Abstract
Background
The objective of this study was to detect the level of olfactory function in patients with systemic lupus erythematosus (SLE) and to explore the relationship between impaired olfactory function and anti-ribosomal P protein antibody (ARPA), disease duration, and age.
Material/Methods
The level of olfactory function in 65 patients with SLE and 50 healthy participants was detected using the Connecticut Chemosensory Clinical Research Center (CCCRC) method; serum ARPA levels in SLE patients and the healthy control group were detected by enzyme-linked immunosorbent assay (ELISA).
Results
CCCRC scores in the active SLE group was lower than that in the inactive SLE and healthy control groups (P<0.01). In SLE patients, the CCCRC scores of ARPA-positive patients were lower than those of ARPA-negative patients (P<0.01). A negative correlation was discovered between CCCRC scores and ARPA serum levels in SLE patients. Multiple linear regression analyses showed a correlation among the CCCRC score, age, and ARPA.
Conclusions
Olfactory dysfunction was found in patients with active SLE; which correlated with SLE disease activity and ARPA levels.
MeSH Keywords: Autoantibodies; Lupus Erythematosus, Systemic; Olfactory Pathways
Background
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect multiple organs such as skin, mucosa, kidneys, and blood as well as the musculoskeletal and nervous systems. Neuropsychiatric SLE (NPSLE) is a particularly serious condition associated with poor quality of life and high mortality among patients with central nervous system involvement. Shoenfeld et al. [1] showed a decrease in the sense of smell in SLE patients compared with healthy people; moreover, the olfactory disruption among NPSLE patients correlated with disease activity and central nervous system involvement. More than 20 non-organ-specific autoantibodies, such as anti-dsDNA and anti-histone, along with anti-extractable nuclear antigens (e.g., anti-Sm, anti-RNP, anti-Ro/SSA, and La/SSB antibodies) can be found in the serum of SLE patients. Disruption to olfactory function is common in brain disease. Bonfa et al. first reported that anti-ribosomal P protein antibody (ARPA) is associated with NPSLE [2]. Therefore, understanding the relationship between the condition of SLE patients and changes in olfactory function and ARPA is crucial.
Therefore, this study was designed to test olfactory function in SLE patients and preliminarily discussed the correlation between olfactory function and anti-ribosomal P protein antibody, disease duration and age.
Material and Methods
Participants
Sixty-five patients with SLE seen at the Department of Rheumatology, Qilu Hospital, Shandong University were included in the study, including 7 males and 58 females. Their ages ranged from 14 to 65 years, with an average of 33.0±11.6 years; the disease duration ranged from 0.2 to 16 years, with an average of 3.3±3.9 years. The diagnosis of SLE was in line with the SLE Classification Criteria revised by the American College of Rheumatology (ACR) in 1997 [3]. The SLE disease activity scale (SLEDAI) was used to evaluate disease activity in SLE patients. Among the participants, 31 patients were in an active stage (SLEDAI ≥10) [4,5] and 34 patients were in an inactive stage (SLEDAI ≤9). The 50 healthy volunteers were included as a control group, with ages between 20 and 66 years and an average age of 35.8±11.7 years, and included 5 males and 45 females. The healthy controls were matched for sex and demographic background. The characteristics of the SLE group and healthy control group are shown in Table 1. The following diseases that may affect olfactory senses were excluded: obstruction; lesion or surgery of nasal cavity; upper respiratory tract infection within the past 2 weeks; brain trauma or tumor; history of allergies; endocrine disorders or active hepatitis; neuropsychiatric disorders that may affect olfaction such as Parkinson’s disease or schizophrenia; neuropsychiatric lupus; and female menstruation or gestation. The study was approved by the local ethics committee (No. KYLL2015011), and all participants signed the informed consent form.
Table 1.
The demographic and clinical characteristics of SLE patients and healthy controls.
| Items | SLE patients | Healthy controls |
|---|---|---|
| N | 65 | 50 |
| Age, year | 33.0±11.6 | 35.8±11.7 |
| Female, n (%) | 58 (89.2) | 45 (90.0) |
| Male, n (%) | 7 (10.8) | 5 (10.0) |
| Active stage (SLEDAI ≥10), n (%) | 31 (47.7) | – |
| Inactive stage (SLEDAI ≤9), n (%) | 34 (52.3) | – |
| Serositis, n (%) | 13 (20.0) | – |
| Arthritis, n (%) | 24 (36.9) | – |
| Lupus nephritis, n (%) | 28 (43.1) | – |
| Hematologic disorders, n (%)* | 29 (44.6) | – |
SLE – systemic lupus erythematosus.
Leukopenia <4000/L and/or thrombocytopenia <100 000/L).
Olfactory test method
The Connecticut Chemosensory Clinical Research Center (CCCRC) test was implemented to test olfactory function [6,7]. The olfactory test was carried out in a ventilated and quiet room. Neither the test implementers nor the participants used odor-laden cleaning products. The CCCRC test kit consist of odor threshold detection and identification test. Detection threshold was measured using 9 serial dilutions of butanol in nanopure-deionized water. Each concentration was presented along with a water control. Threshold was defined as the dilution at which the butanol bottle was correctly identified in 4 consecutive trials. If the water bottle was incorrectly selected in less than 4 trials, the next higher concentration step was tested in a similar fashion. The CCCRC identification test was composed of 7 olfactory stimuli, including talcum powder, chocolate, cinnamon, star anise, camphor, peanut butter, and soap (coffee in the standard CCCRC test method was replaced by star anise because coffee is not popular among middle-aged and elderly women in China). Ammonia was also presented to test trigeminal nerve nasal sensation but are not included in calculating the olfactory function test score. The odor stimuli were placed in jars, and the patients was asked to select the stimulus name from a list of odors. The number of olfactory stimuli correctly identified determined the identification score. The composite CCCRC test score (a maximum of 100 points) was calculated by adding the threshold score (a maximum of 50 points) and identification score (a maximum of 50 points).
ARPA enzyme-linked immunosorbent assay (ELISA)
A peripheral blood sample was collected on the day of olfactory function examination from all participants, and then stored at −20°C until analyzed. ARPA was evaluated with ELISA and a commercial kit provided by Euroimmun Medical Diagnostics, Inc. for in vitro testing. Following the kit’s instructions, the test results were explained as follows: a result ≥20 RU/mL was considered positive; if the result was <20 RU/mL, it was considered negative.
Statistical analysis
All the statistical calculations were performed using SPSS 20.0 software. All numerical data were expressed as mean ± standard deviation; a pairwise comparison of each group’s CCCRC scores and serum ARPA levels was analyzed by an independent sample t-test, and the correlation between the variables was tested using Pearson’s correlation and multiple linear regression analyses. The count data were represented by the number and percentage of cases, and a comparison between the groups was performed using χ2 test. P<0.05 were considered significant.
Results
Impaired olfactory function in active SLE patients
The CCCRC scores of SLE patients in the active group (62.4±15.8) was lower than that in the inactive SLE group (79.7±13.0) and healthy control group (83.4±11.7), and the difference between the active SLE group and healthy control group was statistically significant (P<0.01); the inactive group was compared to the healthy control group, and the difference was not statistically significant (P=0.226).
Impaired olfactory function in APRA-positive SLE patients
The serum ARPA expression in SLE patients (32.47±70.01 U/mL) was significantly higher than that in the healthy control group (2.42±1.37 U/mL), and the difference was statistically significant (P=0.000). Therein, 16 patients in the SLE group were ARPA-positive, with a positivity rate of 25%; 49 patients were ARPR-negative, with a negativity rate of 75%; the healthy control group was negative. The difference in positive rates between the SLE group and the healthy control group was statistically significant (P=0.01). The CCCRC score (61.3±16.7) of the ARPA-positive group was significantly lower than that of the negative group (73.7±15.9), and the difference was statistically significant (P=0.009).
Correlation analysis of olfactory function and ARPA, age, and disease duration in SLE patients
There was a negative correlation between olfactory function and age in the healthy control group (r=0.505; P<0.001). However, the correlation between olfactory function and age in the SLE patients was lower than that in the healthy control group (r=−0.338; r=−0.505, P<0.01, respectively); there was a negative correlation between olfactory function and ARPA in SLE patients (r=−0.327, P=0.008, Figure 1); there was no correlation between olfactory function and disease duration in SLE patients (r=0.141, P>0.05). The effects of age (X1) and ARPA (X2) on CCCRC (Y) were obtained by a multiple linear regression analysis; the linear regression equation was Y=90.808–0.529X1–0.185X2, (F=9.696, P<0.01) (Table 2).
Figure 1.
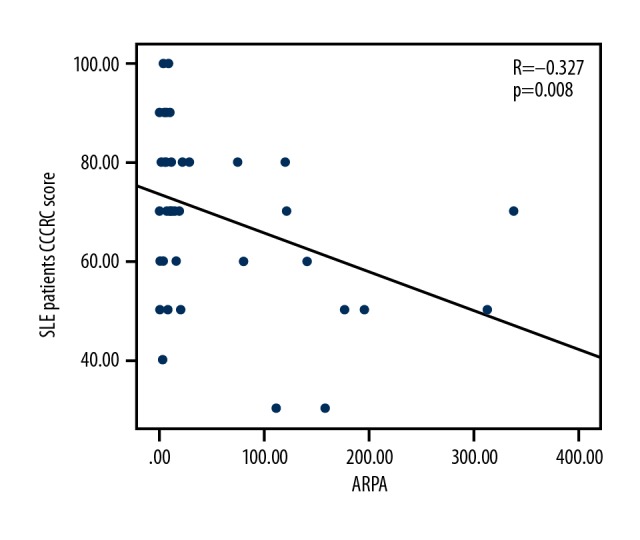
Correlation between CCCRC scores and ARPA levels in SLE patients (n=65). The correlation between CCCRC score and ARPA levels in SLE patients was not strong (r=−0.327, P=0.008). SLE – systemic lupus erythematosus; CCCRC – Connecticut Chemosensory Clinical Research Center; ARPA – anti-ribosomal P protein antibody.
Table 2.
Index of multiple linear correlations for CCCRC score, age, and ARPA.
| Item | B value | Standard coefficient | t value | P value |
|---|---|---|---|---|
| Constant | 90.808 | 15.732 | 0.000 | |
| Age (X1) | −0.529 | −0.363 | −3.267 | 0.002 |
| ARPA(X2) | −0.185 | −0.353 | −3.178 | 0.002 |
CCCRC – Connecticut Chemosensory Clinical Research Center; ARPA – anti-ribosomal P protein antibody.
Discussion
SLE is an autoimmune disease that can lead to multiple system involvement. In recent years, research has found that there is a close relationship between olfaction and emotions, neuropsychiatric disorders, and autoimmune diseases. Our study focused on changes in olfactory function and associated factors in SLE patients.
Shoenfeld et al. [1] reported dysosmia in SLE patients; their test results showed that 46% of SLE patients had heterosmia, which was significantly higher than the 25% observed in the control group, and dysosmia was related to disease activity and neuropsychiatric SLE. Bombini et al. [8] found a higher probability of olfactory abnormalities among SLE patients than among healthy controls, with olfactory abnormalities associated with disease activity, age, and positivity for anti-P antibody. Our results were consistent with the results of previous studies. Cavaco et al. [9] found that olfactory abnormalities were only observed in NPSLE patients and that patients without NPSLE had no abnormalities in their sense of smell. Our study found that patients without NPSLE also experienced significant olfactory impairment. Smell recognition has important cultural aspects, which may be one of the reasons for the observed impact. In addition, although the patients we studied did not have NPSLE, a significant proportion of the patients had elevated SLEDAI scores; prior studies have identified high SLEDAI scores as a factor related to olfactory impairment. Previous studies have indicated that olfactory impairment has no association with course of disease, and one investigation found that the olfactory function of SLE patients remained stable for 2 years, a result consistent with the findings of our study. We conducted a preliminary study on dysosmia in SLE patients. This experiment examined the olfactory function of SLE patients and explored the factors that may cause heterosmia. The olfactory system can be evaluated through several methods [7]. CCCRC test method chosen in our study was simple and easy to operate, the time to answer was short, and the odorants used were well known by the participants. Diseases that may affect olfaction were excluded when the participants were screened, and environmental conditions affecting the participant’s judgement of the odorants were also avoided as much as possible during testing. Due to the limited number of available cases, the effects of menopause, menstrual cycle, and ovulation on olfaction were not considered in this experiment. The experimental results showed that the olfactory function of the active SLE group was significantly lower than that of the inactive SLE group and the healthy control group. This result was consistent with previous studies, suggesting that active SLE patients have heterosmia and that dysosmia may be one of the manifestations of active SLE. However, whether dysosmia can be used as an evaluation index of SLE activity requires further research to confirm.
It has been reported in the literature that injection of ARPA into the lateral ventricle of female C3H mice can cause dysosphresia [10]. SLE patients with olfactory dysfunction had more frequent anti-P antibodies when compared to SLE patients without olfactory impairment [8]. Some studies have shown that the positive ARPA rate found in SLE patients in active disease was higher than that in patients in a stable stage [11,12], and the rate of heterosmia in the active disease stage was also higher than that found in patients with stable disease [1]. In view of the aforementioned animal and clinical research results, it can be speculated that ARPA may be related to heterosmia in SLE patients. In our study, the positive rate of serum ARPA in SLE patients was 24.6%; the olfactory function of the ARPA-positive and the ARPA-negative group were compared, and the olfactory scores in the positive group were lower than that in the negative group. This difference was statistically significant. The ARPA-positive rate in this group of SLE patients was consistent with previous studies [13], but the small sample size may reduce the reliability of the results. It was found that when discussing the correlation between serum levels of ARPA and olfactory scores in SLE patients, the CCCRC scores of SLE patients were only slightly negatively correlated with serum ARPA levels. Although ARPA may be related to heterosmia, there was no close correlation between the 2, which may be related to the low number of ARPA-positive cases, showing that the olfactory factors affecting SLE patients are complicated and need further study.
There exist limitations of our study. Both animal models and human studies have suggested that olfactory impairment in SLE may be associated with central nervous system involvement. In a mouse model of NPSLE established via direct injection of antibodies into mouse brains, mice developed depressive-like behavior and a decreased ability to smell menthol [10,14]. Previous studies have found more significant impairment of olfactory function in SLE patients with central nervous system involvement or NPSLE. Neuropsychiatric SLE is also closely correlated with ARPA [14,15]. However, our study did not include patients with NPSLE. According to ACR standards [16], patients with NPSLE are heterogeneous; their conditions involve not only the central nervous system but also the peripheral nervous system. Olfactory tests require complex skills, including cognitive and verbal processing. It is difficult for us to enroll a sufficient number of homogeneous NPSLE patients for analysis. We will further study heterosmia in patients with neuropsychiatric SLE and its relationship with ARPA in the future. In healthy people, olfaction decreases with age, and there is a significant negative correlation between these 2 factors. In our study, the relationship between olfaction and age in SLE patients was not as close as that in normal people. This finding may be due to the general decline in olfaction caused by SLE; the trend that olfaction decreases with age was not significant.
Conclusions
In conclusion, our study found that patients with highly active SLE had impaired olfactory function. There was no significant difference in olfactory function between SLE patients with relatively low SLEDAI scores and healthy controls. ARPA-positive SLE patients had lower olfactory CCCRC scores than ARPA-negative patients. Olfactory function score was negatively correlated with ARPA titer and age, regardless of the course of disease.
Abbreviations
- ACR
American College of Rheumatology
- ARPA
anti-ribosomal P protein antibody
- CCCRC
Connecticut Chemosensory Clinical Research Center
- ELISA
enzyme-linked immunosorbent assay
- NPSLE
Neuropsychiatric systemic lupus erythematosus
- SLE
systemic lupus erythematosus
Footnotes
Source of support: Departmental sources
References
- 1.Shoenfeld N, Agmon-Levin N, Flitman-Katzevman I, et al. The sense of smell in systemic lupus erythematosus. Arthritis Rheum. 2009;60(5):1484–87. doi: 10.1002/art.24491. [DOI] [PubMed] [Google Scholar]
- 2.Bonfa E, Golombek SJ, Kaufman LD, et al. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987;317:265–71. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 4.Lau CS, Yuen KY, Chan KH, Wong RW. Lack of evidence of active lytic replication of Epstein-Barr and cytomegaloviruses in patients with systemic lupus erythematosus. Chin Med J (Engl) 1998;111(7):660–65. [PubMed] [Google Scholar]
- 5.Lee CS, Hu CY, Tsai HF, et al. Elevated serum decoy receptor 3 with enhanced T cell activation in systemic lupus erythematosus. Clin Exp Immunol. 2008;151(3):383–90. doi: 10.1111/j.1365-2249.2007.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cain WS. Testing olfaction in a clinical setting. Ear Nose Throat J. 1989;68(4):316, 322–28. [PubMed] [Google Scholar]
- 7.Doty RL. Olfactory dysfunction and its measurement in the clinic and workplace. Int Arch Occup Environ Health. 2006;79(4):268–82. doi: 10.1007/s00420-005-0055-6. [DOI] [PubMed] [Google Scholar]
- 8.Bombini MF, Peres FA, Lapa AT, et al. Olfactory function in systemic lupus erythematosus and systemic sclerosis. A longitudinal study and review of the literature. Autoimmun Rev. 2018;17(4):405–12. doi: 10.1016/j.autrev.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Cavaco S, Martins da Silva A, Santos E, et al. Are cognitive and olfactory dysfunctions in neuropsychiatric lupus erythematosus dependent on anxiety or depression? J Rheumatol. 2012;39(4):770–76. doi: 10.3899/jrheum.110574. [DOI] [PubMed] [Google Scholar]
- 10.Katzav A, Ben-Ziv T, Chapman J, et al. Anti-P ribosomal antibodies induce defect in smell capability in a model of CNS -SLE (depression) J Autoimmun. 2008;31(4):393–98. doi: 10.1016/j.jaut.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Uchiumi T, Ozawa T, Kikuchi M, et al. Autoantibodies against ribosomal proteins found with high frequency in patients with systemic lupus erythematosus with active disease. J Rheumatol. 1991;18(11):1681–84. [PubMed] [Google Scholar]
- 12.Massardo L, Burgos P, Martínez ME, et al. Antiribosomal P protein antibodies in Chilean SLE patients: No association with renal disease. Lupus. 2002;11(6):379–83. doi: 10.1191/0961203302lu209oa. [DOI] [PubMed] [Google Scholar]
- 13.Arnett FC, Reveille JD, Moutsopoulos HM, et al. Ribosomal P autoantibodies in systemic lupus erythematosus. Frequencies in different ethnic groups and clinical and immunogenetic associations. Arthritis Rheum. 1996;39(11):1833–39. doi: 10.1002/art.1780391109. [DOI] [PubMed] [Google Scholar]
- 14.Katzav A, Solodeev I, Brodsky O, et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 2007;56(3):938–48. doi: 10.1002/art.22419. [DOI] [PubMed] [Google Scholar]
- 15.Schneebaum AB, Singleton JD, West SG, et al. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am J Med. 1991;90(1):54–62. doi: 10.1016/0002-9343(91)90506-s. [DOI] [PubMed] [Google Scholar]
- 16.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]


