Abstract
The VenaSeal (Medtronic, Minneapolis, Minn) cyanoacrylate closure system is a nonthermal technique for ablating saphenous veins using a proprietary n-butyl cyanoacrylate. One possible side effect is an allergic reaction to cyanoacrylate. We report the case of a 49-year-old woman treated with cyanoacrylate closure who developed a persistent type IV hypersensitivity reaction. The patient elected to have the vein excised, and the histologic features were consistent with a type IV hypersensitivity reaction.
Keywords: VenaSeal, Hypersensitivity, Endovenous ablation, Varicose vein, Allergic reaction
VenaSeal (Medtronic, Minneapolis, Minn) cyanoacrylate closure (CAC) system is a novel technique for ablating saphenous veins using a proprietary n-butyl cyanoacrylate (CA) that polymerizes quickly on contact with blood.1 The purpose of this report was to detail the clinical course of a patient who developed a type IV hypersensitivity to CA after endovenous treatment of the great saphenous vein (GSV) for venous insufficiency. The patient provided written consent for use of images and written descriptions of the case for academic purposes.
Case report
A 49-year-old woman presented for evaluation of persistent ache, tiredness, and swelling in the left leg (Clinical, Etiology, Anatomy, and Pathophysiology class 3; Venous Clinical Severity Score, 7). Venous duplex ultrasound imaging of the left leg demonstrated GSV reflux and numerous branch varicosities. The patient reported a history of allergy to sulfa and penicillin but denied any allergy to CA or adhesives.
The patient elected CAC of the left GSV with 1.3 mL of adhesive used to occlude the GSV from 5 cm distal to the saphenofemoral junction to the proximal calf. In addition, 3 mL of 1% sodium tetradecyl sulfate was injected into the branch veins in multiple injections remote from the GSV CAC in the associated branch varicosities.
On postoperative day (POD) 7, the patient was without complaints, and the ultrasound examination showed that the left GSV was occluded from 2 cm distal from the saphenofemoral junction to the vein access point in the proximal calf. On POD 13, she returned, complaining of leg pain and redness. This was thought to be either phlebitis or an allergic reaction and was treated with oral diphenhydramine (Benadryl) and topical diclofenac (Voltaren) 1% cream. On POD 17, she complained of progressive leg pain, chills, and erythema over the medial thigh. Because of concern about infection, she was treated with cephalexin (Keflex) for 5 days. On POD 21, she described significant improvement in symptoms. On POD 124, she returned, complaining of persistent leg pain, erythema, and swelling. She was prescribed a methylprednisolone (Medrol) dose pack and referred to an allergist. Patch testing was performed to CA, methyl methacrylate 2%, and a negative control. After 48 hours, she had a moderate (2+) reaction to the CA and no reaction to methyl methacrylate 2% or the negative control. At 96 hours, CA reaction remained moderately reactive (2+).
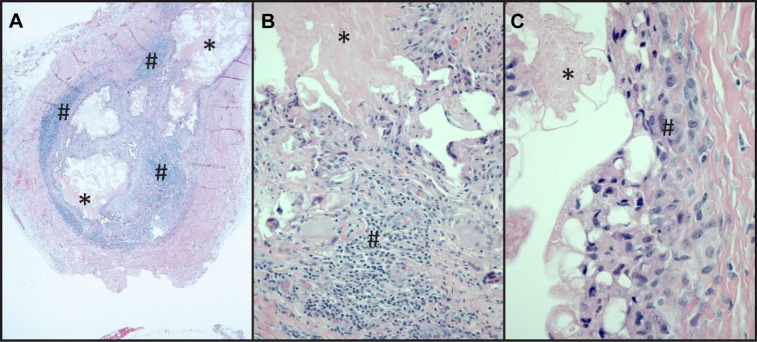
The patient elected to have the vein endoscopically excised on POD 200 (Fig 1). After excision, the patient had symptoms of pain and swelling in the treated limb, although reduced, that persisted for 2 years. Histopathologic evaluation of the removed tissue showed intraluminal foreign material and evidence of mononuclear cell inflammation. There was dense chronic inflammation that was localized to the luminal aspect of the vessel. Trichrome elastin and periodic acid-Schiff stains showed the absence of transmural inflammation, specifically with absence of destructive changes toward the periphery of the vessel. Immunohistochemical stain showed that a majority of the mononuclear cells were T lymphocytes, and most of these were of the T4 subset (Fig 2).
Fig 1.
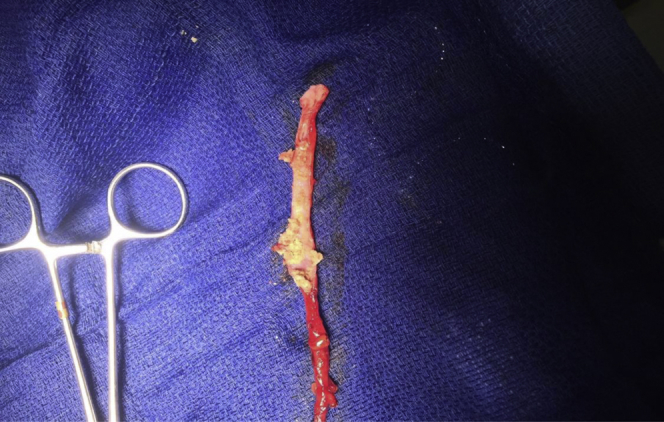
Excised great saphenous vein (GSV) with intraluminal foreign body.
Fig 2.
Histopathologic evaluation of the removed saphenous vein. A, Low-magnification view showing vein with intraluminal foreign material (*) and dense chronic inflammation (#) that was localized to the luminal aspect of the vessel with relative preservation of the periphery. B, Higher magnification view shows intraluminal material (*), some of which is present in the vessel wall within foreign body giant cell macrophages. Small lymphocytes (#) that type predominantly as T4 lymphocytes contribute to the granulomatous inflammation. C, The endothelial surface associated with the intraluminal material (*) shows vacuoles characteristic of injury and with subendothelial histiocytes and reactive fibroblasts (#).
Discussion
CAC was approved by the Food and Drug Administration in 2015 to treat saphenous vein reflux in patients with venous insufficiency. First-in-human studies did not report hypersensitivity reactions.2, 3 In the VenaSeal Sapheon Closure System Pivotal Study (VeClose), none of the 114 subjects randomized to CAC developed cutaneous or deep tissue hypersensitivity reactions.4 As with many new medical devices, more is learned in the postmarket setting after initial regulatory approval. Lawson et al1 described a hypersensitivity reaction with CAC attributed to subcutaneous delivery of the adhesive. In the Lake Washington Vascular VenaSeal Post-Market Evaluation (WAVES) trial, one patient developed full-body urticaria 1 week after the CAC procedure, which resolved after treatment with oral steroids.5 Park et al6 described a phenomenon they termed phlebitis-like abnormal reaction in 25% of patients treated with VenaSeal, which they speculated to be a type IV hypersensitivity reaction. This percentage, however, is far higher than what has otherwise been reported in published studies and is not consistent with what we see in our practice, suggesting the need to more clearly define this condition and to track its incidence in future studies.
In preclinical studies, the main histopathologic manifestation at 3 days to 2 weeks was an acute inflammatory reaction; this progressed to subacute vasculitis at 3 weeks, and a chronic granulomatous foreign body reaction developed at 4 weeks.7 In chronic preparations, the vessel had fibrotic changes with partial vascular recanalization.8, 9 By 60 days, histologic changes were consistent with a chronic foreign body-type inflammatory response.
In this case, the histopathologic evaluation showed intravascular foreign body material with nonspecific inflammation. The findings were not diagnostic of primary vasculitis or infection and suggested the possibility of an immune reaction that targeted the luminal contents or lumen-exposed endothelial or subendothelial tissue. The predominant cell type was T4, with additional nonspecific inflammatory markers consistent with a hypersensitivity reaction.
Cutaneous allergic reactions to CA are well described as type IV hypersensitivity reactions. Type IV hypersensitivity reactions have occurred with topical medical uses of CA, such as Dermabond (Ethicon, Somerville, NJ) for skin closure, and adhesives used for glucose sensor and eyelash extensions and artificial nails.10, 11, 12 Type IV hypersensitivity reactions are not antibody mediated, like the other hypersensitivity reactions, but rather are a T lymphocyte-mediated response to a recognized foreign antigen.
The true incidence and severity of type IV hypersensitivity reactions to CAC are unknown. The manufacturer (Medtronic) has reported in a personal communication with the authors that the observed rate of hypersensitivity reaction resulting in explantation of the treated vein is <1 in 10,000 to date.
Our protocol is to screen patients for allergies in general and known hypersensitivity reactions to adhesives and CA. We take efforts to prevent extravascular CAC from the vein when the catheter is removed from the entry point by “sheathing” the treatment catheter while the sheath is withdrawn through the skin to avoid subcutaneous and dermal CA exposure. Postoperatively, we ask that patients about itching at the treatment site, which may help distinguish a hypersensitivity reaction from phlebitis. If a patient develops a suspected hypersensitivity, we first treat it with topical steroids, followed by oral steroids and antihistamines. If the reaction persists, we refer the patient for hypersensitivity testing to confirm a specific reaction to CAC.
Further postmarket studies are needed to explore the possible role of preoperative CA patch testing. At this time, however, there is insufficient evidence to recommend routine patch test screening before CAC use. Studies are also needed to help define a medical regimen that can optimally treat the symptoms as well as the timing of initiation of such an approach. The best approaches to avoid this complication are likely to be adequate screening of patients and informing them of the risks before the procedures. Patients should be asked about a history of allergic reactions to CA. This includes CA issued in the home, at work, with cosmetic applications, or for previous skin closure.5 Patients who might be considered at higher risk of having or developing allergic sensitization to CAC, nail care industry workers, for example, should be informed of the presence of CA in this closure system and be offered alternative forms of treatment.13
Conclusions
Type IV hypersensitivity reactions to CA are a potential complication of treatment. The treating provider should be aware of this potential complication when selecting patients for this therapy.
Footnotes
Author conflict of interest: A.D.J. and E.M.B. are consultants for Medtronic (Dublin, Ireland).
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Lawson J., Gauw S., van Vlijmen C., Pronk P., Gaastra M., Mooij M. Sapheon: the solution? Phlebology. 2013;28(Suppl 1):2–9. doi: 10.1177/0268355513475970. [DOI] [PubMed] [Google Scholar]
- 2.Almeida J.I., Javier J.J., Mackay E., Bautista C., Proebstle T.M. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg Venous Lymphat Disord. 2013;1:174–180. doi: 10.1016/j.jvsv.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Proebstle T.M., Alm J., Gockeritz O., Wenzel C., Noppeney T., Lebard C. Three-year European follow-up of endovenous radiofrequency-powered segmental thermal ablation of the great saphenous vein with or without treatment of calf varicosities. J Vasc Surg. 2011;54:146–152. doi: 10.1016/j.jvs.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 4.Morrison N., Gibson K., McEnroe S., Goldman M., King T., Weiss R. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose) J Vasc Surg. 2015;61:985–994. doi: 10.1016/j.jvs.2014.11.071. [DOI] [PubMed] [Google Scholar]
- 5.Gibson K., Ferris B. Cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of post-procedure compression: initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study) Vascular. 2017;25:149–156. doi: 10.1177/1708538116651014. [DOI] [PubMed] [Google Scholar]
- 6.Park I., Jeong M.H., Park C.J., Park W.I., Park D.W., Joh J.H. Clinical features and management of "phlebitis-like abnormal reaction" after cyanoacrylate closure for the treatment of incompetent saphenous veins. Ann Vasc Surg. 2019;55:239–245. doi: 10.1016/j.avsg.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 7.Min R.J., Almeida J.I., McLean D.J., Madsen M., Raabe R. Novel vein closure procedure using a proprietary cyanoacrylate adhesive: 30-day swine model results. Phlebology. 2012;27:398–403. doi: 10.1258/phleb.2011.011084. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y.M., Cheng L.F., Li N. Histopathological study of vascular changes after intra-arterial and intravenous injection of N-butyl-2-cyanoacrylate. Chin J Dig Dis. 2006;7:175–179. doi: 10.1111/j.1443-9573.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- 9.Almeida J.I., Min R.J., Raabe R., McLean D.J., Madsen M. Cyanoacrylate adhesive for the closure of truncal veins: 60-day swine model results. Vasc Endovascular Surg. 2011;45:631–635. doi: 10.1177/1538574411413938. [DOI] [PubMed] [Google Scholar]
- 10.Peeters C., Herman A., Goossens A., Bruze M., Mowitz M., Baeck M. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis. 2017;77:426–429. doi: 10.1111/cod.12873. [DOI] [PubMed] [Google Scholar]
- 11.Shanmugam S., Wilkinson M. Allergic contact dermatitis caused by a cyanoacrylate-containing false eyelash glue. Contact Dermatitis. 2012;67:309–310. doi: 10.1111/cod.12000. [DOI] [PubMed] [Google Scholar]
- 12.Toriumi D.M., Bagal A.A. Cyanoacrylate tissue adhesives for skin closure in the outpatient setting. Otolaryngol Clin North Am. 2002;35:103–118, vi-vii. doi: 10.1016/s0030-6665(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch T. Non-thermal endovenous treatment: acrylate adhesion of varicose saphenous veins. Phlebologie. 2017;46:143–147. [Google Scholar]