Abstract
Smith-Magenis Syndrome (SMS) is a contiguous gene syndrome linked to interstitial microdeletion, or mutation of RAI1, within chromosome 17p11.2. Key behavioral features of SMS include intellectual disability (ID), sleep-disturbances, maladaptive, aggressive and self-injurious behaviors, hyperactivity and sudden changes in mood. A distinguishing feature of this syndrome is an inverted pattern of melatonin characterized by elevated daytime and low nighttime melatonin levels. Since the central circadian clock controls the 24-hour rhythm of melatonin, we hypothesized that the clock itself may contribute to the disrupted pattern of melatonin and sleep. In this report, 24-hour patterns of body temperature, a surrogate marker of clock-timing, and continuous wrist activity were collected to examine the links between body temperature, sleep behavior, and the circadian clock. In addition, age-dependent changes in sleep behavior were explored. Actigraphy-estimated sleep time for SMS was one hour less than expected across all ages studied. The timing of the 24-hour body temperature (Tb-24) rhythm was phase advanced, but not inverted. Compared to sibling (SIB) controls, the SMS group had less total night sleep, lower sleep efficiency, earlier sleep onset, earlier final awake times, increased waking after sleep onset (WASO), and increased daytime nap duration. The timing of wake onset varied with age, providing evidence of ongoing developmental sleep changes from childhood thru adolescence. Clarification of the circadian and developmental factors that contribute to the disrupted and variable sleep patterns in this syndrome will be helpful in identifying more effective individualized treatments.
Keywords: Sleep disturbance, circadian disorder, SMS, Del 17p11.2, RAI1, neurobehavioral disorder, intellectual disability, neurodevelopmental disorder, melatonin, body temperature, melatonin
INTRODUCTION
Smith-Magenis Syndrome (SMS; OMIM #182290) is a contiguous gene syndrome linked to interstitial deletion of chromosome 17p11.2 that includes the dosage sensitive gene for retinoic acid induced 1 (RAI1). Approximately 90% of cases are due to heterozygous deletion that includes the RAI1 gene, while the remaining 10% are caused by de novo RAI1 mutation (Girirajan et al. 2006). Phenotypically, SMS overlaps with other neurodevelopmental disorders (NDD) that present with infantile hypotonia, speech and motor delays, cognitive and functional impairment, and behavioral and sensory processing issues that may lead to concomitant diagnosis of autism spectrum disorder (ASD) and/or psychiatric dual diagnosis (Gropman et al. 2006; Hildenbrand &Smith 2012; Laje et al. 2010b). Behaviorally, some of the key features of SMS include sleep-disturbances, hyperactivity and sudden changes in mood, and stereotypic, aggressive and self-injurious behaviors.
Sleep disturbance occurs in virtually all individuals with SMS. Early descriptive studies suggest age-related changes in sleep parameters (Smith et al. 1998b). The few clinical studies of SMS examining single-night EEG sleep measures reported decreased total sleep time, increased nocturnal awakenings, early sleep offset and increased daytime somnolence (Boudreau et al. 2009; De Leersnyder et al. 2001; Greenberg et al. 1996; Potocki et al. 2000). There have been no multi-night EEG sleep studies of day-to-day variability of sleep patterns. While EEG sleep evaluation has many benefits, such studies are difficult to conduct in a challenged population such as SMS in which sensory issues often lead to non-compliance. Further, when patients are asked to sleep in an unfamiliar clinical setting, EEG sleep evaluations often underestimate sleep. This study is therefore designed to objectively investigate these prior reports using multiple days of wrist actigraphy.
Over an extended period of time home sleep can be estimated using wrist-activity watches. Wrist actigraphy is a non-invasive methodology that objectively estimates temporal pattern of human activity-rest patterns for several months at a time (Piazza et al. 1997; Wehr et al. 1979). This method has successfully been used to document activity-rest patterns in medical conditions characterized by motor hyperactivity (Bauer 1991; Cassidy et al. 1998; Teicher 1995), including children with ADHD (Teicher 1995) and SMS (De Leersnyder et al. 2001). Data from activity monitors and sleep logs may provide useful information to guide recommendations with regard to sleep hygiene and improve the quality of sleep in SMS.
The circadian and endocrine factors that contribute to disturbed sleep in SMS are gradually being identified (De Leersnyder et al. 2001; Greenberg et al. 1991; Greenberg et al. 1996; Potocki et al. 2000) and include inversion of the sleep moderating hormone melatonin (De Leersnyder et al. 2001; Potocki et al. 2000), as well as a suggested RAI1-related dysregulation (transcription) of CLOCK gene and other circadian genes (Williams et al. 2012). Thus, it is important to examine multiple circadian processes to evaluate their contribution to sleep disturbance.
In humans, surrogate biological markers of the central circadian clock include the 24-hour rhythms of melatonin and body temperature (Lewy et al. 1984; Shanahan &Czeisler 1991). In SMS, the relationship between the melatonin pattern and the clock may be complex. On the one hand, the inverted melatonin pattern might suggest that the circadian clock itself is inverted. If so, rhythms of body temperature and other hormones controlled by the central clock, might also be inverted.
Because the pattern of melatonin release is abnormal in SMS, the pattern of body temperature was used as a surrogate marker of the clock in the current investigation (Shanahan &Czeisler 1991). Further, since the pattern of sleep is strongly coupled to the circadian rhythm of body temperature (Weitzman 1982), information regarding the timing of the body temperature rhythm would assist our understanding of SMS sleep.
Earlier reports indicated that decreased total sleep begins in infants with SMS by 12 months of age (Gropman et al. 2006). Sleep questionnaires indicated decreased sleep from early childhood through adulthood (Smith et al. 1998b). Preliminary analysis of actigraphy data shows decreased night sleep through late childhood (Gropman et al. 2006). Sleep in SMS is often characterized by a phase-advanced profile (early sleep onset and early morning awakening) (De Leersnyder et al. 2006; De Leersnyder et al. 2001). There have been no developmental EEG studies of sleep patterns in SMS.
The purpose of this investigation is to address three questions regarding the relationship between circadian clock function and sleep in SMS. First, what activity-rest markers characterize disturbance in SMS? Second, is the temporal relationship between the 24-hour pattern of body temperature (Tb-24) and sleep-timing consistent with well described inverted melatonin pattern? Third, what are the developmental changes in sleep during early/late childhood and adolescence (age 10–19years) in the SMS population?
MATERIALS AND METHODS
Study Population Demographics:
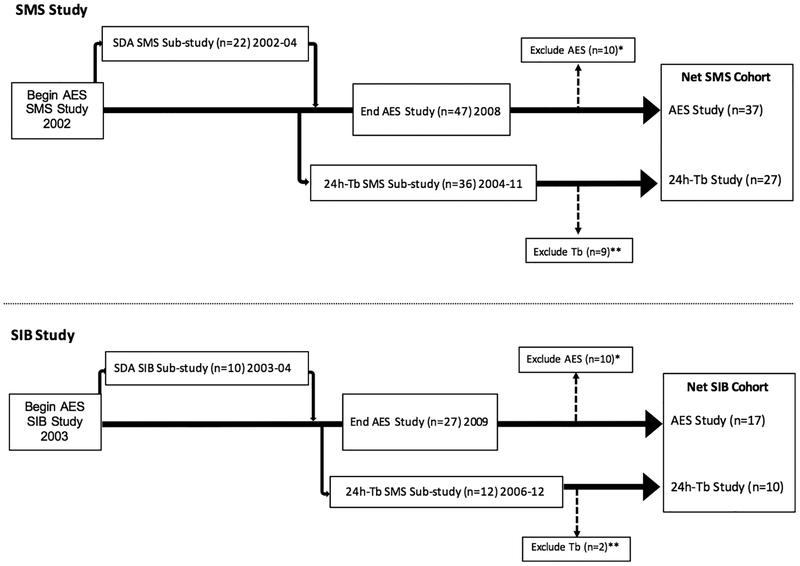
Study subjects were all patients enrolled in the NIH IRB approved SMS Natural History Study () and were recruited between 2002–2011. Subjects who participated in this study agreed to: a) wrist actigraphy, and/or b) Tb-24 assessments. Wrist actigraphy was used to measure activity-rest patterns. Body temperature measurements were taken every 3 hours for 72 hours in willing participants (see below). Unaffected siblings were recruited to participate as the healthy control group. A flowchart of the study design showing showing subjects and recruitment into the different studies is shown in Figure 1.
Figure 1:
Flowchart of patient enrollment detailing the data exclusion and final sample sizes used in different analyses for individuals with A) Smith–Magenis syndrome (SMS; top) and Sibling (SIB; bottom) controls. Data excluded from AES study (*) for noncompliance (2SMS; 1SIB) or incomplete data (8SMS; 9SIB). Reasons for incomplete AES data included missing log; watch off/on; data not retrievable; and/or lost watch. Data excluded from 24-h Tb study (**) for incomplete data (9SMS; 2 SIB). Subjects (3SMS; 5SIB) enrolled after the end of AES data collection period provided Tb data only.
SMS Group:
Fifty SMS subjects (26 females; 24 males) between ages 2 years and 32 years were recruited for the actigraphy and/or body temperature studies (Table I). The final data used for each cohort analysis largely depended on compliance. All SMS subjects had a confirmed diagnosis due to documented 17p11.2 interstitial deletion (n=48) or heterozygous RAI1 mutation (n=2). Due to ethical concerns of withdrawing clinically indicated medications, all SMS subjects continued their existing drug regimen during the study.
Table I:
Subject Demographics
| Parameter | SMS Group* | SIB Group** | ||
|---|---|---|---|---|
| N | Mean Age in years | N | Mean Age in years | |
| Total Recruited Subjects | 50 (26F/24M)* | 9.6y ± 7.1 | 32 (18F/14M)** | 8.2 ± 4.3 |
| Actigraphy | ||||
| Total Subjects | 47 (24F/23M) | 10.2y ± 7.0 | 27 (15F/12M) | 8.2 y ± 4.7 |
| Complete data for AES1 | 37 (20F/17M) | 9.0y ± 6.1 | 17 (10F/7M) | 8.9y ± 3.96 |
| Subgroup for SDA2 | 22 (13F/9M) | 9.2y ± 6.0 | 10 (5F/5M) | 9.1y ± 4.4 |
| Body Temperature | ||||
| Total Subjects | 36 (18F/18M) | 9.7y ± 6.7 | 12 (7F/5M) | 9.8 ± 3.8 |
| Complete data | 27 (12F/15M) | 9.0y ± 5.1 | 10 (6F/4M) | 9.3 ± 3.3 |
SMS (n=50): 20 ATW only; 27 did both; 3 did Tb only.
SIB (n=32): 20 ATW only; 7 did both; 5 Tb only.
Actigraphy Estimated Sleep (AES): no significant differences in gender (p=.7765) or age (p=.9510)
Sleep Dependent Analysis (SDA): no significant differences in gender (p=.7120) or age (p=.9628)
Tb: no significant differences in gender (p=.7112) or age (p=.8642)
Abbreviations: M, male; F, female.
The demographics of the study group are summarized in Table I. Thirty-seven SMS subjects (20F/17M; age 9.0y ± 6.1 (mean ± SD; range 2–24y) provided data for Actigraphy Estimated Sleep (AES) analysis. Ten SMS subjects (4F/6M) were excluded from actigraphy analysis for non-compliance and/or technical problems. Seven of the 37 SMS subjects were drug free at the time of the study, while 21 were on psychoactive medication with/without other medications, and 9 were only on non-psychoactive medications during the study (See Supplemental Materials, Table 1). Actigraphy data (Figure 1 flowchart) from a subgroup of 22 SMS AES subjects (13F/9M; 2.5 to 24.1y) was used to conduct a Sleep Dependent Activity (SDA) analysis, described later. Thirty-six SMS subjects (16F/17M) were enrolled in the 24-hour temperature study; 27 SMS subjects provided complete data; 9 SMS subjects (6F/3M) were excluded due to incomplete data. Psychotropic medication use was reviewed for possible confounds.
Unaffected Siblings Group:
Unaffected (non-SMS) siblings (n=32; 18F/14M) living in the same household as the SMS subjects were recruited to participate as the sibling control group (SIB) (Table I). Seventeen siblings (10F/7M; age range 3.5–15.7y) served as controls for the AES study; one sibling was excluded for non-compliance and nine had incomplete data. Ten siblings (5 females, 5 males; age range 3.5–15.7y) served as controls for the SDA sub-study. Ten (6F/4M; age range 6–16y) siblings served as controls for 24-hour serial body temperature study, including 8 who only provided temperature data. Incomplete Tb-24 data for two siblings was excluded from analysis. Some SIBS were used as controls in several studies.
Data Collection:
Wrist Actigraphy
Wrist activity was collected using a Mini Mitter Actiwatch® that was continuously worn on the non-dominant wrist (with the exception of bathing and swimming) for a period of approximately 4–6 weeks. AES analysis was conducted on this initial sampling period. In some cases, per parent request to provide further feedback regarding their child’s sleep during continuing care and intervention, the child wore the watch on later occasions. A parent-maintained watch log diary was kept for identifying periods of watch removal. Periods of non-compliance and removal were excluded from the data sample. Twenty-four hour patterns of wrist-activity from subjects with SMS were compared to healthy siblings who served as the control group. In addition, ten-day wrist activity samples were selected for a Sleep Dependent Analysis (SDA) of activity levels. Details of these studies are described below.
Twenty-four Hour Body Temperature (Tb-24)
Serial body temperature measurements were obtained in the home setting using a Thermoscan™ thermometer provided to the parent(s). Triplicate temperature readings taken within 1–2 minutes were measured every three hours at home from the forehead for 48–72 hours by parents who were trained in the procedure. Care was taken to measure temperature only during quiet behavior and rest, and efforts were made not to alter the usual sleep/wake pattern. Parents recorded time of day, and described activity at the time of measurement. Acquisition of actigraphy data was not conducted during the serial Tb-24 measurements since Tb-24 measurements would confound activity measurements.
Data Analysis:
Wrist Actigraphy
Activity Estimated Sleep (AES) patterns were analyzed using Actiware sleep software (Mini-Mitter Co, Inc). Specifically, AES was used for calculating Total Night Sleep (minutes), Initial Sleep and Final Waking Onsets (hh:mm), “Waking After Sleep Onset” (WASO), Sleep Efficiency (the percent of time estimated as sleep between Sleep and Waking Onsets), Nap Length (minutes) and Total 24-hour Sleep (minutes). Details of the AES procedure may be found in the Supplemental Material.
Developmental Analysis:
AES results were used to examine age-differences between SMS and SIB subjects < 16 years (Table II; III). Regression analysis between age and AES was conducted in SMS and SIBs <16y of age (no sibs >16y were recruited; Table II). In a secondary analysis, ten day convenience samples of wrist activity were randomly selected from an early cohort of protocol enrollees between 2–24 years (Table 1; Figure 1) for the Sleep Dependent Activity (SDA) Sub-study. These results then compared developmental differences between SMS and SIB groups. Briefly, early (the first three hours after sleep onset) and late (the last three hours before final waking onset) activity counts were summed after adjusting for day-to-day variation in the clock-times of sleep and waking onsets. The technique used for this analysis provides an evoked pattern of motor activity linked to sleep markers (onset, offset) and is similar to the methodological approach used to examine temporal patterns of EEG stage distribution during transitions after sleep onset (Perlis 2001), or to examine central clock-controlled timing of melatonin release in individual subjects (Wehr et al. 2001). In this secondary analysis, the pattern of wrist activity was examined around the transitions of sleep onset and final waking onset. Independent group t tests, corrected for unequal variances were used to compare wrist activity levels between subjects ≤ 10 years with subjects > 10 years, as well as between SMS and sibling subjects. The methods used for AES and SDA analyses are described in more detail in Supplemental Materials and Methods (S1) section.
Table II:
Comparison of Activity Estimated Sleep (AES) in SMS and Sibling Controls Under Age 16 Years1
| SMS (n=30)2 | SIB (n=17) | P value3 | |
|---|---|---|---|
| Gender | 15F/15M | 10F/7M | 0.7617 |
| Mean age (y) | 6.8 ± 3.8 | 8.9 ± 3.96 | 0.0725 |
| Age range (y) | 2 to 15 | 3 to 15 | |
| Total Night Sleep (mins) | 465.4 ± 46.2 | 522.1 ± 25.8 | 0.0001 |
| Sleep Efficiency (%) | 78.8 ± 15.7 | 88.8 ± 2.9 | 0.0129 |
| Sleep Onset (hh:mm) | 20:44 ± 0:54 | 21:58 ± 1:16 | 0.0004 |
| Waking Onset (hh:mm) | 6:19 ± 0:44 | 7:47 ± 1:06 | 0.0001 |
| Waking (WASO, mins) | 108.9 ± 33.4 | 66.5 ± 18.4 | 0.0001 |
| Naps (mins) | 72.0 ± 47.3 | 34.7 ± 38.6 | 0.0082 |
| Total 24-Hour Sleep (mins) | 537.4 ± 50.9 | 556.8 ± 56.4 | 0.2323 |
Excluded 6 SMS subjects >16 years and RAI1 female subject
Mean values +/− SD are given.
Unpaired 2-tailed t-test run for age and AES data; Fishers exact test (Gender)
Abbreviations: M, male; F, female; y, years; mins, minutes; WASO, waking after sleep onset.
Table III:
Age Comparison of AES in SMS and SIBS
| P | |||
|---|---|---|---|
| Under 10 years | SMS (n=24)a,b | SIB (n=8) | (SMS vs. SIB)c |
| Gender | 11F/13M | 5F/3M | 0.6851 |
| Mean age (y) | 5.1 ± 1.8 | 5.4y ± 1.6 | 0.6785 |
| Age range (y) | 2–9 | 3–7 | |
| Total Night Sleep (mins) | 465.7 ± 48.7 | 534.2 ± 18.9 | 0.0006 |
| Sleep Efficiency (%) | 78.6 ± 17.5 | 87.8 ± 2.9 | 0.1534 |
| Sleep Onset (hh:mm) | 20:45 ± 0:59 | 21:16 ± 1:06b | 0.2251 |
| Waking Onset (hh:mm) | 06:15 ± 0:46b | 7:25 ± 1:01 | 0.0018 |
| Waking (WASO, mins) | 104.5 ± 33.2b | 74.9 ± 18.2 | 0.0235 |
| Naps (mins) | 77.3 ± 51.0 | 33.0 ± 21.1 | 0.0245 |
| Total 24-Hour Sleep (mins) | 543.0 ± 54.8 | 567.5 ± 30.0 | 0.2406 |
| 10–15 years | SMS (n=7) | SIB (n=9) | |
| Gender | 5F/2M | 5F/4M | 0.6329 |
| Mean Age (y) | 13.2 ± 1.2 | 12.3 ± 1.9 | 0.8250 |
| Age range (y) | 11 – 14 | 10.2 – 15.7 | |
| Total Night Sleep (mins) | 480.4 ± 54.8 | 511.4 ± 27.3 | 0.1594 |
| Sleep Efficiency (%) | 76.1 ± 4.9 | 89.7 ± 2.8 | 0.0001 |
| Sleep Onset (hh:mm) | 20:40 ± 0:30 | 22:36 ± 1:06 | 0.0009 |
| Waking Onset (hh:mm) | 6:44 ± 0:46 | 8:07 ± 1:07 | 0.0156 |
| Waking (WASO, mins) | 149.3 ± 28.8 | 59.1 ± 16.0 | 0.0001 |
| Naps (mins) | 61.2 ± 32.2 | 36.0 ± 50.9 | 0.2735 |
| Total 24-Hour Sleep (mins) | 541.6 ± 72.8 | 547.4 ± 73.2 | 0.8770 |
| 16 years and older | SMS (n=6) | NAd | |
| Gender | 2M/4F | ||
| Mean Age (y) | 19.9 ± 2.9 | ||
| Age range (y) | 16 – 24 | ||
| Total Night Sleep (mins) | 473.4 ± 81.8 | ||
| Sleep Efficiency (%) | 81.7 ± 9.2 | ||
| Sleep Onset (hh:mm) | 20:40 ± 0:46 | ||
| Waking Onset (hh:mm) | 6:57 ± 0:28 | ||
| Waking (WASO, mins) | 113.5 ± 49.7 | ||
| Naps (mins) | 120.16 ± 77.4 | ||
| Total 24-Hour Sleep (mins) | 593.6 ± 148.8 |
Mean values +/− SD are given for age and sleep parameters.
SMS within-group sleep differences by age significant only for waking onset (<10y and >16y; p=.0453); and WASO (<10y and 10–15y; p=.0031
Between-group (SMS vs. SIB) differences determined by Fisher’s exact test (gender) or unpaired 2-tailed t-test (age and AES sleep data).
NA= No available SIB control group for age 16y and over.
Abbreviations: M, male; F, female; y, years; mins, minutes; WASO, waking after sleep onset.
Twenty-Four Hour Body Temperature (Tb-24) and Wrist Activity Patterns
Tb was measured every three hours for three days. The 24-hour body temperature (Tb-24) was analyzed using the deviation of raw scores from each subject’s 3-day average. The ‘best-fit’ 24-hour curve for each subject group (SMS vs SIBs) was characterized using a least-squares sine wave fit to the deviation scores, using the form Y= Amplitude*sin((2*pi*X/Wavelength) +Phase-Shift). For wrist activity, average 1 minute activity scores from 15 minute activity bins were used to fit the above equation to the 24-hour activity time series. The period (wavelength) was set constant (24 hours), the amplitude and phase parameters were constrained to be >0.25 and >1.0, respectively. Best-fit parameters and their estimates of variation for the amplitude and phase were derived and were then used to compare group differences in-amplitude and phase. Periodogram analysis was used to calculate the period of the wrist activity time series.
Statistics
An unpaired two-tailed t-test evaluated differences between groups. Fisher’s exact test was used to analyze for difference between groups for categorical variables. An alpha criterion of 0.05 was considered significant. All results were expressed as the mean ± standard deviation (SD). Regression analysis was used to examine the age-related linear decline in total sleep for SMS subjects under age 16 years.
RESULTS
Actigraphy-Estimated Sleep (AES)
Nighttime (7PM – 7AM) estimated sleep in the 2–15y SMS group was about 1 hour less than age-matched SIB cohort (465.4 versus 522.1 minutes, respectively; p=0.0001; Table II). Decreased nighttime sleep in SMS was associated with increased WASO compared with SIBS (108.9 minutes versus 66.5 minutes, respectively (p=0.0001). The clock time of sleep onset was significantly earlier in SMS than SIBs (20:44 +/− SD 0:54 versus 21:58 +/− SD 1:16; p=0.0004). The clock-time of morning wake-up time was also significantly earlier in SMS (6:19 +/− SD 0:44) compared with SIBs (7:47 +/− SD 1:06; p=0.0001). Duration of napping in SMS was greater than SIBs (72.0 versus 34.7 minutes; p=0.0082). Total 24-hour sleep (TS24) was less in SMS than SIBS (537.4 versus 556.8 minutes, respectively; but was not significant, p=0.2323, ns).
Developmental Differences between SMS and Siblings
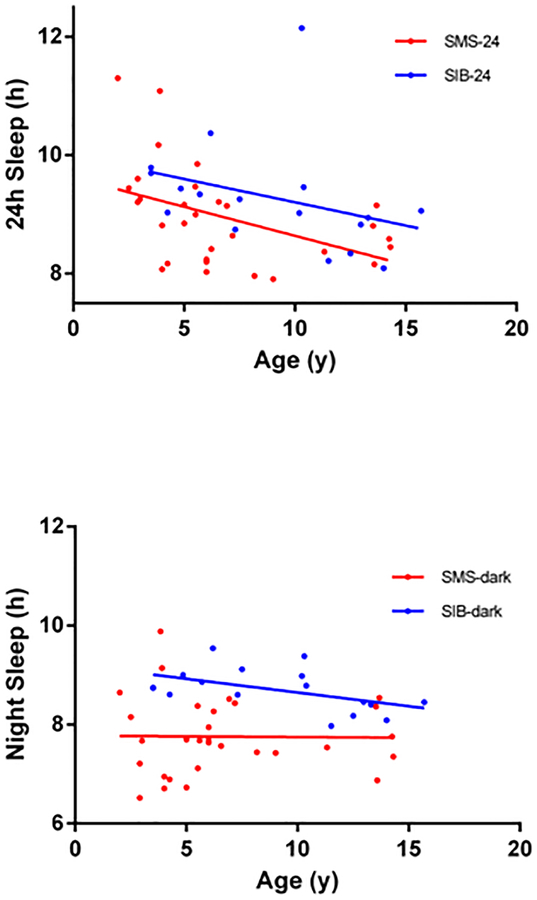
AES measures were examined in SMS and SIB groups under age 16 years (Table II). Regression analysis of age versus night and 24-h sleep totals (Figure 2) showed decreasing levels of 24-h sleep with age in SIBs and SMS (Figure 2 top panel), but in SMS, estimated night sleep did not show an age effect. The different Y-intercepts of these regressions are consistent with lower levels of night or 24-h sleep in SMS versus SIBs (Figure 2). The non-horizontal slopes indicate an age dependent decrease in 24-hour sleep in both SMS and SIB cohorts (Figure 2, top panel). A similar age-related decline in night sleep is present in SIB (F=5.289, DFn, DFd [1, 15], p=.03), but not in SMS night sleep (F=.0046, DFn, DFd [1,28], p=0.94) (Figure 2, bottom panel), in which levels of night sleep remained at a similar low level from 2–15y.
Figure 2:
Panel A (top): The regressions of age on activity-estimated 24h sleep are shown for the SMS group (red line, circles, n=30, under age16y) and for SIB group (blue line, circles, n=17). The y intercepts of the two regression lines are significantly different (p=0.037) but not the slopes (pooled slope=0.0908). Panel B (bottom): The regressions of age in activity-estimated night-sleep are shown for the same groups as above. As above, the y intercepts, of the two lines are significantly different (p<.0001) but not the slopes (pooled slope= 0.022).
Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com_
In the under 10 year SMS and SIB age groups (Table III; AES), the clock times of Sleep Onset were similar (20:45 vs 21:16) (p=0.2251). In contrast, SMS subjects age 10–15 years had significantly earlier Sleep Onset clock times compared to SIBS (20:40 vs 22:36; p<.0009). Clock times for Waking Onset were earlier (p<0.01) for the SMS cohort, regardless of age group (Table III). Among SMS subjects (within group analysis), the clock time of Waking Onset was earlier (p <.02) in younger than older SMS subjects (Table III). WASO was also greater (<.05) in older versus younger SMS subjects.
Twenty-four hour Activity Patterns in SMS and SIBs
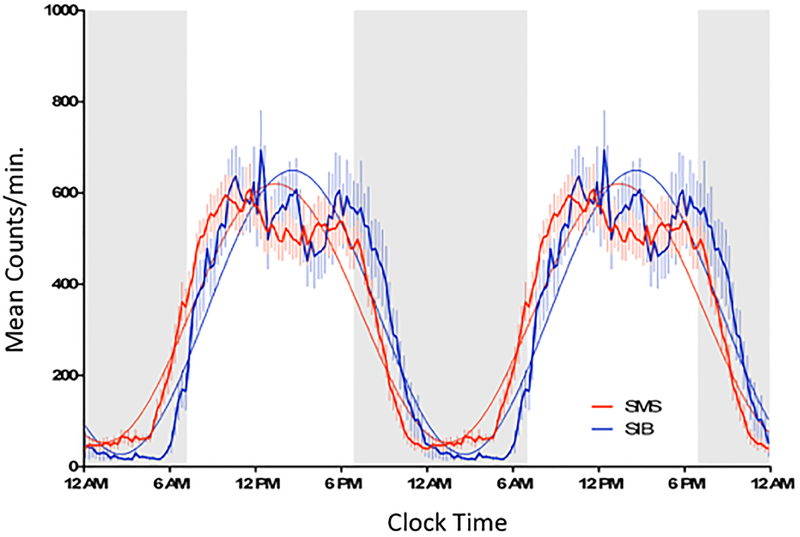
The 24-h clock-time averages (mean +/− SEM) of wrist activity (Figure 3) was shifted earlier for SMS consistent with earlier morning awakening (5AM) and earlier onset of the evening/night (~7–8PM) rest interval compared to SIBs. Curve fits to the 24-hour data indicated that the SMS and SIBs groups differed with respect to both the timing (acrophase= 1:25 pm ± 0:19 versus 2:35 pm±0:27; p<.0001, F=76.06; df 1, 3066) and amplitude (283.4 ±5.9 versus 310.9 ±8.8; (p<.0097, F=6.69; 1,3066; df 1,3066), but not the central (mesor) value. Periodogram estimates of the time series indicated no difference in the period of activity in SMS versus SIBs (24.07±.03 and 24.02±.08 hours respectively [mean ± SEM]; p=.48; t-test).
Figure 3:
The average (15 minute mean ±sem) 24 hour patterns from ten days of wrist activity are shown for SMS (n=22) and SIB (n=10). The 24-hour interval is plotted twice to enable viewing a continuous 48-hour pattern of activity and the transitions between episodes of rest and activity. The shaded area between 7 PM and 7AM represents the time interval when AES and SDA analysis of the activity pattern were conducted. The 24 hour activity pattern of the SMS group is shifted to the left relative to the SIB group.
Twenty-four Hour Body Temperature (Tb-24) Pattern
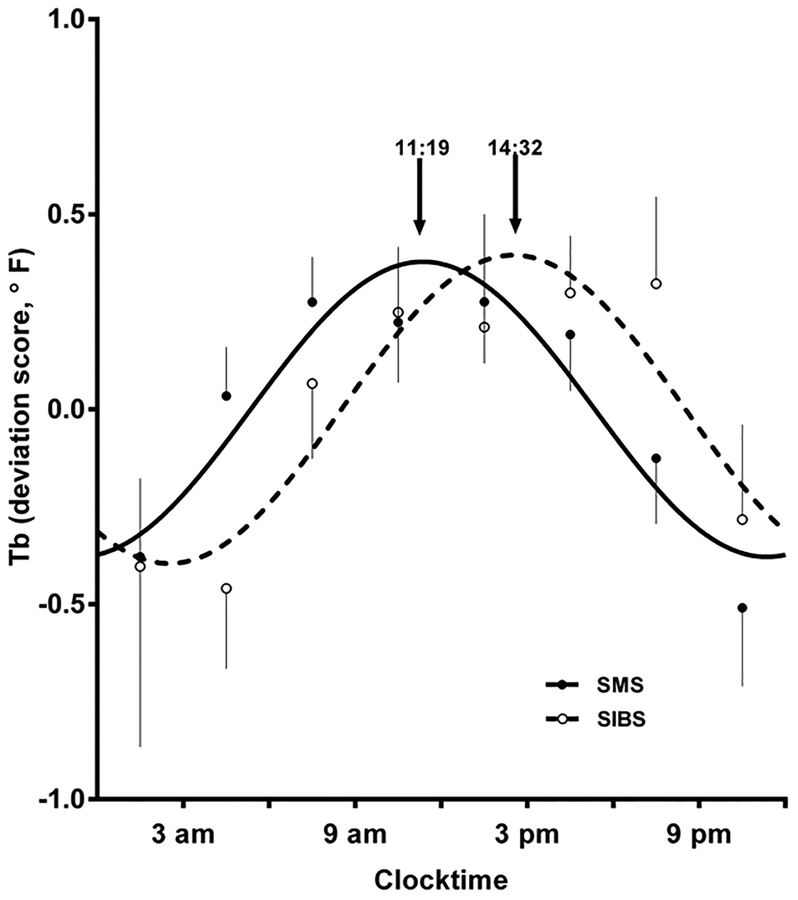
Peak Tb-24 levels were present near mid-day with low levels about midnight. There was some variability within the rising and falling portions of the Tb-24 pattern. In each subject, a circadian rhythm of body temperature was visually identified prior to curve fitting. For both SMS and SIB control subjects, temperature peaks were present during the daytime; troughs were present at night. Average SMS versus SIB Tb deviations (mean +/− SEM) from the Tb-24 mean are shown in Figure 4, along with the best-fit curve to the grouped data. The two curves differ significantly with respect to phase parameter as shown by the earlier (shift to the left) peak of the 24-hour Tb rhythm in SMS (11:22 ± 0:25) relative to SIBs (14:32 ± 0:32; t=4.141, df = 291, p<.0001). For SMS group data, the Tb trough occurred about midnight and Tb values peaked prior to noon. The trough and peak in the SIBS group data were 3–4 hours later than the SMS group (p<.0001; Figure 4). The amplitude of the Tb-24 curve was slightly greater in SIBS but this effect was not significant (Table IV).
Figure 4:
24-hour Body Temperature (Tb-24) in SMS. Tb-24 deviations from the 24-hour mean are shown in a group of SMS individuals (filled circles) compared with a group of siblings (unfilled circles). The two fitted curves (SMS solid line; SIBs dashed line) differ with respect to timing (p<0001). See text. For clarity, the mean is plotted showing either the upper or lower bounds of the 95% confidence interval for SMS (solid curve) and the SIBs (dashed curve).
Table IV.
Best-Fit Parameter Estimates for 24-hour Temperature
| Parameter | SMS (n= 27) | SIBS (n=10) | p value |
|---|---|---|---|
| Amplitude | 0.378 ± 0.041 | 0.395 ± 0.059 | p =0.8274 |
| Phase | 11:22 ± 0.25 | 14:32 ± 0.34 | p < .0001 |
Curve-fits of Tb-24 patterns in young SMS subjects (≤10 years) were also compared with older SMS subjects (>10 years). There was no difference in the timing between young (mean = 11:13 ± 00:25) and older (mean = 11:44 ± 00:59) age groups, although there was a trend (p<.09) for the Tb-24 amplitude to be greater in the young (mean = 0.4347 ± 0.04847) than the older (mean = 0.2854 ± 0.07366) SMS cohort. Three SMS subjects within the Tb-24 study cohort were older than 16 years; their exclusion did not alter the results.
Sleep Dependent Activity (SDA)
In a secondary analysis, the average daily level of activity counts before and after sleep/wake onset for each subject was calculated to examine the temporal pattern of activity levels in the hours of ‘sleep’ that precede and follow the onsets of morning waking and evening sleep respectively. For the analysis of SDA levels (Figure 1 flowchart), convenience samples (above and below 10 years) were used: SMS (n=22, 13F/9M, 9.2y ± 6.0) and SIB (n=10, 5F/5M, age 9.1y ± 4.4y).
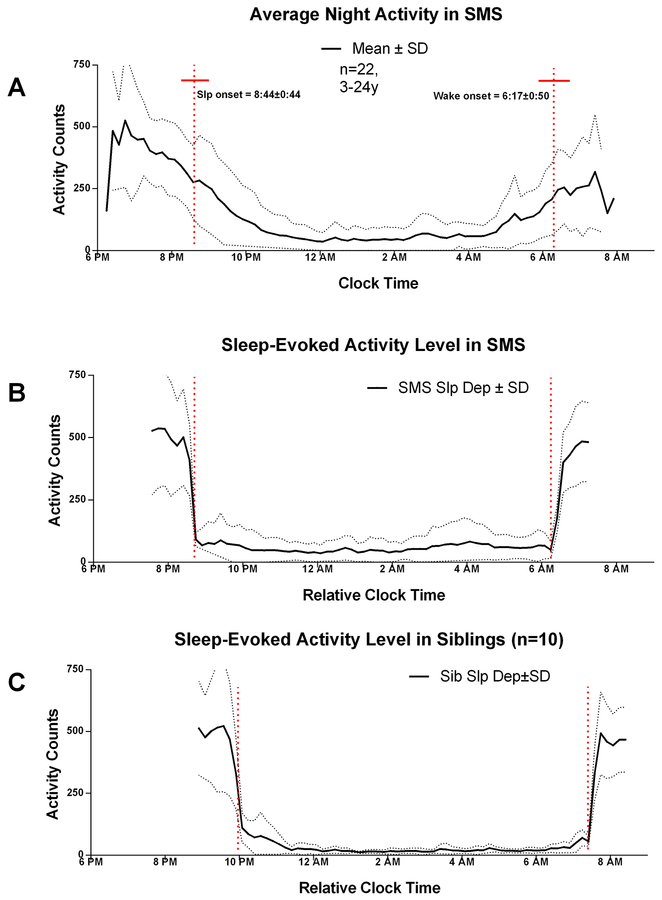
As a comparison with the SDA result, clock-time averages of SMS wrist activity within a narrow window of time that includes the major sleep period (6PM-8AM) show activity levels gradually decreasing from 7PM through the average clock-time of sleep onset (20:44 ± 00:44, hh:mm), until midnight, when minimum levels are observed (Figure 5, panel A). There was a small but noticeable change in the level of activity at average sleep onset. After a 2–4 hour trough during the mid-sleep phase, activity levels gradually rise, through the average clock-time of waking onset (06:17 ± 00:50; hh:mm) until a peak between 7–8AM (Figure 5). There was also a negligible change in level of activity counts at the clock-time of waking onset.
Figure 5:
The relationship between external (clock) and internal (sleep onset) averaged night-time wrist activity in SMS. Panel A: Clock time averaged night activity level in SMS (mean: solid line ± sem: dashed lines; n=22; 2.5–24 y). Panel B: Sleep-onset/offset averaged night activity in SMS (n=22; 2.5–24 y). The red vertical dashed lines indicate the time of sleep onset (8:44 pm ±0:44 and wake onset (6:17 am ± 0:50). The red horizontal bar corresponds to the SEM of sleep onset and offset times. Panel C: Sleep-onset/offset averaged night activity in SIB (n=10; age 3.5–15.7 years). Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com_
Not apparent in the clock-time analysis, SDA indicated a rapid decrease in average wrist activity counts after sleep onset, and a gradual increase in counts prior to waking onset, [Figure 5 panel B], after controlling for day-to-day variation in the clock times of sleep and waking onset.
In the SMS cohort used for SDA analysis, the clock-times of sleep-onset and wake-onset were phase advanced relative to SIBs [Figure 5, panels B versus C]. The calculated clock-times of SMS AES sleep onset (8:44 pm ± 0:09; mean ± sem) and waking onset (6:17 am ± 0:11) are significantly earlier (p=.006 and p=.0032, two-tailed t-test) respectively, than the clock-times of AES estimates in SIBs (9:56 pm± 0:32 and 7:31 am ± 0:16). Between the times of sleep onset and wake-onset, SDA (Figure 5) indicated small increases in wrist activity after sleep onset in both SMS and SIB groups. Between these times (10PM-3AM) there were higher levels of SDA in SMS compared with SIB, and there was a moderate rise in SDA beginning about 4AM in SMS relative to SIB. As discussed next, there were significant age and group related differences of total activity in the three hours after sleep onset and prior to waking onset (Figure 6; Figure 7). In both groups, the level of SDA before sleep onset and after waking onset appeared similar (~500 counts), indicating that the night-time increase in SMS was sleep specific.
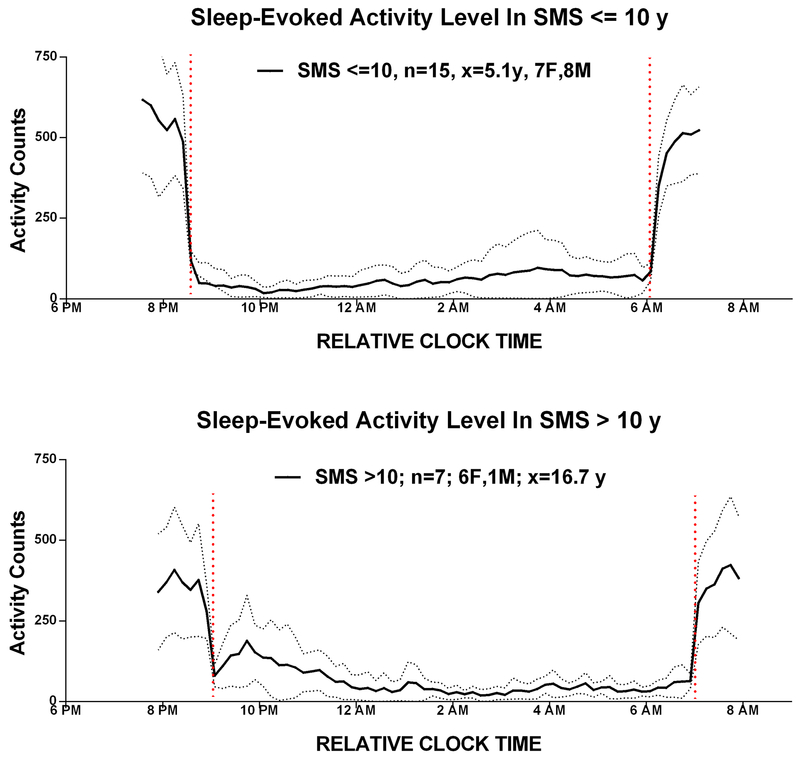
Figure 6:
Age dependent sleep pattern: The relationship between activity patterns in SMS youths above and below 10 years.
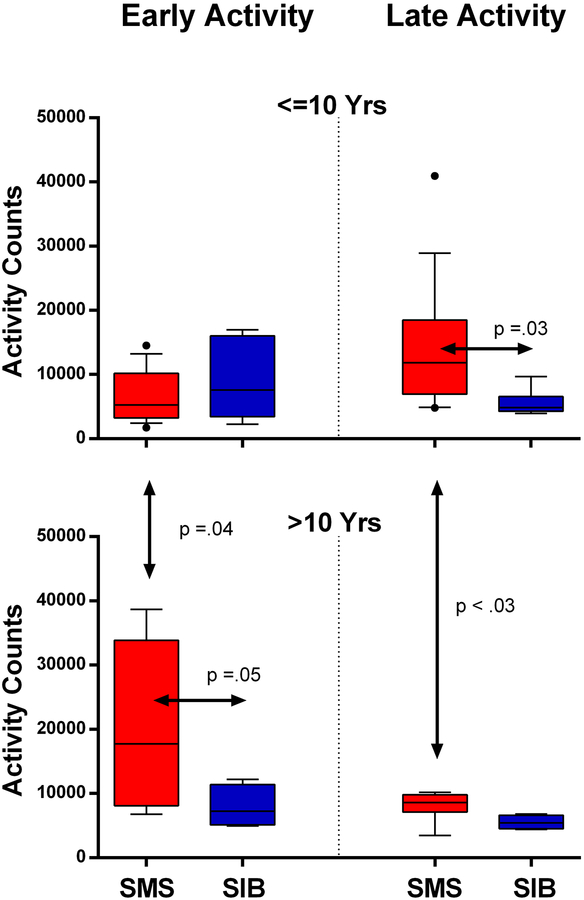
Figure 7:
The relationship between early and late night-time wrist activity in SMS persons and siblings younger or older than ten years illustrated by box-whisker plot (upper and lower quartiles and median). Top Panels: For subjects 10 years and younger, early (left) and late (right) levels of night time wrist activity in patients with SMS (red) or their siblings (blue) are shown. For this age group, children with SMS had significantly more late activity than siblings of SMS children; there was no difference in levels of early activity. Bottom panels: For subjects over 10 years, early activity (left) was significantly higher in older SMS persons compared with the younger SMS group, or with the >10 year sibling group. The older SMS group had significantly less late night activity (right) than the younger SMS group. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com_
SDA in the first 3 hours and last 3 hours of sleep was compared between SMS and SIB. SDA total activity counts during the first three hours (Early Act), and the last three hours (Late Act) of sleep were also compared in young versus older SMS and SIB cohorts after adjusting for unequal variances (Figure 7). Early Act was similar in SIB and SMS groups less than 10 years but increased in in the SMS cohort above 10 years. In contrast, Late Act was higher in the under 10-years SMS versus the under 10 years SIB group (p=0.03), but not different when over 10 years.
Psychotropic medication use
Psychotropic medication use (See online Supplemental Materials, Table 1) differed across the three SMS age groups (one-way ANOVA p=0.005; F=6.06). Use of these medications by SMS subjects under 10y was significantly different from the oldest age group (unpaired t-test p=.002 p<0.01); no significant differences were seen between age <10y and 10–15y (unpaired t-test p=.0.07), or 10–15y vs 16y and over (unpaired t-test p=.26).
DISCUSSION
The current 24-hour actigraphy and body temperature results extends current understanding of circadian underpinnings of SMS. The Tb-24 rhythm was phase advanced, but not inverted (Figure 4; Table IV), thus consistent with the disrupted and phase-advanced sleep pattern. Consistent with earlier reports, estimated sleep was about one hour less than expected for the SMS group (Table II). Compared to SIB controls, the SMS group had less total night sleep, lower sleep efficiency, earlier sleep onset, earlier final awake times, increased waking after sleep onset (WASO), and increased daytime nap duration (Table II). The timing of wake onset (but not sleep onset) and WASO varied with age (Table III), providing evidence of ongoing developmental sleep changes from childhood, adolescence to adulthood.
This investigation of sleep patterns in SMS combines objective activity-based measurement of home sleep with a study of Tb-24. The sleep study cohort represents the largest home-based wrist-activity study of sleep in Smith-Magenis Syndrome (SMS) to date. The Tb-24 study is the first report of circadian variation of Tb-24 in SMS, a patient population with an inverted pattern of melatonin. The investigation documents decreased night sleep and increased day sleep in SMS, consistent with the literature of sleep disturbance in other neurodevelopmental disorders (NDD), including the high prevelance reported in children with ASD (Johnson &Malow 2008; Malow et al. 2006),. Further, the Tb-24 SMS study indicates a phase-advanced rhythm of body temperature. Early sleep and terminal waking onsets are consistent with this phase advance (Table II and III), suggesting that the circadian clock is phase-advanced (Morgenthaler et al. 2007), significantly contributing to the sleep disturbance. The extent to which this phase-advance is unique to SMS when compared with other NDD generally remains to be fully examined.
Specifically related to decreased night sleep, minutes of WASO are increased, and overall sleep efficiency is decreased. Daytime napping is elevated. The clock-times of both sleep and waking onset are earlier in SMS, and late-night activity levels are higher, in comparison to a sibling control group. Tb-24 is phase advanced 3–4 hours relative to siblings, consistent with these advanced onsets. Early waking onset was more pronounced in younger children, and became less pronounced as individuals approached adolescence (Table III).
This investigation provides a biological basis of the critical factors that underlie the sleep disturbance in SMS, from both a developmental and biological perspective.
Actigraphy Estimated Sleep (AES)
Wrist-actigraphy provides an objective estimate of sleep patterns when used over multiple days (Ancoli-Israel et al. 2003). The method has strengths (e.g., non-invasive, long-term and continuous measurement) and limitations (e.g. AES is not a substitute for EEG/PSG, especially in populations with disturbed sleep). For this study, in which multiple days of continuous home measurements were obtained, activity watches were a useful alternative to EEG studies that might otherwise contribute to disrupted sleep in a sensory challenged SMS population (Hildenbrand &Smith 2012). Individuals with SMS were 80% compliant with wrist actigraphy monitoring (similar to the SIB group), when asked to wear a watch for an extended time (Table I). Nevertheless, while wrist activity monitoring was relatively well-suited to SMS, the results do not represent the sleep disorder present in the non-compliant (20%) part of the SMS population who did not wear a watch and were not part of the actigraphy database. The sleep in this non-compliant cohort may be quite different, and possibly more disrupted than in the compliant cohort examined here.
Wrist activity provides an estimate of sleep when compared with the gold standard EEG based methods. Therefore, throughout this report (Table II & III) the term ‘sleep’ specifically refers to “actigraphy estimated sleep” (AES). Several studies indicate that actigraphy based devices often overestimate sleep (Pollak et al. 2001), especially in populations with poor sleep (Gropman et al. 2006; Johnson et al. 2007; Kushida et al. 2001), but this result varies with developmental age and sleep disorder status (Meltzer et al. 2012). The AES SMS findings may overestimate ‘true’ sleep, and underestimate ‘true’ waking minutes. Formal comparison with EEG has not been conducted. Nevertheless, these results represent the most comprehensive and objective summary of sleep in SMS in a home environment to date.
In the watch-compliant group, 79% of the interval between estimated sleep onset and waking onset was spent asleep compared with 89% in the sibling control group. This decrease in sleep efficiency (Table II) translated to about one hour less (465.4 versus 522.1 minutes) sleep per night in the SMS versus the sibling groups. The decline in estimated sleep was related to a near doubling of (170%) the amount of waking after sleep onset (WASO) in SMS versus their siblings (108.9 versus 66.5 minutes) as opposed to a contraction of the sleep episode defined by sleep onset and terminal waking onset. Thus, shifts in the time of sleep and waking onset account for a 5-minute decrement in potential sleep time by the SMS group (Table II).
Total nap time was greater in the SMS group than in siblings (72 versus 34.7 minutes respectively; Table II). Increased napping and sleep debt is often a consequence of deficient night sleep. Sleep debt, and elevated daytime melatonin alters daytime EEG sleep (Dijk et al. 1995), but its role on increasing daytime sleep totals is less clear in SMS. Together however, absence of nocturnal melatonin and increased night waking, may contribute to a sleep debt driven cycle of elevated day-sleep that contributes to a further increase in night waking and greater daytime napping.
Clock-times of sleep and waking onsets (20:44 and 06:19, respectively) were earlier in SMS relative to SIBS (21:58 and 07:47) (Table II), consistent with phase-advanced timing of the Tb-24 rhythm in SMS.
Twenty-Four Hour Body Temperature Rhythm (Tb-24)
In contrast to the inverted 24h pattern of melatonin, often used as a surrogate marker central circadian clock timing (Lewy &Sack 1989; Lewy et al. 1986; Shanahan &Czeisler 1991), the Tb-24 rhythm was not inverted, suggesting the central circadian clock is not inverted. It is likely that the melatonin pattern is inverted by disordered timekeeping downstream from the central clock (Klein 1991; Klein et al. 1983). This is consistent with the non-inverted timing of the circadian rhythms of cortisol and growth hormone in SMS (De Leersnyder et al. 2006; De Leersnyder et al. 2001). It is possible that the inverted pattern of melatonin is more associated with inverted secondary oscillators. The fact that individuals with SMS fall asleep early, and wake-up early, is consistent with a sleep phase advance (Wehr et al. 1979; Weitzman 1982; Wirz-Justice 2007). The possibility that the abnormal rhythm of melatonin per se, or another intrinsic disorder (phase-advance) of the circadian clock, might contribute to dysregulated sleep patterns has been suggested (De Leersnyder et al. 2006; De Leersnyder et al. 2001).
The Tb-24 rhythm in SMS was phase advanced by about 3 hours relative to the sibling control group (Figure 4; Table IV). No evidence of free-running temperature rhythms was observed, either in the Tb-24 rhythms, or in the extended wrist activity patterns themselves. The phase-advanced pattern of Tb-24 appears to be relatively consistent; Tb-24 patterns were consistently characterized by elevated day and low night values without the ‘inverted’ pattern as described for melatonin. One should ask whether the Tb-24 rhythm accurately represents the timing of the clock, or might ‘masking’ effects of behavior/sleep underlie the apparent phase-advance? While the circadian rhythm of Tb-24 is strongly affected (masked) by the sleep-wake pattern (Minors &Waterhouse 1992), and it is likely that sleep had ‘masking’ effects on body temperature in this study, it is unlikely that the 3-hour phase difference in Tb in SMS vs. SIBs can be fully explained by difference in sleep timing. The clock-time differences in both sleep and wake onsets were about 1.25h, i.e. much smaller than the 3-hour Tb phase change. This would suggest a difference in central clock timing in SMS vs. siblings, as opposed to a ‘masking’ effect of sleep on body temperature.
If the central clock is phase advanced in SMS, the mechanism of this advance remains to be clarified. The phase-advanced circadian rhythm in SMS is consistent with the advanced sleep and wake-up times in SMS, low sleep efficiency, and increased early morning activity, and may have a behavioral, endocrine, or molecular basis (Novakova et al. 2012; Williams et al. 2012), with negative impact of health and well-being.
The three-hour shift in central circadian timekeeping corresponds in magnitude to the jet-lag produced by traveling in the US from Pacific Coast to the Eastern time zones. However, unlike the transient effects of jet-lag, SMS internal phase-shift is a persisting condition linked to dysfunctional internal biological timing. The resulting sleep debt accumulates daily, with little respite, and has clear costs and health implications for the affected individual. Chronic disruptions of circadian function leading to loss of synchrony between circadian clock and environment correlate with a variety of clinical pathologies, including obesity, hypertension and cardiovascular disease, diabetes, ulcers, and psychologic disorders (Di Milia et al. 2013; Edelman et al. 2007; Gibson et al. 2009; Grandner et al. 2013; Hillman &Lack 2013; Shochat et al. 2014)
Sleep Dependent Activity (SDA)
In the 3 hours immediately preceding waking onset, activity levels were higher in SMS subjects relative to siblings (Figure 5). In contrast, in the 3 hours immediately following sleep onset, activity counts were similar in SMS and SIBS. The fact that during waking, (i.e., just before sleep onset or after waking onset) activity levels were similar in SMS and SIBS, suggests that SMS vs SIB activity differences were: a) sleep-dependent, and b) phase-dependent, showing largest effects occurring in the hours prior to final awakening (during the ‘sleep’ period), and during the second half of the night. This is consistent with advanced timing of the circadian clock. The increased second half activity might also be related to sleep stage (REM) specific arousals since this sleep state predominates during the second half of the night. In either case, there is a developmental effect since activity levels vary with age and time of night.
Developmental Effects
The fact that the timing of Waking Onset and the duration of Waking After Sleep Onset (WASO) vary with age in SMS (Table III; Figures 6 and 7), provides evidence of ongoing developmental changes in timekeeping mechanisms, from childhood thru adolescence to adulthood. Such changes have been documented in healthy individuals (Crowley et al. 2014). The present study is the first to objectively document such changes in the SMS population. Future study will be required to determine whether increasing use of psychoactive drug use by older age groups might contribute to the age-related effects (see Study Limitations and Special Considerations).
Age differences were associated with different sleep patterns between children and adolescents within the SMS cohort, and also distinguished the sleep between SMS and the sibling cohort. For example, for those younger than 10 years, late night activity was greater in SMS than their siblings, and lessened in those persons over 10 years (Figure 6). Interestingly, the decrease in late night activity in persons above 10 years was accompanied by an increase in early night activity (“settling to sleep”). This might be associated with the poor settling pattern and delayed sleep pattern present in many non-SMS adolescents (Carskadon 1993; Moore 2012). Whether this shift in early versus late night SDA is related to a change in the timing of the 24h Tb rhythm is unclear since there was no difference in the timing of young versus older subjects, although there was a trend in circadian amplitude. While the amount of night-time and 24h sleep decreased (Figure 2) in the SMS population between 5–16 years, the effect of increased medication use in older SMS subjects cannot be eliminated. This decline in SMS sleep during the first two decades is similar to a decline in non-SMS populations, although total night sleep is consistently lower in the SMS population, across all age groups.
Putative Mechanisms
A phase-advance of the Tb-24 rhythm (Figure 4) is consistent with late night increased activity, and with the advanced sleep and waking onsets. Together, these findings are consistent with advance timing of the central circadian clock. The underlying mechanism(s) contributing to an advance may be complex, involving molecular, endocrine (melatonin) and or behavioral factors. Relative to melatonin, it is important to note that while endogenous melatonin pattern is inverted in virtually all studied (>90%) cases (Boudreau et al. 2009; Chik et al. 2010), the disrupted sleep that occurs in SMS is not solely attributed to the abnormal diurnal melatonin secretion (Boudreau et al. 2009; Potocki et al. 2000). Moreover, while the molecular cause of the circadian defect in SMS is unknown, recent evidence suggests clock-associated molecules might be involved (Williams et al. 2012).
Disordered central circadian clock timing is consistent with dysregulated clock associated molecules previously described in many sleep disorders (Landolt 2008; Wirz-Justice 2012), some consistent with phase advance of the clock (Novakova et al. 2012; Williams et al. 2012). Molecular level irregularities have also been identified within SMS (Williams et al. 2012). A second possibility is that poor sleep hygiene, augmented by both the inverted melatonin pattern and DD, reduces night sleep, causing increased sleep debt and napping, which leads to reduced night sleep, increased late night activity and the earlier morning wake-up time. Third, elevated daytime melatonin could directly signal the clock to phase-shift (Pevet 2014) via melatonin receptors within the central clock (SCN)(Pevet 2016; Wu et al. 2013), and the effect of daytime melatonin to alter central timekeeping (Lewy et al. 1992; Lewy et al. 2002). Along these lines, daytime melatonin administration has been shown to synchronize non-twenty-four hour sleep-wake cycles in blind individuals (Sack et al. 2000). While phase shifting by melatonin has not been demonstrated in SMS, the fact that beta-blockers block daytime melatonin release and improve sleep quality (De Leersnyder et al. 2003), supports this possibility. Whether beta-blockers phase-advanced the Tb-24 curve is unknown.
Potential Treatment Interventions
Sleep disturbances and phase-advanced circadian rhythm in body temperature may be related to a complex biology that stems from the interstitial deletion or mutation of RAI1 within chromosome 17p11.2. The current findings suggest that the phase-advanced Tb-24 rhythm contributes to the sleep disturbance. In addition to the advanced sleep pattern, the inversed melatonin pattern, the disrupted sleep associated with intellectual disability features of autism spectrum (Elrod &Hood 2015), and developmental disability, the individual differences associated with deletion size and/or RAI1 mutation (Edelman et al. 2007; Girirajan et al. 2005), as well the more general developmental effects on sleep all play a role in shaping the complex sleep pattern of SMS, making a single treatment intervention for all patients quite challenging.
Interventions that normalize the phase-advance might improve SMS sleep disturbance specifically by altering the timing of the central clock. The treatments require prior assessment of circadian phase to determine properly timed delivery of a phase-resetting agent (either bright light [BL] or melatonin) that will phase-delay of the circadian clock (Lewy et al. 1992; Minors et al. 1991; St Hilaire et al. 2012). In a healthy individual, bright light delays the timing of the clock when presented during the late afternoon thus causing later wake-up and sleep onset times (St Hilaire et al. 2012). A similar effect of light was observed in a SMS subject (See Case 1, online Supplemental Material). Alternatively, the use of carefully timed morning melatonin dose has also been shown to delay the timing of the circadian clock and sleep-wake cycle (Lewy et al. 1992). Timing of these interventions is very important and specific to each intervention. Anecdotal case studies suggest benefit from these interventions (See Case 2 and Case 3, online Supplemental Material), but formal studies are needed.
Limitations and Special Considerations
Due to the unique study population, numerous challenges were encountered in the conduct of these experiments. The SMS population exhibits major behavioral issues, sensory challenges, as well as ongoing pharmacological interventions (Dykens &Smith 1998; Hildenbrand &Smith 2012; Laje et al. 2010a; Smith et al. 1998a). These unique challenges required adjustments to both experimental design and methodologies. For example, many of the subjects received maintenance behavioral medications, and the possibility cannot be excluded that such interventions might have affected developmental results, since fewer younger than older children were treated with psychotropic medications (See the online Supplemental Table 1). Melatonin treatment did not differ by SMS age group; however, the use of 2 or more psychoactive medications (polypharmacy) varied across the three SMS age groups. It cannot clearly (or definitively) be determined from this study whether age-related sleep changes are associated with incidence of medication use, or indeed reflect previously described difference in age-related sleep. Noncompliance by some SMS individuals represents a potential bias in estimated sleep by excluding data acquisition from individuals with possibly greater sensory issues and therefore unable to tolerate wearing the watch. In addition, the measurement of Tb-24 patterns did not utilize a constant routine in which levels of behavior, posture and food consumption was kept constant thru 24 hours in order to prevent their ‘evoked’ effects on the pattern of Tb-24; such a methodology is not practical with this population. Finally, as stated earlier, actigraphy is not a substitute for PSG EEG measures which may uncover specific PSG sleep related challenges in SMS (Boudreau et al. 2009; Potocki et al. 2000). However, as designed, the methods provide an objective measurement of some of the factors that impact the sleep of this challenging population, and further provide the basis for treatment interventions that might be employed to more effectively improve the health and lives of individuals and families living with SMS.
In summary, this study represents the largest home-based wrist-activity study of sleep in SMS. It significantly adds to the current understanding of the circadian underpinnings of the syndrome. These circadian related effects are likely to underlie the sleep disturbance and contribute to behavioral challenges. This is the first description of a phase-advance Tb-24 rhythm sleep pattern, consistent with the disrupted phase advance sleep pattern well recognized in the syndrome. Actigraphy-based estimated sleep was one hour less than expected across all ages, with evidence of ongoing developmental sleep changes from early childhood through adolescence (age 10–19years). Compared to SIB controls, the SMS group exhibited less total night sleep, lower sleep efficiency, earlier sleep onset, earlier final awake times, increased night awakenings (WASO), and increased daytime nap duration. Knowledge about the circadian and developmental factors that contribute to the disrupted and variable sleep patterns in SMS has the potential to lead to more effective treatments.
Supplementary Material
Supplemental Figure 8: Effects of afternoon light on 24-hr Tb pattern for Case 1 is shown. Peak Tb at baseline (pre-therapy Feb 27–29; top panel) was at 10:48 AM. Use of afternoon light (administered ~18:00 for 45 minutes) over the next two months was associated with a delay in timing of peak Tb to 13:42 (April 23–25; bottom panel). Comparison of actigraphy data using 10-day samples preceding light treatment and during light use indicated later sleep onsets (20:31 FEB; 21:50 APR); decreased waking minutes (68min. FEB; versus 50min. APR); and decreased motor activity evidenced by a 23.5% decline in activity counts. The left and right vertical dotted lines correspond to peak Tb at baseline and treatment intervals, respectively.
ACKNOWLEDGEMENTS
This study was supported by the Intramural Research Programs of the National Human Genome Research Institute (HG000215–15) and National Institute of Mental Health NIH, USDHHS. A National Institutes of Health Clinical Center Bench-to-Bedside grant awarded to principal investigator (ACMS) and coauthor (WD) provided partial funding support for a research assistant (RM). The authors also express their gratitude to the individuals and their families who participated in this study.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPLEMENTAL MATERIALS
This article contains supplementary material, which may be viewed at the American Journal of Medical Genetics website at http://www.interscience.wiley.com.
REFERENCES
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. 2003. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26(3):342–392. [DOI] [PubMed] [Google Scholar]
- Bauer M. 1991. Independent assessment of manic and depressive symptoms by self-rating. Scale characteristics and implications for the study of mania. Arch Gen Psychiatry 48(9):807–812. [DOI] [PubMed] [Google Scholar]
- Boudreau EA, Johnson KP, Jackman AR, Blancato J, Huizing M, Bendavid C, Jones M, Chandrasekharappa SC, Lewy AJ, Smith AC, Magenis RE. 2009. Review of disrupted sleep patterns in Smith-Magenis syndrome and normal melatonin secretion in a patient with an atypical interstitial 17p11.2 deletion. Am J Med Genet A 149A(7):1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Arebo C. 1993. Association between puberty and delayed phase preference. Sleep 16:258–262. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Forest K, Murry E, Carroll BJ. 1998. A factor analysis of the signs and symptoms of mania. Archives of general psychiatry 55(1):27–32. [DOI] [PubMed] [Google Scholar]
- Chik CL, Rollag MD, Duncan WC, Smith AC. 2010. Diagnostic utility of daytime salivary melatonin levels in Smith-Magenis syndrome. Am J Med Genet A 152A(1):96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Van Reen E, LeBourgeois MK, Acebo C, Tarokh L, Seifer R, Barker DH, Carskadon MA. 2014. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PloS one 9(11):e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leersnyder H, Bresson JL, de Blois MC, Souberbielle JC, Mogenet A, Delhotal-Landes B, Salefranque F, Munnich A. 2003. Beta 1-adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith-Magenis syndrome. J Med Genet 40(1):74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leersnyder H, Claustrat B, Munnich A, Verloes A. 2006. Circadian rhythm disorder in a rare disease: Smith-Magenis syndrome. Mol Cell Endocrinol 252(1–2):88–91. [DOI] [PubMed] [Google Scholar]
- De Leersnyder H, De Blois MC, Claustrat B, Romana S, Albrecht U, Von Kleist-Retzow JC, Delobel B, Viot G, Lyonnet S, Vekemans M, Munnich A. 2001. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J Pediatr 139(1):111–116. [DOI] [PubMed] [Google Scholar]
- Di Milia L, Vandelanotte C, Duncan MJ. 2013. The association between short sleep and obesity after controlling for demographic, lifestyle, work and health related factors. Sleep Med 14(4):319–323. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Roth C, Landolt HP, Werth E, Aeppli M, Achermann P, Borbely AA. 1995. Melatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neuroscience letters 201(1):13–16. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Smith AC. 1998. Distinctiveness and correlates of maladaptive behaviour in children and adolescents with Smith-Magenis syndrome. J Intellect Disabil Res 42 (Pt 6):481–489. [DOI] [PubMed] [Google Scholar]
- Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith AC, Elsea SH. 2007. Gender, genotype, and phenotype differences in Smith-Magenis syndrome: a meta-analysis of 105 cases. Clin Genet 71(6):540–550. [DOI] [PubMed] [Google Scholar]
- Elrod MG, Hood BS. 2015. Sleep Differences Among Children With Autism Spectrum Disorders and Typically Developing Peers: A Meta-analysis. Journal of developmental and behavioral pediatrics : JDBP 36(3):166–177. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Williams WP 3rd, Kriegsfeld LJ. 2009. Aging in the circadian system: considerations for health, disease prevention and longevity. Exp Gerontol 44(1–2):51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girirajan S, Elsas LJ 2nd, Devriendt K, Elsea SH. 2005. RAI1 variations in Smith-Magenis syndrome patients without 17p11.2 deletions. J Med Genet 42(11):820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girirajan S, Vlangos CN, Szomju BB, Edelman E, Trevors CD, Dupuis L, Nezarati M, Bunyan DJ, Elsea SH. 2006. Genotype-phenotype correlation in Smith-Magenis syndrome: evidence that multiple genes in 17p11.2 contribute to the clinical spectrum. Genet Med 8(7):417–427. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. 2013. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nature and science of sleep 5:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg F, Guzzetta V, Montes de Oca-Luna R, Magenis RE, Smith AC, Richter SF, Kondo I, Dobyns WB, Patel PI, Lupski JR. 1991. Molecular analysis of the Smith-Magenis syndrome: a possible contiguous-gene syndrome associated with del(17)(p11.2). Am J Hum Genet 49(6):1207–1218. [PMC free article] [PubMed] [Google Scholar]
- Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, Murphy MA, Williamson D, Brown F, Dutton R, McCluggage C, Friedman E, Sulek M, Lupski JR. 1996. Multi-disciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2). Am J Med Genet 62(3):247–254. [DOI] [PubMed] [Google Scholar]
- Gropman AL, Duncan WC, Smith AC. 2006. Neurologic and developmental features of the Smith-Magenis syndrome (del 17p11.2). Pediatr Neurol 34(5):337–350. [DOI] [PubMed] [Google Scholar]
- Hildenbrand HL, Smith AC. 2012. Analysis of the sensory profile in children with Smith-Magenis syndrome. Phys Occup Ther Pediatr 32(1):48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman DR, Lack LC. 2013. Public health implications of sleep loss: the community burden. The Medical journal of Australia 199(8):S7–10. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Malow BA. 2008. Sleep in children with autism spectrum disorders. Curr Neurol Neurosci Rep 8(2):155–161. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Kirchner HL, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, Emancipator JL, Kibler AM, Redline S. 2007. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. Sleep 30(7):899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC. 1991. Suprachiasmatic nucleus: the mind’s clock. New York: Oxford. [Google Scholar]
- Klein DC, Smoot R, Weller JL, Higa S, Markey SP, Creed GJ, Jacobowitz DM. 1983. Lesions of the paraventricular area of the hypothalamus disrupt the suprachiasmatic-spinal cord circuit in melatonin rhythm generating system. Brain Res Bull 10:647–652. [DOI] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. 2001. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Medicine 2:389–396. [DOI] [PubMed] [Google Scholar]
- Laje G, Bernert R, Morse R, Pao M, Smith AC. 2010a. Pharmacological treatment of disruptive behavior in Smith-Magenis syndrome. Am J Med Genet C Semin Med Genet 154C(4):463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje G, Morse R, Richter W, Ball J, Pao M, Smith AC. 2010b. Autism spectrum features in Smith-Magenis syndrome. Am J Med Genet C Semin Med Genet 154C(4):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP. 2008. Genotype-dependent differences in sleep, vigilance, and response to stimulants. Current Pharmaceutical Design 14(32):3396–3407. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Ahmed S, Jackson JM, Sack RL. 1992. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int 9(5):380–392. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. 2002. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int 19(3):649–658. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RA, Singer CL. 1984. Assessment and treatment of chronobiologic disorders using plasma melatonin levels and bright light exposure: the clock-gate model and the phase response curve. Psychopharmacol Bull 20(3):561–565. [PubMed] [Google Scholar]
- Lewy AJ, Sack RL. 1989. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int 6(1):93–102. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Miller LS, Hoban TM, Singer CM, Samples JR, Krauss GL. 1986. The use of plasma melatonin levels and light in the assessment and treatment of chronobiologic sleep and mood disorders. J Neural Transm Suppl 21(311):311–322. [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. 2006. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep 29(12):1563–1571. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Walsh CM, Traylor J, Westin AM. 2012. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep 35(1):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM. 1992. Investigating the endogenous component of human circadian rhythms: a review of some simple alternatives to constant routines. Chronobiol Int 9(1):55–78. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. 1991. A human phase-response curve to light. Neuroscience letters 133(1):36–40. [DOI] [PubMed] [Google Scholar]
- Moore M. 2012. Behavioral sleep problems in children and adolescents. Journal of clinical psychology in medical settings 19(1):77–83. [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, Brown T, Chesson AL Jr., Kapur V, Maganti R, Owens J, Pancer J, Swick TJ, Zak R. 2007. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 30(11):1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova M, Nevsimalova S, Prihodova I, Sladek M, Sumova A. 2012. Alteration of the circadian clock in children with Smith-Magenis syndrome. J Clin Endocrinol Metab 97(2):E312–318. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Kehr E, Smith MT, Andrews PJ, Orff H, Giles DE 2001. Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleepers. J Sleep Res 10:93–104. [DOI] [PubMed] [Google Scholar]
- Pevet P. 2014. The internal time-giver role of melatonin. A key for our health. Rev Neurol (Paris) 170(11):646–652. [DOI] [PubMed] [Google Scholar]
- Pevet P. 2016. Melatonin receptors as therapeutic targets in the suprachiasmatic nucleus. Expert Opin Ther Targets 20(10):1209–1218. [DOI] [PubMed] [Google Scholar]
- Piazza CC, Fisher WW, Sherer M. 1997. Treatment of multiple sleep problems in children with developmental disabilities: faded bedtime with response cost versus bedtime scheduling. Dev Med Child Neurol 39(6):414–418. [DOI] [PubMed] [Google Scholar]
- Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. 2001. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep 24(8):957–965. [DOI] [PubMed] [Google Scholar]
- Potocki L, Glaze D, Tan DX, Park SS, Kashork CD, Shaffer LG, Reiter RJ, Lupski JR. 2000. Circadian rhythm abnormalities of melatonin in Smith-Magenis syndrome. J Med Genet 37(6):428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Brandes RW, Kendall AR, Lewy AJ. 2000. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med 343:1070–1077. [DOI] [PubMed] [Google Scholar]
- Shanahan TL, Czeisler CA. 1991. Light exposure induces equivalent phase-shifts of the endogenous circadian rhythms of circulating plasma melatonin and core body temperature in men. J Clin Endocrin Metab 73:227–235. [DOI] [PubMed] [Google Scholar]
- Shochat T, Cohen-Zion M, Tzischinsky O. 2014. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep medicine reviews 18(1):75–87. [DOI] [PubMed] [Google Scholar]
- Smith AC, Dykens E, Greenberg F. 1998a. Behavioral phenotype of Smith-Magenis syndrome (del 17p11.2). Am J Med Genet 81(2):179–185. [DOI] [PubMed] [Google Scholar]
- Smith AC, Dykens E, Greenberg F. 1998b. Sleep disturbance in Smith-Magenis syndrome (del 17 p11.2). Am J Med Genet 81(2):186–191. [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. 2012. Human phase response curve to a 1 h pulse of bright white light. The Journal of physiology 590(Pt 13):3035–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH. 1995. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry 3(1):18–35. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Duncan WC Jr., Sher L, Aeschbach D, Schwartz PJ, Turner EH, Postolache TT, Rosenthal NE. 2001. A circadian signal of change of season in patients with seasonal affective disorder. Arch Gen Psychiatry 58(12):1108–1114. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A, Goodwin FK, Duncan W, Gillin JC. 1979. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science 206(4419):710–713. [DOI] [PubMed] [Google Scholar]
- Weitzman ED. 1982. Chronobiology of man. Sleep, temperature and neuroendocrine rhythms. Hum Neurobiol 1:173–183. [PubMed] [Google Scholar]
- Williams SR, Zies D, Mullegama SV, Grotewiel MS, Elsea SH. 2012. Smith-Magenis syndrome results in disruption of CLOCK gene transcription and reveals an integral role for RAI1 in the maintenance of circadian rhythmicity. American journal of human genetics 90(6):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A. 2007. Chronobiology and psychiatry. Sleep medicine reviews 11(6):423–427. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. 2012. Temporal organization as a therapeutic target. Dialogues in clinical neuroscience 14(4):335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Ursinus J, Zhou JN, Scheer FA, Ai-Min B, Jockers R, van Heerikhuize J, Swaab DF. 2013. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord 148(2–3):357–367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 8: Effects of afternoon light on 24-hr Tb pattern for Case 1 is shown. Peak Tb at baseline (pre-therapy Feb 27–29; top panel) was at 10:48 AM. Use of afternoon light (administered ~18:00 for 45 minutes) over the next two months was associated with a delay in timing of peak Tb to 13:42 (April 23–25; bottom panel). Comparison of actigraphy data using 10-day samples preceding light treatment and during light use indicated later sleep onsets (20:31 FEB; 21:50 APR); decreased waking minutes (68min. FEB; versus 50min. APR); and decreased motor activity evidenced by a 23.5% decline in activity counts. The left and right vertical dotted lines correspond to peak Tb at baseline and treatment intervals, respectively.