Abstract
Purpose
In this study, we evaluated the feasibility of recovering the corneal surface integrity in a patient suffering from unilateral LSCD through the transplantation of cultured autologous corneal epithelial cells.
Methods
Human corneal epithelial cells (HCECs) were isolated from a limbal biopsy of the contralateral eye of a patient with unilateral LSCD and cultured in monolayer in the presence of an irradiated human fibroblasts feeder layer (iHFL). To produce a cultured autologous corneal epithelium (CACE), HCECs were seeded on a fibrin substrate and maintained in culture until confluence. The in vitro obtained CACE was then used to treat the affected eye of the patient. Two years later, a successful penetrating keratoplasty was performed.
Results
Efficient restoration of the corneal epithelium was achieved following transplantation of CACE indicating probable re-colonization of the cornea by stem cells. Corneal transparency was restored after removing the scarred stroma by performing a penetrating keratoplasty.
Conclusion
CACE produced in vitro was shown to restore a normal corneal surface capable of sustaining a viable and clear penetrating keratoplasty and reestablished a near normal vision in a unilateral LSCD patient.
Keywords: Cornea, Tissue engineering, Limbal stem cell deficiency, Autologous graft, Penetrating keratoplasty
1. Introduction
Loss of limbal epithelial stem cells in the palisades of Vogt can lead to a condition known as limbal stem cell deficiency (LSCD).1 It can be either complete, or partial, in which case some stem cells still have the potential to recolonize the cornea. LSCD can also be congenital as in Congenital Aniridia.2 The acquired entities include chemical or thermal burns, severe allergic conjunctivitis, neurotrophic keratitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, mucous membrane pemphigoid, chronic use of ophthalmic drops, long term contact lens wear, and idiopathic etiologies.3 As of today, the incidence of LSCD yet remains evasive primarily because it has various etiologies and because of the absence of an appropriate clinical framework for diagnosis. For patients diagnosed with complete LSCD, healthy renewal of the corneal epithelium is no longer possible. This leads to compromised vision, chronic pain, inflammation of the ocular surface, persistent corneal defects, thinning, scarring, conjunctivalization and neovascularization of the cornea.4 Partial LSCD can be treated with constant eye lubrication and topical steroids. In contrast, severe LSCD defies conservative medical approaches. Surgical limbal stem cell replacement is often necessary to adequately improve the patient's ocular surface in order to allow restoration of vision. For unilateral LSCD, a full-size limbal autograft can be attempted from the contralateral eye. This, however, bears the potential of depleting the donor's eye of its own limbal stem cells.5 Alternatives such as conjunctival-limbal autografting (CLAu), cultivated limbal epithelial transplantation (CLET) and simple limbal epithelial transplantation (SLET) have also been successfully used, although their long-term success rates vary from one another6, 7, 8(also reviewed in9). The transplantation of a limbal stem cell substitute cultured from a very small piece of limbal tissue can provide a long-term solution for the LSCD eye without compromising the surface of the patient's contralateral eye.10
The purpose of the present work was to regenerate a healthy ocular surface on the eye of a patient suffering from unilateral LSCD by transplantation of a cultured autologous corneal epithelium (CACE). The restored surface was shown to successfully sustain a viable penetrating keratoplasty and a clear cornea.
2. Materials and methods
2.1. Cell culture, graft preparation and immunohistochemistry
The culture was initiated from the limbal biopsy harvested from the controlateral eye (see section 3). The biopsy was processed within 5 h of removal from the limbus. The isolation and culture of human cornea epithelial cells (HCECs) was performed as previously described.11 Briefly, the limbus was incubated in 2 mg/ml dispase II (Roche Diagnostics, Laval, Qc, Canada) in HEPES buffer (MD Biomedicals, Montreal, Québec, Canada) overnight at 4 °C. The epithelium of the limbal region was removed and digested in trypsin for 15 min at 37 °C. Cells were then transferred to tissue culture flasks containing a lethally irradiated human fibroblasts feeder layer (iHFL) seeded 7 days before. HCECs were cultured with Dulbecco-Vogt modified Eagle medium: Ham's F12, ratio 3:1, 24.3 μg/mL adenine (Corning), 5 μg/mL insulin (Sigma-Aldrich), 0.4 μg/mL hydrocortisone (Teva Canada Ltd., Scarborough, ON, Canada), 0.212 μg/mL isoproterenol hydrochloride (Sandoz Canada, Boucherville, QC, Canada), 5% bovine HyClone FetalClone II serum (GE Healthcare), 10 ng/mL human epidermal growth factor (Austral biologicals, San Ramon, CA, USA), 100 U/mL penicillin and 25 μg/mL gentamicin as previously reported.11, 12, 13 A fibrin gel (Tisseel® kit VH, Baxter Hyland Immuno, Mississauga, Ont., Canada) was prepared in a culture petri dish (7 cm2) as described.14 Subconfluent (40%) primary cultures were trypsinized and plated at a cell density of 6 × 103 HCECs/cm2 on a fibrin gel previously seeded with iHFL 7 days before. The culture medium was supplemented with the serine protease inhibitor aprotinin (130 kIU/ml, Bayer, Mississauga, Ontario, Canada) to prevent degradation of the fibrin gel. When HCECs reached confluence, the CACE was produced by cutting the fibrin gel and the epithelium with a 14 mm trephine blade. The CACE was placed in a sterile contact lens holder, transferred at room temperature to the surgical theater and grafted within 4 h. Primary culture of HCECs and CACE preparation required 15 days.
The CACE was processed for histology. Specimens were fixed in 1% Histochoice (Amresco, Solon, Ohio, USA) and embedded in paraffin. Cross-sections (5 μm) were stained with Masson's trichrome staining and analyzed with a microscope.
3. Case report
A 72-year-old lady was referred to the Cornea Service of our Institution for a spontaneous left corneal perforation. She was well known in the Service for a 20-year history of marked Herpes Zoster Ophthalmicus neurotrophic keratitis with secondary calcific band keratopathy and progressive thinning (Fig. 1A) of the whole cornea in the left eye. At the initial examination, there was a large central descemetocele without infiltrate. The anterior chamber was flat and aqueous humor was egressing through a 3 mm wide central corneal perforation as confirmed by fluorescein coloration (positive Seidel test). The cornea had thinned out progressively over the years to reach a maximum thickness of not more than 100 μm from one limbus to the other. Microbial cultures were negative.
Fig. 1.
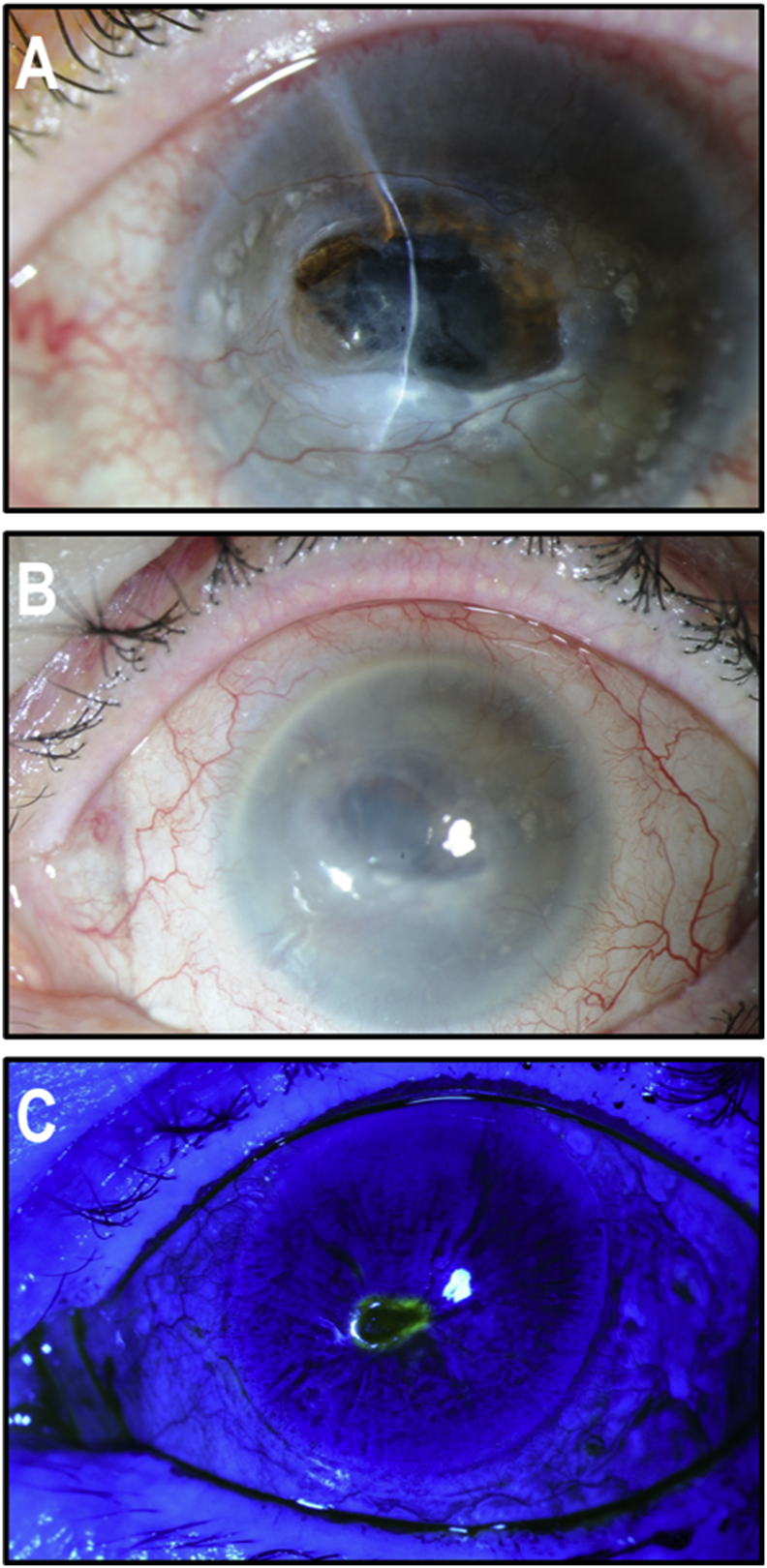
Clinical presentation. (A) Perforated central descemetocele with flat anterior chamber and very thin peripheral cornea extending to the limbus over 360°. (B) Tectonic corneoscleral graft in place. (C) Fluorescein staining showing persistent epithelial defect caused by LSCD.
Under general anesthesia, we removed the loose epithelial cells surrounding the area of descemetocele with microsponges. A 360° peritomy was performed with conjunctival recession. The peripheral corneal epithelial cells were removed with a swab soaked in 4% cocaine. A large 18 mm corneoscleral graft was prepared from a donor eye. The endothelium was removed and the tectonic graft was placed over the de-epithelialized cornea and limbus. The graft was sutured with interrupted 10-0 Nylon sutures. The tectonic graft sealed the aqueous humor leak and readily attached to the patient's residual stroma (Fig. 1B). However, the postoperative course was complicated by chronic epithelial defects secondary to a complete corneal stem cell deficiency (Fig. 1C).
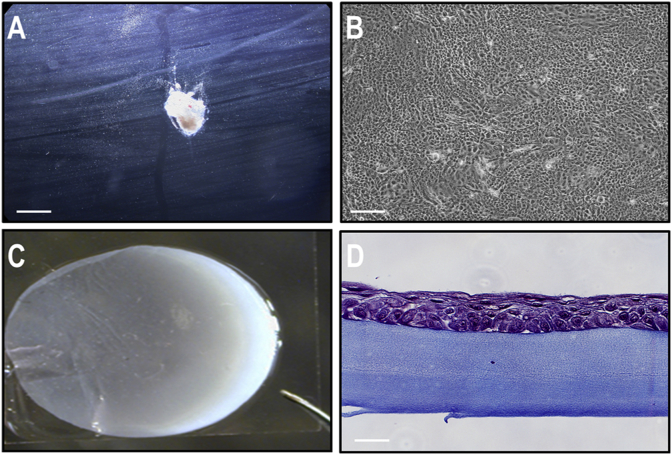
The limbal biopsy was performed under topical (proparacaine 0,5%) and local anesthesia (subconjunctival xylocaïne 2% without epinephrine) three months after the initial 13 mm graft. A 2 mm by 1 mm (2 mm2) flap of tissue was removed from the superior limbus of the contralateral right eye through the palisades of Vogt (Fig. 2A). The epithelial cells were cultured (Fig. 2B) as described above. Three weeks later, the patient was brought to the operating room. Under local anesthesia and mild sedation, a 360° limbal peritomy was performed on the patient's diseased left eye and the fibrovascular corneal pannus carefully removed. A circular sheet of CACE with a diameter of 14 mm (Fig. 2C) was transferred to the surface of the freshly de-epithelialized cornea, completely covering the limbus. It was sutured with interrupted 10-0 polyglactin 910 (Vicryl™). In the CACE, epithelial cells generated a pseudo-stratified epithelium composed of 3–4 layers (Fig. 2D). The graft was completely covered with an amniotic membrane also attached with interrupted 10-0 polyglactin 910 (Vicryl™) sutures (Fig. 3A and B). A bandage soft contact lens was finally put in place. Moxifloxacin and dexamethasone drops were used 4 times a day for the first week and tapered off gradually over a period of a few weeks.
Fig. 2.
Cell culture, graft preparation and immunohistochemistry. (A) Limbal biopsy taken from the limbus in the superior quadrant of the patient's contralateral cornea, observed using a dissecting microscope. Bar: 1 mm. (B) Patient's corneal epithelial cells co-cultured with an iHFL composed of irradiated human dermal fibroblasts, before seeding on fibrin substrate. Bar: 200 μm. (C) Preoperative photograph of the fibrin-cultured epithelial sheet (CACE) trimmed to the appropriate dimension using a 14 mm trephine blade. (D) Histology (Masson's Trichrome staining) of the tissue-engineered epithelium, showing a differentiated epithelium on a fibrin substrate. Bar: 10 μm.
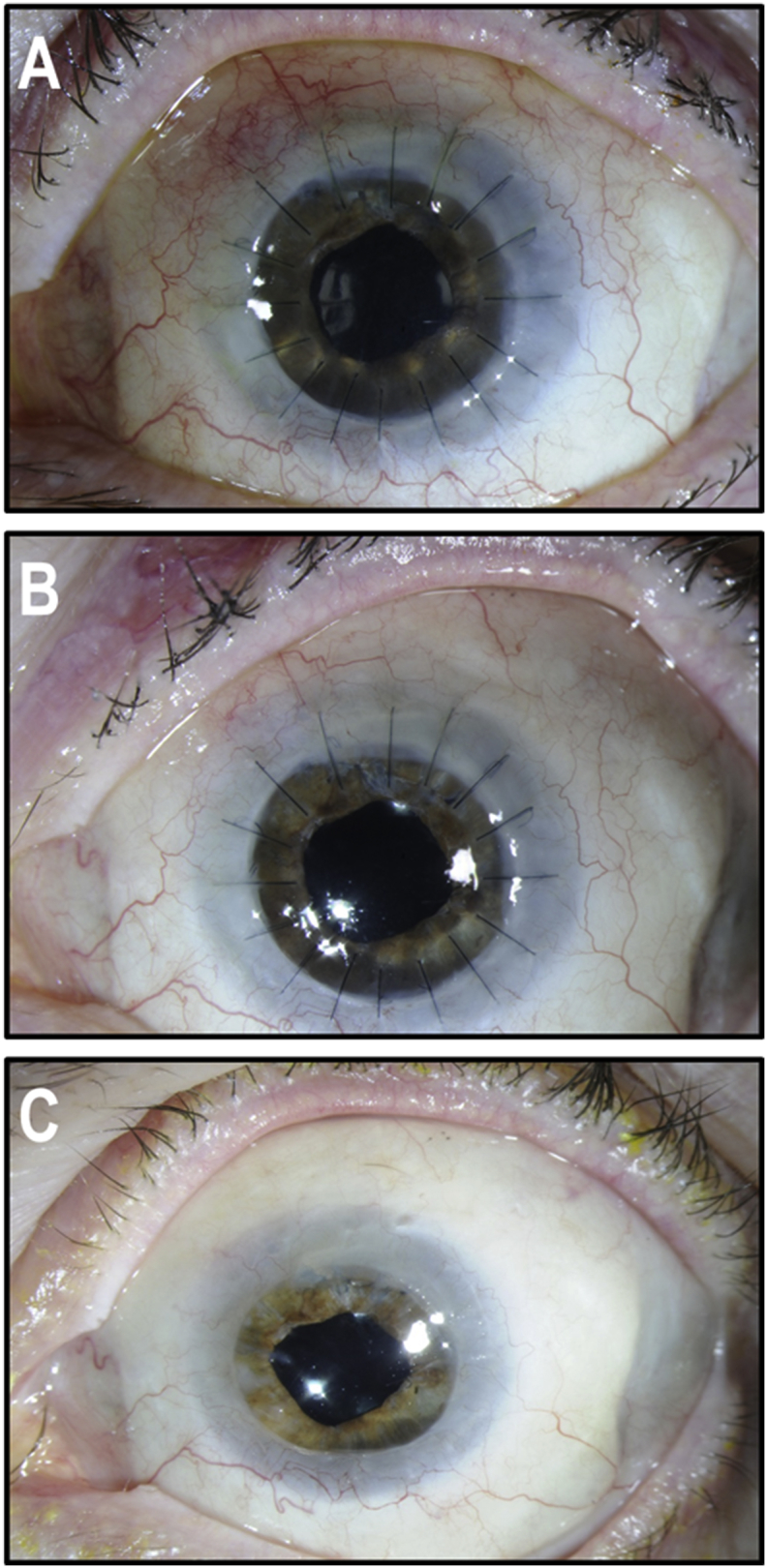
Fig. 3.
Anterior segment of the patient's left eye after grafting of the fibrin-cultured epithelial sheet. (A) One day postoperative photograph showing the epithelial sheet graft coated with an amniotic membrane. (B) Nine days, post-operative photograph showing proper healing of CACE graft. (C) Exposition corneal ulcer that has developed 50 days after the graft in the inferior quadrant of the cornea. (D) Slit-lamp examination of the corneal surface at 5 months post-CACE graft showing adequate corneal surface and the healed corneal ulcer scar.
A month and a half after the CACE graft, the patient developed a Staphiloccocus Epidermidis corneal ulcer, inferiorly, in an area of corneal exposure ((Fig. 3C). It healed rapidly using moxifloxacin, fortified Cefazolin, and fortified Tobramycin. Because of the residual inflammation, it took 1.5 months for the corneal epithelium to heal completely (Fig. 3D). The patient was then closely followed for 2 years looking for signs of epithelial abnormalities. The ocular surface preserved its normal aspect during the whole period of time and signs of stem cell deficiency were never found.
Finally, in order to restore vision, it was decided to perform a penetrating keratoplasty 2 years after the CACE grafting. The healing of the corneal graft took place normally, eventually leading to a best corrected visual acuity of 20/30. After the initial healing phase of the corneal transplant, where prednisolone acetate 1% was used, the patient was kept on low dose topical corticosteroids (Fluorometholone 0.1%) for 3 years. The graft remained clear with a normal corneal surface for the 3-year period (Fig. 4A–C), at which time the patient developed a typical endothelial rejection episode with keratic precipitates ending in secondary graft failure. The patient, then aged 80, did not want to undergo a regraft.
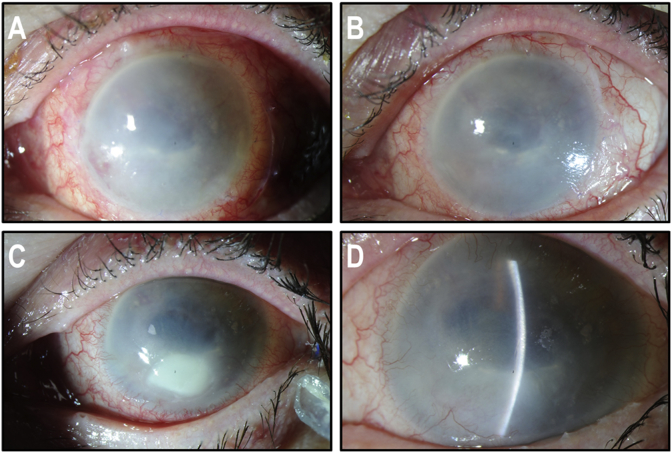
Fig. 4.
Anterior segment of the patient's left eye after penetrating keratoplasty. (A) Post-operative photograph showing the adequate transparency of the graft with intact corneal sutures. (B) One year post-operative photograph showing wound healing of the graft, with excellent transparency. (C) Examination of fully healed penetrating keratoplasty after complete suture removal. (A, B and C: post-operative photographs taken 2, 3 and 4 years post-CACE, respectively).
4. Discussion
In the present study, we have shown that HCECs cultured on iHFL can produce a 3–4 epithelial cell layers of CACE that can be used to successfully treat unilateral LSCD. A clear vision was restored after a subsequent penetrating keratoplasty.
LSCD patients can have their corneal epithelial phenotype restored by one of the three most currently used limbal stem cells transplantation procedures: CLAu, CLET and SLET. CLAu (Conjunctival-Limbal Autologous transplantation) was first described by Kenyon and Tseng in 1989 for the treatment of patients with various types of corneal problems (chronic chemical injury, thermal burns, contact lens-induced keratopathy and ocular surface failure after multiple surgical procedures).6 CLET (Ex vivo Cultivated Limbal Epithelial Transplantation) was reported for the first time in 1997 as a novel technique for reconstructing and improving ocular surface of a patient with unilateral LSCD through transplantation of an autologous cultivated corneal epithelium.7 The most recent technique, SLET (Simple Limbal Epithelial Transplantation), rely on in vivo expansion of the limbal tissue and therefore does not require the cell culture facility needed for CLET or as much tissue as CLAU.8 Overall, these transplantation procedures proved to share very similar success rates for the treatment of patients with LSCD (up to 77-, 78- and 81% for CLAU, SLET and CLAu, respectively).9,15,16
The HCECs graft that is the subject of this case report is not the first that have been conducted. Indeed, the first graft with HCECs cultured on fibrin gel was performed on a patient with LSCD in 19957 by Pellegrini et al. leading to a first clinical trial conducted in 2001.15 As discussed above, the efficacy of this treatment to restore a functional ocular surface was 76.6%.16 In the present study, we modified the culture method to replace murine cells with a human feeder layer and also substituted cholera toxin by isoproterenol, a culture medium developed for epithelial cells and autologous tissue-engineered skin for the treatment of burn patients.12
The emergency corneoscleral tectonic graft, performed on the patient, led to the unilateral iatrogenic loss of the limbal stem cells. It was treated successfully with a CACE graft. The improvement of the corneal surface was sufficient to allow a normal penetrating keratoplasty to thrive and re-establish normal corneal transparency leading to a best corrected visual acuity of 20/30. A normal ocular surface was maintained for 5 years after CACE grafting (3 years after keratoplasty) without any epithelial defects or ulceration, indicating that limbal stem cells were likely preserved in the newly grafted CACE. Our first successful procedure demonstrated the feasibility of this innovative approach to treat unilateral LSCD and allowed us to begin a clinical study, currently in phase 2A, involving fifteen patients with unilateral LSCD.
5. Conclusions
Each day, about 2000 U.S. workers have a job-related eye injury requiring medical treatment. Moreover, according to a recent WHO study, up to 55 million injuries per year involving the ophthalmic system occur worldwide.17,18 Corneal wounds account for 37% of all visual disabilities in North America. More than 10 million patients worldwide are in need of a corneal transplant. Of these, 1.5–2.0 million patients annually have untreated corneal blindness primarily because of the shortage of corneal donors.19 In this study, we have shown that CACE has the ability to recolonize the corneal surface including the limbus once grafted to an eye with LSCD, thus providing permanent restoration of a self-renewing corneal epithelium. Combined with the penetrating keratoplasty, the two procedures reestablished a useful vision to the patient. The methods used in this case report can therefore provide a solution to unilateral LSCD patients.
Patient consent
The patient was enrolled through Health Canada, Special Access Program (SAP). CACE was produced at the Centre de recherche du CHU de Québec - Université Laval. In Canada, SAP is designed to provide access to non-marketed drugs or health products for patients with extremely serious or life-threatening conditions who require emergency and/or compassionate therapies, when other treatments/therapies have failed, are unsuitable or are not available. Proper informed consent for CACE treatment was obtained from the patient, as required by institutional policies and guidelines.
Funding
This work was supported by the Canadian Institutes for Health Research (CIHR) grant MOP-53170 and FDN-143213 (L.G.), the Stem Cell Network of the National Centre of Excellence, the Fonds de Recherche du Québec - Santé (FRQS), and the Québec Cell, Tissue and Gene Therapy Network – ThéCell (a thematic network supported by the FRQS). L.P.G. was supported by a studentship from the Faculty of Medicine of Laval University. L.G. is the recipient of a Tier 1 Canadian Research Chair in Stem Cells and Tissue Engineering and a Research Chair on Tissue-Engineered Organs and Translational Medicine from the Fondation de l’Université Laval.
Conflicts of interest
None.
Financial disclosure
None.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
The authors are grateful to the research professionals from the LOEX involved in the production of CACE, Amélie Lavoie, Rina Guignard and Danielle Larouche. We would also like to thank Jocelyne Boivin, our research nurse, and the staff of the CUO/CHU de Québec - Université Laval who provided dedicated care to the patient.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.100532.
Contributor Information
Lucie Germain, Email: lucie.germain@fmed.ulaval.ca.
Sylvain L. Guérin, Email: Sylvain.Guerin@fmed.ulaval.ca.
Richard Bazin, Email: bazin988@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Daniels J.T., Dart J.K., Tuft S.J., Khaw P.T. Corneal stem cells in review. Wound Repair Regen. 2001;9:483–494. doi: 10.1046/j.1524-475x.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 2.Skeens H.M., Brooks B.P., Holland E.J. Congenital aniridia variant: minimally abnormal irides with severe limbal stem cell deficiency. Ophthalmology. 2011;118:1260–1264. doi: 10.1016/j.ophtha.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellegrini G., Rama P., Di Rocco A., Panaras A., De Luca M. Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells. 2014;32:26–34. doi: 10.1002/stem.1517. [DOI] [PubMed] [Google Scholar]
- 4.Dua H.S., Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins C., Tuft S., Liu C., Buckley R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye. 1993;7(Pt 5):629–633. doi: 10.1038/eye.1993.145. [DOI] [PubMed] [Google Scholar]
- 6.Kenyon K.R., Tseng S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. discussion 722-3. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrini G., Traverso C.E., Franzi A.T., Zingirian M., Cancedda R., De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 8.Sangwan V.S., Basu S., MacNeil S., Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–934. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 9.Shanbhag S.S., Nikpoor N., Rao Donthineni P., Singh V., Chodosh J., Basu S. Autologous limbal stem cell transplantation: a systematic review of clinical outcomes with different surgical techniques. Br J Ophthalmol. 2019 doi: 10.1136/bjophthalmol-2019-314081. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Yin J., Jurkunas U. Limbal stem cell transplantation and complications. Semin Ophthalmol. 2018;33:134–141. doi: 10.1080/08820538.2017.1353834. [DOI] [PubMed] [Google Scholar]
- 11.Germain L., Auger F.A., Grandbois E. Reconstructed human cornea produced in vitro by tissue engineering. Pathobiology. 1999;67:140–147. doi: 10.1159/000028064. [DOI] [PubMed] [Google Scholar]
- 12.Germain L., Larouche D., Nedelec B. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: a case series of 14 severely burned patients indicating clinical effectiveness. Eur Cells Mater. 2018;36:128–141. doi: 10.22203/eCM.v036a10. [DOI] [PubMed] [Google Scholar]
- 13.Black A.F., Bouez C., Perrier E., Schlotmann K., Chapuis F., Damour O. Optimization and characterization of an engineered human skin equivalent. Tissue Eng. 2005;11:723–733. doi: 10.1089/ten.2005.11.723. [DOI] [PubMed] [Google Scholar]
- 14.Larouche D., Paquet C., Fradette J., Carrier P., Auger F.A., Germain L. Regeneration of skin and cornea by tissue engineering. Methods Mol Biol. 2009;482:233–256. doi: 10.1007/978-1-59745-060-7_15. [DOI] [PubMed] [Google Scholar]
- 15.Rama P., Bonini S., Lambiase A. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–1485. doi: 10.1097/00007890-200111150-00002. [DOI] [PubMed] [Google Scholar]
- 16.Rama P., Matuska S., Paganoni G., Spinelli A., De Luca M., Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 17.Pascolini D., Mariotti S.P. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 18.Aghadoost D. Ocular trauma: an overview. Arch Trauma Res. 2014;3 doi: 10.5812/atr.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitcher J.P., Srinivasan M., Upadhyay M.P. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.