Abstract
Case summary
A 4-year-old neutered male cat was presented with a 2-month history of intermittent constipation that progressed to obstipation. Primary clinical findings included a large, multi lobulated mass in the caudodorsal abdomen, peripheral eosinophilia and hyperglobulinemia. Abdominal imaging revealed a multilobulated, cavitated mass in the sublumbar region. Exploratory celiotomy revealed multiple firm masses in the sublumbar retroperitoneal space causing ventral displacement and compression of the descending colon with extension of the masses into the pelvic canal. Histopathology was consistent with feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF). Aerobic culture was positive for Staphylococcus aureus. The cat was treated with prednisolone (2 mg/kg PO q24h), lactulose (0.5 g/kg PO q8h), amoxicillin/clavulanic acid (62.5 mg/cat PO q12h for 1 month) and fenbendazole (50 mg/kg PO q24h for 5 days). Six months postoperatively, the cat had no recurrence of clinical signs. Repeat evaluation and imaging at day 732 postoperatively revealed marked improvement of the abdominal mass, resolution of peripheral eosinophilia and no clinical signs with continued prednisolone therapy (0.5 mg/kg PO q24h).
Relevance and novel information
This is a report of a primary extramural FGESF lesion, and the first description of characteristics of FGESF on CT. Previous evidence suggests that the most favorable outcomes require immunosuppressive therapy and complete surgical excision; however, this case demonstrates a favorable outcome with medical management alone.
Keywords: Gastrointestinal, eosinophilic sclerosing fibroplasia, surgery, medical
Case description
A 4-year-old male neutered domestic medium hair cat, weighing 4.1 kg, was presented to Purdue University Veterinary Teaching Hospital (PUVTH) for intermittent episodes of constipation for 2 months. Prior to referral, the cat had been assessed by the primary veterinarian and management included polyethylene glycol 3350 (½ tsp PO q12h [MiraLAX; Bayer)] and a prescription canned gastrointestinal diet (Royal Canin Gastro-intestinal High Energy; Royal Canin). When clinical signs progressed, the frequency of polyethylene glycol 3350 administration was increased to q8h, and lactulose (Patrin Pharma) was added at 3 ml (~0.5 g/kg [PO q8h]). Progression of clinical signs also prompted further diagnostics, including abdominal radiographs, which revealed a sublumbar, space-occupying soft tissue mass with ventral deviation and displacement with partial obstruction of the descending colon. Formed heterogeneous feces and colonic distention was noted in the descending colon cranial to the mass effect. Blood was submitted for feline leukemia virus (FeLV) immunofluorescence assay, feline immunodeficiency virus (FIV) Western blot and Bartonella Western blot. The cat was negative for FeLV/FIV but positive for Bartonella species (3+ on a 4+ scale) and was prescribed azithromycin (9.1 mg/kg PO q24h for 1 month [Teva Pharmaceuticals]). Referral to PUVTH was recommended after 7–10 days of suspected obstipation.
Physical examination revealed tachycardia (heart rate 230 beats per min [bpm]; reference interval [RI] 160–220 bpm), tachypnea (70 breaths per min; RI 10–40 breaths/min), body condition score 3/9 and an unkempt coat. A firm sublumbar mass was palpated in the caudodorsal abdomen, with soft stool palpated in the region cranial to the mass. No pain was elicited on abdominal palpation. The sublumbar mass was palpable on rectal examination, with no other abnormalities noted. Results of laboratory analysis of blood samples revealed a leukocytosis of 24.2 K/µl (RI 6.0–18.0 K/µl) characterized by a mature neutrophilia of 17.4 K/µl (RI 3.0–12.0 K/µl) and eosinophilia of 2.2 K/µl (RI 0.1–1.5 K/µl), and hyperproteinemia (8.5 g/dl; RI 5.5–7.1 g/dl) characterized by hyperglobulinemia (5.3 g/dl; RI 2.3–3.8 g/dl). Serum total T4 was measured and T4 concentrations were 1.6 µg/dl (RI 2.4–4.6 µg/dl), consistent with probable sick euthyroid syndrome. Histoplasma antigen enzyme immunoassay was negative. Serum protein electrophoresis revealed a polyclonal gammopathy, characterized by elevations in alpha-2 (1.2 g/dl; RI 0.4–0.9 g/dl), beta (1.0 g/dl; RI 0.3–0.9 g/dl) and gamma (2.7 g/dl; RI 0.3–2.5 g/dl) fractions.
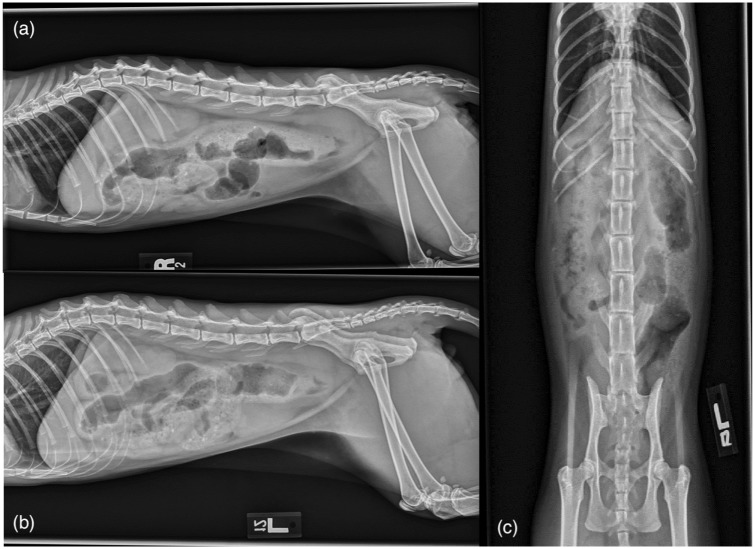
Thoracic and abdominal radiographs were performed. Thoracic radiographs revealed an ill-defined rounded mild increase in soft tissue opacity dorsal to the second sternebra, suggestive of sternal lymphadeno-pathy. The remainder of the thoracic radiographs were within normal limits. Abdominal radiographs (Figure 1) revealed a large, well-defined, slightly lobulated, ovoid soft tissue mass in the caudal abdomen ventral to L7–S1 with focal ventral displacement of the descending colon and cranial displacement of the urinary bladder. The mass measured approximately 10 cm × 3 cm in the right lateral abdominal view. The colon contained a large amount of heterogeneous feces and gas, which markedly narrowed at the level of the mass.
Figure 1.
(a) Right lateral, (b) left lateral and (c) ventrodorsal radiographic projections of the abdomen. The descending colon is ventrally displaced and the urinary bladder is cranially displaced. The colon contains a large amount of heterogeneous feces and gas, and becomes narrowed at the level of the lesion
Focal abdominal ultrasound was performed to better determine the extent of the abdominal mass and to obtain cytologic samples. The mass was lobulated and heterogeneous with many hypoechoic-to-anechoic regions within the center. The mass was moderately vascular on color Doppler in the areas surrounding the center of the mass. Cytologic sampling via ultrasound-guided fine-needle aspiration with a 22 G needle revealed marked inflammatory cells, which consisted mostly of degenerate neutrophils and moderately vacuolated macrophages. Within some neutrophils, cocci bacteria were observed, consistent with marked septic suppurative inflammation.
Abdominal CT with triple-phase contrast and 1.25 mm slice thickness was performed (Figure 2), which revealed an extensive (10.2 cm × 3.0 cm), multi-lobulated, heterogeneous, soft tissue mass present in the caudal abdomen extending into the pelvic canal dorsal to the rectum and urethra and continuing caudally just cranial to the anus. Multiple lobules of the mass were peripherally contrast enhancing with non-enhancing areas centrally. A few small mineral-attenuating foci were present within the center of the mass. The cranial aspect of the mass displaced the right ureter ventrally. At the level of the sacrum, the descending colon was displaced ventrally and narrowed, and the dorsal margin was confluent with the mass. The colon orad to the mass was feces-filled. The mass extended into the gluteal muscles at the level of the caudal sacrum, dorsal to the ileum bilaterally. There was medial iliac, inguinal, jejunal, gluteal and colic lymphadenomegaly with no contrast enhancement seen. A mild amount of peritoneal effusion was also noted.
Figure 2.
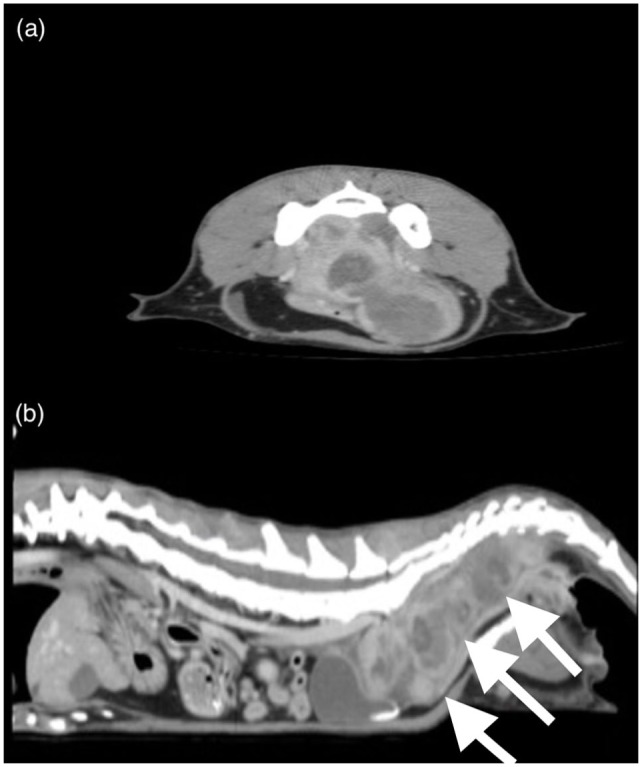
Examples of a (a) transverse and (b) sagittal slice obtained by abdominal CT. The multilobulated, heterogeneous soft tissue mass (white arrows) present in the caudal abdomen extends into the pelvic canal dorsal to the rectum and urethra and continues caudally just cranial to the anus
A definitive diagnosis was unable to be obtained via cytology, and proceeding with exploratory celiotomy was elected. The cat was pre-medicated with fentanyl (5 µg/kg IV; Hospira) and general anesthesia was induced with propofol (4 mg/kg IV; Hospira). General anesthesia was maintained with isoflurane inhalant 1–2% (Piramal Critical Care) and fentanyl continuous rate infusion (10 µg/kg/h IV). The cat received perioperative intravenous isotonic crystalloids (5 ml/kg/h IV [Normosol-R; Hospira]) and ampicillin/sulbactam (30 mg/kg IV q90mins [Unasyn; Pfizer]). The cat was positioned in dorsal recumbency and the ventral abdomen was clipped and aseptically prepared.
Following ventral midline incision from xiphoid to pubis, exploratory celiotomy revealed the organs to be grossly normal other than the abnormalities described. Multiple firm, round soft tissue masses were palpated just dorsal to the colon in the sublumbar region extending into the pelvic canal. Each mass was white to pale pink, measured 2–3 cm in diameter, and was adhered to adjacent masses and the surrounding soft tissues by multiple fibrous adhesions, which formed a pseudocapsule. Upon digital palpation, the masses entered the pelvic canal adjacent to the descending colon and rectum with no obvious complete obstruction palpated. Multiple 4 mm punch biopsies were obtained from the sublumbar masses. Impression smears of the punch biopsy samples were prepared and submitted for cytology. After biopsy, a small amount of thick, purulent material was noted to be coming from the sites. Blunt and sharp dissection was then performed to remove the most cranial sublumbar mass (measuring 3 cm × 2 cm × 2 cm) for excisional biopsy and aerobic, anaerobic and fungal culture.
The jejunal and colic lymph nodes were enlarged but of normal consistency on palpation. Blunt dissection was performed to isolate a colic lymph node for histopathologic analysis. 3-0 polydioxanone (Ethicon) was used to ligate and separate the lymph node from its attachment to the mesentery using a guillotine technique.
Following completion of all procedures, gloves and instruments were changed. The surgical site was lavaged with warm saline and closed routinely. The cat recovered uneventfully from general anesthesia.
Postoperatively, the cat remained in hospital for 3 days and was treated with lactulose (0.5 g/kg PO q8h), ampicillin/sulbactam (30 mg/kg IV q8h) until transitioned to amoxicillin/clavulanic acid (PO 62.5 mg/cat [~16 mg/kg] PO q12h [Clavamox; Pfizer]), fenbendazole (50 mg/kg PO q24h for 5 days [MSD Animal Health]), fentanyl (5 µg/kg/h IV) until transitioned to buprenorphine (0.02 mg/kg buccally q8h [Buprenex; Reckitt Benckiser Healthcare]) and isotonic crystalloids at 60 ml/kg/day. At the time of discharge, the cat was comfortable, eating, drinking and urinating normally. Defecation was noted twice and consisted of a soft ribbon-like consistency. The cat was sent home on lactulose (0.5 g/kg PO q8h), polyethylene glycol 3350 (¼–½ tsp PO q8h), buprenorphine (0.02 mg/kg buccally q8h), amoxicillin/clavulanic acid (62.5 mg/cat PO q8h) and fenbendazole (50 mg/kg PO q24h) to complete the 5-day course.
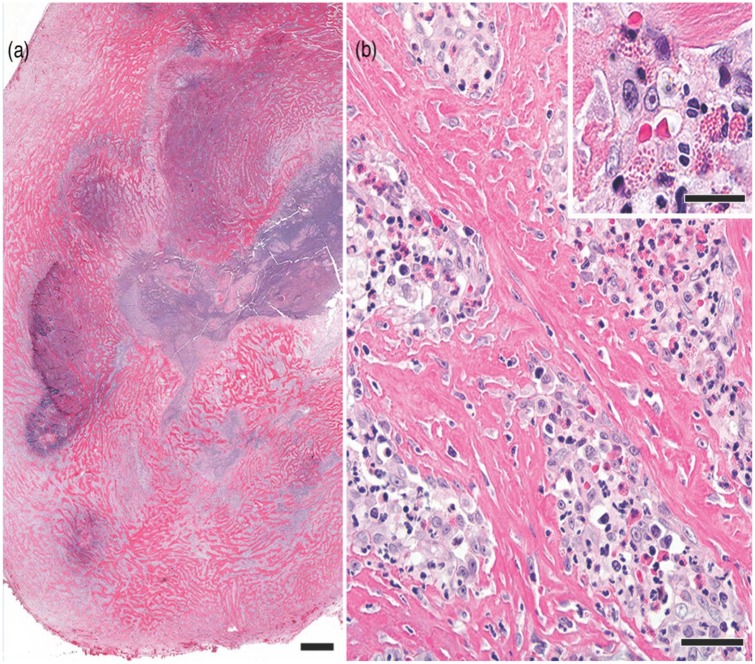
Impression smears taken at the time of surgery were of low cellularity, with non-degenerate neutrophils and fibroblasts seen, and were considered non-diagnostic. The formalin-fixed excisional biopsy specimen of a sublumbar mass was firm to hard with central cavitations. Histologic sections (Figure 3) consisted of interlacing bands of dense fibrous tissue with hypertrophied fibroblasts. Neither nuclear atypia nor mitotic figures were noted in the fibroblasts. Aggregates of eosinophils, with fewer neutrophils, macrophages, lymphocytes and plasma cells, were scattered through the sclerotic tissue. Some aggregates contained Gram-positive cocci. No other microorganisms were detected with Gram stain, Giemsa stain or Grocott’s methenamine silver. Despite the numerous eosinophils, mast cells were few, individualized and well differentiated. The cavitations noted grossly were foci of necrosis with numerous neutrophils. Despite the lack of gastrointestinal tissue in the biopsy specimen, the histologic findings were typical of feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF) lesions; therefore, a diagnosis of FGESF was made.
Figure 3.
Photomicrographs of an excisional biopsy specimen of one of the sublumbar masses in the cat. (a) The well-circumscribed mass consists of sclerotic fibrous tissue with central necrosis. Hematoxylin and eosin stain; bar = 1 mm. (b) Hypertrophied fibroblasts are in the sclerotic tissue and in the scattered foci of eosinophilic inflammation. Hematoxylin and eosin stain; bar = 60 μm. (inset) Higher magnification of an aggregate of eosinophils mixed with other leukocytes and fibroblasts. Hematoxylin and eosin stain; bar = 25 μm
The colic lymph node was reactive with prominent follicles, increased trabecular fibrous tissue and a 1 mm diameter medullary nodule of eosinophils with fewer macrophages and fibroblasts. Focally, trabecular or capsular fibrosis was more severe and infiltrated by eosinophils, but typical lesions of eosinophilic sclerosing fibroplasia were not observed in the nodal sections. Eosinophilic lymphadenitis was diagnosed. Aerobic culture grew many Staphylococcus aureus, with susceptibility to amoxicillin/clavulanic acid (minimum inhibitory concentration = 0.25). Anaerobic and fungal cultures revealed no growth.
After histopathology results were obtained, the cat was prescribed long-term prednisolone therapy (2 mg/kg PO q24h; Akorn). The owner was advised to continue amoxicillin/clavulanic acid (62.5 mg/cat PO q12h) for 1 month to treat the S aureus isolated from the cavitated mass. Repeat abdominal ultrasound at PUVTH was recommended 3 months postoperatively; however, the owner elected to pursue all postoperative follow-up care with the primary veterinarian. The cat continued to have consistent, ribbon-like stool production through the postoperative period and developed no postoperative complications. Three weeks postoperatively, the cat was eating well and had gained approximately 0.9 kg. Seven weeks postoperatively, the abdominal mass was no longer palpable. Six months postoperatively, the cat was alive and doing well, with normal appetite, drinking habits and urination, and production of soft, ribbon-like stools daily. At that time, the cat was medically managed on polyethylene glycol 3350 (~0.5 g/kg PO q24h) and prednisolone (2 mg/kg PO q24h).
The cat was presented for an additional follow-up evaluation 732 days postoperatively. The cat was eating, drinking, urinating and defecating normally. Within the first year post-diagnosis, the cat’s prednisolone dosage was tapered to 0.5 mg/kg PO q24h, and it has been maintained on this dosage long term. Physical examination revealed a body condition score of 4/9 and 0.7 kg weight gain. Abdominal palpation revealed no mass, while rectal palpation revealed a persistent firm mass dorsally. Repeat blood work revealed a lymphopenia of 1.2 K/µl (RI 1.5–1.7 K/µl) and no other abnormalities.
Repeat abdominal CT with triple-phase contrast and 1.2 mm slice thickness was performed (Figure 4), which revealed the absence of peritoneal effusion. The previously described massive multi-lobulated, heterogeneous soft tissue mass was markedly reduced in size. One ovoid soft tissue-attenuating mass, measuring approximately 2.8 cm in length, remained dorsal to the rectum from the level of the first sacral vertebra to the third coccygeal vertebra, which was causing ventral displacement and narrowing of the rectum at the level of the sacrum. On a sagittal view, the approximate size of the mass was previously 22.6 cm2 vs 4.93 cm2 on repeat CT scan. The previous mineral foci were absent. Sacral and colic lymphadenomegaly was present, but the remainder of the previously described lymph nodes were of normal size.
Figure 4.
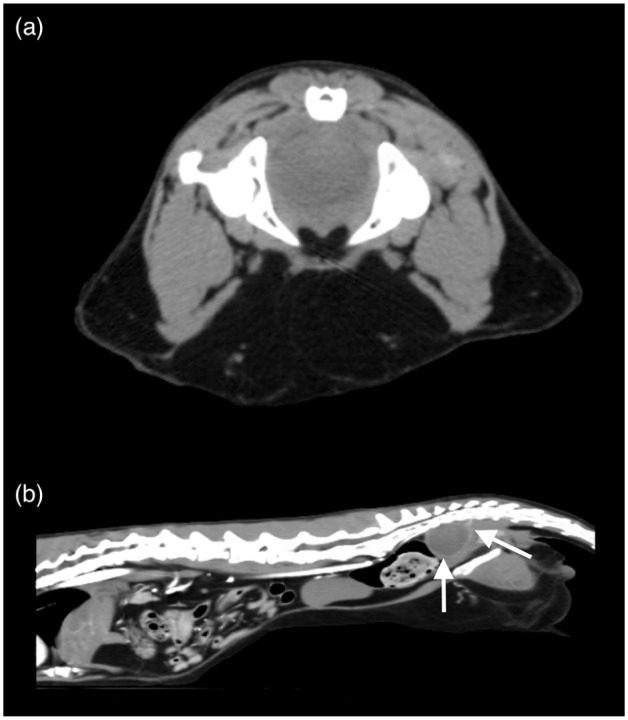
(a) Transverse and (b) sagittal post-contrast abdominal CT images at 732 days postoperatively. There is a single ovoid contrast-enhancing, soft tissue-attenuating mass (white arrows) present within the pelvic canal causing ventral displacement and narrowing of the rectum at the level of the sacrum
Discussion
FGESF is an inflammatory condition of unknown etiology in domestic cats.1 It has a worldwide distribution, and has been diagnosed in the USA, Europe, Japan and New Zealand.1–7 FGESF affects cats ranging in age from 14 weeks to >16 years, with the median age being 7–8 years.1,3 Males and Ragdoll cats may be over-represented.3 Clinical signs commonly include chronic vomiting and/or diarrhea, weight loss, lethargy and anorexia.1,3,7 As in the described case, a palpable abdominal mass is found in the majority (~85%) of cats, in addition to abdominal pain.3 Other findings may include pyrexia and peripheral eosinophilia (~58%).1,3 Diagnostic imaging such as abdominal radiography or ultrasonography may be performed to identify an abdominal mass, but they cannot be used to distinguish FGESF from intestinal neoplasia.7
Macroscopically, FGESF typically manifests as a nodular, ulcerated, intramural gastrointestinal mass most commonly affecting the pylorus or ileocecocolic junction, although masses in the duodenum, jejunum and colon have been described.1–7 Involvement of regional abdominal lymph nodes and eosinophilic inflammation within adjacent tissues, such as the pancreas or liver, has also been described.1–3 Extension into the mesenteric and cranial mediastinal lymph nodes has been reported in one cat.5 Gross lesions may be confused with lymphoma, adenocarcinoma, granulomatous disease or soft tissue sarcoma.1,3,7 Microscopically, FGESF is characterized by trabeculae of dense sclerotic collagen, large, reactive fibroblasts and mixed inflammation of many eosinophils with fewer well-granulated mast cells, neutrophils, plasma cells, lymphocytes and/or macrophages.1–8 Histologically, these inflammatory lesions have been mistaken for sclerosing mast cell tumors and intestinal osteosarcomas in some cats.9 Examination of biopsies sampled from the described cat’s caudal abdominal mass was consistent with previously reported histologic findings. Biopsy of a regional lymph node revealed eosinophilic lymphadenitis, indicating probable extension of this lesion into local lymph nodes, as reported.5
Although a specific cause has not been identified, proposed pathogeneses include inherited eosinophil dysregulation, food hypersensitivity, penetrating wound from a migrating foreign body, herpesvirus infection or bacterial infection.1–8 Certain cats may be genetically predisposed to significant eosinophilic inflammation in response to antigenic stimulation from bacteria, fungi or parasites.8 Bacteria are isolated from lesions in about 56% of cases; however, it is unclear whether the bacteria have a primary or secondary role.1 Most commonly, Gram-negative rods are isolated; however, Gram-positive rods and Gram-positive cocci, as isolated in the described case, have been reported. One case report previously identified phycomycetes in a domestic cat with FGESF, and similar lesions have been found in the pylorus of pumas infected with intestinal nematodes (Cyclospirura species), which may indicate this sclerotic reaction is a felid-specific inflammatory response to infection of varying causes.8 A recent study aimed to explore the possible involvement of feline herpesvirus-1 (FHV-1) and feline coronavirus (FCoV)/feline infectious peritonitis in the development of FGESF, and concluded that 12/13 cats diagnosed with FGESF tested negative for FHV-1 and FCoV, making it unlikely that the viruses contributed to the development of FGESF.3
Treatment of FGESF typically involves a multimodal approach, including prednisolone at immunosuppressive doses with other immunomodulatory drugs as needed, and surgical resection of the mass, if possible.1,3,5,7 Despite bacterial involvement in the majority of cases, antibiotics appear to be ineffective and have not been shown to increase survival time.1 Prognosis is variable depending on the location of the mass (cats with distal lesions tend to have longer survival times than those with pyloric lesions) and the type of treatment instituted.1 Cats treated with a multimodal approach may live for several years after initial diagnosis.3,7
The presented case provides further understanding of the clinical findings of this rare inflammatory condition in cats and describes a unique feature of the disease process. FGESF lesions have been described throughout the gastrointestinal tract and have been found to involve several tissues outside of the gastrointestinal tract, including regional lymph nodes, pancreas and liver. However, in the majority of FGESF cases currently described in the veterinary literature, the primary FGESF lesion consists of an intramural mass.
To our knowledge, this is the second case describing an extramural FGESF lesion. In the other case reported, a cat was diagnosed with FGESF within the mesentery and no masses were found on an abdominal CT scan.10 In the case presented here, the mass did not extend from the colonic wall, but instead originated in the retroperitoneal soft tissues, resulting in displacement and compression of the descending colon. Although the available literature indicates that multimodal therapy including immunosuppressive medication and surgical excision provides the best outcome, complete surgical excision of the primary mass was not pursued in this case. This was following a discussion with the owner prior to surgery who expressed a desire to avoid significant morbidity associated with pubic symphysiotomy, which would have been required to attempt complete resection of the masses. Given the current clinical signs and an almost 4.6-fold reduction in the size of the mass, medical management was considered successful.
Conclusions
This report broadens the spectrum of potential lesions and locations in which FGESF has been noted to occur, characterizes the appearance of FGESF on CT, shows that FGESF cannot be ruled out solely owing to an extra-mural location of an abdominal mass and indicates that patients with FGESF can have a good quality of life, and potentially equivalent survival times with medical management alone when complete surgical excision is not possible or declined.
Footnotes
Accepted: 16 June 2019
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrew D Woolcock  https://orcid.org/0000-0001-7102-3224
https://orcid.org/0000-0001-7102-3224
References
- 1. Craig LE, Hardam EE, Hertzke DMet al. Feline gastrointestinal eosinophilic sclerosing fibroplasia. Vet Pathol 2009; 46: 63–70. [DOI] [PubMed] [Google Scholar]
- 2. Grau-Roma L, Galindo-Cardiel I, Isidoro-Ayza Met al. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia associated with phycomycetes. J Comp Pathol 2014; 151: 318–321. [DOI] [PubMed] [Google Scholar]
- 3. Linton M, Nimmo JS, Norris JMet al. Feline gastrointestinal eosinophilic sclerosing fibroplasia: 13 cases and review of an emerging clinical entity. J Feline Med Surg 2015; 17: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munday J, Martinez A, Soo M. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia mimicking metastatic neoplasia. N Z Vet J 2014; 62: 356–360. [DOI] [PubMed] [Google Scholar]
- 5. Sihvo HK, Simola OT, Vainionpaa MHet al. Pathology in practice. Severe chronic multifocal intramural fibrosing and eosinophilic enteritis, with occasional intralesional bacteria, consistent with feline gastrointestinal eosinophilic sclerosing fibroplasia. J Am Vet Med Assoc 2011; 238: 585–587. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki M, Onchi M, Ozaki M. A case of feline gastro-intestinal eosinophilic sclerosing fibroplasia. J Toxicol Pathol 2013; 26: 51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weissman A, Penninck D, Webster Cet al. Ultrasonographic and clinicopathological features of feline gastrointestinal eosinophilic sclerosing fibroplasia in four cats. J Feline Med Surg 2013; 15: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eckstrand CD, Barr BC, Woods LWet al. Nematode-associated intramural alimentary nodules in pumas are histologically similar to gastrointestinal eosinophilic sclerosing fibroplasia of domestic cats. J Comp Pathol 2013; 148: 405–409. [DOI] [PubMed] [Google Scholar]
- 9. Halsey CH, Powers BE, Kamstock DA. Feline intestinal sclerosing mast cell tumour: 50 cases (1997–2008). Vet Comp Oncol 2010; 8: 72–79. [DOI] [PubMed] [Google Scholar]
- 10. Kambe N, Okabe R, Osada Het al. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia limited to the mesentery. J Small Anim Pract. Epub ahead of print 30 April 2018. DOI: 10.1111/jsap.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]