Abstract
Previous experiments demonstrated that survival and proliferation of chronic lymphocytic leukemia (CLL) cells depends upon complex cross-talk between CLL cells and accessory cells in the tissue microenvironment. To further dissect these interactions in situ, we analyzed lymph nodes from 43 different patients infiltrated by CLL cells for expression of the chemokine CCL3, Ki-67, macrophages, and T cell subsets by immunohistochemistry. CCL3 expression was detected in 24 of 43 cases (56%), particularly in prolymphocytes and paraimmunoblasts within the proliferation centers. Significantly higher numbers of CD3+ T cells and CD57+ cells were noticed in CCL3 positive cases. Furthermore, denser infiltration of CLL lymph node tissues by CD57+ cells correlated with higher proliferation rates of the CLL cells. In conclusion, we demonstrate an association of CCL3 expression by CLL cells with increased numbers of CD3+ T cells and CD57+ cells in the lymph node microenvironment, which may promote CLL cell survival and proliferation.
Keywords: CD57, CCL3, CLL, lymph node microenvironment, T cells
Introduction
B cell chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) is the most common leukemia in adults in Europe and the United States [1]. It is incurable with current chemotherapeutic approaches; however, considerable differences in the clinical course have long been noticed, with some patients displaying an indolent clinical course, while others rapidly succumb to the disease despite aggressive therapy [2]. CLL is characterized by the presence of a monoclonal population of lymphocytes with a phenotype of mature, antigenexperienced B lymphocytes recirculating between the peripheral blood and the bone marrow as well as the secondary lymphoid organs, where they proliferate in response to antigenic stimulation and interaction with the microenvironment [1,2].
In the past, it has become increasingly clear that B cell receptor (BCR) stimulation, stimulation via cytokines and interaction with accessory cells in the tumor microenvironment, such as bone marrow stromal cells, monocyte derived so-called nurse-like cells (NLC) and T cells, provide important proliferative and survival signals to the CLL cells, and may be involved in drug resistance [3-8].
Several recent studies have highlighted BCR signaling as crucial activating stimulus for the CLL cells, which can be altered pharmacologically, e.g. by inhibitors of Bruton’s tyrosine kinase (BTK), which is an essential component of B-cell-receptor signaling, like ibrutinib [8-12]. Based on gene expression microarray experiments, we have previously found the chemokine CCL3 among the most strongly upregulated genes in CLL cells after co-culture with NLC as well as after BCR stimulation [13], indicating that this chemokine is involved in the crosstalk of the CLL cells with the microenvironment. CCL3, also known as macrophage inflammatory protein 1 alpha (MIP-1α), has been shown to be a potent chemoattractant for monocytes as well as T lymphocyte subsets and NK cells [14-16]. In a previous study, we could demonstrate strong differences regarding the CCL3 plasma levels between individual CLL patients, and its utility as a prognostic marker [17].
A characteristic histomorphological feature of CLL lymph node infiltrates is the formation of so-called pseudofollicles or proliferation centers (PCs), which are pale appearing areas of variable size which contain numerous prolymphocytes and paraimmunoblasts [1], and it has been suggested that the PCs may be the predominant site for clonal expansion of the CLL cells [18]. Recently, a gene expression profiling-based study demonstrated the crucial role of the CLL lymph node microenvironmental interactions in vivo, with upregulation of gene signatures associated with B cell receptor signaling, NFκB activation and increased proliferation in the CLL cells obtained from lymph nodes compared to peripheral blood [11]. In line with our aforementioned in vitro findings, the chemokine CCL3 was among the most strongly upregulated genes in the CLL cells from the lymph node compartment in this study.
To further dissect the interactions between the CLL tumor cells and the microenvironment in situ, we investigated CCL3 protein expression by immunohistochemistry in 43 sections from lymph nodes infiltrated by CLL. Moreover, in order to explore the presence and spatial distribution of the microenvironmental bystander cells in CLL lymph nodes, in particular in relation to CCL3 expression, we have performed a histomorphological analysis combined with immunohistochemical stainings for different T lymphocyte subsets and macrophages as well as for Ki-67, as a marker of the proliferation fraction.
Material and methods
CLL cases
Forty-three samples of lymph nodes infiltrated by CLL/SLL were selected from the files of the Institute of Pathology, University of Würzburg. The cases were reviewed regarding morphology and immunophenotype by an experienced hematopathologist (A.R.), and all included cases matched the diagnostic criteria of the World Health Organization classification of hematopoietic and lymphoid tumors [1]. A subset of this cohort was characterized for CCL3 expression in a previous study [17]. The patients included 24 men and 19 women, with a median age of 63 years at diagnosis (range 41–91 years) [Table I], without available additional clinical data. Ethics approval for this study was granted by the Ethics Committee of the Medical Faculty of the University of Würzburg.
Table I.
Basic clinical features of the 43 investigated chronic lymphocytic leukemia /small lymphocytic lymphoma cases.
| Sex | |
| Male | 24 (56%) |
| Female | 19 (44%) |
| Age at diagnosis (years) | |
| Mean | 63.4 |
| Median | 64.0 |
| Range | 41–91 |
Immunohistochemical staining methods
The CCL3 immunohistochemical stainings were performed on deparaffinized, formalin-fixed tissue sections using a CCL3 polyclonal goat IgG antibody (clone BAF270; R&D Systems, 1:50) and the Histostain plus kit (Zymed/Invitrogen), according to the manufacturer’s protocol. After antigen retrieval by pressure cooking with DAKO target retrieval solution, blocking was performed using rabbit serum (Zymed/Invitrogen) for 10 min, followed by incubation with the CCL3 antibody for 1 h. Subsequently, the slides were incubated with the biotinylated secondary antibody (polyclonal rabbit anti goat, 1:400; Dako Cytomation) for 20 min, followed by incubation with an enzyme conjugate with streptavidin and horseradish peroxidase. Visualization was performed with the diaminobenzidine system. After each incubation step, the slides were washed three times in phosphate-buffered saline.
All other stainings were performed on deparaffinized, formalin-fixed tissue sections following standard procedures by automated processing using the Tecan Evo Freedom stainer and the Dako Advance system according to the manufacturer’s protocol. Visualization was performed with the diaminobenzidine system.
Detailed information about the primary antibodies used is given in Table II. The CCL3 stains were evaluated by three independent investigators; all other stainings were scored by two independent investigators that were blinded regarding the CCL3 status.
Table II.
Immunohistochemical antibody panel.
| Antibody | Clone | Company/developer | Dilution |
|---|---|---|---|
| CCL3 | BAF270 | R&D Systems | 1:50 |
| CD20 | L26 | DAKO | 1:500 |
| CD3 | PS1 | Novocastra/Leica | 1:80 |
| CD4 | 1F6 | Novocastra/Leica | 1:40 |
| CD8 | C8144B | DAKO | 1:80 |
| CD57 | NK1 | BD Bioscience | 1:800 |
| Ki67 | MIB1 | DAKO | 1:800 |
| FOXP3 | 236A/E7 | Abcam | 1:50 |
| CD68 | Ki-M1p | Developed in Kiel | 1:80000 |
| PD1 | Developed by Roncador et al., described in detail in [28] | 1:200 |
Immunohistochemical and histomorphological analysis
We developed a three-tiered semiquantitative scoring system for CD3, CD4, CD8, CD57 and FOXP3, subclassifying cases with few, intermediate and dense infiltration of the lymph nodes by CD3, CD4, CD8, CD57 and FOXP3 positive cells, respectively.
In addition, we analyzed the size of the proliferation centers (PCs), which were classified as “not prominent”, “prominent” and “prominent and expanded” (according to [19]). The Ki-67 level inside and outside the proliferation centers was evaluated by counting 2 × 100 cells each.
Furthermore, the distribution patterns of all of the above-mentioned cell subsets, particularly in respect to the proliferation centers, were examined histomorphologically.
Images were captured with an Olympus BX43 microscope and a ColorView digital camera, and processed with the cellSens imaging software (all Olympus).
Immunofluorescence staining methods and analysis
Immunofluorescence double stainings for CD57 with CD4 and CD8 were performed according to standard protocols [20]. The images were evaluated using confocal laser scanning microscopy with the Leica confocal software (Leica TCS SP2). Analysis and cell counting was performed in 400× magnification plus 2× Zoom.
Statistical analysis
Statistical analysis was performed using the χ2 test, t-test and ANOVA; p values less than 0.05 were considered statistically significant. Statistical tests were performed using IBM SPSS Statistics 22.
Results
CCL3 protein expression in CLL lymph node infiltrates
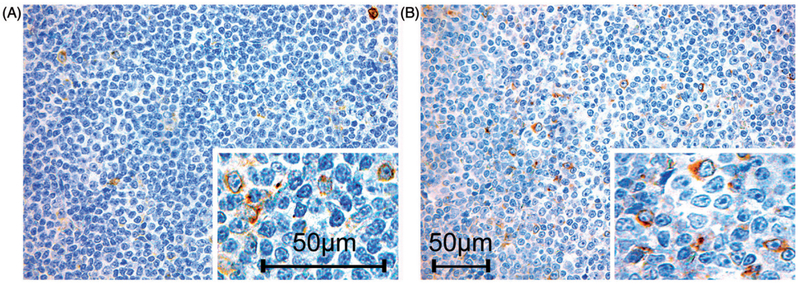
To determine CCL3 protein expression in sifu, three independent pathologists investigated CCL3 expression by immunohistochemistry (IHC) in 43 CLL lymph node infiltrates. Overall, we detected CCL3 expression in about half of the investigated cases; specifically, we found 24 CCL3 positive (56%) and 19 CCL3 negative (44%) cases. We observed a weak to moderate, predominantly membranous CCL3 staining with slight intercellular positivity giving the morphologic impression of secretion to the environment [Fig. 1]. However, in the positive cases not all of the CLL tumor cells showed a noticeable staining for CLL3, but especially prolymphocytes and paraimmunoblasts particularly in the proliferation centers were positive. In the negative cases, we found very sparse and weakly positive cells corresponding to macrophages, which served as internal control.
Figure 1.
Variable CCL3 protein expression in different chronic lymphocytic leukemia (CLL) lymph node infiltrates. Pictures show two cases with immunohistochemical evidence of CCL3 protein expression. The insets demonstrate the membranous staining, and illustrate the impression of secretion of CCL3 to the environment. Pictures were originally photographed at 400× magnification.
Differences in T cell numbers in association with CCL3 expression
In order to explore the presence and spatial distribution of different T lymphocyte subsets in CLL lymph nodes, particularly in relation to CCL3 expression, we performed IHC stainings for CD3, CD4, CD8 as well as FOXP3 and CD57 in this series of 43 CLL lymph node tissue samples.
Increased numbers of bystander T lymphocytes in the CCL3 positive cases
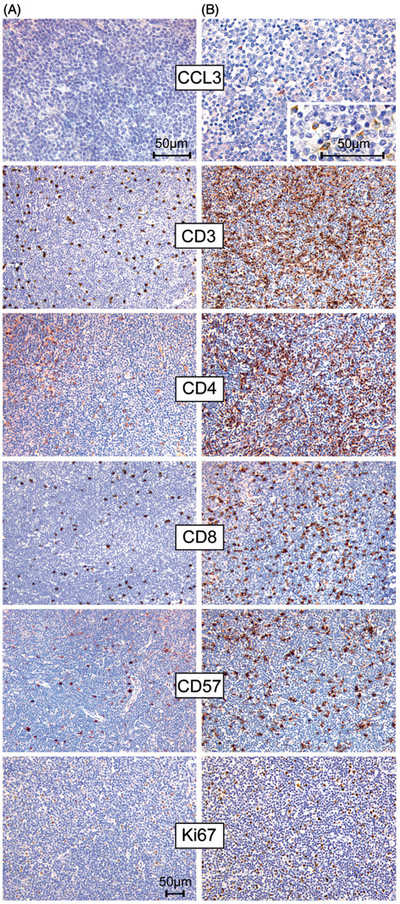
Analysis of CD3 staining displayed a variable reactive T cell component within the CLL tumor infiltrate, with 19% (8/43) of the cases containing very few T lymphocytes, 44% (19/43) with an intermediate T cell infiltrate, and 37% (16/43) showing dense infiltration by CD3 positive T cells. Interestingly, we found significantly more CD3 positive T lymphocytes in CCL3 positive cases compared to the CCL3 negative cases (p = 0.014) [Fig. 2, Supplementary Fig. 2 [available online], Table III].
Figure 2.
Association of CCL3 protein expression with T cell density in chronic lymphocytic leukemia (CLL) infiltrated lymph nodes. Staining results from a case without evidence of CCL3 expression (A) and a CCL3 positive case (B). The CCL3 positive case (B) shows a much more prominent T cell infiltrate (stainings for CD3, CD4 and CD8) than the CCL3 negative case (A). Moreover, in (B) a higher number of CD57 positive cells and a higher proliferation fraction, measured by Ki-67, are observed. Pictures of all immunostains were originally photographed at 200× magnification, except for CCL3 stainings, which were originally photographed at 400× magnification.
Table III.
Distribution of CD3+ (a) and CD57+ (b) cell density groups in CCL3 positive and negative cases as well as mean Ki-67 in the CD57+ cell density groups (b).
| CD3+ cells |
||||||
|---|---|---|---|---|---|---|
| few | intermediate | dense | total | |||
| (a) | ||||||
| CCL3 | negative | 7 | 8 | 4 | 19 | |
| positive | 1 | 11 | 12 | 24 | ||
| total | 8 | 19 | 16 | 43 | p = 0.014 | |
| (b) | ||||||
| CD3+ cells |
||||||
| few | intermediate | dense | total | |||
| CCL3 | negative | 9 | 6 | 4 | 19 | |
| positive | 6 | 2 | 16 | 24 | ||
| total | 15 | 8 | 20 | 43 | p = 0.009 | |
| Mean Ki-67 | 9.4 | 11.8 | 14 | p = 0.019 | ||
In the majority of the cases (77%, 33/43) the CD3 positive T cells were diffusely scattered throughout the lymph node. However, in eight cases (18%) we found more CD3 positive T cells within the PCs than in the surrounding infiltrate (equally distributed between CCL3 positive and negative cases) and in two cases (5%) a tendency towards more T cells outside the PCs was observed.
Distribution of CD4 and CD8 positive T cells
In the vast majority of the cases the distribution of CD4 and CD8 positive T cells followed approximately the density and the pattern of the CD3 staining results; however, we found no clear association between the number of CD4+ T cells or CD8+ T cells and CCL3 status (data not shown). In most cases we observed slightly more CD4 than CD8 positive cells according to a physiological CD4/CD8 ratio.
Histomorphological analysis in respect to CCL3 expression
Histomorphologically, we often noted a more prominent clustering of CCL3 positive cells within pseudofollicles/PCs, but this was not statistically significant when correlating CCL3 distribution pattern with its localization inside or outside of PCs. We noted a trend towards higher CLL cell proliferation in CCL3 positive cases, measured by Ki-67 staining of CLL cells, which, however, did not reach statistical significance (p = 0.065).
Analysis of CD57 positive cells in CLL lymph node infiltrates
Increased density of CD57 positive cells was associated with CCL3 positivity and an increased proliferation of the CLL cells. In the analysis of CD57 immunohistochemical stainings, CD57 positive cells were mainly scattered diffusely throughout the tissue (86% of the cases). However, we found significantly more CD57 positive cells in CCL3 positive cases when compared to CCL3 negative cases (p = 0.009) [Fig. 2]. In addition, we observed a significantly higher proliferation fraction measured by Ki-67 immunohistochemistry in cases with a dense tumor infiltration by CD57 positive cells (p = 0.019) [Fig. 2, Supplementary Fig. 2, Table III].
Increased number of CD57 and CCL3 positive cells in a CLL case with prolymphocytic progression and increased proliferation
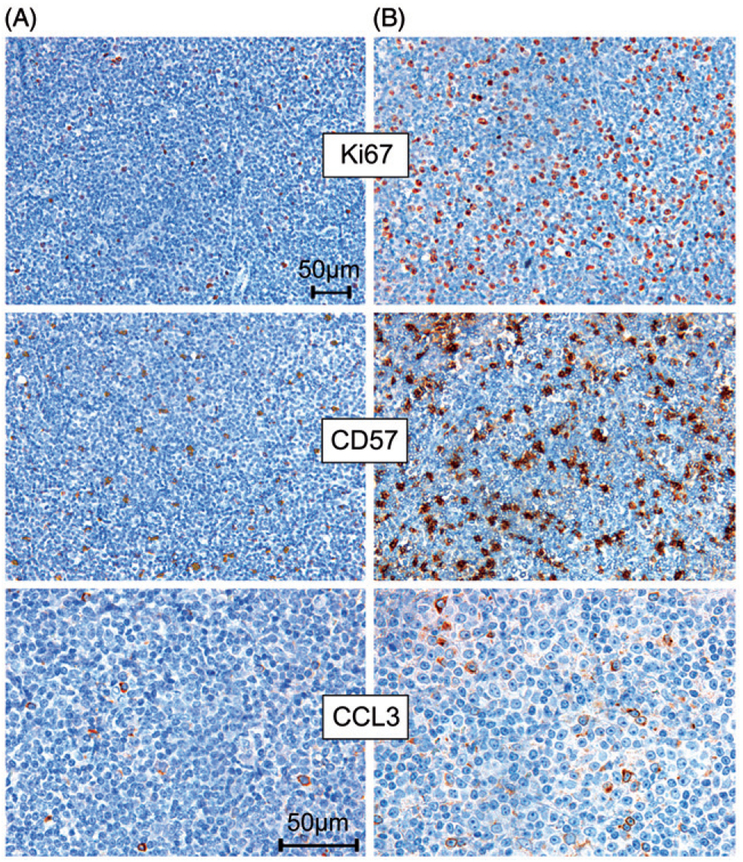
Our series included one case with two sequential excisional lymph node biopsies. In the chronologically later biopsy specimen with prolymphocytic progression and increased proliferation (Ki-67 focally up to 40%), we found a particularly high number of CCL3 positive cells and a dense infiltration with CD57 positive cells. In contrast, fewer and more focal CCL3 positive cells and fewer CD57 positive cells as well as a lower proliferation fraction (Ki-67 up to 15%) were observed in a lymph node biopsy from the same patient taken 2.5 months earlier [Fig. 3].
Figure 3.
Increased CCL3 protein expression and a higher number of CD57 positive cells in a case with prolymphocytic progression and increased proliferation (measured by Ki-67 immunohistochemistry) (B), compared with an earlier lymph node excisional biopsy from the same patient with lower proliferation (A). Pictures of Ki-67 and CD57 immunostains were originally photographed at 200× magnification and pictures of CCL3 immunohistochemical staining were originally photographed at 400× magnification.
Analysis of the CD57 positive cell population by immunofluorescence double stainings
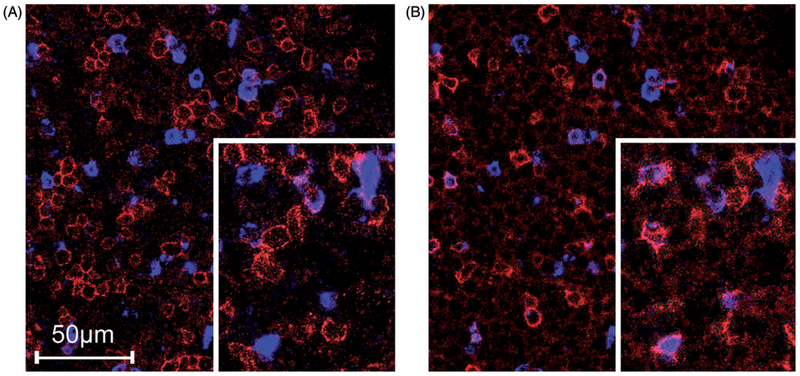
In order to explore the proportion of CD4 and CD8 positive cells in the CD57 positive population, immunofluorescence double stainings of CD57 with CD4 and CD8 were performed in a subset of 11 cases. In these cases, we observed a mixture of CD57/CD4 positive and CD57/CD8 positive cells, and also a population which showed only positivity for CD57, presumably representing NK cells. In eight of the 11 cases, a slight but not statistically significant predominance of CD8/CD57 positive cells over the CD4/CD57 positive cells (mean 10.4 CD8/CD57 positive cells versus mean 7.6 CD4/CD57 positive cells per view) was noticed (p = 0.13). In the majority of the cases we observed lower numbers of CD57 only positive cells (mean 5.9 per view) compared to the aforementioned CD8/CD57 and CD4/CD57 positive cells [Fig. 4].
Figure 4.
Immunofluorescence double stainings in a chronic lymphocytic leukemia (CLL) lymph node infiltrate. (A) shows CD4 expression (labeled red) and CD57 expression (labeled blue), whereas (B) shows CD8 expression (labeled red) and again CD57 expression (labeled blue).
Analysis of further T cell subset markers and macrophages in CLL
To further characterize infiltrating macrophages and T cells we analyzed the content of FOXP3, PD1 and CD68 (KiM-1p) positive cells. FOXP3 is used as a surrogate marker for regulatory T cells (Tregs) [21], which are important regulators of T-cell activation. In analogy to CD57 and CD3, we also used a three-tiered scoring system for quantification of FOXP3 positive cells, and analyzed the infiltration pattern. We observed a diffuse infiltration pattern of the FOXP3 positive cells in the majority of the cases (84%) and differences in the density of FOXP3 positive cells. However, we found no significant association between the density of intratumoral FOXP3 positive cells and CCL3 or the proliferation fraction (measured by Ki-67 IHC) (data not shown).
PD1 is a transmembrane protein belonging to the CD28-B7 signaling family of receptors which can be expressed by different immune cells (T and B cells as well as monocytes) upon activation [22]. Particularly, PD1 expression on different subsets of T cells is intensively described in the literature, where on the one hand, it is described as marker of T cell exhaustion in chronic viral infection [23,24], and on the other hand as a marker of follicular T helper cells [25-27]. Moreover, Xerri and co-workers described PD1 expression also on tumor cells in CLL/SLL using a self-generated PD1-antibody [27]. Using an antibody developed and described by Roncador and colleagues [28] in our series, PD1 expression was predominantly found in T cells; however, we also noticed a weak PD1 staining in few interspersed CLL cells in the majority of the cases. In five cases we found a particular prominent PD1 positivity in CLL cells accentuated in the proliferation centers [Supplementary Fig. 1, available online]. Staining was predominantly membranous and cytoplasmic with slight variations in intensity; however, in most cases the staining intensity was weaker than in residual germinal centers. The expression of PD1 showed no clear correlation with the CCL3 status, the proliferation fraction or other investigated immunohistochemical markers.
In order to investigate the intratumoral macrophages, we also performed IHC stainings for CD68 (Ki-M1p); however, we could not detect a significant difference in number or spatial distribution of macrophages in our series (data not shown).
Discussion
In the last decade, in vitro, but also in vivo data demonstrated that the interaction of CLL cells with the microenvironment in the bone marrow and lymph nodes, e.g. bone marrow stromal cells, so-called nurselike cells (NLC) and T cells, plays an important role in the pathogenesis of this disease.
Based on microarray experiments, we previously found that the chemokine CCL3 is involved in crosstalk between CLL cells and their microenvironment, being induced in CLL cells after co-culture with NLC and upon B cell receptor (BCR) signaling [13]. In subsequent studies, we verified the expression and secretion of CCL3 protein by CLL cells, demonstrated that high CCL3 plasma levels in CLL patients are associated with poor prognosis [17], and that elevated CCL3 plasma levels in CLL patients rapidly normalized during therapy with kinase inhibitors that target BCR signaling, such as the BTK inhibitor ibrutinib [29] and the PI3Kδ inhibitor idelalisib [30]. Recently, by comparative gene expression analysis, Herishanu and co-workers identified the lymph nodes as the main site of CLL cell activation and CLL cell proliferation in vivo [11]. Interestingly, these authors also reported that CCL3 was among the most strongly upregulated genes in the CLL cells from the lymph node compartment, corroborating our earlier findings.
In this present study, we demonstrate variable expression of CCL3 protein in CLL lymph node infiltrates by immunohistochemistry, with 56% CCL3 positive cases. Despite well-known general problems with interobserver variability when analyzing immunohistochemical stains [31], we believe that CCL3 expression was robustly scored in our series, since three independent pathologists arrived at highly concordant results. Particularly prolymphocytes and paraimmunoblasts in the proliferation centers showed CCL3 staining. The CCL3 staining pattern was found to be predominantly membranous with the morphologic impression of secretion to the environment.
Furthermore, a significant association between CCL3 expression and an increased density of CD3 positive T lymphocytes in the CLL infiltrated lymph nodes was observed. Moreover, there was a trend towards higher average CLL cell proliferation measured by Ki-67 in the CCL3 positive cases. Our data suggest an interaction between these cell types in vivo, which in turn might provide survival and proliferation signals to the CLL cells. This is in line with recently published data that showed that specific T cells are important mediators of CLL cell proliferation [6,32]. In more detail, Bagnara et al. demonstrated that autologous T cells were key mediators of leukemic cell proliferation in a xenograft CLL transfer mouse model [32], and that administration of anti CD3 mAbs or anti CD4 mAbs aborted or at least diminished growth of the CLL cells, respectively. In addition, Os et al. reported activation of CLL cells in response to specific CD4+ T helper cells [6].
However, although we observed significant differences in the number of infiltrating CD3 positive T lymphocytes between the CCL3 positive and negative cases, we did not find substantial differences regarding the density and pattern of CD4 or CD8 positive cells by histomorphological analysis in our series.
It is known that the T cell compartment in the peripheral blood of CLL patients is altered, with an increase of CD8 positive T cells which display a diminished repertoire with oligoclonal expansions and upre gulation of CD57 [8,33,34]. The CD57 antigen can be principally expressed on CD4 and CD8 T cells as well as on NK cells. On T cells, expression of CD57 is characteristic for their chronic activation in conditions of persistent immune stimulation [35]. CD8+CD57+ T cells are supposed to be antigen-specific, terminally differentiated, memory/effector T cells, however, divergent reports exist regarding their function, with some studies describing them as cytotoxic whereas others ascribe them immunosuppressive activity [35]. Furthermore, also CD4+CD57+ T lymphocytes have been described, and these cells constitute a T cell subset in the germinal centers of lymphoid tissues were they may provide important helper activity to the B cells [25,36].
Several reports have indicated that in particular CD8+CD57+, but also CD4+CD57+ T cells may be an important source of IFN-γ production [37-39]. IFN-γ levels are increased in CLL patients, and IFN-γ prevents apoptosis in CLL cells in vitro [40]. Podhorecka and colleagues reported a correlation of CD3+/CD8+ IFN-γ producing T lymphocytes with unfavorable clinical features of CLL by flow cytometry analyses in peripheral blood [41], and very recently, Bürgler et al. have shown upregulation of CD38 in CLL cells in response to specific T helper 1-derived IFN-γ, which were attracted by chemokines such as CCL3 [42].
Interestingly, we found in our series a higher density of CD57 positive cells in the CCL3 positive cases. In addition, a high number of CD57 positive cells was significantly associated with a higher proliferation rate of the CLL cells. Moreover, we observed a shift towards a more prominent CCL3 but also CD57 positive cell infiltrate in one case with prolymphocytic progression and increased proliferation in comparison to a previous lymph node excisional biopsy from the same patients a few months earlier. These findings suggest that a high number of CD57 positive cells in the lymph node compartment may be associated with an unfavorable, more aggressive clinical and biological behavior in CLL, potentially through secretion of IFN-γ.
Several studies have described an increase of CD57 positive cells in association with malignant tumors [35]; however, in solid tumors the data is divergent in view of the prognostic role of the CD57 positive tumor infiltrate (e.g. [43-45]). In follicular lymphomas, Alvaro and colleagues showed that a high number of CD57 positive cells is associated with a more aggressive clinical behavior [46], and Focosi and Petrini formulated the idea of CD57 expression in the lymphoma microenvironment as a prognostic marker related to immune dysfunction [47].
In order to further elucidate the CD57 positive cell subpopulations, we performed immunofluorescence double stainings and found that the CD57 positive cells consist of a mixture of CD4 and CD8 positive T cells and NK cells, with a slight predominance of CD8/CD57 positive cells over the CD4/CD57 positive cells, and fewer NK cells. However, further immunological studies are needed to gain more insights in the specific features of the CD57 positive T cell subtypes and their capacities in the lymph node microenvironment in CLL.
In conclusion, our data shows an association of CCL3 expression by CLL cells with increased numbers of CD3 positive T cells and CD57 positive cells in the lymph node microenvironment in vivo, where they might provide important proliferation and survival signals to the CLL cells via cognate T-B cell interactions. Moreover, our data indicate that high numbers of CD57 positive cells in CLL lymph node infiltrates are associated with unfavorable clinical and biological features of the disease. However, the precise identity and features of the CLL-associated T lymphocyte subsets, and, particularly, the role of the CD57-positive accessory cell population in the CLL microenvironment remain to be further defined.
Supplementary Material
Acknowledgements
We thank Theodora Nedeva and Sabine Roth for excellent technical assistance. This study was supported by a CLL Global Research Foundation grant (Title: Cellular characterization of the microenvironment in chronic lymphocytic leukemia (CLL): Who are the key players and how do they look?).
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- [1].Swerdlow SH, Campo E, Harris NL, et al. The WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC;2008. [Google Scholar]
- [2].Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005;352: 804–815. [DOI] [PubMed] [Google Scholar]
- [3].Burger JA, Tsukada N, Burger M, et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 2000;96: 2655–2663. [PubMed] [Google Scholar]
- [4].Ghia P, Strola G, Granziero L, et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L + T cells by producing CCL22. Eur J Immunol 2002;32: 1403–1413. [DOI] [PubMed] [Google Scholar]
- [5].Lagneaux L, Delforge A, Bron D, et al. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood 1998;91: 2387–2396. [PubMed] [Google Scholar]
- [6].Os A, Burgler S, Ribes AP, et al. Chronic lymphocytic leukemia cells are activated and proliferate in response to specific T helper cells. Cell Rep 2013;4: 566–577. [DOI] [PubMed] [Google Scholar]
- [7].Patten PE, Buggins AG, Richards J, et al. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood 2008; 111: 5173–5181. [DOI] [PubMed] [Google Scholar]
- [8].Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol 2014;24: 71–81. [DOI] [PubMed] [Google Scholar]
- [9].Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Herishanu Y, Katz BZ, Lipsky A, et al. Biology of chronic lymphocytic leukemia in different microenvironments: clinical and therapeutic implications. Hematol Oncol Clin North Am 2013;27: 173–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011; 117: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol 2014;15: 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Burger JA, Quiroga MP, Hartmann E, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood 2009; 113: 3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schall TJ, Bacon K, Camp RD, et al. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med 1993; 177: 1821–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Taub DD, Lloyd AR, Wang JM, et al. The effects of human recombinant MIP-1 alpha, MIP-1 beta, and RANTES on the chemotaxis and adhesion of T cell subsets. Adv Exp Med Biol 1993; 351: 139–146. [DOI] [PubMed] [Google Scholar]
- [16].Tregoning JS, Pribul PK, Pennycook AM, et al. The chemokine MIP1alpha/CCL3 determines pathology in primary RSV infection by regulating the balance of T cell populations in the murine lung. PLoS One 2010;5: e9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sivina M, Hartmann E, Kipps TJ, et al. CCL3 (MIP-1alpha) plasma levels and the risk for disease progression in chronic lymphocytic leukemia. Blood 2011;117: 1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Caligaris-Cappio F, Ghia P. Novel insights in chronic lymphocytic leukemia: are we getting closer to under-standing the pathogenesis of the disease? J Clin Oncol 2008; 26: 4497–4503. [DOI] [PubMed] [Google Scholar]
- [19].Gine E, Martinez A, Villamor N, et al. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica 2010;95: 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brighenti A, Andrulis M, Geissinger E, et al. Extrafollicular proliferation of B cells in the absence of follicular hyperplasia: a distinct reaction pattern in lymph nodes correlated with primary or recall type responses. Histopathology 2005;47: 90–100. [DOI] [PubMed] [Google Scholar]
- [21].Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299: 1057–1061. [PubMed] [Google Scholar]
- [22].Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996;8: 765–772. [DOI] [PubMed] [Google Scholar]
- [23].Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443: 350–354. [DOI] [PubMed] [Google Scholar]
- [24].Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12: 492–499. [DOI] [PubMed] [Google Scholar]
- [25].Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol 2015; 16: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dorfman DM, Brown JA, Shahsafaei A, et al. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol 2006;30: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xerri L, Chetaille B, Serriari N, et al. Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol 2008;39: 1050–1058. [DOI] [PubMed] [Google Scholar]
- [28].Roncador G, Garcia Verdes-Montenegro JF, Tedoldi S, et al. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica 2007;92: 1059–1066. [DOI] [PubMed] [Google Scholar]
- [29].Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012;119: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 2011; 118: 3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].de Jong D, Rosenwald A, Chhanabhai M, et al. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications – a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol 2007;25: 805–812. [DOI] [PubMed] [Google Scholar]
- [32].Bagnara D, Kaufman MS, Calissano C, et al. A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood 2011; 117: 5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Goolsby CL, Kuchnio M, Finn WG, et al. Expansions of clonal and oligoclonal T cells in B-cell chronic lymphocytic leukemia are primarily restricted to the CD3(+)CD8(+) T-cell population. Cytometry 2000;42: 188–195. [PubMed] [Google Scholar]
- [34].Serrano D, Monteiro J, Allen SL, et al. Clonal expansion wthin the CD4+CD57+ and CD8+CD57+ T cell subsets in chronic lymphocytic leukemia. J Immunol 1997;158: 1482–1489. [PubMed] [Google Scholar]
- [35].Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology 2011; 134: 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim JR, Lim HW, Kang SG, et al. Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination. BMC Immunol 2005; 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bandres E, Merino J, Vazquez B, et al. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high)CD28(−)CD57(+) subpopulation. Clin Immunol 2000; 96: 230–235. [DOI] [PubMed] [Google Scholar]
- [38].Sun Z, Zhong W, Lu X, et al. Association of Graves’ disease and prevalence of circulating IFN-gamma-producing CD28(−) T cells. J Clin Immunol 2008; 28: 464–472. [DOI] [PubMed] [Google Scholar]
- [39].Eylar EH, Lefranc CE, Yamamura Y, et al. HIV infection and aging: enhanced interferon- and tumor necrosis factoralpha production by the CD8+ CD28- T subset. BMC Immunol 2001;2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Buschle M, Campana D, Carding SR, et al. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. J Exp Med 1993;177: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Podhorecka M, Dmoszynska A, Rolinski J. Intracellular IFN-gamma expression by CD3+/CD8+ cell subset in B-CLL patients correlates with stage of the disease. Eur J Haematol 2004; 73: 29–35. [DOI] [PubMed] [Google Scholar]
- [42].Bürgler S, Gimeno A, Parente-Ribes A, et al. Chronic lymphocytic leukemia cells express CD38 in response to Th1 cell-derived IFN-gamma by a T-bet-dependent mechanism. J Immunol 2015;194: 827–835. [DOI] [PubMed] [Google Scholar]
- [43].Chaput N, Svrcek M, Auperin A, et al. Tumour-infiltrating CD68+ and CD57+ cells predict patient outcome in stage II-III colorectal cancer. Br J Cancer 2013;109: 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Iida M, Takayama E, Naganawa K, et al. Increase of peripheral blood CD57+ T-cells in patients with oral squamous cell carcinoma. Anticancer Res 2014; 34: 5729–5734. [PubMed] [Google Scholar]
- [45].Akagi J, Baba H. Prognostic value of CD57(+) T lymphocytes in the peripheral blood of patients with advanced gastric cancer. Int J Clin Oncol 2008;13: 528–535. [DOI] [PubMed] [Google Scholar]
- [46].Alvaro T, Lejeune M, Salvado MT, et al. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol 2006; 24: 5350–5357. [DOI] [PubMed] [Google Scholar]
- [47].Focosi D, Petrini M. CD57 expression on lymphoma microenvironment as a new prognostic marker related to immune dysfunction. J Clin Oncol 2007;25: 1289–12891; author reply 91–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.