability using 0.4% trypan blue. Nucleated cell count, cell viability, and, therefore, SVF dosing varied in all patients as expected with an autologous biologic product.
The IC deployment protocol provided for half of the final 10 cc. SVF product to be used intravenously for systemic treatment and the other half of the isolated SVF to be injected regionally into the pelvic floor targets. For women, these targets included the trigone, bladder neck, and para-urethral tissue, and for men there was either no regional injection or in a few cases, a trans-abdominal CT-guided injection into the trigone.
Results
Patients from 18 different affiliate research centers self-reported results through an automated online patient tracking system (Regentrak™) at baseline and every 3 months using O’Leary-Sant and PUF questionnaires as well as a visual analog pain score and also a subjective quality of life question on whether their condition was improved by the treatment. Patients fill out results of their outcomes testing from their home devices without the influence of clinical team presence (the ‘Hawthorne’ effect) and research results are obtained merely by query of the database.
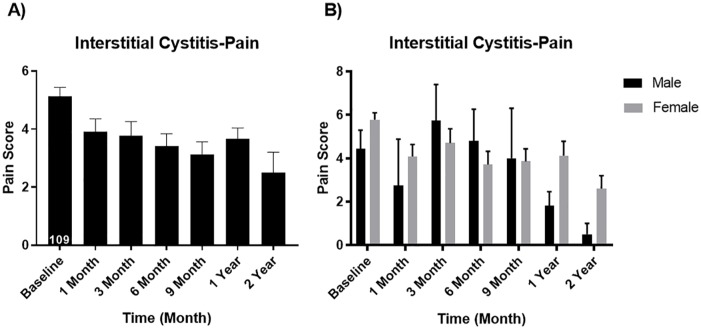
Overall visual analog pain scores decreased from a mean of 5.14 baseline to 3.67 at 12 months (p < 0.05). For men, the pain scores went from 4.44 down to 1.80 (p < 0.05) and for women, the pain scores went down from 5.78 down to 4.13 (p < 0.05); see Figure 1.
Figure 1.
Visual analog pain score (A) overall and (B) for males vs. females. Male baseline mean ± SEM: 4.44 ± 0.85 and 1 year mean ± SEM: 1.80 ± 0.66 (p value 0.0447 significantly different). Female baseline mean ± SEM: 5.78 ± 0.32 and 1 year mean ± SEM: 4.13 ± 0.05 (p value 0.047 significantly different).
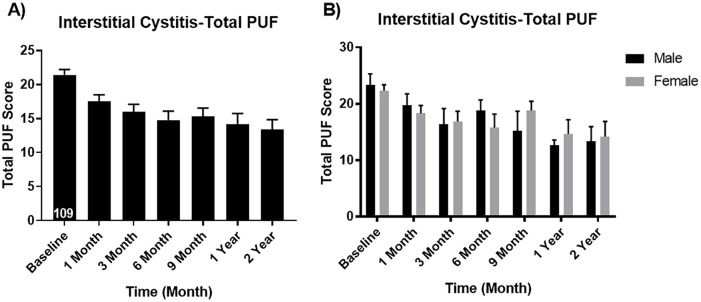
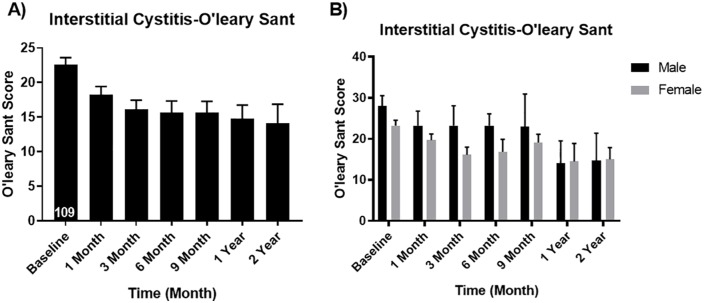
Results also demonstrated that symptom and bother scores all improved at 12 months. The PUF scores decreased from a baseline of 21.42 down to 14.20 at 12 months (p < 0.05). There were no significant gender differences on the PUF scores (Figure 2). The O’Leary-Sant scores decreased from baseline 22.59–14.76 at 1 year (p < 0.05). Again, there were no significant differences between men and women on the O’Leary-Sant scores (Figure 3).
Figure 2.
Pelvic Pain and Urgency/Frequency Patient Symptom Scale (A) overall and (B) for males vs. females. Male baseline mean ± SEM: 23.30 ± 1.99 and 1 year mean ± SEM: 12.67 ± 0.88 (p value 0.017 significantly different). Female baseline mean ± SEM: 22.33 ± 1.04 and 1 year mean ± SEM: 14.63 ± 2.52 (p value 0.006 significantly different).
Figure 3.
O’Leary-Sant scores (A) overall and (B) for males vs. females. Male baseline mean ± SEM: 28.00 ± 2.57 and 1 year mean ± SEM: 14.00 ± 5.51 (p value 0.028 significantly different). Female baseline mean ± SEM: 23.26 ± 1.29 and 1 year mean ± SEM: 14.5 ± 4.40 (p value 0.029 significantly different).
Both pain scores and symptom/bother metrics continued to decrease after 12 months with improvement seen at 24 months, but these lacked statistical significance owing to the low reporting numbers of patients at the 2 year interval.
When asked whether their condition was improved by the treatment, results showed that 78 out of 109 patients (71.5%) reported improvement in their IC/PBS (Figure 4). Men and women had similar response rates with 66 out of 91 female patients (72.4%) reporting improvement and 12 out of 18 male patients (67%) reporting improvement.
Figure 4.
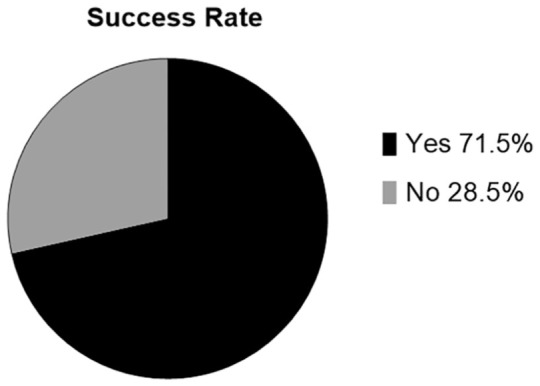
Overall patients reporting that their condition was improved by SVF deployment.
Several minor adverse events were reported by patients to the database. One patient complained of lumps in her back from the lipoharvesting procedure. Three patients complained of pain associated with the harvest and one of those reported it as ‘extremely painful’. One patient developed urinary tract infection after the SVF procedure. One patient had urinary retention lasting 2 days until she was able to spontaneously void. No long-term side effects were reported. There were no serious adverse events associated with SVF harvesting or deployment.
Discussion
IC/PBS is a chronic and debilitating disease associated with pelvic pain, severe voiding dysfunction, sexual dysfunction, and severe psychosocial impact.1,2 Women are affected by IC 10 times more frequently than men and 3.3 to 7.9 million women in the United States are estimated to have IC/PBS.3 IC represents a significant diagnostic and therapeutic challenge for clinicians. Identification of the exact cause of IC/PBS has been the topic of much debate and research, and despite several theories there remains a lack of consensus on the exact etiology. The association of IC/PBS with other pain syndromes such as vulvodynia, fibromyalgia, chronic pelvic pain, and irritable bowel syndrome may suggest involvement of the nervous system in the genesis of IC/PBS. One large multicenter study elucidated the psycho-physiologic and autonomic dysfunction characteristics of IC/PBS.4 Another popular etiological theory involves pelvic organ mast cells suspected as an inflammatory effector cell. Urothelial mast cell activation syndrome may be a factor in the pathogenesis of the condition,5 but it is unclear whether it is the cause or just part of a biologic cascade. One of the most compelling explanations for IC/PBS is based on defective urothelial permeability. In this explanation theory, a permeability deficiency exists in which the integrity of the glycosaminoglycan (GAG) mucus barrier that protects the bladder epithelial surface.6 In this model, the bladder becomes vulnerable to the leakage of irritative solutes. Potassium, the most irritative leaking solute, potentially causes depolarization of bladder nerves and muscle resulting in the symptoms of urgency, frequency, pain, or urinary incontinence in any combination.7 The abnormally increased urothelial permeability also results in local tissue inflammation, which may affect surrounding local organs promoting pelvic floor muscle spasm associated with myofascial pain. The subsequent overstimulation of pelvic afferent sensory nerve fibers that occurs is associated with chronic pelvic pain syndromes.
There appears to be a significant autoimmune component to IC/PBS, which is associated with other autoimmune conditions including inflammatory bowel disease (Crohn’s disease and ulcerative colitis), systemic lupus erythematosus, and Sjögren’s syndrome.8 Autoimmune problems may interfere with growth factor mediated systems responsible for ongoing support and maintenance of the GAG barrier. One such peptide growth factor is vascular endothelial growth factor (VEGF), which is a permeability factor that is known to modulate bladder inflammation and neuronal responses to stimuli and bladder neuroplasticity.5
Providing effective treatment for IC/PBS has remained problematic for clinicians. IC/PBS is known for its insidious progression and resistance to conventional therapies. The American Urologic Association treatment guideline algorithms are notoriously complex and convoluted and include six levels of diagnostic and interventional treatments for IC/PBS.9 Many of the current IC/PBS treatments clinically focus on masking symptoms of pain, contributing to patient reliance on narcotics. In addition to chronic opioid addiction, IC patients are known to endure multiple invasive medical and surgical procedures. Overall, there appear to be few treatment options for associated with high levels of evidence of efficacy.10 Consequently, many patients and clinicians designate IC/PBS as ‘incurable’ and the condition is often associated with depression, suicidal ideation, and the adverse financial effects of chronic pain and disability. Despite increases in awareness and research funding, as well as a growing clinical experience with detection and diagnosis, viable and effective approved treatments for IC/PBS remain elusive.11
Early research on the use of stem cell therapy for IC has mostly been limited to animal studies. Stem cells have been used in rats in an attempt to regenerate bladder epithelial integrity because the IC/PBS urothelium shows altered synthesis of several proteoglycans and tight junction proteins for cell adhesion.12–15 One study described the benefit of conditioned medium derived from mesenchymal stem cell (MSC) culture as an intravesical therapy for IC.16 In another study using a rat IC model (defective bladder voiding function, epithelium denudation, abnormal increased inflammation), treatment with human umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) provided experimental evidence of significantly improved irregular voiding and frequency and improved histologic changes.17 The mechanism of action was considered regeneration of the bladder epithelium through stimulation of Wnt-EGF (epidermal growth factor) signaling.
SVF is an autologous biologic product derived in surgery from the enzymatic digestion of adipose tissue, which is split into its fat fraction (adipocytes) and stromal and vascular fractions (containing regenerative cells). After disposing of the fatty adipocyte fraction, the remaining fraction contains stem cells and cytokines that are associated with regenerative, immunomodulatory, antinociceptive, and anti-inflammatory properties. Our research group has used SVF extensively, especially for orthopedic conditions and we hypothesized that SVF may safely mitigate some of the symptoms of IC.
In considering the potential for this type of stem cell therapy to treat IC/PBS, one must define the therapeutic clinical concepts behind deployment of autologous SVF for personal cell therapy. The key regenerative ingredient in SVF is a collection of adult stem cells that are functionally identical to those in bone marrow but are locked in a subcutaneous collagen matrix where natural functions include healing local lacerations or responding to caloric challenge by forming and supporting adipocytes. Lipoharvesting (mechanical release) followed by collagenase incubation (enzymatic digestion) liberates these cells by separating them from their collagen matrix, making them bioaccessible to damaged tissue in other parts of the body. Once stem cells in SVF are deployed into target tissue, they mimic natural processes and equivalently function as all autologous stem cells in the damaged milieu to effect cellular repair. Providing large numbers of autologous stem cells in the proximity of damaged tissues by clinical deployment effectively increases the numbers of healing cells available to the injured or diseased condition acting as a biologic repair ‘surge’ in an effort to promote healing.
The mechanism of action stem cells use to effect repair is multifaceted. One common mechanism is actual target cell formation by direct stem cell engraftment and trans-differentiation guided by the cellular damage milieu. Another mechanism is through the ‘paracrine’ effect, wherein stem cells effect repair to damaged target cells through complex cellular signaling systems, which may lead to increased angiogenesis, promotion of cell survival, and prevention of cellular apoptosis in damaged tissues. Growth factors communicating between healthy stem cells and damaged target cells may also modulate the microenvironments of the damaged target tissues making them more favorable for the regeneration process and ultimately enhancing the regenerative potency of the damaged tissues.18–21 In addition to peptide signaling molecules, cell signals may also be mediated by exosomes that may contain proteins, mRNA, miRNA (regulatory RNA), and other genetic material.22,23 New data have now shown that the paracrine effect can also be manifested by the direct transfer of mitochondria from healthy stem cells into damaged cells to restore oxidative phosphorylation and rejuvenate the energy production capabilities of unhealthy and damaged cells.24 It is likely that the paracrine effect of stem cells in SVF may play a very significant role in its clinical efficacy by mitigating cellular damage.25
In addition to a unique mixture of four different types of autologous stem cells in an extremely rich cytokine milieu, autologous SVF also contains T-reg cells that may be helpful in immunomodulation. One analytic study performed on SVF cells derived from our institution used flow cytometry to demonstrate these four subtypes, which included a robust line of hematopoietic stem cells (HSCs) in the SVF in addition to the traditional expected MSCs. Interestingly, the ratio of MSCs to HSCs in the SVF appeared to affect clinical cell therapy efficacy.26 Fresh SVF as an autologous biological product is only useful for personal cell therapy used at the surgical point of care and, therefore, it can never be mass produced and distributed as a pharmaceutical.
Autologous SVF containing stem cells in this IC study were deployed both systemically through intravenous infusion and also regionally into pelvic floor targets. The SVF may have had positive effects on subjective outcomes in this pilot series by potentially decreasing inflammation, decreasing afferent pain fiber transmission in pelvic nerves, repairing local damage to bladder and surrounding tissues, and immunomodulating the immune system. In this study, patients were asked whether the treatment helped them and 71.5% of the patients reported improvement in quality of life. One study limitation was that since no severity stratification such as evaluating for the presence of Hunner’s ulcers was performed at the time of SVF deployment, it is not possible to draw firm conclusions about clinical outcomes in this patient population.
The stem cells in autologous adipose-derived SVF are adult stem cells and, unlike embryonic stem cells, have been widely considered practical and safe sources for stem cell therapy.27–30 As the source of SVF in this study was autologous rather than allogeneic, there are no issues with immediate or delayed immune rejection and there are also no issues with risk of viral or prion transmission that may potentially occur through insufficient donor screening methods. Adult stem cells are derived from a variety of adult (i.e. bone marrow adipose tissue, peripheral blood, and dental pulp) and fetal tissues (i.e. umbilical cord blood, placenta, and amniotic fluid).
Autologous adipose-derived SVF containing multipotent adult stem cells is associated with many clinical advantages including ease of harvesting, high stem cell yield, and easy access to human fat because of its abundance. In addition, the cells in autologous SVF can also be easily stored and culture expanded into personalized cell lines of mesenchymal lineage for future use. Other advantages of SVF adult stem cells include lack of malignant potential and lack of ethical sourcing issues as opposed to human embryonic stem cells. For these reasons, SVF as a regenerative product has gained widespread popularity, however recent regulatory issues have emerged in the United States, which may affect the ability of physicians to use SVF.
Like most SVF-based treatments under investigation, a combination of regional and systemic deployment appears to provide the best clinical results for IC/PBS. Intravesical deployment of cellular therapy initially proved futile because of the poor urinary dwell time that results in the relative wasting of the vital therapeutic cells. Nevertheless, it is possible, and in some cases advisable, to inject SVF cells directly into bladder lesions such as Hunner’s ulcers. The regional deployment in this study consisted of transvaginal SVF injection into the trigone and also around the periurethral and perivesicle tissues. Occasionally, pelvic floor trigger points were also included. All of these targets can be approached in the office with a local anesthetic block in women; however, in men, it is necessary to use interventional radiology and ultrasound or computed-tomography-guided injections for the trigone deployments if required. Every patient also received an intravenous infusion of SVF for systemic immunomodulatory and anti-inflammatory effects because IC is associated with systemic autoimmune features as well as significant regional inflammation of the pelvic floor and associated tissues.
Conclusion
IC/PBS syndrome is a disease characterized by chronic pelvic pain associated with urinary urgency and frequency with a significant negative effect on quality of life. Lack of consensus on disease etiology, pathophysiology, and on effective treatment modalities has made this condition particularly difficult to treat for clinicians. Conventional treatments for IC/PBS have often been focused on symptom mitigation and long-term outcomes have traditionally been poor.
This 18-center study is the largest human study to date using cell therapy to treat IC and is the first to use autologous stem cells for the mitigation of IC.
Overall, 71.5% of the patients reported that they felt better after the SVF treatment and also patients reported significant decreases in overall mean pain scores. Patients also reported significant decreases in mean PUF and O’Leary-Sant symptom and bother scores at 1 year. Placebo studies are indicated to further elucidate whether this therapy will be effective for IC, but one drawback to such studies is that because this is a surgical procedure, it would require the performance of a ‘sham liposuction surgery’, which results in some ethical considerations. In view of the lack of long-term data, it is difficult to draw conclusions regarding the use of autologous SVF containing stem cells with IC/PBS, but the safety and efficacy results of this form of personalized stem cell therapy are promising and warrant larger, more extensive controlled trials.
Acknowledgments
The following subinvestigator affiliates of the Cell Surgical Network participated in patient treatment and data collection.
| CSN contributing affiliates | ||
|---|---|---|
| Treatment Center | ||
| Arizona Stem Cell Research Center | Paul Holden, MD | |
| California Stem Cell Treatment Center | Elliot Lander, MD | Mark Berman, MD |
| Colorado Stem Cell Treatment Center | Ken Oleszek, MD | Greta Mclaren, MD |
| East Bay Stem Cell Center | Paul Kim, MD | |
| Gulf Coast Stem Cell and Regenerative Center | Hazem Barmada, MD | |
| Indiana Stem Cell Treatment Center Team | Robert Jackson, MD | Chris Lowery, MD |
| Innovations Medical Stem Cell Treatment Center | Bill Johnson, MD | |
| Little Rock Stem Cell Treatment Center | Ken Martin, MD | |
| New Health Regenerative Medicine Center | Ekwensi Griffith, DO | |
| New Jersey Stem Cell Treatment Center | Erica Song, MD | |
| Newport Beach Stem Cell Treatment Center | Michael Elam, MD | |
| New Zealand Stem Cell Treatment Centre | Peter Chapman-Smith, MD | |
| Ohio Stem Cell Treatment Center | Mark Foglietti, DO | |
| San Francisco Stem Cell Treatment Center | Ahvie Herskowitz, MD | |
| Sparrow Regenerative Medical Team | Leslye Pace, MD | |
| Stem Cell Center of Georgia Team | Jamie Walraven, MD | |
| Stem Cell Treatment Team of San Antonio | Adrienne Askew, MD | Tamyra Rogers, MD |
| Vero Beach Stem Cell Treatment Center Team | David Griffin, MD | John Atwell, MD |
Special thanks go to Shawntae Dowell who participated in data management.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Dr. Lander, Dr. Berman, and Dr. See have ownership in Cell Surgical Network.
Ethical statement: Our study was approved by the International Cell Surgical Society IRB (approval # ICSS-2018-005). All patients provided written IRB-approved informed consent prior to enrollment in the study.
ORCID iD: Elliot B. Lander  https://orcid.org/0000-0003-4357-3397
https://orcid.org/0000-0003-4357-3397
Contributor Information
Elliot B. Lander, Cell Surgical Network, 72780 Country Club Drive #301, Rancho Mirage, CA 92270, USA.
Mark H. Berman, Cell Surgical Network, Rancho Mirage, CA, USA
Jackie R. See, Cell Surgical Network, Rancho Mirage, CA, USA
References
- 1. Nickel JC, Tripp DA, Pontari M. Psychosocial phenotyping in women with interstitial cystitis/painful bladder syndrome: a case control study. J Urol 2010; 183: 167–172. [DOI] [PubMed] [Google Scholar]
- 2. Propert KJ, Schaeffer AJ, Brensinger CMet al. A prospective study of interstitial cystitis: results of longitudinal followup of the interstitial cystitis database cohort. The interstitial cystitis data base study group. J Urol 2000; 163: 1434–1439. [DOI] [PubMed] [Google Scholar]
- 3. Berry S, Elliott M, Suttorp Met al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011; 186: 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chelimsky T, Chelimsky G, McCabe NPet al. Interstitial cystitis-elucidation of psychophysiologic and autonomic characteristics (the ICEPAC study): design and methods. J Pain Res 2014; 7: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sant GR, Saban R. Focused issue on interstitial cystitis/blader pain syndrome (IC/BPS). Transl Androl Urol 2015; 4: 592–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teichman JM, Moldwin R. The role of the bladder surface in interstitial cystitis/painful bladder syndrome. Can J Urol 2007; 14: 3599–3607. [PubMed] [Google Scholar]
- 7. Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU Int 2011; 107: 370–375. [DOI] [PubMed] [Google Scholar]
- 8. Van de Merwe JP, Yamada T, Sakamoto Y. Systemic aspects of interstitial cystitis, immunology and linkage with autoimmune disorders. Int J Urol 2003; 10: S35–S38. [DOI] [PubMed] [Google Scholar]
- 9. Hanno PM, Burks DA, Clemens JQet al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011; 185: 2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cox A, Golda N, Nadeau Get al. CUA guideline: diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Can Urol Assoc J 2016; 10: E136–E155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ratner V. The interstitial cystitis association of America: lessons learned over the past 30 years. Transl Androl Urol 2015; 4: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim A, Shin DM, Choo MS. Stem cell therapy for interstitial cystitis/bladder pain syndrome. Curr Urol Rep 2016; 17: 1–9. [DOI] [PubMed] [Google Scholar]
- 13. Zhao W, Zhang C, Jin Cet al. Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur Urol 2011; 59: 155–163. [DOI] [PubMed] [Google Scholar]
- 14. Huang YC, Shindel AW, Ning Het al. Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol 2010; 183: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang H, Qiu X, Shindel AWet al. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev 2012; 21: 1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adamowicz J, Pokrywczynska M, Drewa T. Conditioned medium derived from mesenchymal stem cells culture as a intravesical therapy for cystitis interstitials. Med Hypotheses 2014; 82: 670–673. [DOI] [PubMed] [Google Scholar]
- 17. Song M, Lim J, Yu HYet al. Mesenchymal stem cell therapy alleviates interstitial cystitis by activating Wnt signaling pathway. Stem Cells Dev 2015; 24: 1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng AS, Yau TM. Paracrine effects of cell transplantation: strategies to augment the efficacy of cell therapies. Semin Thorac Cardiovasc Surg 2008; 20: 94–101. [DOI] [PubMed] [Google Scholar]
- 19. Mirotsou M, Jayawardena TM, Schmeckpeper Jet al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 2011; 50: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gnecchi M, Danieli P, Malpasso Get al. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol 2016; 1416: 123–146. [DOI] [PubMed] [Google Scholar]
- 21. Song M, Heo J, Chun JYet al. The paracrine effects of mesenchymal stem cells stimulate the regeneration capacity of endogenous stem cells in the repair of a bladder-outlet-obstruction-induced overactive bladder. Stem Cells Dev 2014; 23: 654–663. [DOI] [PubMed] [Google Scholar]
- 22. Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 2015; 40: 82–88. [DOI] [PubMed] [Google Scholar]
- 23. Basu J, Ludlow JW. Exosomes for repair, regeneration and rejuvenation. Expert Opin Biol Ther 2016; 16: 489–506. [DOI] [PubMed] [Google Scholar]
- 24. Paliwal S, Chaudhuri R, Agrawal Aet al. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 2018; 25: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gharaibeh B, Lavasani M, Cummins JHet al. Terminal differentiation is not a major determinant for the success of stem cell therapy—cross-talk between muscle-derived stem cells and host cells. Stem Cell Res Ther 2011; 2: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kilinc O, Santidrian A, Minev Iet al. The ratio of ADSCs to HSC-progenitors in adipose tissue derived SVF may provide the key to predict the outcome of stem cell therapy. Clin Trans Med 2018; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Pham P. Clinical trials for stem cell transplantation: when are they needed? Stem Cell Res Ther 2016; 7: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berman M, Lander E. A prospective safety study of autologous adipose-derived stromal vascular fraction using a specialized surgical processing system. Am J Surg 2017; 34: 129–142. [Google Scholar]
- 29. Michalek J, Moster R, Lukac Let al. Stromal vascular fraction cells of adipose and connective tissue in people with osteoarthritis: a case control prospective multi-centric non-randomized study. Glob Surg. 2017; 3: 1–9. [Google Scholar]
- 30. Comella K, Parlo M, Daly Ret al. Safety analysis of autologous stem cell therapy in a variety of degenerative diseases and injuries using the stromal vascular fraction. J Clin Med Res 2017; 9: 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]