Abstract
We report on a patient who underwent magnetic resonance guided focused ultrasound (MRgFUS) thalamotomy to treat tremor 3 years after a stereotactic radiosurgery (SRS) thalamotomy. The SRS produced only limited and transient improvements and was associated with a persistent hyperintensity on T2-FLAIR MR images. The MRgFUS thalamotomy was successful, with tremor improvement at 3 months, no adverse effects, and radiological appearance of the MRgFUS lesion similar to other patients undergoing this therapy. We also observed that the SRS-related T2-FLAIR hyperintensity had increased signal intensity 1 day post-MRgFUS, but appeared completely resolved 3 months post-MRgFUS. In conclusion, the case demonstrates that MRgFUS thalamotomy may effectively control tremor in patients with a history of SRS thalamotomy. We also speculate on the potential mechanisms of the apparent resolution of radiation-related change, and discuss possible applications of MRgFUS to reduce persistent SRS-related inflammation.
Keywords: Magnetic resonance guided focused ultrasound, High intensity focused ultrasound, Radiation, Thalamotomy, Tremor
Introduction
When medically refractory, neurosurgical treatments for tremor are considered. Minimally invasive options include stereotactic radiosurgery (SRS) and magnetic resonance guided focused ultrasound (MRgFUS), the latter being an emerging technology with promising early results [1]. MRgFUS thalamotomy has many potential advantages relative to SRS thalamotomy, including immediate feedback regarding tremor reduction [1], [2], [3]. In contrast, there is no method for monitoring SRS thalamotomy performance during the procedure, with clinical effects generally taking months to evolve [4]. SRS has also been associated with persistent perilesional edema or inflammation in some patients [4], [5], [6], [7].
We report on a patient who had previously undergone SRS thalamotomy for tremor, which resulted in only limited and transient improvement. Three years later, the patient underwent MRgFUS thalamotomy, which was successful in controlling tremor without adverse effects. We also observed apparent MRgFUS-related resolution of SRS-induced edema/inflammation of the thalamus.
Case report
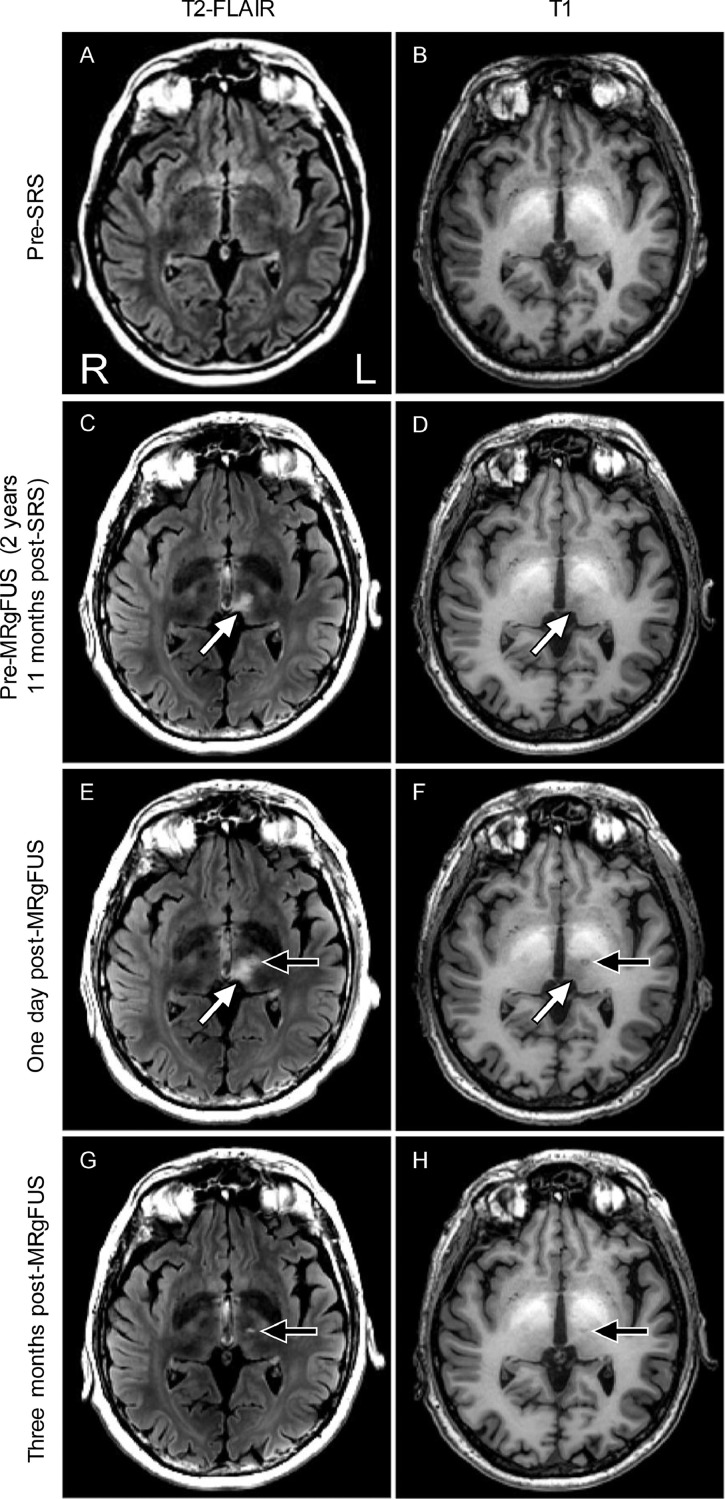
In 2015, a 62-year-old right-handed man with debilitating neuropathic tremor and chronic inflammatory demyelinating polyneuropathy underwent SRS thalamotomy at our center. Chronic inflammatory demyelinating polyneuropathy was diagnosed in 1996 and treated intermittently with monthly courses of IVIg and methylprednisolone courses every 6 weeks. He was also on thyroid replacement therapy. The patient's tremor was refractory to medications and disabling, preventing employment. He also had a history of thrombocytopenia and splenomegaly. Because transfusions did not correct his platelet counts, only noninvasive methods of tremor management were entertained. T2-weighted fluid attenuated inversion recovery images (T2-FLAIR) acquired using 1.5 T magnetic resonance imaging (MRI; Siemens Magnetom Aera, Erlangen, Germany) acquired approximately 1 year and 8 months pre-SRS did not reveal any abnormal findings in the thalamus (Fig. 1A). Similarly, T1-weighted images acquired at 3 T (General Electric Discovery 750, Waukesha, WI) approximately 1 month pre-SRS did not reveal any thalamic abnormalities (Fig. 1B).
Fig. 1.
MRI evidence of resolution of radiation related change post-MRgFUS. All images are presented in radiological orientation. (A) Pre-SRS T2-FLAIR and (B) T1-weighted MR images show no evidence of inflammation in the thalamus. (C) The pre-MRgFUS (post-SRS) T2-FLAIR image shows evidence of SRS-related hyperintensity (white arrow). (D) The pre-MRgFUS (post-SRS) T1-weighted image shows evidence of SRS-related hypointensity (white arrow). (E) One day post-MRgFUS, the T2-FLAIR image shows apparent enhancement of the SRS-related hyperintensity (white arrow), as well as a typical-appearing MRgFUS lesion (black arrow). (F) One day post-MRgFUS, the T1-weighted image shows apparent enhancement of the SRS-related hypointensity (white arrow), as well as a typical-appearing MRgFUS lesion (black arrow). (G) Three months post-MRgFUS, the T2-FLAIR image shows the SRS-related hyperintensity is resolved; a typical-appearing MRgFUS lesion remains (black arrow). (H) Three months post-MRgFUS, the T1-weighted image shows the SRS-related hypointensity is resolved; a typical-appearing MRgFUS lesion remains (black arrow). The hyperintense signal in the basal ganglia visible on the T1-weighted images is thought to be related to the patient's history of cirrhosis.
SRS left thalamotomy was performed on a LINAC (Novalis, BrainLab, Germany) with a dose of 130 Gy. The ventral intermediate nucleus (Vim) target was selected on MRI based on the Schaltenbrand and Wahren brain atlas [8] relative to the anterior and posterior commissure and internal capsule. Pre-SRS, the patient had a clinical rating scale for tremor (CRST) score of 67 [9]. His CRST score was 60 and 51 at 1 and 2 years post-SRS, respectively, with no side effects observed.
In 2018, the patient was enrolled in a study at our center investigating MRgFUS thalamotomy for tremor. Written informed consent was obtained. At this time, his CRST score was 66.5. The only change to his immunomodulating treatment in the period preceding the MRgFUS thalamotomy was a small, gradual decrease in IVIg dosage 1 month prior to focused ultrasound thalamotomy. MRI was performed pre-MRgFUS using 3 T and an aftermarket 32 channel receive-only head coil (Nova Medical, Wilmington, MA), approximately 2 years and 11 months after the SRS. At this time, an irregular area of hyperintense signal, approximately 862 mm3, was observed in inferior-medial aspect of the thalamus on T2-FLAIR images (Fig. 1C). T1-weighted images showed corresponding signal hypointensity (Fig. 1D).
Three years after the SRS, the left Vim of the thalamus was targeted for lesioning with high intensity transcranial focused ultrasound (ExAblate 4000, InSightec, Haifa, Israel) guided by MRI. The patient's skull density ratio was 0.38 [10]. The initial sonication target was 1 mm superior to the standard Vim target [1]. Adjustments to the target were made by the neurosurgeon based on sensory percepts and tremor control associated with the initial sonications. The procedure entailed a total of 21 sonications, with the peak temperature reaching 60.2°C as measured by MR thermometry. No adverse effects were seen.
One day after MRgFUS thalamotomy, 3 T MR imaging was repeated (note: no postcontrast imaging was performed). On the T1-weighted image, we observed a small but typical-appearing MRgFUS lesion at the expected location, with a combined zone I and II [11], [12] volume of 71 mm3 (Fig. 1E and F). The MRgFUS lesion overlapped with the pre-MRgFUS SRS-related hyperintense region by 29 mm3. Medial to the target location, we observed an 8.5% increased signal enhancement and an apparent expansion of the SRS-related T2-FLAIR hyperintense region, although this expanded hyperintense area was contiguous with zone III [11] of the MRgFUS lesion (Fig. 1B). Approximately 3 months post-MRgFUS, CRST and 3 T MR imaging was repeated. Total CRST score was reduced to 39 (41% improvement) relative to the pre-MRgFUS evaluation. The SRS-related T2-FLAIR hyperintensity was no longer visible (Fig. 1G; signal intensity reduced by 28.0 % relative to pre-MRgFUS T2-FLAIR). The T1-weighted image demonstrated a small focus of hypointensity at the MRgFUS target, typical of patients 3 months post-MRgFUS (Fig. 1H).
We sought to understand the mechanism of the apparent resolution of the persistent SRS-related inflammation. We calculated the accumulated thermal dose (ATD) in cumulative equivalent minutes at 43°Celsius (CEM43) [13] in the SRS-associated T2-FLAIR hyperintensity as it appeared pre-MRgFUS, as well as in the MRgFUS-generated lesion as it appeared 1 day post-MRgFUS. In the pre-MRgFUS SRS-associated hyperintense area (excluding the 29 mm3 that overlapped with the MRgFUS lesion), the average ATD was 7.4 CEM43. In contrast, the MRgFUS lesion had an average ATD of 360.6 CEM43.
Discussion
To our knowledge, this is the first report of successful MRgFUS thalamotomy following SRS thalamotomy for tremor, suggesting that patients with a history of SRS thalamotomy can be safely and successfully treated with MRgFUS thalamotomy. The SRS thalamotomy was associated with persistent edema/inflammation, as observed on T2-FLAIR imaging approximately 3 years post-SRS. This persistent SRS-related hyperintense signal appeared worsened 1-day post-MRgFUS treatment, but resolved after 3 months such that the SRS-related hyperintensities were no longer readily visible. The reduction in hyperintense signal/edema we observed appears similar to cases in which radiation necrosis was treated with laser interstitial thermal therapy [14].
It has been speculated that persistent SRS-related edema/inflammation is related to permanent radiation damage [15] and can be associated with clinical side effects [4], [5], [6], [7], although this patient showed none. It has recently been proposed that the immune-stimulating effects of high intensity focused ultrasound could be combined with radiation therapy (RT) for cancer in order to reduce RT-related inflammation and associated side effects [16]. We hypothesize that the thermal dose experienced by the MRgFUS target produced an immune response that infiltrated and reactivated healing of the neighboring SRS-associated hyperintense area. While we cannot rule out an effect of the thermal dose experienced by the SRS-associated hyperintense area itself, these values were relatively low, suggesting the primary mechanism of inflammation resolution was related to the much higher thermal dose achieved in neighboring regions at the MRgFUS target.
While cautious interpretation is required, it is possible that the case described here provides the first evidence in a human for the potential value of high intensity focused ultrasound in reducing radiation-related inflammation. Combining high intensity focused ultrasound with RT may be a promising approach for improving clinical outcomes for both oncology treatment [16] and for patients with persistent adverse effects after SRS.
Footnotes
Declaration of Competing Interest: None.
Acknowledgments: Thanks are extended to Dr Richard Falkenstein for anesthesiology support and Ms Tamara Trainor for help with MRI data collection. This work was funded by the Canadian Foundation for Innovation (CFI-36073), Cumming Medical Research Fund, Canadian Institutes forHealth Research (FDN-143290), CampusAlberta InnovatesChair, and Natural Science and Engineering Research Council (NSERC 04126-2017).
References
- 1.Elias W.J., Lipsman N., Ondo W.G., Ghanouni P., Kim Y.G., Lee W. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375:730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- 2.Lipsman N., Schwartz M.L., Huang Y., Lee L., Sankar T., Chapman M. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12:462–468. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 3.Martin E., Jeanmonod D., Morel A., Zadicario E., Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66:858–861. doi: 10.1002/ana.21801. [DOI] [PubMed] [Google Scholar]
- 4.Niranjan A., Raju S.S., Kooshkabadi A., Monaco E., Flickinger J.C., Lunsford L.D. Stereotactic radiosurgery for essential tremor: retrospective analysis of a 19-year experience. Mov Disord. 2017;32:769–777. doi: 10.1002/mds.26925. [DOI] [PubMed] [Google Scholar]
- 5.Elaimy A.L., Demakas J.J., Arthurs B.J., Cooke B.S., Fairbanks R.K., Lamoreaux W.T. Gamma knife radiosurgery for essential tremor: a case report and review of the literature. World J Surg Oncol. 2010;8:20. doi: 10.1186/1477-7819-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kooshkabadi A., Lunsford L.D., Tonetti D., Flickinger J.C., Kondziolka D. Gamma knife thalamotomy for tremor in the magnetic resonance imaging era: clinical article. J Neurosurg. 2013;118:713–718. doi: 10.3171/2013.1.JNS121111. [DOI] [PubMed] [Google Scholar]
- 7.Steiner L., Forster D., Leksell L., Meyerson B.A., Boëthius J. Gammathalamotomy in intractable pain. Acta Neurochir. 1980;52:173–184. doi: 10.1007/BF01402072. [DOI] [PubMed] [Google Scholar]
- 8.Schaltenbrand G., Wahren W. 2nd ed. Thieme; Stuttgart: 1977. Atlas for stereotaxy of the human brain. [Google Scholar]
- 9.Fahn S., Tolosa E., Marín C. Parkinson's disease and movement disorders. 2nd ed. Williams and Wilkins; Baltimore: 1993. Clinical rating scale for tremor; pp. 225–234. [Google Scholar]
- 10.Chang W.S., Jung H.H., Zadicario E., Rachmilevitch I., Tlusty T., Vitek S. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J Neurosurg. 2016;124:411–416. doi: 10.3171/2015.3.JNS142592. [DOI] [PubMed] [Google Scholar]
- 11.Wintermark M., Druzgal J., Huss D.S., Khaled M.A., Monteith S., Raghavan P. Imaging findings in MR imaging-guided focused ultrasound treatment for patients with essential tremor. Am J Neuroradiol. 2014;35:891–896. doi: 10.3174/ajnr.A3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutet A., Ranjan M., Zhong J., Germann J., Xu D., Schwartz M.L. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain J Neurol. 2018;141:3405–3414. doi: 10.1093/brain/awy278. [DOI] [PubMed] [Google Scholar]
- 13.Sapareto S.A., Dewey W.C. Thermal dose determination in cancer therapy. Int J Radiat Oncol. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M., Balasubramanian S., Silva D., Barnett G.H., Mohammadi A.M. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: an overview. Expert Rev Neurother. 2016;16:223–232. doi: 10.1586/14737175.2016.1135736. [DOI] [PubMed] [Google Scholar]
- 15.Plowman P.N. Stereotactic radiosurgery VIII. The classification of postradiation reactions. Br J Neurosurg. 1999;13:256–264. doi: 10.1080/02688699943655. [DOI] [PubMed] [Google Scholar]
- 16.Cirincione R., Di Maggio F.M., Forte G.I., Minafra L., Bravatà V., Castiglia L. High-intensity focused ultrasound– and radiation therapy–induced immuno-modulation: comparison and potential opportunities. Ultrasound Med Biol. 2017;43:398–411. doi: 10.1016/j.ultrasmedbio.2016.09.020. [DOI] [PubMed] [Google Scholar]