Abstract
Skeletal tissue development and regeneration in mammals are intricate, multistep, and highly regulated processes. Various signaling pathways have been implicated in the regulation of these processes, including Notch. Notch signaling is a highly conserved, intercellular signaling pathway that regulates cell proliferation and differentiation, determines cell fate decision, and participates in cellular process in embryonic and adult tissue. Here, we review recent data showing the regulation of Notch signaling in osteogenesis, osteoclastogenesis, and angiogenesis. These processes are cell-context–dependent via direct or indirect mechanisms. Furthermore, Notch signaling may be highly beneficial for efficient coupling of osteogenesis and angiogenesis for tissue engineering and skeletal repair, which is critical to develop clinically therapeutic options.
Notch signaling is a highly conserved, intercellular signaling pathway system that regulates cell proliferation and differentiation, determines cell fate decisions, and participates in cellular processes in both embryonic and adult tissue, including skeletal tissue development and regeneration.1, 2, 3 Notch signaling consists of four parts: ligand, receptor, DNA binding protein, and downstream transcription genes. In humans and mice, there are four single-pass transmembrane Notch receptors (Notch1–4). These receptors are initiated after direct binding of ligands Jagged1 or Jagged2, or Delta-like 1, 3, or 4 of neighboring cells.4 This interaction causes proteolytic cleavage of a Notch receptor via the γ-secretase complex; as a result, the Notch intracellular domain (NICD) is released from the cellular membrane into the cytoplasm.5 Then the NICD activates canonical and noncanonical Notch signaling mechanisms. During canonical Notch signaling the NICD translocates to the nucleus and binds with CSL transcription factors [recombination signal binding protein for immunoglobulin kappa J region (RBPjκ)/suppressor of hairless/lag-1] and co-activators, such as Mastermind-like proteins, forming a complex that induces the transcription of downstream target genes.4, 6 These genes include basic helix-loop-helix family transcription factors, such as hairy enhancer of split family genes (Hes1, Hes5, and Hes7) and HES-related with YRPF motif family genes (Hey1, Hey2, and HeyL).7
Canonical Notch signaling is identified as a critical regulator of stem cell proliferation, differentiation, and self-renewal in the pancreatic, hematopoietic, neural, and skeletal muscle systems.8, 9, 10, 11, 12 Here, we highlight recent insights into the physiological role of Notch signaling in regulating skeletal tissue development and regeneration.
Notch Signaling in Skeletal Tissue Development
Skeletal tissue development in mammals is intricate, involving multiple steps and highly regulated processes by which the collagenous mesenchymal tissues are replaced by bone. This process is performed during two distinct and important mineralization types called intramembranous ossification and endochondral ossification.13, 14 Both are needed to coordinate growth, differentiation, and interaction of various cells. Endochondral ossification occurs when cartilage is replaced systematically by bone at a healing fracture or at the site of epiphyseal growth plates.15 This process occurs in limb bone and axial bone, such as the femur, tibia, vertebrae, and pelvis. This is also an important process during the healing of fractures when treated by cast immobilization or certain types of internal fixation.16 Intramembranous ossification is characterized by which mesenchymal tissue is replaced directly by bone and does not need the step of intermediate cartilage formation.17 This process occurs most notably in the bones of the skull, clavicle, and mandible. It also occurs during the healing process of fractures when the bones are treated by anatomic open reduction and fixation with a metal plate and screws.16 Notch signaling may interact directly or indirectly with other signaling pathways such as Wingless-type MMTV integration site family (Wnt) and bone morphogenetic protein in osteoblasts, osteocytes, and osteoclasts to regulate skeletal tissue development18 (Figure 1).
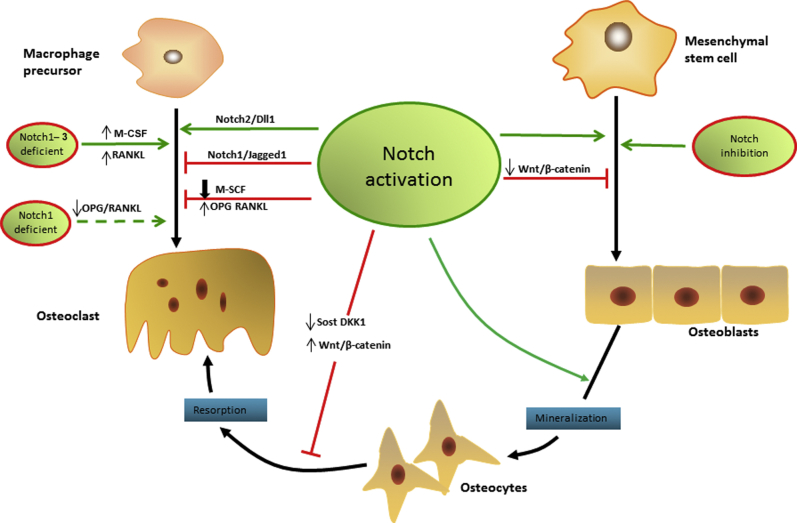
Figure 1.
Notch signaling in bone tissue development. Notch signaling has dual effects on osteogenesis and osteoclastogenesis. Notch signaling activation not only enhances osteogenic differentiation and the bone mineralization process, but also inhibits the osteogenesis by suppressing Wnt/β-catenin signaling. Meanwhile, Notch signaling inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) can enhance the osteogenesis process. Furthermore, Notch activation in osteocytes can suppress bone resorption and increase bone volume by reducing sclerostin (Sost) and dickkopf WNT signaling pathway inhibitor 1 (Dkk1), as well as up-regulation of Wnt signaling. Notch1–3 deficiency directly promotes osteoclastic differentiation, and Notch1 deficiency can promote this process indirectly by decreasing the osteoprotegerin (OPG) and receptor activator of nuclear factor κB ligand (RANKL) ratio. The Notch1/Jagged1 axis suppresses osteoclastogenesis, whereas the Notch2/Delta-like (Dll) 1 axis has the opposite effect. Notch activation has a negative effect of osteoclast formation by strongly inhibiting the macrophage colony-stimulating factor (M-CSF).
Notch Signaling and Osteogenesis
Osteoblasts are derived from the mesenchymal stromal cells in the bone microenvironment, and are responsible for the synthesis and mineralization of bone formation in postnatal development and adult life.19, 20 The role of Notch signaling in osteogenesis has been examined in several in vitro and in vivo studies.21, 22, 23
In vitro studies have shown that Notch signaling has dual effects of induction and inhibition on stromal cell osteoblastic differentiation, which may depend on cell type, cell differentiation stage, and the timing of Notch activation. Tezuka et al21 examined the role of Notch activation in various types of osteoblastic cells in vitro. A significant increase in calcified nodule formation is observed in long-term cultures when a Notch1 cytoplasmic domain was delivered by an adenovirus vector to osteoblastic MC3T3-E1 cells. Consistent with this finding, our study further showed that Notch ligand Jagged1-mediated primary osteoblast mineralization was enhanced through decreased cell apoptosis in a caspase 3–dependent manner.24 A similar effect also was observed in cultures of primary human bone marrow–derived mesenchymal stem cells (MSCs). When human bone marrow–derived MSCs were transduced with lentiviral vectors containing either human NICD1 or ligand jagged1, the cultures showed an enhanced mineralization. However, when dominant-negative mastermind1 was co-expressed with NICD1 or jagged1 to inhibit Notch signaling, the enhanced cell osteogenic differentiation process was partially delayed.22 In contrast, when Notch signaling was activated in stromal cells, a negative effect of Notch signaling on osteogenic differentiation was observed. Deregowski et al23 found that Notch1 overexpression inhibits osteoblastogenesis in ST-2 stromal cells by suppressing Wnt/β-catenin signaling. Sciaudone et al25 further showed that overexpression of NICD1 not only impaired the mature osteoblastic phenotype in MC3T3-E1 cells, but also enhanced adipogenesis in murine ST-2 stromal cells. Our previous findings also support this notion by showing enhanced limb-bud stromal cell osteogenic, adipogenic, and chondrogenic differentiation when Notch signaling was reduced by inhibitor N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT). This strongly suggests that the Notch pathway is a general suppressor of stromal differentiation that does not bias lineage allocation.26
To further confirm these in vitro effects, a variety of Notch gain-of-function and loss-of function mouse models have been developed. Transgenic mice overexpressing NICD1 driven by the 2.3-kb fragment of the collagen type 1 α1 promoter in committed osteoblastic cells causes severe osteosclerosis owing to the increased proliferation of immature osteoblasts.27 Tao et al28 further reported that Notch inhibition by selective deletion of Notch nuclear effector Rbpjκ in mice osteoblasts completely suppressed Notch-induced osteosclerotic and growth-retardation phenotypes, indicating that the effect of Notch signaling in osteoblasts depends solely on Rbpjκ activity. Although conditional null Notch1 mice under the control of the osteocalcin promoter had no obvious skeletal phenotype, deletion of both Notch1 and Notch2 in osteoblasts showed increased alkaline phosphatase activity, suggesting a possible rescue by Notch2 in Notch1 null mice.29 More importantly, in vivo data generated from conditional Notch gain-of-function and loss-of function specific in MSCs from our laboratory clearly show for the first time that the RBPjκ-dependent Notch signaling pathway is a crucial enhancer of MSC proliferation and suppressor of differentiation during skeletal tissue development. In addition, Hes1 was identified as an RBPjκ-dependent Notch target gene important for MSC maintenance and the suppression of osteogenesis.26
Notch Signaling and Osteocytes
Osteocytes are derived from terminally differentiated osteoblasts, located deep within the mineralized matrix, to support bone structure and metabolism.30 Osteocytes are dendritic cells that communicate through a canalicular network with neighboring osteocytes, as well as surrounding osteoblasts and osteoclasts.31 An in vitro study showed that dysregulation of Notch in osteocyte differentiation spontaneously can deposit calcium phosphate, disturb transportation of intracellular mineral vesicles, alter mineral crystal structure, decrease bonding force between minerals and organic matrix, and suppress dendrite development coupled with decreased expression of E11. This suggests that Notch plays a critical role in osteocyte differentiation and bone mineralization from an intracellular mineral package to extracellular mineral deposition.32 Shao et al33 showed that Notch signaling regulates osteocyte differentiation by promoting the expression of important osteocyte markers. Furthermore, the antagonism of Notch and Wnt signaling during the late differentiation stage of osteocytes is mediated by regulating phosphorylation of Akt and aggregation of β-catenin. Zanotti and Canalis34 reported that fluid shear stress activates Notch signaling in osteocytic cell lines and an in vivo study supported similar outcomes. Canalis et al35 further examined Notch effects in osteocytes by using dentin matrix protein 1 promoter Cre. When Notch signaling was overexpressed in osteocytes, suppressed bone resorption and increased bone volume were observed. Consistent with this, when Notch signaling is activated preferentially in osteocytes by crossing the dentin matrix protein 1–Cre transgenics with Rosa-Notch mice, dentin matrix protein 1–Cre+/-; Rosa-Notch mice had an increase in trabecular bone volume, an increase in cortical bone, as well as an enhancement of Wnt signaling.36 Similarly, another in vivo transgenic mice study found that Notch activation in osteocytes can suppress bone resorption and increase bone volume, resulting in reduced expression of sclerostin (Sost) and dickkopf WNT signaling pathway inhibitor 1 (Dkk1), as well as up-regulation of Wnt signaling.37
Notch Signaling and Osteoclasts
Osteoclasts are multinucleated cells that differentiate from macrophage precursors and are responsible for bone resorption.38 Osteoclastogenesis has been reported to be regulated by several molecules, induced by two critical cytokines, namely receptor activator of nuclear factor κB ligand (RANKL) and macrophage colony-stimulating factor, and inhibited by a decoy receptor of RANKL, osteoprotegerin.39 The effect of Notch signaling on osteoclast differentiation and function is cell-context–dependent via direct and indirect mechanisms. Deletion of Notch1–3 in murine bone marrow–derived macrophages directly promotes differentiation toward the osteoclastic linages. As a result, these osteoclast precursors are more sensitive and proliferate more rapidly than the wild type in response to RANKL and macrophage colony-stimulating factor. Furthermore, Notch1 deletion indirectly promotes osteoclast differentiation by enhancing osteoblastic lineage cell–mediated stimulation of osteoclastogenesis.40 Constitutive activation of Notch1 in mesenchymal cells leads to an increased expression of osteoprotegerin and RANKL; however, the expression of macrophage colony-stimulating factor strongly is inhibited, reducing the supportive effect of osteoclast development and a negative effect of osteoclast formation.41 In addition, an in vitro study showed that NF-κB promotes RANKL-induced osteoclast differentiation and resorption in RAW264.7 cells, while enhanced transcription factor Hes1 also was observed.42 In contrast, Canalis et al43 found that enhanced osteoclast number, osteoclast differentiation, and bone resorption are detected in Notch2 mutation mice, when Notch signaling is suppressed by the γ-secretase inhibitor. Furthermore, the differential regulation of osteoclastogenesis may involve different Notch receptors and ligands because it has been reported that Notch1/Jagged1 axis suppresses, while Notch2/Delta-like 1 axis promotes, osteoclastogenesis.44 Together, the effect of Notch signaling on osteoclastogenesis largely depends on the differentiation status of cells and the expression of specific ligands and receptors.
Notch Signaling and Skeletal Tissue Regeneration
Skeletal tissue regeneration is a dynamic process that balances the breakdown of old bones, the formation of new bones, and the infiltration of blood vessels in these areas, which is important during normal fracture healing.45 Bone repair is considered the prototypic physiological model for bone regeneration that generates new bone at the site of injury via intramembranous or endochondral ossification.
Numerous studies have shown that bone repair is promoted via manipulation of Notch signaling activity. Previous studies have shown that Notch signaling regulates both endochondral and intramembranous bone healing in experimental models of fracture healing.46 Recently, a genetic model study reported the requirement for Notch and bone-marrow–derived MSCs in fracture repair. Wang et al47 performed nonstabilized and stabilized fracture models in Notch-deficient mice with targeted deletion of RBPjκ in skeletal progenitors. They found reduced Notch signaling in bone-marrow–derived MSCs, and the subsequent depletion of this population leads to fracture nonunion. Our previous study found that Notch activation by Jagged1 in human bone-marrow–derived MSCs decreases cellular senescence and cell-cycle arrest, therefore allograft transplantation with Notch activation of a human bone-marrow–derived MSC sheet significantly enhances callus formation and biomechanical properties.48 In contrast, a single administration of γ-secretase inhibitors coupled with the effects on MSC accelerates bone repair in a mouse tibial fracture model. This can be explained by the increase of osteoclastogenesis and enhancement of bone remodeling via inhibited Notch signaling.49 Interestingly, when a transgenic mouse model [MX dynamin like GTPase 1 (Mx1)-Cre; dn Mastermind-like f/−] is performed to impair RBPjκ-mediated canonical Notch signaling, increased bone volume fraction and trabecular thickness are observed.50 These findings suggest that Notch signaling plays a critical and complex role in bone tissue repair.
Notch Signaling and Angiogenesis
Angiogenesis is critical for skeletal tissue regeneration, and impaired angiogenesis will lead to poor skeletal tissue regeneration. In addition to osteoblasts, osteoclasts, and chondrocytes, endothelial cells also greatly influence skeletal tissue regeneration. Skeletal tissue regeneration in the absence of endothelial cells will affect bone morphology, length, and mass. Endothelial progenitor cells are a specialized subset of immature cells circulating in adult bone marrow and peripheral circulation with the ability to differentiate into mature endothelial cells. A cell lineage tracing study showed that immature endothelial precursors in the coupled vascular invasion are essential to endochondral bone repair.51
Notch signaling also plays an important and complex role in angiogenesis. It has been reported that Notch signaling directly or indirectly regulates angiogenesis through receptors for vascular endothelial growth factor.52, 53 In a murine retinal model, it was suggested that Delta-like 4 acts downstream of vascular endothelial growth factor as a negative regulator on vascular endothelial growth factor–mediated angiogenesis.54 Another in vitro study showed Notch ligand Jagged1 is a potent proangiogenic regulator that antagonizes Delta-like 4–mediated Notch signaling in angiogenesis.55 Recent studies by Ramasamy et al56 found genetic disruption of Notch signaling in mice damages bone vessel morphology and growth, and reduces osteogenesis, resulting in shorter long bones and loss of trabecular bone. In addition, Liao et al57 showed that bone morphogenetic protein 9 up-regulates the expression of NICD1 and angiogenic regulator vascular endothelial growth factor-α in mouse adipose-derived progenitor cells when used for ectopic bone formation, which was blocked effectively by dominant-negative Notch1 (Figure 2).
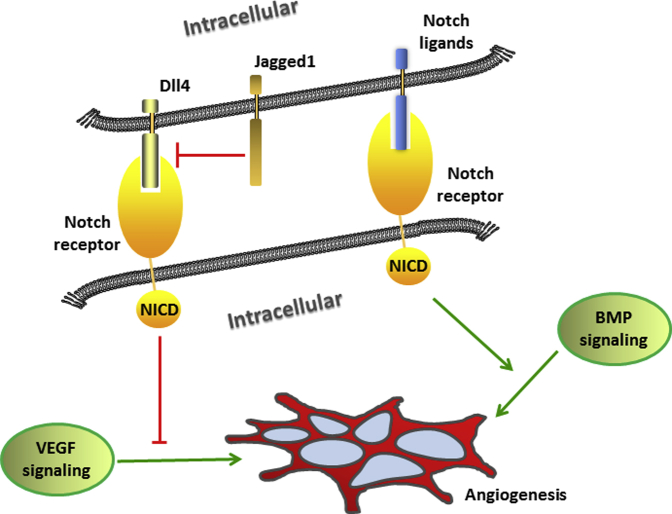
Figure 2.
Notch effect on angiogenesis. Delta-like (Dll) 4 negatively regulates vascular endothelial growth factor (VEGF)-mediated angiogenesis, while Jagged1 is a proangiogenic regulator that antagonizes Dll4-mediated Notch signaling in angiogenesis. Notch intracellular domain (NICD1) enhances bone morphogenetic protein (BMP)-induced angiogenesis.
Conclusions
Notch signaling plays an important and complex role in skeletal tissue development and regeneration. The regulations of Notch signaling in osteogenesis, osteoclastogenesis, and angiogenesis processes are cell-context–dependent via direct or indirect mechanisms. Because Notch signaling is the key factor for the coupling of osteogenesis and angiogenesis in skeletal repair, targeting Notch signaling may be an efficient way to develop novel therapeutic options for bone tissue regeneration.
Acknowledgments
Z.L., Y.D., X.S., and C.G.K. obtained funding; X.S., Z.L., G.W., and H.Z. drafted the manuscript; P.A.M., S.R.B., and C.G.K. edited the manuscript; all authors wrote the paper and had final approval of the submitted versions.
Footnotes
Supported by Natural Science Foundation of Anhui Province grant 1808085QH242 (Z.L.), Anhui Provincial Foreign Science and Cooperation grant 1503062022 (X.S.), National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH grant R01AR073277-01A1 (Y.D.), and Institutional Development Award P20GM121307 from the National Institute of General Medical Sciences of the NIH (C.G.K.).
Disclosures: None declared.
Contributor Information
Xifu Shang, Email: shangxifu@163.com.
Yufeng Dong, Email: ydong@lsuhsc.edu.
References
- 1.Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 3.Lai E.C. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 4.Tian Y., Xu Y., Fu Q., Chang M., Wang Y., Shang X., Wan C., Marymont J.V., Dong Y. Notch inhibits chondrogenic differentiation of mesenchymal progenitor cells by targeting Twist1. Mol Cell Endocrinol. 2015;403:30–38. doi: 10.1016/j.mce.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeter E.H., Kisslinger J.A., Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 6.Sikandar S.S., Pate K.T., Anderson S., Dizon D., Edwards R.A., Waterman M.L., Lipkin S.M. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70:1469–1478. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S., Lee B.H., Bae Y. Notch signaling in skeletal stem cells. Calcif Tissue Int. 2014;94:68–77. doi: 10.1007/s00223-013-9773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y., Long T., Wang C., Mirando A.J., Chen J., O'Keefe R.J., Hilton M.J. NOTCH-mediated maintenance and expansion of human bone marrow stromal/stem cells: a technology designed for orthopedic regenerative medicine. Stem Cells Transl Med. 2014;3:1456–1466. doi: 10.5966/sctm.2014-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apelqvist Å., Li H., Sommer L., Beatus P., Anderson D.J., Honjo T., de Angelis M.H., Lendahl U., Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 10.Stier S., Cheng T., Dombkowski D., Carlesso N., Scadden D.T. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99:2369–2378. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- 11.Imayoshi I., Sakamoto M., Yamaguchi M., Mori K., Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mourikis P., Sambasivan R., Castel D., Rocheteau P., Bizzarro V., Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 13.Runyan C.M., Gabrick K.S. Biology of bone formation, fracture healing, and distraction osteogenesis. J Craniofac Surg. 2017;28:1380–1389. doi: 10.1097/SCS.0000000000003625. [DOI] [PubMed] [Google Scholar]
- 14.Berendsen A.D., Olsen B.R. Bone development. Bone. 2015;80:14–18. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackie E., Ahmed Y., Tatarczuch L., Chen K.-S., Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Marsell R., Einhorn T.A. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franz-Odendaal T.A. Induction and patterning of intramembranous bone. Front Biosci. 2011;16:2734–2746. doi: 10.2741/3882. [DOI] [PubMed] [Google Scholar]
- 18.Regan J., Long F. Notch signaling and bone remodeling. Curr Osteoporos Rep. 2013;11:126–129. doi: 10.1007/s11914-013-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanotti S., Canalis E. Notch and the skeleton. Mol Cell Biol. 2010;30:886–896. doi: 10.1128/MCB.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canalis E. The fate of circulating osteoblasts. N Engl J Med. 2005;352:2014–2016. doi: 10.1056/NEJMe058080. [DOI] [PubMed] [Google Scholar]
- 21.Tezuka K.I., Yasuda M., Watanabe N., Morimura N., Kuroda K., Miyatani S., Hozumi N. Stimulation of osteoblastic cell differentiation by Notch. J Bone Miner Res. 2002;17:231–239. doi: 10.1359/jbmr.2002.17.2.231. [DOI] [PubMed] [Google Scholar]
- 22.Ugarte F., Ryser M., Thieme S., Fierro F.A., Navratiel K., Bornhäuser M., Brenner S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. 2009;37:867–875. doi: 10.1016/j.exphem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Deregowski V., Gazzerro E., Priest L., Rydziel S., Canalis E. Notch1 overexpression inhibits osteoblastogenesis by suppressing Wnt/β-catenin but not bone morphogenetic protein signaling. J Biol Chem. 2006;281:6203–6210. doi: 10.1074/jbc.M508370200. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y., Shu B., Tian Y., Chelly M., Morandi M.M., Barton S., Shang X., Dong Y. Notch activation promotes osteoblast mineralization by inhibition of apoptosis. J Cell Physiol. 2018;233:6921–6928. doi: 10.1002/jcp.26592. [DOI] [PubMed] [Google Scholar]
- 25.Sciaudone M., Gazzerro E., Priest L., Delany A.M., Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–5639. doi: 10.1210/en.2003-0463. [DOI] [PubMed] [Google Scholar]
- 26.Dong Y., Jesse A.M., Kohn A., Gunnell L.M., Honjo T., Zuscik M.J., O'Keefe R.J., Hilton M.J. RBPjκ-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development. 2010;137:1461–1471. doi: 10.1242/dev.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engin F., Yao Z., Yang T., Zhou G., Bertin T., Jiang M.M., Chen Y., Wang L., Zheng H., Sutton R.E. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao J., Chen S., Yang T., Dawson B., Munivez E., Bertin T., Lee B. Osteosclerosis owing to Notch gain of function is solely Rbpj-dependent. J Bone Miner Res. 2010;25:2175–2183. doi: 10.1002/jbmr.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanotti S., Smerdel-Ramoya A., Stadmeyer L., Durant D., Radtke F., Canalis E. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology. 2008;149:3890–3899. doi: 10.1210/en.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonewald L.F. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonewald L. Generation and function of osteocyte dendritic processes. J Musculoskelet Neuronal Interact. 2005;5:321–324. [PubMed] [Google Scholar]
- 32.Shao J., Zhou Y., Lin J., Nguyen T.D., Huang R., Gu Y., Friis T., Crawford R., Xiao Y. Notch expressed by osteocytes plays a critical role in mineralisation. J Mol Med. 2018;96:333–347. doi: 10.1007/s00109-018-1625-x. [DOI] [PubMed] [Google Scholar]
- 33.Shao J., Zhou Y., Xiao Y. The regulatory roles of Notch in osteocyte differentiation via the crosstalk with canonical Wnt pathways during the transition of osteoblasts to osteocytes. Bone. 2018;108:165–178. doi: 10.1016/j.bone.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Zanotti S., Canalis E. Notch signaling and the skeleton. Endocr Rev. 2016;37:223–253. doi: 10.1210/er.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canalis E., Parker K., Feng J.Q., Zanotti S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology. 2012;154:623–634. doi: 10.1210/en.2012-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canalis E., Adams D.J., Boskey A., Parker K., Kranz L., Zanotti S. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. J Biol Chem. 2013;288:25614–25625. doi: 10.1074/jbc.M113.470492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canalis E., Bridgewater D., Schilling L., Zanotti S. Canonical Notch activation in osteocytes causes osteopetrosis. Am J Physiol Endocrinol Metab. 2015;310:E171–E182. doi: 10.1152/ajpendo.00395.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teitelbaum S.L. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W., Zhang X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues. Mol Med Rep. 2015;11:3212–3218. doi: 10.3892/mmr.2015.3152. [DOI] [PubMed] [Google Scholar]
- 40.Bai S., Kopan R., Zou W., Hilton M.J., Ong C.-T., Long F., Ross F.P., Teitelbaum S.L. NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem. 2008;283:6509–6518. doi: 10.1074/jbc.M707000200. [DOI] [PubMed] [Google Scholar]
- 41.Yamada T., Yamazaki H., Yamane T., Yoshino M., Okuyama H., Tsuneto M., Kurino T., Hayashi S.-I., Sakano S. Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood. 2003;101:2227–2234. doi: 10.1182/blood-2002-06-1740. [DOI] [PubMed] [Google Scholar]
- 42.Duan L., de Vos P., Fan M., Ren Y. Notch is activated in RANKL-induced osteoclast differentiation and resorption. Front Biosci. 2008;13:7064–7071. doi: 10.2741/3210. [DOI] [PubMed] [Google Scholar]
- 43.Canalis E., Schilling L., Yee S.-P., Lee S.-K., Zanotti S. Hajdu Cheney mouse mutants exhibit osteopenia, increased osteoclastogenesis, and bone resorption. J Biol Chem. 2016;291:1538–1551. doi: 10.1074/jbc.M115.685453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekine C., Koyanagi A., Koyama N., Hozumi K., Chiba S., Yagita H. Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axis. Arthritis Res Ther. 2012;14:R45. doi: 10.1186/ar3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimitriou R., Tsiridis E., Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Dishowitz M.I., Terkhorn S.P., Bostic S.A., Hankenson K.D. Notch signaling components are upregulated during both endochondral and intramembranous bone regeneration. J Orthop Res. 2012;30:296–303. doi: 10.1002/jor.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C., Inzana J.A., Mirando A.J., Ren Y., Liu Z., Shen J., O'Keefe R.J., Awad H.A., Hilton M.J. NOTCH signaling in skeletal progenitors is critical for fracture repair. J Clin Invest. 2016;126:1471–1481. doi: 10.1172/JCI80672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian Y., Xu Y., Xue T., Chen L., Shi B., Shu B., Xie C., Morandi M.M., Jaeblon T., Marymont J.V. Notch activation enhances mesenchymal stem cell sheet osteogenic potential by inhibition of cellular senescence. Cell Death Dis. 2017;8:e2595. doi: 10.1038/cddis.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C., Shen J., Yukata K., Inzana J.A., O'Keefe R.J., Awad H.A., Hilton M.J. Transient gamma-secretase inhibition accelerates and enhances fracture repair likely via Notch signaling modulation. Bone. 2015;73:77–89. doi: 10.1016/j.bone.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dishowitz M.I., Mutyaba P.L., Takacs J.D., Barr A.M., Engiles J.B., Ahn J., Hankenson K.D. Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing. PLoS One. 2013;8:e68726. doi: 10.1371/journal.pone.0068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maes C., Kobayashi T., Selig M.K., Torrekens S., Roth S.I., Mackem S., Carmeliet G., Kronenberg H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kofler N.M., Shawber C.J., Kangsamaksin T., Reed H.O., Galatioto J., Kitajewski J. Notch signaling in developmental and tumor angiogenesis. Genes Cancer. 2011;2:1106–1116. doi: 10.1177/1947601911423030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia A., Kandel J.J. Notch: a key regulator of tumor angiogenesis and metastasis. Histol Histopathol. 2012;27:151–156. doi: 10.14670/hh-27.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suchting S., Freitas C., le Noble F., Benedito R., Bréant C., Duarte A., Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benedito R., Roca C., Sörensen I., Adams S., Gossler A., Fruttiger M., Adams R.H. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 56.Ramasamy S.K., Kusumbe A.P., Wang L., Adams R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao J., Wei Q., Zou Y., Fan J., Song D., Cui J., Zhang W., Zhu Y., Ma C., Hu X. Notch signaling augments BMP9-induced bone formation by promoting the osteogenesis-angiogenesis coupling process in mesenchymal stem cells (MSCs) Cell Physiol Biochem. 2017;41:1905–1923. doi: 10.1159/000471945. [DOI] [PubMed] [Google Scholar]