Abstract
Background
This study aimed to assess the prevalence, clinical characteristics, and risk factors for sinistral portal hypertension in patients with moderate or severe acute pancreatitis.
Material/Methods
A retrospective study included 825 patients with moderate or severe acute pancreatitis. Clinical and demographic data, the Acute Physiology and Chronic Health Evaluation (APACHE II) and the Ranson scores for severity of acute pancreatitis, and the computed tomography (CT) severity index (CTSI) were evaluated. The formation of collateral vessels, bleeding, splenomegaly, hypersplenism during hospitalization or follow-up, and early anticoagulation and the occurrence of sinistral portal hypertension were evaluated.
Results
Of the 825 patients with moderate or severe acute pancreatitis, 103 patients (12.5%) developed sinistral portal hypertension. The median time to diagnosis was 8 months, and the median patient age was 39 years. The most common causes of pancreatitis were biliary (46.3%), hypertriglyceridemia (31.5%), alcohol (14.9%), and others (7.3%). Independent risk factors for sinistral portal hypertension were male gender (OR, 4.666; 95% CI, 2.54–8.572; P<0.001), recurrent acute pancreatitis (OR, 9.556; 95% CI, 5.218–17.5; P<0.001), hypertriglyceridemia (OR, 2.056; 95% CI, 1.184–3.57; P=0.001), glucose >10 mmol/L (OR, 6.965; 95% CI, 4.027–12.045; P<0.001), smoking (OR, 6.32; 95% CI, 3.544–11.269; P<0.001), and infection of walled-off necrosis (OR=1.637; 95% CI, 1.061–2.524; P=0.015). Anticoagulation during hospitalization was not significantly associated with sinistral portal hypertension.
Conclusions
Hypertriglyceridemia, hyperglycemia, infection of walled-off necrosis, recurrent acute pancreatitis, and smoking were risk factors for sinistral portal hypertension, and early anticoagulation did not prevent the occurrence.
MeSH Keywords: Hypersplenism; Hypertension, Portal; Venous Thrombosis
Background
Sinistral portal hypertension, also known as left-sided portal hypertension, is characterized by isolated gastric varices caused by splenic vein thrombosis or occlusion following acute or chronic pancreatitis, pancreatic cyst, walled-off pancreatic necrosis, or pancreatic tumors, with normal liver function [1–3]. Acute or chronic pancreatitis is a major cause of sinistral portal hypertension [4].
Anatomically, the splenic vein lies against the posterior surface of the pancreatic tail. Injury from persistent pancreatic inflammation and external compression from pancreatic necrosis or pseudocyst leads to stenosis, thrombosis, or occlusion of the splenic vein and thus the development of collateral vessels. Sinistral portal hypertension usually occurs following splenic vein thrombosis. The interval from the onset of acute pancreatitis to the diagnosis of sinistral portal hypertension is reported to range from 10 days to nine years, which makes it unlikely to be discovered during hospitalization or recognized during early follow-up. Most patients with sinistral portal hypertension are asymptomatic or have chronic abdominal pain, but upper gastrointestinal bleeding is a life-threatening clinical manifestation that is reported in between 4–17% of cases [5–8]. The frequency of fatal bleeding ranges from 1.2–14.5% [9,10].
In a recent meta-analysis conducted by Guo et al., the pooled prevalence of splanchnic vein thrombosis, mainly splenic vein thrombosis, was 16.6% in patients with acute pancreatitis and 11.6% in those with chronic pancreatitis [11]. However, because recent studies have mainly focused on the clinical course of splenic vein thrombosis, the natural history of sinistral portal hypertension induced by acute pancreatitis remains unclear. The aim of the present retrospective cohort study was to report the prevalence and clinical characteristics of sinistral portal hypertension in the setting of acute pancreatitis. Potential risk factors for sinistral portal hypertension were also studied.
Material and Methods
Patients
This retrospective single-center study was performed at Shengjing Hospital of China Medical University. The clinical records of patients who were discharged with acute pancreatitis were reviewed, and we found that all cases of acute pancreatitis-induced sinistral portal hypertension occurred in patients with moderate or severe acute pancreatitis, except for two with mild disease. Therefore, we included only patients with moderate or severe acute pancreatitis in this study. Patients aged <18 years, pregnancy, acute-on-chronic pancreatitis, or pancreatic tumor were excluded, as were those who did not have regular follow-up until complete absorption of peripancreatic collections. The study protocol was approved by the Institutional Review Board of Shengjing Hospital (Approval No. 2019PS409K, dated April 2nd, 2019).
Diagnosis and definitions
Acute pancreatitis was diagnosed by acute onset of persistent, severe, epigastric pain often radiating to the back, serum lipase level or amylase level at least three times greater than the upper limit of normal, and characteristic findings of acute pancreatitis on computed tomography (CT) [12].
According to the revised Atlanta criteria, moderate acute pancreatitis was defined as transient organ failure (<48 h) or local complications including acute peripancreatic fluid collection, pancreatic pseudocyst, and acute necrosis or walled-off necrosis. Severe acute pancreatitis was defined as local complications accompanied by organ failure lasting more than 48 hours [13]. The modified Marshall scoring system [14] was used to diagnose organ failure, as described in the revised Atlanta criteria.
The diagnostic criteria for sinistral portal hypertension included newly-formed collateral vessels, confirmed by contrast-enhanced computed tomography (CT) or gastric fundal varices confirmed by endoscopy, the presence of splenomegaly or hypersplenism, and the exclusion of other causes of portal hypertension, such as hepatic cirrhosis or idiopathic portal hypertension. The definition of infection of walled-off pancreatic necrosis was by positive culture of fluid in the cavity by fine needle aspiration or the presence of gas bubbles within the cavity on CT. Persistent sepsis or progressive clinical deterioration was also suggestive of infection [15].
Acute biliary pancreatitis was defined as the presence of gallstones or biliary sludge in the biliary tree or the gallbladder. Hypertriglyceridemia was diagnosed when the serum triglyceride level was >11.3 mmol/L or between 5.65–11.3 mmol/L (500–1000 mg/mL) with hyperlipemic serum [16]. Hyperglycemia was defined as blood glucose levels >10 mmol/L in more than two random samples. Other causes included drug abuse or a history of endoscopic retrograde cholangiopancreatography (ERCP) or abdominal surgery [17]. Patients were considered to be smokers if they were currently active smokers or if they had a history of active smoking (at least three cigarettes daily). Alcohol consumption was defined as drinking 20–40 g of alcohol daily for women, and 40–60 g of alcohol daily for men [18].
Data collection
Patient characteristics, including age, gender, cause and recurrence of acute pancreatitis, smoking and drinking habits, and clinical parameters, including the Acute Physiology and Chronic Health Evaluation (APACHE II) severity score and the Ranson scores for severity of acute pancreatitis were evaluated on hospital admission. The CT severity index (CTSI) was recorded at one week after onset. C-reactive protein (CRP), D-dimer, and platelet levels were also analyzed during the most severe episodes of acute pancreatitis. Complications of acute pancreatitis, including organ failure, pancreatic infection, and mortality, were recorded. Characteristics associated with sinistral portal hypertension included the formation of collateral vessels, bleeding, splenomegaly, and hypersplenism during the course of hospitalization or follow-up were also evaluated.
Management and follow-up
All patients received medical treatment, including necessary intensive care, fluid therapy, pain control, nutritional support, and antibiotics for patients with proven infection. Percutaneous drainage was conducted in patients with infected walled-off pancreatic necrosis unresponsive to antibiotics. Subcutaneous low molecular weight heparin (38000–76000 IU/day) by subcutaneous injection was routinely used in patients with hypercoagulability.
Serial contrast-enhanced CT was routinely performed for patients with acute pancreatitis. CT evaluation was performed on multidetector helical CT scanners with 64 detector rows. Contiguous 3-mm axial sections were displayed from the diaphragm to the symphysis pubis. Patients received non-ionic intravenous contrast material with Loversol injection (Jiangsu Hengrui Pharmaceutical Co., Ltd.) that was administered at 5 mL/s at a volume of 100–150 mL. All patients were imaged in the portal venous dominant phase. Patients who were discharged with a local collection had regular CT scans between one to two months until the collection was completely absorbed. For patients with gastrointestinal bleeding or splenomegaly, contrast-enhanced CT or gastroendoscopy were required to confirm the diagnosis of sinistral portal hypertension.
Statistical analysis
Descriptive data were expressed as absolute numbers and percentages for qualitative variables and as medians and ranges for quantitative variables. The independent samples t-test was used for continuous variables, and the chi-squared or Fisher’s exact test were used for categorical or discrete variables. Univariate analysis was performed, and those variables with P<0.05 were considered to be statistically significant. Multivariate analysis consisted of forward logistic regression according to the Wald statistic. Variables with a P<0.05 remained in the final logistic model. The adjusted odds ratios (ORs) and their respective 95% confidence intervals (CI) were presented in the final model. All statistical calculations were performed using SPSS statistical software, version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Descriptive analysis
A total of 3,154 patients were admitted to our hospital for treatment of acute pancreatitis from January 2011 to July 2018. There were 105 patients who developed sinistral portal hypertension. Only two patients had sinistral portal hypertension with mild acute pancreatitis, compared with 103 patients who had sinistral portal hypertension with moderate and severe acute pancreatitis. Because of the very low prevalence of sinistral portal hypertension in mild acute pancreatitis, we included only patients with moderate and severe acute pancreatitis in the study. Finally, a total of 825 patients (26%) who met the criteria for moderate and severe acute pancreatitis, including 594 patients (72%) with moderate acute pancreatitis and 231 patients (28%) with severe acute pancreatitis underwent regular follow-up (Figure 1). Among these, 103 patients (12.5%) developed sinistral portal hypertension. The median time to the diagnosis of sinistral portal hypertension was 8 months (range, 0.3–84 months). Sinistral portal hypertension was diagnosed in 15 patients (14.6%) during hospitalization, in 62 patients (60.2%) within one year of the latest episode of acute pancreatitis, and in 26 patients (25.2%) after more than one year. A total of 131 patients (15.9%) had recurrent acute pancreatitis, and the clinical data analyzed in these patients were from the most recent episode before the diagnosis of sinistral portal hypertension.
Figure 1.
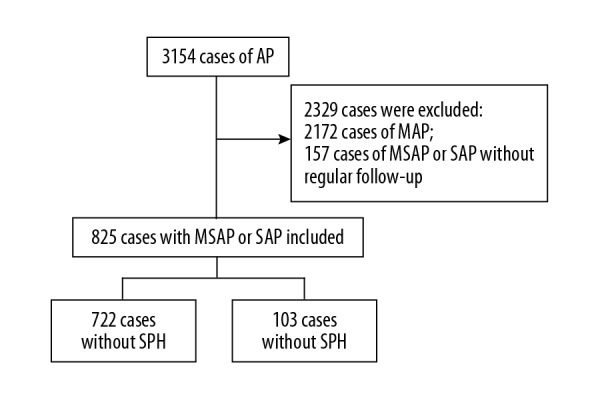
The flowchart of patients with acute pancreatitis included in the study.
The median age of the patients was 39 years. The most common causes of acute pancreatitis were biliary (46.3%), hypertriglyceridemia (31.5%), alcohol (14.9%), and others (7.3%). All cases of sinistral portal hypertension were confirmed by contrast-enhanced computed tomography (CT), and 77 patients (74.8%) with collateral vessels on CT underwent endoscopy. Fifty-eight patients (56.3%) had varices in the fundus at endoscopy, 13 patients (12.6%) had bleeding, 72 patients (70%) had splenomegaly, and seven patients (6.8%) had hypersplenism. Most cases of sinistral portal hypertension were asymptomatic, but three patients had life-threatening hematemesis requiring surgery (one case is shown in Figure 2). Infected walled-off pancreatic necrosis was identified in 228 patients (27.6%). Organ failure, including acute respiratory distress syndrome (ARDS), renal failure, and shock occurred in 178 patients (21.6%), 157 patients (19%), and 107 patients (13%), respectively. Mortality in acute pancreatitis with sinistral portal hypertension and without sinistral portal hypertension occurred in 28 patients (3.9%) and five patients (4.9%), respectively.
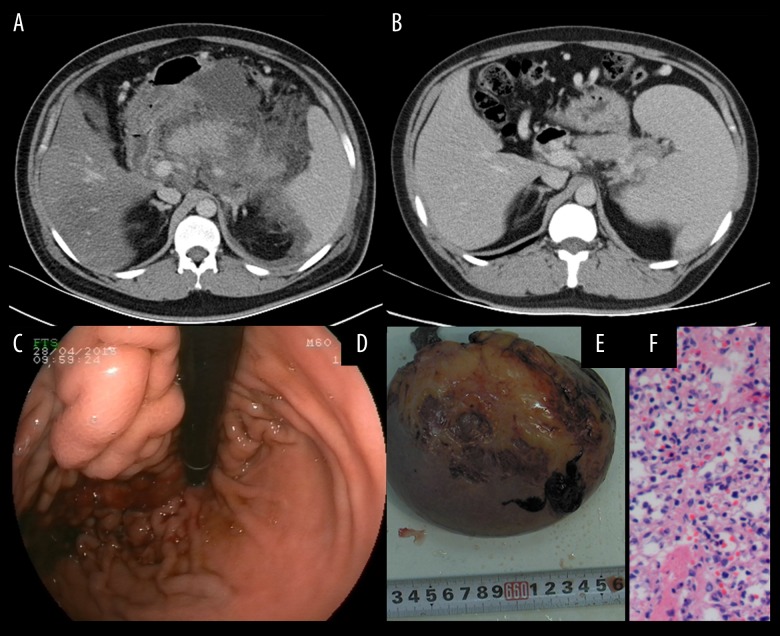
Figure 2.
A 30-year-old male patient was diagnosed with severe acute pancreatitis associated with hyperlipidemia. (A) A contrast-enhanced computed tomography (CT) scan was performed at one week after onset. The pancreas was surrounded by diffuse inflammation. (B) Repeat CT was performed one year later and an enlarged spleen was present. (C) The varices at the fundus of the stomach were complicated by bleeding as shown on endoscopy. (D) Splenectomy and pericardial devascularization were performed due to severe upper gastrointestinal bleeding. (E) Photomicrograph of the histology of the spleen shows congestion with atrophy and expansion of splenic sinus. Hematoxylin and eosin (H&E).
Risk factors for sinistral portal hypertension
On univariate analysis, the factors that were significantly associated with sinistral portal hypertension were male gender (P<0.001), recurrent acute pancreatitis (P<0.001), hypertriglyceridemia (P<0.001), hyperglycemia (P<0.001), smoking (P=0.001), and infected walled-off pancreatic necrosis (P=0.026) (Tables 1, 2). These variables underwent multivariate logistic regression analysis and were independently associated with the risk of sinistral portal hypertension (Table 3). These risk factors included male gender (OR, 4.666; 95% CI, 2.54–8.572; P<0.001), recurrent acute pancreatitis (OR, 9.556; 95% CI, 5.218–17.5; P<0.001), hypertriglyceridemia (OR, 2.056; 95% CI, 1.184–3.57; P=0.001), hyperglycemia (OR, 6.965; 95% CI, 4.027–12.045; P<0.001), smoking (OR, 6.32; 95% CI, 3.544–11.269; P<0.001), and infected walled-off pancreatic necrosis (OR, 1.637; 95% CI, 1.061–2.524; P=0.015). A total of 541 patients underwent anticoagulation therapy during hospitalization, but there was no significant difference between the patients with or without sinistral portal hypertension.
Table 1.
Clinical characteristics of patients with moderate or severe acute pancreatitis and sinistral portal hypertension (SPH).
| Total (n=825) | No SPH (n=722) | SPH (n=103) | P-value | |
|---|---|---|---|---|
| Age, yrs (range) | 39 (19–80) | 39 (19–80) | 36 (20–78) | 0.235 |
| Gender (Male, %) | 462 (56) | 380 (52.6) | 82 (79.6) | <0.001* |
| Recurrent (%) | 131 (15.9) | 81 (11.2) | 50 (48.5) | <0.001* |
| Etiology | ||||
| Biliary (%) | 382 (46.3) | 347 (48.1) | 35 (34) | 0.068 |
| HTG (%) | 260 (31.5) | 211 (29.2) | 49 (47.6) | <0.001* |
| Alcoholic (%) | 123 (14.9) | 113 (15.4) | 10 (9.7) | 0.075 |
| Others (%) | 60 (7.3) | 51 (7.1) | 9 (8.7) | 0.121 |
| Hyperglycemia (%) | 214 (25.9) | 141 (19.5) | 73 (70.9) | <0.001* |
| Smoking (%) | 168 (20.4) | 110 (15.2) | 58 (56.3) | 0.001* |
| APACHE II | 10 (8–15) | 10 (8–15) | 10 (8–15) | 0.798 |
| Ranson score | 6 (3–6) | 6 (3–6) | 6 (4–6) | 0.065 |
| CTSI | 6 (4–6) | 6 (4–6) | 6 (4–6) | 0.378 |
| CRP | 221 (61–673) | 196 (67–504) | 245 (67–673) | 0.263 |
| DD | 2342 (122–8909) | 2587 (353–8909) | 3241 (122–7685) | 0.315 |
| PLT | 252 (84–702) | 206 (98–702) | 317 (84–671) | 0.223 |
Qualitative variables are expressed as n (%); quantitative variables are expressed as the median (range).
Significant at <0.05.
HTG – hypertriglyceridemia; hyperglycemia refers to serum glucose >10 mmol/L in two random samples; CTSI, computed tomography (CT) severity index; CRP – C-reactive protein; DD – D-dimer; PLT – platelets.
Table 2.
Disease severity, complications, and anticoagulation in patients with or without acute pancreatitis (AP) and sinistral portal hypertension (SPH).
| Total (n=825) | No SPH (n=722) | SPH (n=103) | P-value | |
|---|---|---|---|---|
| Moderate AP (%) | 594 (72) | 510 (70.6) | 84 (81.6) | 0.851 |
| Infection of WOPN | 228 (27.6) | 190 (26.3) | 38 (36.9) | 0.026* |
| Organ failure ARDS (%) | 178 (21.6) | 156 (21.6) | 22 (21.4) | 0.827 |
| Renal failure (%) | 157 (19) | 136 (18.8) | 21 (20.4) | 0.738 |
| Shock | 107 (13) | 99 (13.7) | 8 (7.8) | 0.097 |
| Anticoagulation | 541 (65.6) | 472 (65.4) | 69 (67) | 0.741 |
Qualitative variables are expressed as n (%); quantitative variables are expressed as median (range).
Significant at <0.05.
AP – acute pancreatitis; WOPN – walled-off pancreatic necrosis; ARDS – acute respiratory distress syndrome.
Table 3.
Multivariate logistic regression analysis for sinistral portal hypertension.
| OR | 95% Cl | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Gender (male) | 4.666 | 2.54 | 8.572 | <0.001* |
| Recurrent | 9.556 | 5.218 | 17.5 | <0.001* |
| Hypertriglyceridemia | 2.056 | 1.184 | 3.57 | 0.01* |
| Hyperglycemia (%) | 6.965 | 4.027 | 12.045 | <0.001* |
| Smoking | 6.32 | 3.544 | 11.269 | <0.001* |
| Infection | 1.637 | 1.061 | 2.524 | 0.015* |
OR – odds ratio; CI – confidence interval.
Significant at <0.05.
Discussion
In the present study, we analyzed the clinical characteristics and risk factors for sinistral portal hypertension induced by moderate or severe acute pancreatitis. The rate of sinistral portal hypertension in all patients with acute pancreatitis was 3.3%. Because the prevalence of sinistral portal hypertension was very low in patients with mild acute pancreatitis, our study included only those with moderate and severe acute pancreatitis (n=825); the rate of sinistral portal hypertension in this group was 12.5%.
Data for the prevalence of sinistral portal hypertension associated with sinistral portal hypertension are limited and differ among studies. Splenic vein thrombosis is reported in 16.4–36.4% of cases of acute necrotizing pancreatitis [19–21]. The incidence of sinistral portal hypertension associated with splenic vein thrombosis has been reported to be 57% in patients with pancreatitis [22], and the frequency of gastrointestinal bleeding ranges from 37–100% [23–25]. In the present study, sinistral portal hypertension occurred in 3.3% of all cases of acute pancreatitis, and in 12.5% of cases of moderate or severe acute pancreatitis, which is similar to previous studies [19,20].
The time to recognition of sinistral portal hypertension following acute pancreatitis is not well elaborated, which may be due to the late onset of sinistral portal hypertension during the course of follow-up for acute pancreatitis. Harris et al. summarized the time to diagnosis of splenic vein thrombosis in 45 patients with acute pancreatitis and found that 18% were diagnosed with splenic vein thrombosis at presentation, 42% had thrombosis within one month, and 40% were diagnosed within one year of the onset of acute pancreatitis [26]. All of these patients had ongoing acute pancreatitis, which could be the cause of splenic vein thrombosis, and 47% of patients who had sinistral portal hypertension were asymptomatic [26]. In our study, the time interval between the diagnosis of sinistral portal hypertension and the last episode of acute pancreatitis was 8 months (range, 0.3–84 months). Sinistral portal hypertension was diagnosed in 14.6% of patients during hospitalization, in 60.2% during the first year after the latest episode of acute pancreatitis, and in 25.2% after more than one year. Most of the patients were asymptomatic, and the chronic development of clinical features explain the difficulty in recognizing the condition during acute hospitalization.
Collateral circulation and splenomegaly are the typical clinical characteristics of sinistral portal hypertension, unlike other causes of sinistral portal hypertension, including hepatic cirrhosis, where symptoms such as ascites, abdominal distention, and pain are not common. Varices are reported in 53.0% of cases, 12.3% of which are associated with gastrointestinal bleeding, and splenomegaly has been reported in 51.9% of patients [7]. Zhou et al. retrospectively studied 104 patients with acute necrotizing pancreatitis complicated by splenic vein thrombosis and found that the incidence of variceal bleeding, persistent ascites, and intolerance to enteral feeding was 1.92%, 18.3%, and 13.5%, respectively [27]. In the present study, 74.8% of patients with sinistral portal hypertension had collateral blood vessels on computed tomography (CT) scan, and 56.3% had varices on endoscopy. Although most affected patients have splenomegaly, few patients have splenic pain or develop leukopenia or thrombocytopenia [2], and in our study, only 6.8% had hypersplenism.
The reported incidence of gastrointestinal bleeding varies, ranging from 1.92–18.0%, and in one study with a relatively long follow-up period, bleeding occurred in 3.8% of 53 patients [26,27]. The variations in bleeding rates may reflect the differences in follow-up periods [7]. Therefore, for patients with acute pancreatitis, especially those with risk factors for sinistral portal hypertension, prolonged monitoring may be suggested, while for those with known sinistral portal hypertension, preventive measures such as appropriate dietary restrictions and patient education for early recognition of gastrointestinal bleeding should be provided.
In the present study, male gender, recurrent acute pancreatitis, hypertriglyceridemia, hyperglycemia (serum glucose >10 mmol/L in at least two random samples), infected walled-off pancreatic necrosis, and smoking were independent predictors of sinistral portal hypertension in acute pancreatitis The finding that sinistral portal hypertension was more common in men (79.6%; P <0.001) was supported by the findings from a previous study by Roach et al., which also showed a higher risk of splenic vein thrombosis in men [28]. Nearly half (48.5%) of the patients with sinistral portal hypertension in our study had recurrent acute pancreatitis. Recurrent acute pancreatitis increased the risk of sinistral portal hypertension by a factor of 9.56. Similarly, a prospective study by Rebours et al. showed that splenic vein thrombosis occurred in 40% of patients with recurrent acute pancreatitis, and 68% of patients with splenic vein thrombosis due to recurrent acute pancreatitis or chronic pancreatitis developed sinistral portal hypertension during a median follow-up of 1.79 years (range, 0.48–5.36 years). Splenic vein thrombosis is a frequent complication of recurrent acute pancreatitis, and is associated with local inflammatory changes, but not with prothrombotic states [29].
Although the leading causes of moderate and severe acute pancreatitis in our study were gallstones (46.3%), hypertriglyceridemia (31.5%), alcohol (14.9%), and other causes (7.3%) that included drugs, endoscopic retrograde cholangiopancreatography (ERCP), abdominal surgery, and viral infection, only hypertriglyceridemia, and not biliary or alcoholic acute pancreatitis, was identified as a risk factor for sinistral portal hypertension in our study population. Infected walled-off pancreatic necrosis in moderate and severe acute pancreatitis also increased the risk of the development of sinistral portal hypertension by a factor of 1.64. The incidence of sinistral portal hypertension in patients with infected walled-off pancreatic necrosis was 36.9%. This finding might be explained by the increased local inflammatory reaction surrounding the splenic vein during infection. Sinistral portal hypertension was also more common in smokers, which is supported by the findings from a previous study by Toqué et al., which indicating that heavy smoking was a risk factor for thrombosis [18]. An earlier study from Severinsen et al. also showed an increased risk of thrombosis beyond thresholds of 20 g/day tobacco for women and 30 g/day for men [30].
There is limited information about anticoagulation in patients with sinistral portal hypertension. Although the majority of patients with acute splenic vein thrombosis have received anticoagulation with heparin and warfarin, the decision to anticoagulate is still controversial because of the low recanalization rate and the increased risk from bleeding [18,26,31,32]. In our study, there was no correlation between sinistral portal hypertension and early anticoagulation during hospitalization for acute pancreatitis, which suggests that the formation of collateral vessels is a chronic process that is mainly promoted by local inflammation rather than by the hypercoagulability that is common in severe acute pancreatitis.
This study has several limitations. First, as a retrospective study, a certain number of patients might have been missed during follow-up, which reduces the estimated incidence of sinistral portal hypertension. Second, although we were unable to find an association between the development of sinistral portal hypertension and early anticoagulation, further prospective studies are needed to clarify whether other interventions, such as early drainage of the pancreatic collection, can reduce the risk of sinistral portal hypertension. Despite these limitations, we identified risk factors for sinistral portal hypertension in the setting of severe acute pancreatitis in one of the largest study cohorts to date. Our study provides an account of the natural history of sinistral portal hypertension and a valuable assessment of the risk factors, which are useful for advancing awareness and prevention of sinistral portal hypertension in selected patients.
Conclusions
Sinistral portal hypertension is commonly asymptomatic and occurs in moderate or severe acute pancreatitis and has a male preponderance. Hypertriglyceridemia, hyperglycemia, infection of walled-off pancreatic necrosis, recurrent relapse, and smoking are all independent predictors of sinistral portal hypertension. In this study, there was no association between early anticoagulation and sinistral portal hypertension. Sinistral portal hypertension was a delayed complication of acute pancreatitis, and prolonged monitoring during follow-up is suggested in cases of moderate or severe acute pancreatitis with the risk factors identified in this study.
Footnotes
Source of support: Departmental sources
References
- 1.Moossa AR, Gadd MA. Isolated splenic vein thrombosis. World J Surg. 1985;9:384–90. doi: 10.1007/BF01655272. [DOI] [PubMed] [Google Scholar]
- 2.Madsen MS, Petersen TH, Sommer H. Segmental portal hypertension. Ann Surg. 1986;204:72–77. doi: 10.1097/00000658-198607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito K, Kudo A, Nakamura N, et al. Left-sided portal hypertension caused by serous cystadenoma of the pancreas: Report of a case. Surg Today. 2008;38:184–87. doi: 10.1007/s00595-007-3600-y. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes A, Almeida N, Ferreira AM, et al. Left-sided portal hypertension: A sinister entity. GE Port J Gastroenterol. 2015;22:234–39. doi: 10.1016/j.jpge.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakorafas GH, Sarr MG, Farley DR, Farnell MB. The significance of sinistral portal hypertension complicating chronic pancreatitis. Am J Surg. 2000;179:129–33. doi: 10.1016/s0002-9610(00)00250-6. [DOI] [PubMed] [Google Scholar]
- 6.Butler JR, Eckert GJ, Zyromski NJ, et al. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HBP (Oxford) 2011;13:839–45. doi: 10.1111/j.1477-2574.2011.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heider TR, Azeem S, Galanko JA, Behrns KE. The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg. 2004;239:876–82. doi: 10.1097/01.sla.0000128685.74686.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal AK, Raj Kumar K, Agarwal S, Singh S. Significance of splenic vein thrombosis in chronic pancreatitis. Am J Surg. 2008;196:149–54. doi: 10.1016/j.amjsurg.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Flati G, Andrén-Sandberg A, La Pinta M, et al. Potentially fatal bleeding in acute pancreatitis: pathophysiology, prevention, and treatment. Pancreas. 2003;26:8. doi: 10.1097/00006676-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Stabile BE, Wilson SE, Debas HT. Reduced mortality from bleeding pseudocysts and pseudoaneurysms caused by pancreatitis. Arch Surg. 1983;118:45–51. doi: 10.1001/archsurg.1983.01390010035009. [DOI] [PubMed] [Google Scholar]
- 11.Xu W, Qi X, Chen J, Su C, Guo X. Prevalence of splanchnic vein thrombosis in pancreatitis: A systematic review and meta-analysis of observational studies. Gastroenterol Res Pract Gastroenterol Res Pract. 2015;2015 doi: 10.1155/2015/245460. 245460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 13.Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–82. doi: 10.1053/j.gastro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Isayama H, Nakai Y, Rerknimitr R, et al. Asian consensus statements on endoscopic management of walled-off necrosis Part 1: Epidemiology, diagnosis, and treatment. J Gastroenterol Hepatol. 2016;31:1546–54. doi: 10.1111/jgh.13394. [DOI] [PubMed] [Google Scholar]
- 16.Tsuang W, Navaneethan U, Ruiz L, et al. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104:984–91. doi: 10.1038/ajg.2009.27. [DOI] [PubMed] [Google Scholar]
- 17.Sharp HT. The acute abdomen during pregnancy. Clin Obstet Gynecol. 2002;45:405–13. doi: 10.1097/00003081-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Toqué L, Hamy A, Hamel JF, et al. Predictive factors of splanchnic vein thrombosis in acute pancreatitis: A 6-year single-center experience. J Dig Dis. 2015;16:734–40. doi: 10.1111/1751-2980.12298. [DOI] [PubMed] [Google Scholar]
- 19.Mortele KJ, Mergo PJ, Taylor HM, et al. Peripancreatic vascular abnormalities complicating acute pancreatitis: Contrast-enhanced helical CT findings. Eur J Radiol. 2004;52:67–72. doi: 10.1016/j.ejrad.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Dorffel T, Wruck T, Ruckert RI, et al. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas. 2000;21:126–33. doi: 10.1097/00006676-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Ke L, Tong Z, et al. Risk factors and outcome of splanchnic venous thrombosis in patients with necrotizing acute pancreatitis. Thromb Res. 2015;135:68–72. doi: 10.1016/j.thromres.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Evans GR, Yellin AE, Weaver FA, Stain SC. Sinistral (left-sided) portal hypertension. Am Surg. 1990;56:758–63. [PubMed] [Google Scholar]
- 23.Johnston FR, Myers RT. Etiologic factors and consequences of splenic vein obstruction. Ann Surg. 1973;177:736–39. doi: 10.1097/00000658-197306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little AG, Moossa AR. Gastrointestinal hemorrhage from left-sided portal hypertension. An unappreciated complication of pancreatitis. Am J Surg. 1981;141:153–58. doi: 10.1016/0002-9610(81)90029-5. [DOI] [PubMed] [Google Scholar]
- 25.Keith RG, Mustard RA, Jr, Saibil EA. Gastric variceal bleeding due to occlusion of splenic vein in pancreatic disease. Can J Surg. 1982;25:301–4. [PubMed] [Google Scholar]
- 26.Harris S, Nadkarni NA, Naina HV, Vege SS. Splanchnic vein thrombosis in acute pancreatitis: A single-center experience. Pancreas. 2013;42:1251–54. doi: 10.1097/MPA.0b013e3182968ff5. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Ke L, Yang D, et al. Predicting the clinical manifestations in necrotizing acute pancreatitis patients with splanchnic vein thrombosis. Pancreatology. 2016;16:973–78. doi: 10.1016/j.pan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Roach RE, Cannegieter SC, Lijfering WM. Differential risks in men and women for first and recurrent venous thrombosis: The role of genes and environment. J Thromb Haemost. 2014;12:1593–600. doi: 10.1111/jth.12678. [DOI] [PubMed] [Google Scholar]
- 29.Rebours V, Boudaoud L, Vullierme MP, et al. Extrahepatic portal venous system thrombosis in recurrent acute and chronic alcoholic pancreatitis is caused by local inflammation and not thrombophilia. Am J Gastroenterol. 2012;107(10):1579–85. doi: 10.1038/ajg.2012.231. [DOI] [PubMed] [Google Scholar]
- 30.Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: A Danish follow-up study. J Thromb Haemost. 2009;7:1297–303. doi: 10.1111/j.1538-7836.2009.03490.x. [DOI] [PubMed] [Google Scholar]
- 31.Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: A prospective multicenter follow-up study. Hepatology. 2010;51:210–18. doi: 10.1002/hep.23259. [DOI] [PubMed] [Google Scholar]
- 32.De Stefano V, Martinelli I. Splanchnic vein thrombosis: Clinical presentation, risk factors and treatment. Int Emerg Med. 2010;5:487–94. doi: 10.1007/s11739-010-0413-6. [DOI] [PubMed] [Google Scholar]